Construction of Microbial Consortium to Enhance Cellulose Degradation in Corn Straw during Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Purification Microbial Strains
2.2. Screening and Identification of Microbial Strains
2.2.1. Primary Screening
2.2.2. Rescreening
2.3. Sequencing and Identification
2.4. Compatibility Test
2.5. Construction of Microbial Strain Consortium
2.5.1. Enzyme Activity and Cellulose Degradation Rate of Two-Microbial-Strain Combinations
2.5.2. Enzyme Activity and Cellulose Degradation Rate of Three-Microbial-Strain Combinations
2.6. pH Changes of Combined Microbial Strains
2.7. Composting Effect of Selected Microbial Strain Combinations for In Situ Fermentation
2.8. Statistical Analysis
3. Results
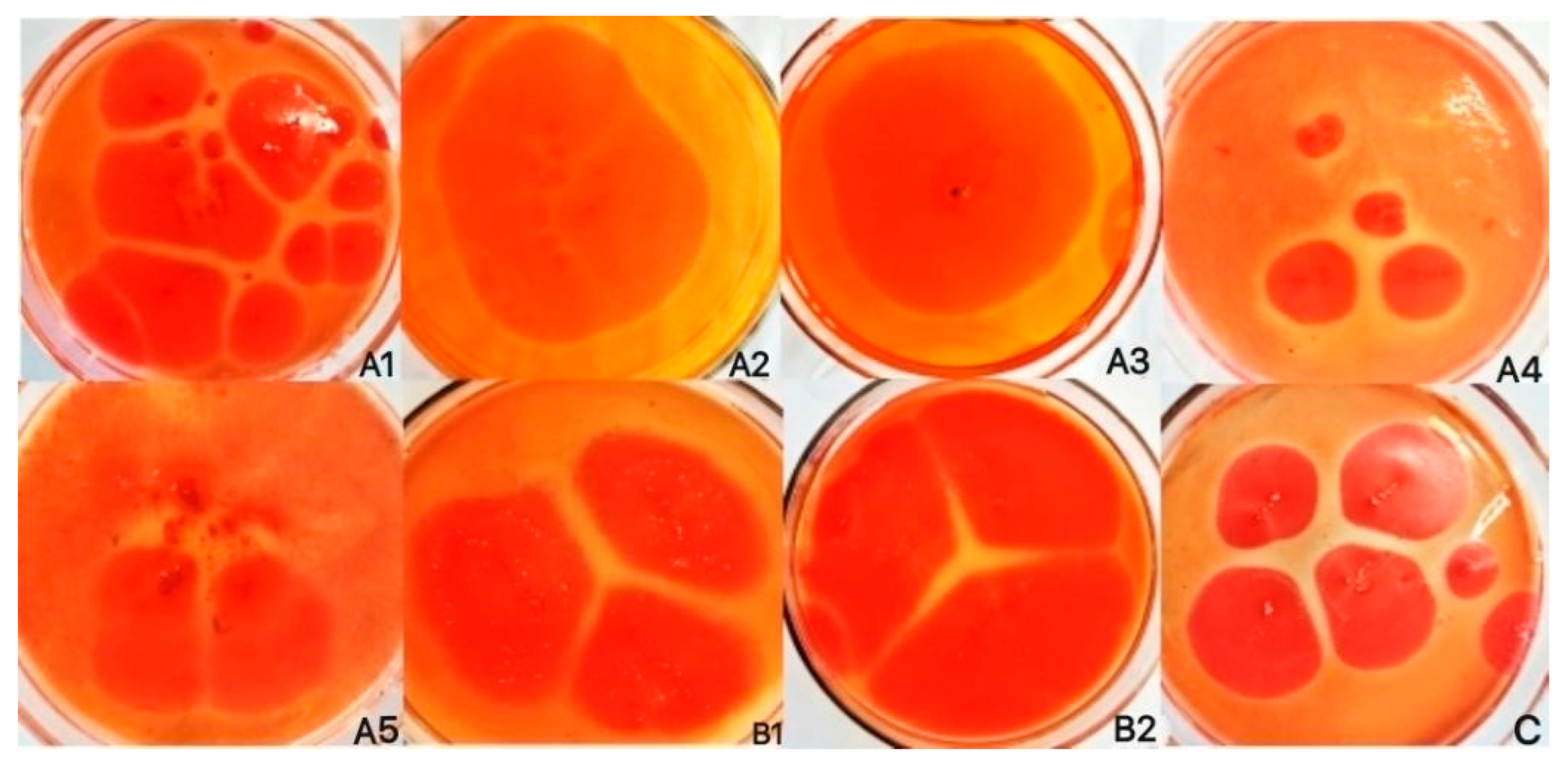
3.1. Screening and Identification of Corn-Straw-Degrading Microbial Strains
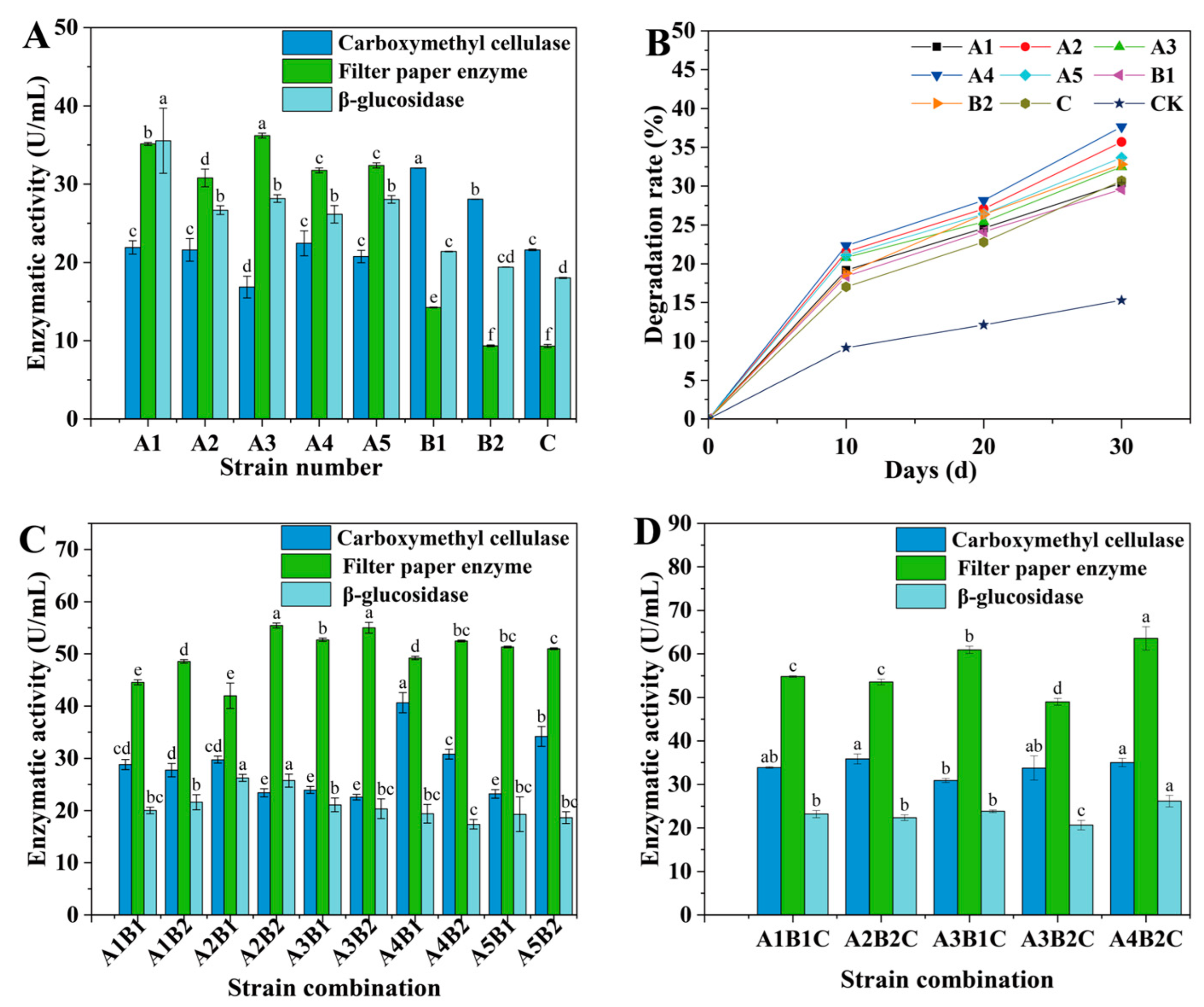
3.2. Enzyme Activity and Degradation Rate of Corn Straw Using Single Strain
3.3. Compatibility Test and Enzyme Activity of Combined Microbial Strains
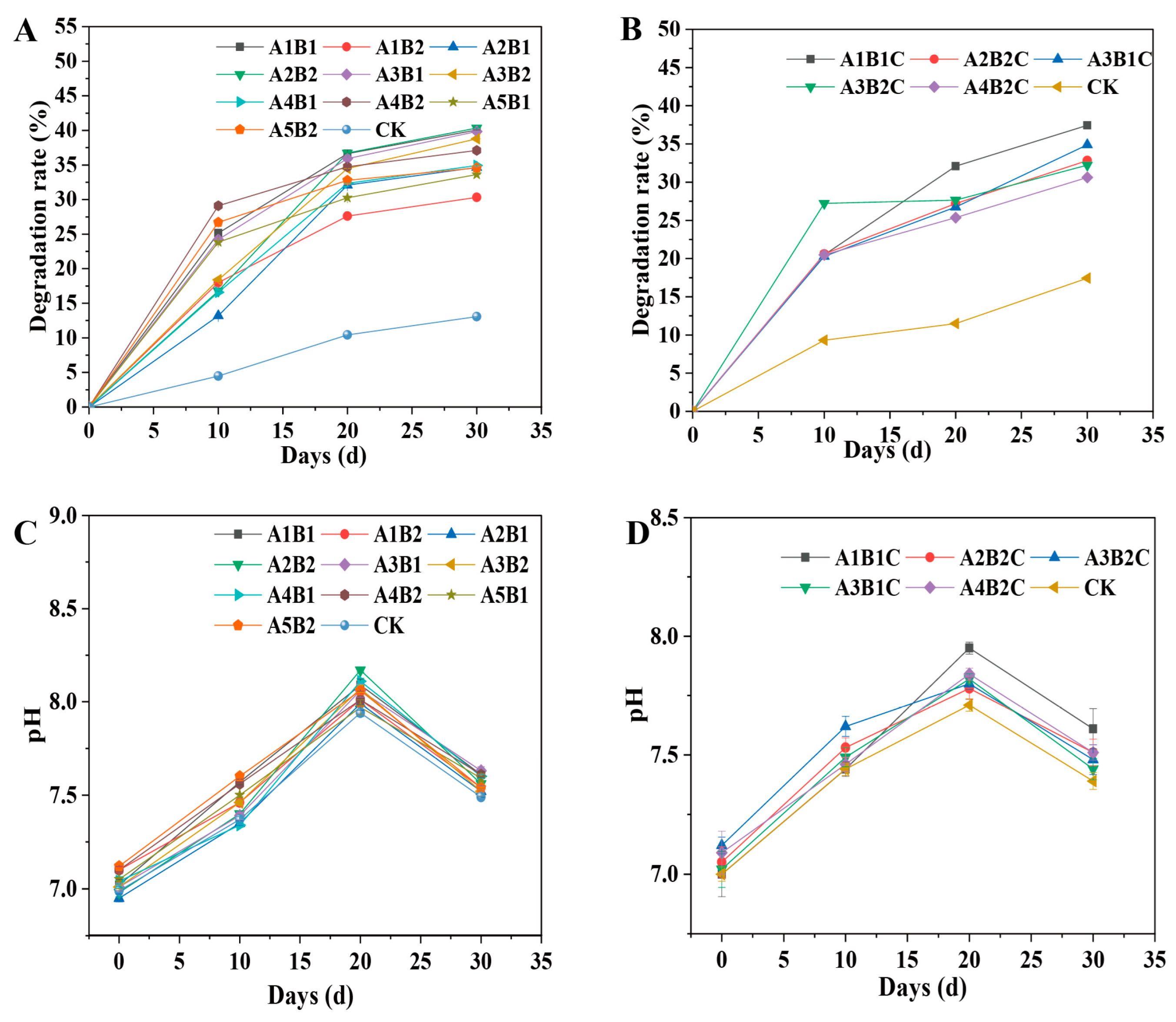
3.4. Degradation Rate of Corn Straw Using Combined Microbial Strains
3.5. pH Value Changes during the Degradation of Corn Straw by Combined Microbial Strains
3.6. Changes in Physicochemical Properties during In Situ Corn Straw Composting
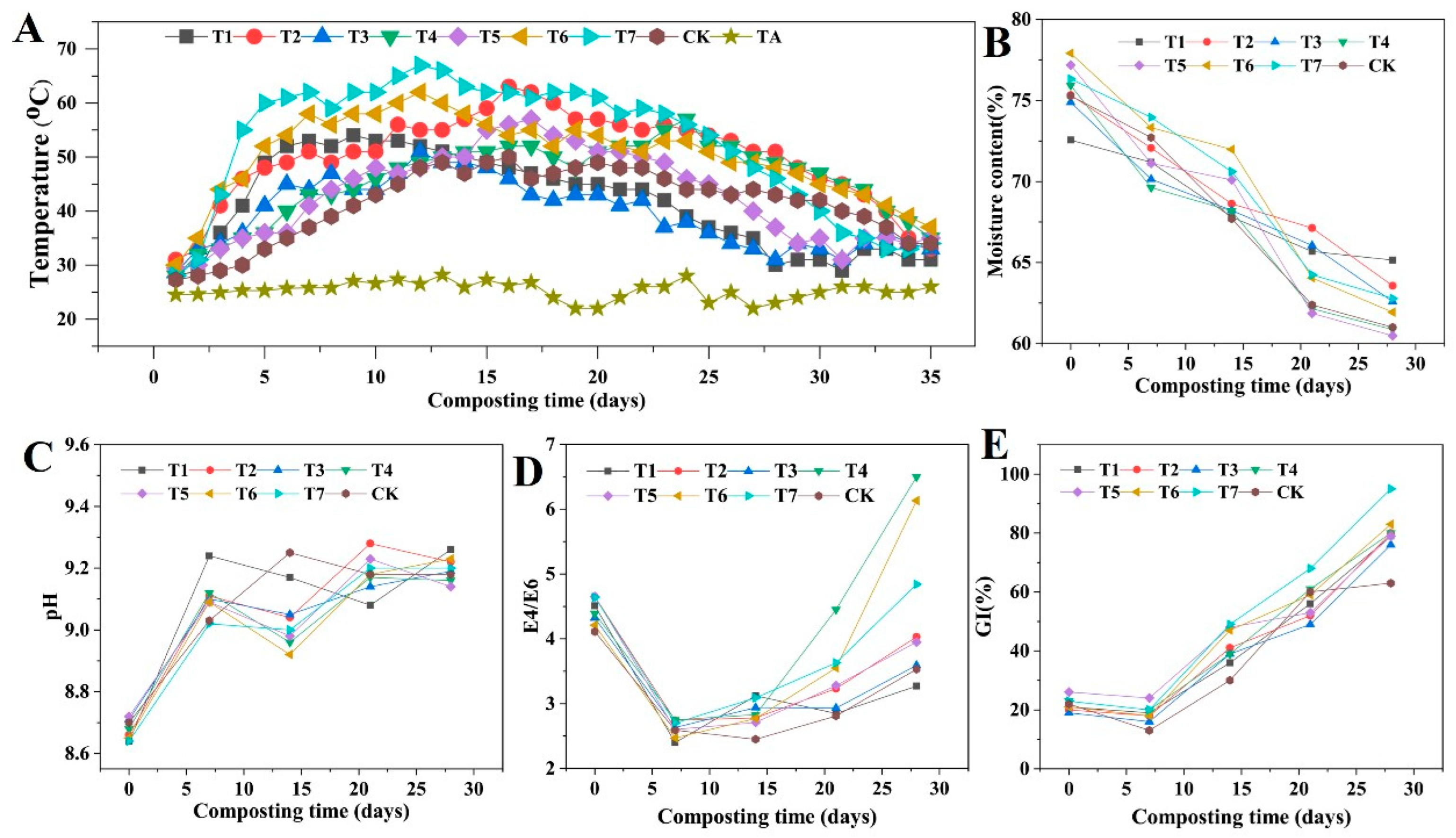
3.6.1. Temperature Changes with Different Microbial Inoculants
3.6.2. Moisture Content Changes with Different Microbial Inoculants
3.6.3. pH Changes during with Different Microbial Inoculants
3.6.4. E4/E6 Changes Value with Different Microbial Inoculants
3.6.5. Seed Germination Index Changes with Different Microbial Inoculants
4. Discussion
4.1. Identification and Screening of Microbial Strains
4.2. Corn Straw Composting In Situ
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, R.; Zhu, X.; Wang, C.; Yue, J.; Pan, L.; Song, C.; Zhao, Y. Effect of NH4+ and NO3− cooperatively regulated carbon to nitrogen ratio on organic nitrogen fractions during rice straw composting. Bioresour. Technol. 2024, 395, 130316. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Q.; Zhu, X.H.; Wu, J.; Guo, D.Y.; Zhang, L.H.; Feng, Y. Dynamics of microbial diversity during the composting of agricultural straw. J. Integr. Agric. 2021, 20, 1121–1136. [Google Scholar] [CrossRef]
