Myrosin Cells and Myrosinase Expression Pattern in Nasturtium (Tropaeolum majus L.)
Abstract
1. Introduction
2. Materials and Methods
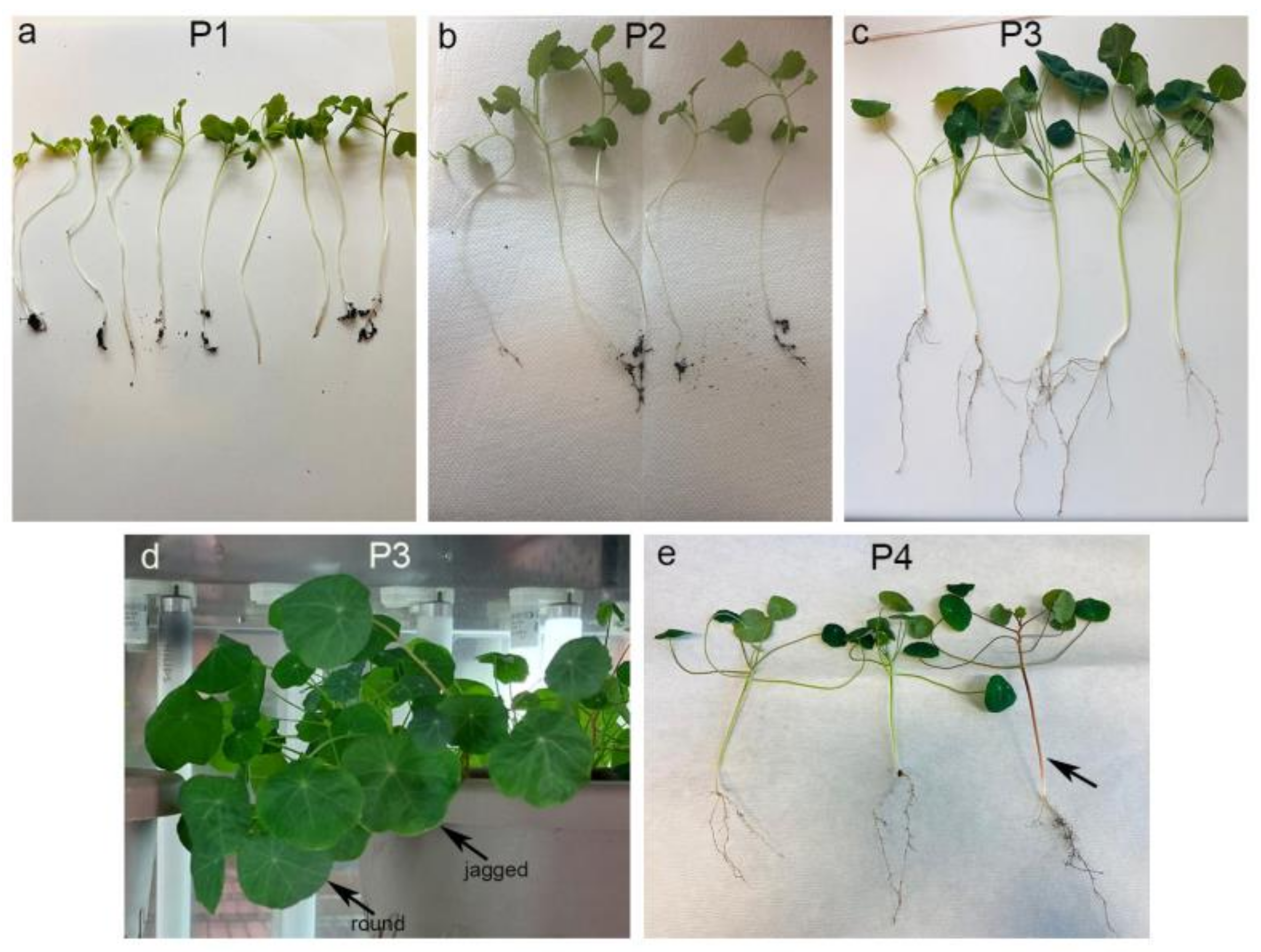
2.1. Planting and Growing of Nasturtium
2.2. Immunofluorescence and Immunohistochemistry Procedures
2.2.1. Immunofluorescence Staining
2.2.2. Immunohistochemical Staining
2.3. Statistical Analysis
2.4. Transmission Electron Microscopy (TEM) Procedure
3. Results
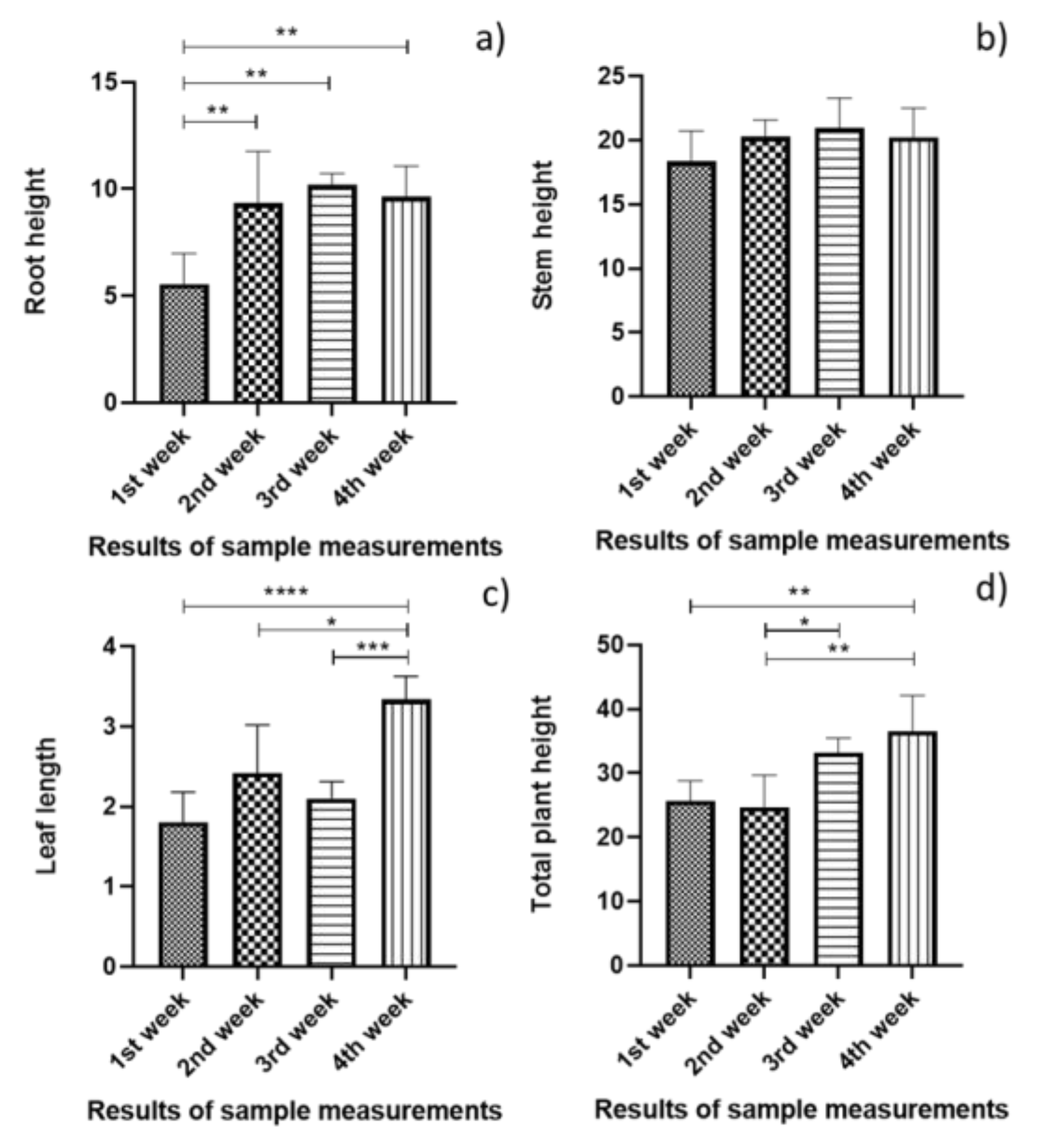
3.1. Statistical Analysis of the Plant Growth Measurement
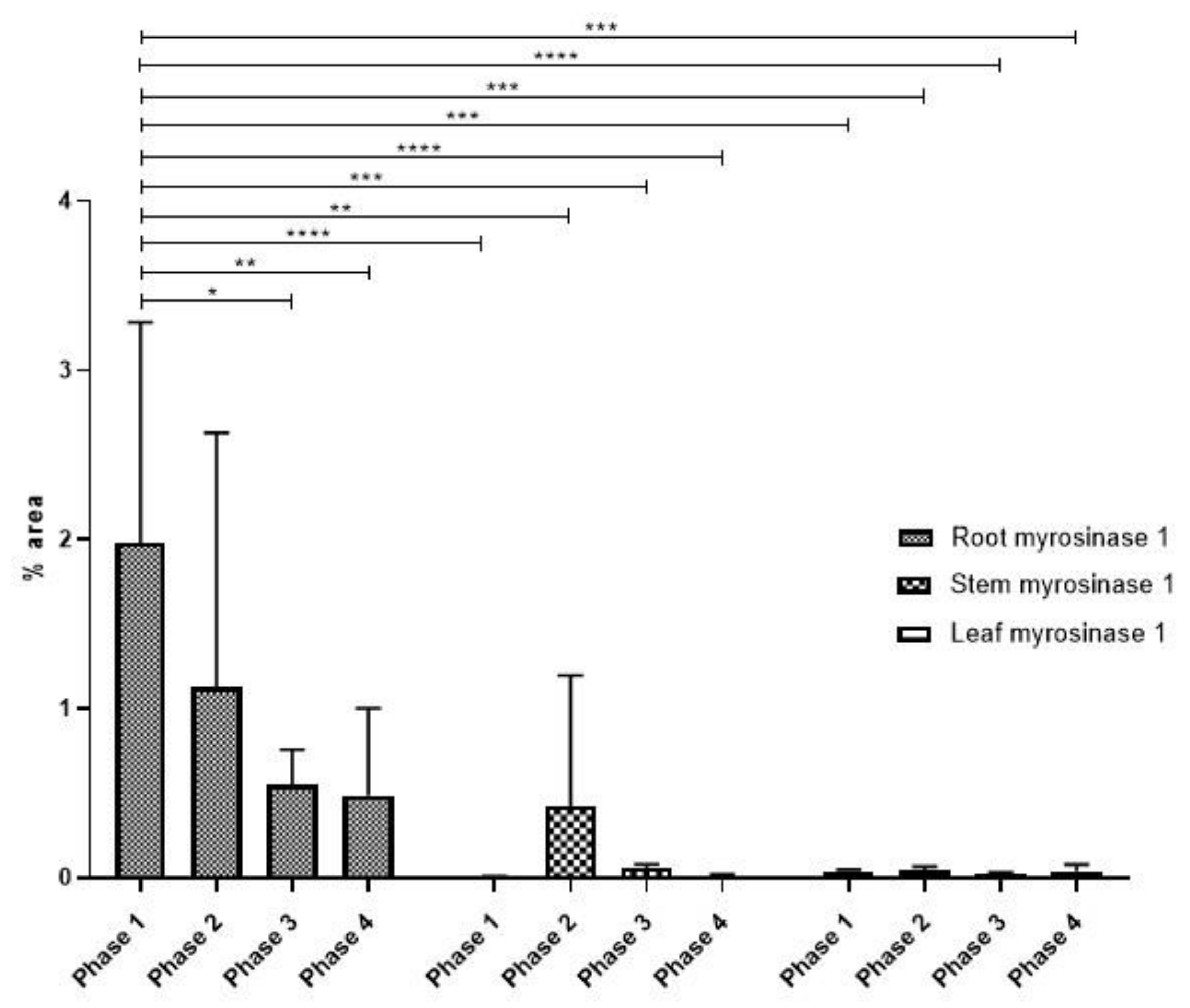
3.2. Myrosinase Type 1 Expression Pattern
Statistical Analysis of Myrosinase Type I Expression
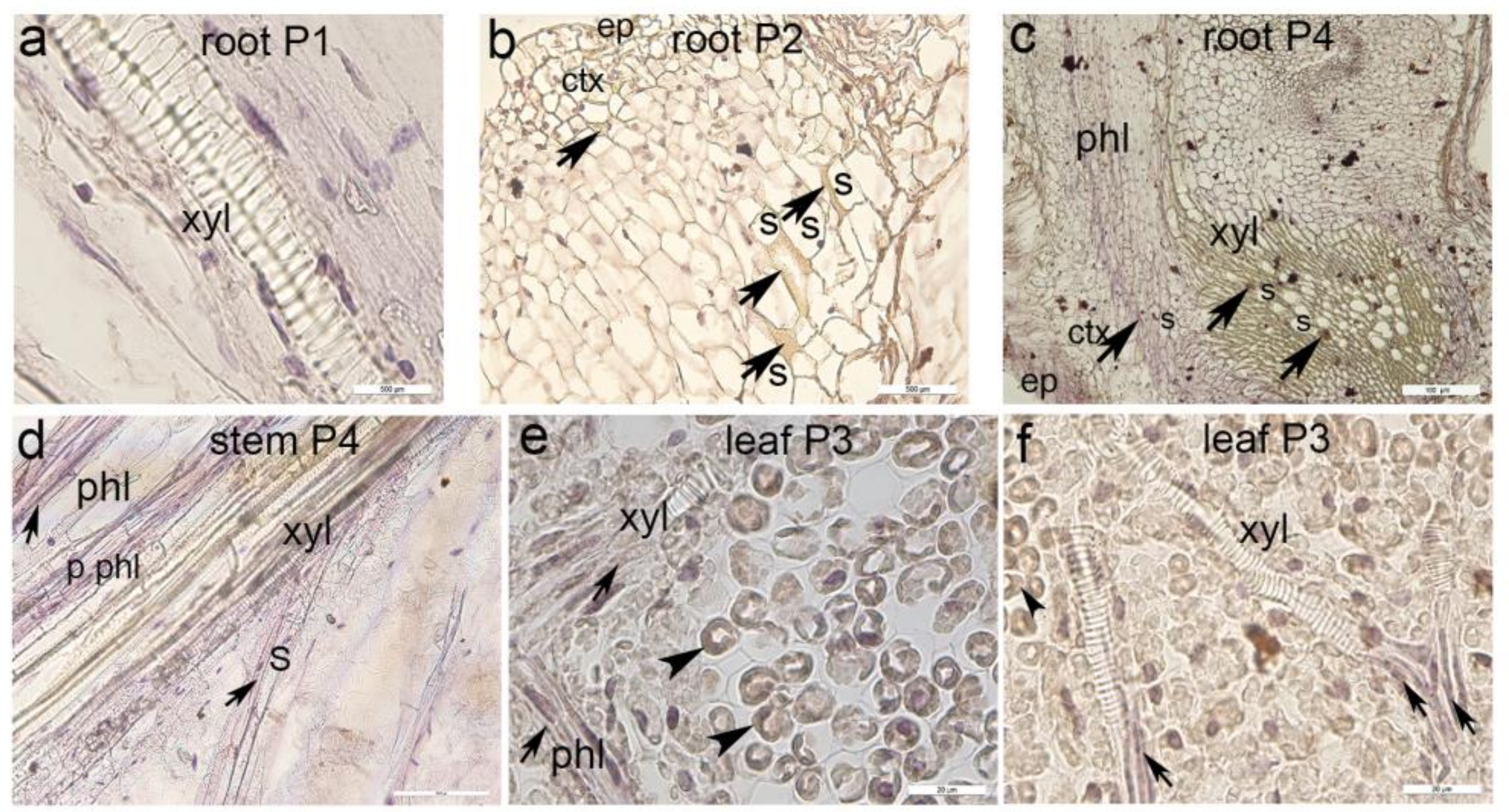
3.3. Immunohistochemical Staining of Myrosinase Type 1
3.4. Ultrastructure of Myrosin Cells in Nasturtium
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boligon, A.A.; Janovik, V.; Boligon, A.A.; Pivetta, C.R.; Pereira, R.P.; da Rocha, J.B.T.; Athayde, M.L. HPLC Analysis of Polyphenolic Compounds and Antioxidant Activity in Nasturtium officinale. Int. J. Food Prop. 2013, 16, 61–69. [Google Scholar] [CrossRef]
- Brondani, J.C.; Cuelho, C.H.F.; Marangoni, L.D.; de Lima, R.; Guex, C.G.; Bonilha, I.D.; Manfron, M.P. Traditional usages, botany, phytochemistry, biological activity and toxicology of Tropaeolum majus L.—A review. Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 264–273. [Google Scholar]
- Garzón, G.A.; Wrolstad, R.E. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Janda, K.; Watychowicz, K.; Lukasiak, J.; Wolska, J. Garden nasturtium (Tropaeolum majus L.)—A source of mineral elements and bioactive compounds. Rocz. Panstw. Zakl. Hig. 2018, 69, 119–126. [Google Scholar] [PubMed]
- Vrca, I.; Ramic, D.; Fredotovic, Z.; Mozina, S.S.; Blazevic, I.; Bilusic, T. Chemical composition and biological activity of essential oil and extract from the seeds of Tropaeolum majus L. var. altum. Food Technol. Biotech 2022, 60, 533–542. [Google Scholar] [CrossRef]
- Andréasson, E.; Jorgensen, L.B.; Höglund, A.S.; Rask, L.; Meijer, J. Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol. 2001, 127, 1750–1763. [Google Scholar] [CrossRef]
- Niu, Y.X.; Rogiewicz, A.; Wan, C.Y.; Guo, M.; Huang, F.H.; Slominski, B.A. Effect of microwave treatment on the efficacy of expeller pressing of Brassica napus rapeseed and Brassica juncea mustard seeds. J. Agric. Food Chem. 2015, 63, 3078–3084. [Google Scholar] [CrossRef]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Cottaz, S.; Rollin, P.; Vasella, A.; Henrissat, B. High resolution x-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J. Biol. Chem. 2000, 275, 39385–39393. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The ‘mustard oil bomb’: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Angelino, D.; Dosz, E.B.; Sun, J.; Hoeflinger, J.L.; Van Tassell, M.L.; Chen, P.; Harnly, J.M.; Miller, M.J.; Jeffery, E.H. Myrosinase-dependent and –independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front. Plant Sci. 2015, 6, 831. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.; Thomas, R.M.; Palmieri, S.; Luisi, P.L. An improved method for the purification of myrosinase and its physicochemical characterization. Arch. Biochem. Biophys. 1990, 280, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.P.; Knol, W.; Bol, J. Glucosinolate degradation by Aspergillus clavatus and Fusarium oxysporum in liquid and solid-state fermentation. Appl. Microbiol. Biotechnol. 1993, 38, 696–701. [Google Scholar] [CrossRef]