- Chen, J.M.; Cai, Y.F.; Wang, Z.; Xu, Z.Z.; Li, J.; Ma, X.T.; Zhuang, W.; Liu, D.; Wang, S.L.; Song, A.D.; et al. Construction of a synthetic microbial community based on multiomics linkage technology and analysis of the mechanism of lignocellulose degradation. Bioresour. Technol. 2023, 389, 129799. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Han, D.; Zhao, Y.; Li, R.; Wu, Y. Environmental evaluation of a distributed-centralized biomass pyrolysis system: A case study in Shandong, China. Sci. Total Environ. 2020, 716, 136915. [Google Scholar] [CrossRef]
- Xu, Z.P.; Tang, Y.Z.; Wang, Q.S.; Xu, Y.; Yuan, X.L.; Ma, Q.; Wang, G.X.; Liu, M.Q.; Hao, H.L. Energy based optimization of regional straw comprehensive utilization scheme. J. Clean. Prod. 2021, 297, 126638. [Google Scholar] [CrossRef]
- Zheng, W.; Ma, Y.; Wang, X.; Wang, X.; Li, J.; Tian, Y.; Zhang, X. Producing high-quality cultivation substrates for cucumber production by in-situ composting of corn straw blocks amended with biochar and earthworm casts. Waste Manag. 2022, 139, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Vizhimalar, A.S.; Vasanthy, M.; Thamaraiselvi, C.; Biruntha, M.; Paul, J.; Thirupathi, A.; Chang, S.W.; Xu, Z.; Al-Rashed, S.; Munuswamy-Ramanujam, G.; et al. Greener production of compost from agricultural biomass residues amended with mule dung for agronomic application. Chemosphere 2021, 288, 132561. [Google Scholar] [CrossRef]
- Niu, C.T.; Xing, X.L.; Yang, X.H.; Zheng, F.Y.; Liu, C.F.; Wang, J.J.; Li, Q. Isolation, identification and application of Aspergillus oryzae BL18 with high protease activity as starter culture in doubanjiang (broad bean paste) fermentation. Food Biosci. 2023, 51, 102225. [Google Scholar] [CrossRef]
- Guo, H.W.; Chang, J.; Yin, Q.Q.; Wang, P.; Lu, M.; Wang, X.; Dang, X.W. Effect of the combined physical and chemical treatments with microbial fermentation on corn straw degradation. Bioresour. Technol. 2013, 148, 361–365. [Google Scholar] [CrossRef]