- Tierens, K.; Thomma, B.P.H.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Cammue, B.P.A.; Broekaert, W.F. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef]
- Francis, F.; Lognay, G.; Wathelet, J.P.; Haubruge, E. Characterisation of aphid myrosinase and degradation studies of glucosinolates. Arch. Insect Biochem. Physiol. 2002, 50, 173–182. [Google Scholar] [CrossRef]
- Husebye, H.; Chadchawan, S.; Winge, P.; Thangstad, O.P.; Bones, A.M. Guard cell- and phloem idioblast-specific expression of thioglucoside glucohydrolase 1 (myrosinase) in Arabidopsis. Plant Physiol. 2002, 128, 1180–1188. [Google Scholar] [CrossRef]
- Bones, A.M.; Thangstad, O.P.; Haugen, O.A.; Espevik, T. Fate of myrosin cells: Characterization of monoclonal antibodies against myrosinase. J. Exp. Bot. 1991, 42, 1541–1549. [Google Scholar] [CrossRef]
- Shirakawa, M.; Hara-Nishimura, I. Specialized vacuoles of myrosin cells: Chemical defense strategy in Brassicales plants. Plan. Cell Physiol. 2022, 59, 1309–1316. [Google Scholar] [CrossRef]
- Thangstad, O.P.; Gilde, B.; Chadchawan, S.; Seem, M.; Husebye, H.; Bradley, D.; Bones, A.M. Cell specific, cross-species expression of myrosinases in Brassica napus, Arabidopsis thaliana and Nicotiana tabacum. Plant Mol. Biol. 2004, 54, 597–611. [Google Scholar] [CrossRef]
- Rask, L.; Andréasson, E.; Ekbom, B.; Eriksson, S.; Pontoppidan, B.; Meijer, J. Myrosinase: Gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 2000, 42, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, O.A.; Davies, A.; Deeken, R.; Thorpe, M.R.; Tomos, A.D.; Hedrich, R. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol. 2000, 124, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, A.S.; Lenman, M.; Falk, A.; Rask, L. Distribution of myrosinase in rapeseed tissues. Plant Physiol. 1991, 95, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Bones, A.; Rossiter, J.T. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta 1998, 206, 370–377. [Google Scholar] [CrossRef]
- Thangstad, O.P.; Evjen, K.; Bones, A. Immunogold-EM localization of myrosinase in Brassicaceae. Protoplasma 1991, 161, 85–93. [Google Scholar] [CrossRef]
- Thangstad, O.P.; Iversen, T.H.; Slupphaug, G.; Bones, A. Immunocytochemical localization of myrosinase in Brassica napus L. Planta 1990, 180, 245–248. [Google Scholar] [CrossRef]
- Xue, J.P.; Jorgensen, M.; Pihlgren, U.; Rask, L. The myrosinase gene family in Arabidopsis thaliana—Gene organization, expression and evolution. Plant Mol. Biol. 1995, 27, 911–922. [Google Scholar] [CrossRef]
- Hara, M.; Fujii, Y.; Sasada, Y.; Kuboi, T. cDNA cloning of radish (Raphanus sativus) myrosinase and tissue-specific expression in root. Plant Cell Physiol. 2000, 41, 1102–1109. [Google Scholar] [CrossRef]
- Hoglund, A.S.; Lenman, M.; Rask, L. Myrosinase is localized to the interior of myrosin grains and is not associated to the surrounding tonoplast membrane. Plant Sci. 1992, 85, 165–170. [Google Scholar] [CrossRef]
- Bellostas, N.; Sorensen, J.C.; Sorensen, H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 2007, 87, 1586–1594. [Google Scholar] [CrossRef]
- Charron, C.S.; Sams, C.E. Glucosinolate content and myrosinase activity in rapid-cycling Brassica oleracea grown in a controlled environment. J. Am. Soc. Hortic. Sci. 2004, 129, 321–330. [Google Scholar] [CrossRef]
- ElSayed, S.T.; Jwanny, E.W.; Rashad, M.M.; Mahmoud, A.E.; Abdallah, N.M. Glycosidases in plant tissues of some Brassicaceae—Screening of different cruciferous plants for glycosidases production. Appl. Biochem. Biotechnol. 1995, 55, 219–230. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, Y.Y.; Zhu, X.W.; Liu, Z.; Gong, Y.Q.; Xu, L.; Gong, M.Y.; Liu, L.W. Molecular characterization and expression profiles of myrosinase gene (RsMyr2) in radish (Raphanus sativus L.). J. Integr. Agric. 2014, 13, 1877–1888. [Google Scholar] [CrossRef]
- Petersen, B.L.; Chen, S.X.; Hansen, C.H.; Olsen, C.E.; Halkier, B.A. Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 2002, 214, 562–571. [Google Scholar] [CrossRef]
- Kević, N.; Restović, I.; Bočina, I. Ultrastructural and immunofluorescence features of the epidermal cells and its secretory granules in the amphioxus Branchiostoma lanceolatum L. Period. Biol. 2023, 125, 35–42. [Google Scholar] [CrossRef]
- Restović, I.; Vučemilo, M.; Obad, M.; Kević, N.; Kelam, N.; Racetin, A.; Bočina, I. Expression of dendrin, neurofilament and glial fibrillary acidic protein in the brain of the dogfish Scyliorhinus canicula L. Period. Biol. 2023, 125, 43–55. [Google Scholar] [CrossRef]
- Racetin, A.; Filipović, N.; Lozić, M.; Ogata, M.; Gudelj Ensor, L.; Kelam, N.; Kovačević, P.; Watanabe, K.; Katsuyama, Y.; Saraga-Babić, M.; et al. A homozygous Dab1(-/-) is a potential novel cause of autosomal recessive congenital anomalies of the mice kidney and urinary tract. Biomolecules 2021, 11, 609. [Google Scholar] [CrossRef]
- Restović, I.; Vukojević, K.; Paladin, A.; Saraga-Babić, M.; Bočina, I. Immunohistochemical studies of cytoskeletal and extracellular matrix components in dogfish Scyliorhinus canicula L. notochordal cells. Anat. Rec. 2015, 298, 1700–1709. [Google Scholar] [CrossRef]