- Yang, J.C.; Chen, R.J.; Zhang, Q.G.; Zhang, L.H.; Li, Q.C.; Zhang, Z.Y.; Wang, Y.C.; Qu, B. Green and chemical-free pretreatment of corn straw using cold isostatic pressure for methane production. Sci. Total Environ. 2023, 897, 165442. [Google Scholar] [CrossRef]
- Wang, P.; Chang, J.; Yin, Q.Q.; Wang, E.Z.; Zhu, Q.; Song, A.D.; Lu, F.S. Effects of thermo-chemical pretreatment plus microbial fermentation and enzymatic hydrolysis on saccharification and lignocellulose degradation of corn straw. Bioresour. Technol. 2015, 194, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.H.; Eom, I.Y.; Joo, J.C.; Yu, J.H.; Song, B.K.; Lee, S.H.; Hong, S.H.; Park, S.J. Recent advances in development of biomass pretreatment technologies used in biorefinery for the production of bio-based fuels, chemicals and polymers. Korean J. Chem. Eng. 2015, 32, 1945–1959. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhao, L.L.; He, X.L. Degradation potential of different lignocellulosic residues by Trichoderma longibrachiatum and Trichoderma afroharzianum under solid state fermentation. Process Biochem. 2022, 112, 6–17. [Google Scholar] [CrossRef]
- Han, S.C.; Zhang, S.N.; Ouyang, Y.; Yu, X.F.; Hu, S.P.; Borjigin, Q.G.; Gao, J.L. An improved method for corn stalk in-situ degrading synthetic bacterial consortium construction in a cold region of China. Biocatal. Agric. Biotechnol. 2023, 50, 102648. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Himmel, E.M.; Mielenz, R.J. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef]
- Qinggerer; Gao, J.L.; Yu, X.F.; Zhang, B.L.; Wang, Z.G.; Naoganchaolu, B.; Hu, S.P.; Sun, J.Y.; Xie, M.; Wang, Z. Screening of a microbial consortium with efficient corn stover degradation ability at low temperature. J. Integr. Agric. 2016, 15, 2369–2379. [Google Scholar] [CrossRef]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Naik, S.K.; Thombare, N.; Singh, A.K. Potential of lignocellulose degrading microorganisms for agricultural residue decomposition in soil: A review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Sun, J.; Wu, J.; Huizi, L. Screening and construction of a novel microbial consortium SSA-6 enriched from the gut symbionts of wood-feeding termite, Coptotermes formosanus and its biomass-based biorefineries. Fuel 2019, 236, 1128–1145. [Google Scholar] [CrossRef]