- Lozić, M.; Filipović, N.; Jurić, M.; Kosović, I.; Benzon, B.; Šolić, I.; Kelam, N.; Racetin, A.; Watanabe, K.; Katsuyama, Y.; et al. Alteration of Cx37, Cx40, Cx43, Cx45, Panx1, and renin mexpression patterns in postnatal kidneys of Dab1-/- (yotari) mice. Int. J. Mol. Sci. 2021, 22, 1284. [Google Scholar] [CrossRef]
- Restovic, I.; Vukojevic, K.; Saraga-Babic, M.; Bocina, I. Ultrastructural features of the dogfish Scyliorhinus canicula (Pisces: Scyliorhinidae) notochordal cells and the notochordal sheath. Ital. J. Zool. 2016, 83, 329–337. [Google Scholar] [CrossRef]
- Vitlov Uljević, M.; Starčević, K.; Mašek, T.; Bočina, I.; Restović, I.; Kević, N.; Racetin, A.; Kretzschmar, G.; Grobe, M.; Vukojević, K.; et al. Dietary DHA/EPA supplementation ameliorates diabetic nephropathy by protecting from distal tubular cell damage. Cell Tissue Res. 2019, 378, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Kleinwächter, M.; Schnug, E.; Selmar, D. The glucosinolate-myrosinase system in Nasturtium (Tropaeolum majus L.): Variability of biochemical parameters and screening for clones feasible for pharmaceutical utilization. J. Agric. Food Chem. 2008, 56, 11165–11170. [Google Scholar] [CrossRef] [PubMed]
- Vrca, I.; Jug, B.; Fredotovic, Z.; Vuko, E.; Brkan, V.; Sestic, L.; Juretic, L.; Dunkic, V.; Nazlic, M.; Ramic, D.; et al. Significant benefits of environmentally friendly hydrosols from Tropaeolum majus L. seeds with multiple biological activities. Plants 2023, 12, 3897. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Andreasson, E. Update on glucosinolate metabolism and transport. Plant Physiol. Biochem. 2001, 39, 743–758. [Google Scholar] [CrossRef]