- Li, F.; Ghanizadeh, H.; Cui, G.L.; Liu, J.Y.; Miao, S.; Liu, C.; Song, W.W.; Chen, X.L.; Cheng, M.Z.; Wang, P.W.; et al. Microbiome-based agents can optimize composting of agricultural wastes by modifying microbial communities. Bioresour. Technol. 2023, 374, 128765. [Google Scholar] [CrossRef]
- Yu, X.F.; Borjigin, Q.G.; Gao, J.L.; Wang, Z.G.; Hu, S.P.; Borjigin, N.; Wang, Z.; Sun, J.Y.; Han, S.C. Exploration of the key microbes and composition stability of microbial consortium GF-20 with efficiently decomposes corn stover at low temperatures. J. Integr. Agric. 2019, 18, 1893–1904. [Google Scholar] [CrossRef]
- Chu, X.D.; Kumar Awasthi, M.; Liu, Y.Y.N.; Cheng, Q.S.; Qu, J.B.; Sun, Y. Studies on the degradation of corn straw by combined bacterial cultures. Bioresour. Technol. 2020, 320, 124174. [Google Scholar] [CrossRef] [PubMed]
- Sharker, B.; Islam, M.A.; Hossain, M.A.A.; Ahmad, I.; Al Mamun, A.; Ghosh, S.; Rahman, A.; Hossain, M.S.; Ashik, M.A.; Hoque, M.R.; et al. Characterization of lignin and hemicellulose degrading bacteria isolated from cow rumen and forest soil: Unveiling a novel enzymatic model for rice straw deconstruction. Sci. Total Environ. 2023, 904, 166704. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, X.; Xiang, Y. Structure-function elucidation of a microbial consortium in degrading rice straw and producing acetic and butyric acids via metagenome combining 16S rDNA sequencing. Bioresour. Technol. 2021, 340, 125709. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.S.; Li, W.; Song, Z.; Wang, S.W.; Geng, S.J.; Jiang, H.; Wang, Z.H.; Tian, P.; Wu, Z.H.; Yang, M.Y. Straw Incorporation with Exogenous Degrading Bacteria (ZJW-6): An Integrated Greener Approach to Enhance Straw Degradation and Improve Rice Growth. Int. J. Mol. Sci. 2024, 25, 7835. [Google Scholar] [CrossRef]
- Liu, X.F.; Qi, Y.N.; Lian, J.; Song, J.; Zhang, S.; Zhang, G.; Fan, J.; Zhang, N. Construction of actinomycetes complex flora in degrading corn straw and an evaluation of their degradative effects. Biotechnol. Lett. 2022, 44, 1477–1493. [Google Scholar] [CrossRef]
- Phukongchai, W.; Kaewpradit, W.; Rasche, F. Inoculation of cellulolytic and ligninolytic microorganisms accelerates decomposition of high c/n and cellulose rich sugarcane straw in tropical sandy soils. Appl. Soil Ecol. 2022, 172, 104355. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Nada, B.; Baker, S.E.; Evans, J.E.; Tian, C.G.; Benz, J.P.; Tamayo, E. Unveiling a classical mutant in the context of the GH3 β-glucosidase family in Neurospora crassa. AMB Express 2024, 14, 4. [Google Scholar] [CrossRef]
- Junghare, M.; Manavalan, T.; Fredriksen, L.; Leiros, I.; Altermark, B.; Eijsink, V.G.H.; Vaaje-Kolstad, G. Biochemical and structural characterisation of a family GH5 cellulase from endosymbiont of shipworm P. megotara. Biotechnol. Biofuels Bioprod. 2023, 16, 61. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharya, S.; Panchanan, G.; Navya, B.S.; Nambiar, P. Production, characterization and Congo red dye decolourizing efficiency of a laccase from Pleurotus ostreatus MTCC 142 cultivated on co-substrates of paddy straw and corn husk. J. Genet. Eng. Biotechnol. 2016, 14, 281–288. [Google Scholar] [CrossRef]
- Lakhesar, D.P.S.; Backhouse, D.; Kristiansen, P. Nutritional constraints on displacement of Fusarium pseudograminearum from cereal straw by antagonists. Biol. Control 2010, 55, 241–247. [Google Scholar] [CrossRef]
- Wang, M.M.; Wu, Y.C.; Zhao, J.Y.; Liu, Y.; Gao, L.; Jiang, Z.K.; Zhang, J.B.; Tian, W. Comparison of composting factors, heavy metal immobilization, and microbial activity after biochar or lime application in straw-manure composting. Bioresour. Technol. 2022, 363, 127872. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Zhao, Y.Y.; Li, F.; Liu, P. Active polysaccharides from Lentinula edodes and Pleurotus ostreatus by addition of corn straw and xylosma sawdust through solid-state fermentation. Int. J. Biol. Macromol. 2023, 228, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F.; Forte, M.; Monaco, A.; Bertoldi, D.E. Biological evaluation of compost maturity. Biocycle 1981, 4, 27–29. [Google Scholar]