- Chen, S.X.; Halkier, B.A. Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Protein Expr. Purif. 1999, 17, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Nintemann, S.J.; Hunziker, P.; Andersen, T.G.; Schulz, A.; Burow, M.; Halkier, B.A. Localization of the glucosinolate biosynthetic enzymes reveals distinct spatial patterns for the biosynthesis of indole and aliphatic glucosinolates. Physiol. Plant 2018, 163, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Rodman, J.E. A taxonomic analysis of glucosinolate-producing plants. Part 1: Phenetics. Syst. Bot. 1991, 16, 598–618. [Google Scholar] [CrossRef]
- Shirakawa, M.; Ueda, H.; Shimada, T.; Hara-Nishimura, I. Myrosin cells are differentiated directly from ground meristem cells and are developmentally independent of the vasculature in Arabidopsis leaves. Plant Signal. Behav. 2016, 11, e1150403. [Google Scholar] [CrossRef]
- Shirakawa, M.; Ueda, H.; Nagano, A.J.; Shimada, T.; Kohchi, T.; Hara-Nishimura, I. FAMA Is an essential component for the differentiation of two distinct cell types, myrosin cells and guard cells, in Arabidopsis. Plant Cell 2014, 26, 4039–4052. [Google Scholar] [CrossRef]
- Li, M.; Sack, F.D. Myrosin idioblast cell fate and development are regulated by the Arabidopsis transcription factor FAMA, the auxin pathway, and vesicular trafficking. Plant Cell 2014, 26, 4053–4066. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.; Iversen, T.H. Myrosin cells and myrosinase. Isr. J. Plant Sci. 1985, 34, 351–376. [Google Scholar]
- Hunziker, P.; Halkier, B.A.; Schulz, A. Arabidopsis glucosinolate storage cells transform into phloem fibres at late stages of development. J. Exp. Bot. 2019, 70, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.B. Myrosin cells and dilated cisternae of the endoplasmic reticulum in the order Capparales. Nord. J. Bot. 1981, 1, 433–445. [Google Scholar] [CrossRef]