- Kaur, J.; Chugh, P.; Soni, R.; Soni, S.K. A low-cost approach for the generation of enhanced sugars and ethanol from rice straw using in-house produced cellulase-hemicellulase consortium from A. niger P-19. Bioresour. Technol. Rep. 2020, 11, 100469. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Deswal, D.; Karp, M.; Kuhad, R.C. Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel 2014, 124, 183–189. [Google Scholar] [CrossRef]
- Lepcha, K.; Ghosh, S. Glycoside hydrolases from a thermophilic microbial consortium and their implication in the saccharification of agroresidues. Biocatal. Agric. Biotechnol. 2018, 15, 160–166. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Chang, N.; Sun, L.; Bello, A.; Deng, L.; Zhao, L.; Egbeagu, U.U.; Wang, B.; Zhao, Y.; et al. Exploration of β-glucosidase-producing microorganisms community structure and key communities driving cellulose degradation during composting of pure corn straw by multi-interaction analysis. J. Environ. Manag. 2023, 325, 116694. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Dong, Y.J. Design and application of an efficient cellulose-degrading microbial consortium and carboxymethyl cellulase production optimization. Front. Microbiol. 2022, 13, 957444. [Google Scholar] [CrossRef]
- Li, C.N.; Li, H.Y.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J.H.; Lan, X.J.; Zhang, Y.C.; Yang, X.M.; et al. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef]
- Bao, J.F.; Li, S.X.; Qv, M.X.; Wang, W.; Wu, Q.R.; Nugroho, Y.K.; Huang, L.Z.; Zhu, L.D. Urea addition as an enhanced strategy for degradation of petroleum contaminants during co-composting of straw and pig manure: Evidences from microbial community and enzyme activity evaluation. Bioresour. Technol. 2024, 393, 130135. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z.; Wei, Y.; Wu, D.; et al. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; He, X.S.; Tang, J.; Li, X.; Zhao, R.; Tao, Y.Q.; Wang, C.; Qiu, Z.P. Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 2021, 264, 128549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Geng, X.Y.; Shi, M.Z.; Chen, X.M.; Wei, Z.M. Effect of different moisture contents on hydrogen sulfide malodorous gas emission during composting. Bioresour. Technol. 2023, 380, 129093. [Google Scholar] [CrossRef]
- Liu, X.L.; Sun, X.; Li, J.; Yan, X.M.; Wei, Y.Q.; Zheng, S.F.; Chen, M.; Kan, H.C.; Wang, W.; Li, S.Y.; et al. Effects of microbial agents on nitrogen conversion and bacterial succession during pig manure composting. Process Biochem. 2023, 134, 101–111. [Google Scholar] [CrossRef]
- Niu, K.; Chao, C.; Zhang, X.X.; An, Z.G.; Zhou, J.Y.; Yang, L.G. Effects of different microbial agents on bedding treatment of ectopic fermentation of buffalo manure. Front. Microbiol. 2022, 13, 1080650. [Google Scholar] [CrossRef]
- Meng, X.Y.; Yan, J.; Zuo, B.; Wang, Y.H.; Yuan, X.F.; Cui, Z.J. Full-scale of composting process of biogas residues from corn stover anaerobic digestion: Physical-chemical, biology parameters and maturity indexes during whole process. Bioresour. Technol. 2020, 302, 122742. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.H.; Zhang, D.W.; Tian, Y.; Nan, J.; Feng, Y.J. Hydrothermal pretreatment and compound microbial agents promoting high-quality kitchen waste compost: Superior humification degree and reduction of odour. Sci. Total Environ. 2023, 862, 160657. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.B.; Wen, J.X.; Zhao, C.; Dong, L.; Liu, H. Underestimated humic acids release and influence on anaerobic digestion during sludge thermal hydrolysis. Water Res. 2021, 201, 117310. [Google Scholar] [CrossRef]
- Ma, S.S.; Shen, Y.J.; Ding, J.T.; Cheng, H.S.; Zhou, H.B.; Ge, M.S.; Wang, J.; Cheng, Q.Y.; Zhang, D.L.; Zhang, Y.; et al. Effects of biochar and volcanic rock addition on humification and microbial community during aerobic composting of cow manure. Bioresour. Technol. 2024, 391, 129973. [Google Scholar] [CrossRef]