- Bones, A.M. Distribution of β-thioglucosidase activity in intact plants, cell and tissue cultures and regenerated plants of Brassica napus L. J. Exp. Bot. 1990, 41, 737–744. [Google Scholar] [CrossRef]
- Andersson, D.; Chakrabarty, R.; Bejai, S.; Zhang, J.M.; Rask, L.; Meijer, J. Myrosinases from root and leaves of Arabidopsis thaliana have different catalytic properties. Phytochemistry 2009, 70, 1345–1354. [Google Scholar] [CrossRef]
- Shirakawa, M.; Tanida, M.; Ito, T. The cell differentiation of idioblast myrosin cells: Similarities with vascular and guard cells. Front. Plant Sci. 2022, 12, 829541. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restović, I.; Kević, N.; Kurić, L.; Bočina, I.; Vuko, E.; Vrca, I. Myrosin Cells and Myrosinase Expression Pattern in Nasturtium (Tropaeolum majus L.). Agronomy 2024, 14, 2108. https://doi.org/10.3390/agronomy14092108
Restović I, Kević N, Kurić L, Bočina I, Vuko E, Vrca I. Myrosin Cells and Myrosinase Expression Pattern in Nasturtium (Tropaeolum majus L.). Agronomy. 2024; 14(9):2108. https://doi.org/10.3390/agronomy14092108
Chicago/Turabian StyleRestović, Ivana, Nives Kević, Laura Kurić, Ivana Bočina, Elma Vuko, and Ivana Vrca. 2024. "Myrosin Cells and Myrosinase Expression Pattern in Nasturtium (Tropaeolum majus L.)" Agronomy 14, no. 9: 2108. https://doi.org/10.3390/agronomy14092108
APA StyleRestović, I., Kević, N., Kurić, L., Bočina, I., Vuko, E., & Vrca, I. (2024). Myrosin Cells and Myrosinase Expression Pattern in Nasturtium (Tropaeolum majus L.). Agronomy, 14(9), 2108. https://doi.org/10.3390/agronomy14092108