- Ge, M.S.; Shen, Y.J.; Ding, J.T.; Meng, H.B.; Zhou, H.B.; Zhou, J.; Cheng, H.S.; Zhang, X.; Wang, J.; Wang, H.H.; et al. New insight into the impact of moisture content and pH on dissolved organic matter and microbial dynamics during cattle manure composting, Bioresour. Technol. 2022, 344, 126236. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, N.A.; Perminova, I.V. Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules 2021, 26, 2706. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.J.; Wang, G.; Fan, Y.D.; Wang, X.Q.; Liu, J.; Guo, M.Z.; Chen, H.; Zhang, S.T.; Chen, G. Enhancing the compost maturation of deer manure and corn straw by supplementation via black liquor. Heliyon 2023, 2, e13246. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.Y.; Wang, X.; Liu, S.M.; Hua, Y.T.; Zhou, S.X.; Jiang, Z.X. Combined use of biochar and microbial agent can promote lignocellulose degradation and humic acid formation during sewage sludge-reed straw composting. Bioresour. Technol. 2023, 370, 128525. [Google Scholar] [CrossRef]
| Inoculant | Microbial Strains | ||
|---|---|---|---|
| Fungi | Bacteria | Actinomycetes | |
| 1 (T1) | A1 + A4 + A5 | B5 + B6 | C |
| 2 (T2) | A1 + A4 + A5 | B5 + B7 | C |
| 3 (T3) | A1 + A4 + A5 | B6 + B7 | C |
| 4 (T4) | A1 + A4 | B5 + B6 + B7 | C |
| 5 (T5) | A1 + A5 | B5 + B6 + B7 | C |
| 6 (T6) | A4 + A5 | B5 + B6 + B7 | C |
| 7 (T7) | A1 + A4 + A5 | B5 + B6 + B7 | C |
| CK | Distilled water | ||
| Strain Label | D/d (cm) |
|---|---|
| A1 | 5.95 ± 0.63 bc |
| A2 | 5.89 ± 0.70 bc |
| A3 | 7.37 ± 1.16 abc |
| A4 | 5.06 ± 1.93 c |
| A5 | 6.78 ± 1.07 abc |
| B1 | 3.11 ± 0.12 b |
| B2 | 3.20 ± 0.12 b |
| C | 2.60 ± 0.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, J.; Yang, R.; Yang, P.; Fu, H.; Yang, Y.; Liu, C. Construction of Microbial Consortium to Enhance Cellulose Degradation in Corn Straw during Composting. Agronomy 2024, 14, 2107. https://doi.org/10.3390/agronomy14092107
Li J, Li J, Yang R, Yang P, Fu H, Yang Y, Liu C. Construction of Microbial Consortium to Enhance Cellulose Degradation in Corn Straw during Composting. Agronomy. 2024; 14(9):2107. https://doi.org/10.3390/agronomy14092107
Chicago/Turabian StyleLi, Jie, Juan Li, Ruopeng Yang, Ping Yang, Hongbo Fu, Yongchao Yang, and Chaowei Liu. 2024. "Construction of Microbial Consortium to Enhance Cellulose Degradation in Corn Straw during Composting" Agronomy 14, no. 9: 2107. https://doi.org/10.3390/agronomy14092107






