Synthesis, Herbicidal Activity, and Molecular Mode of Action Evaluation of Novel Quinazolinone—Phenoxypropionate Hybrids Containing a Diester Moiety
Abstract
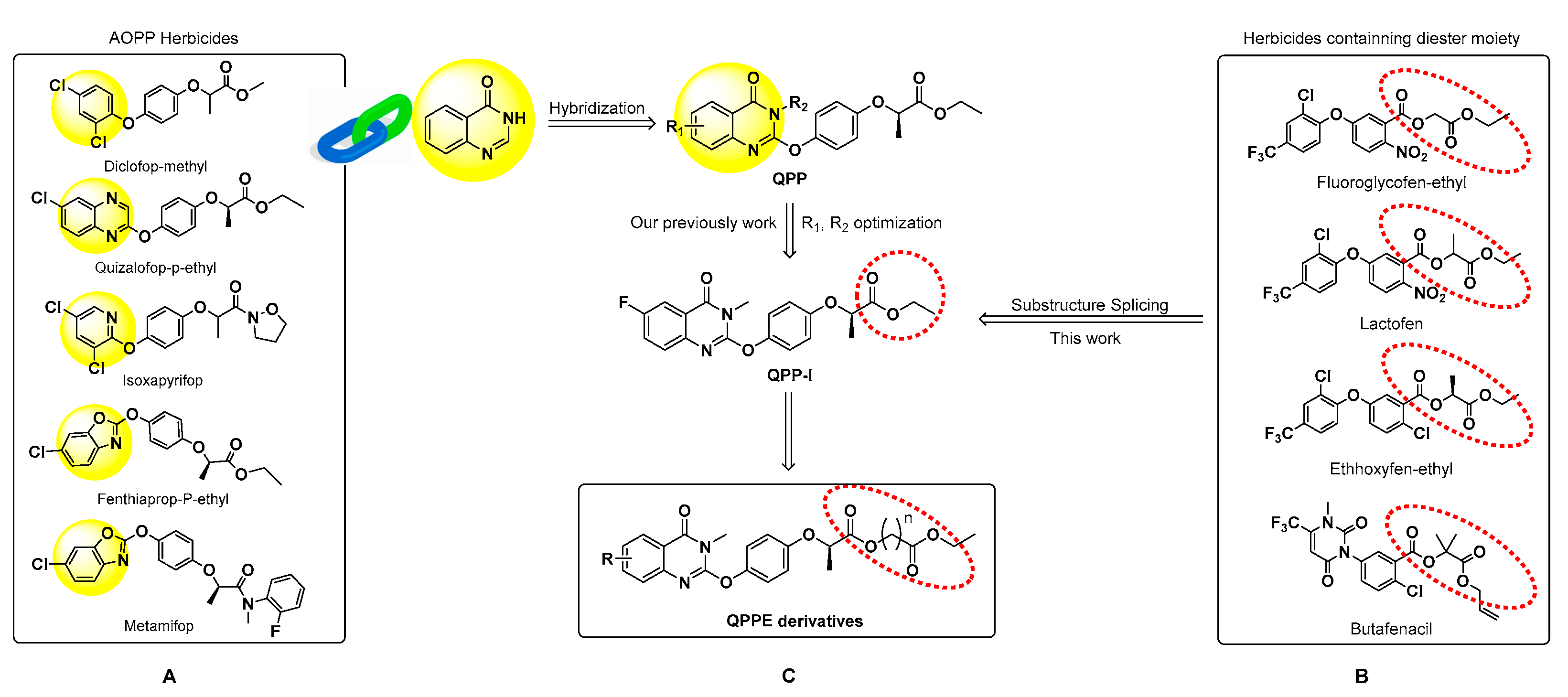
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis Procedures of Target Compounds
2.2.1. General Procedure for Preparation of Intermediates 2a-2d
2.2.2. General Procedure for Preparation of Intermediates 3a-3d
2.2.3. General Procedure for Preparation of Series Target Compounds QPPE-I to QPPE-IV
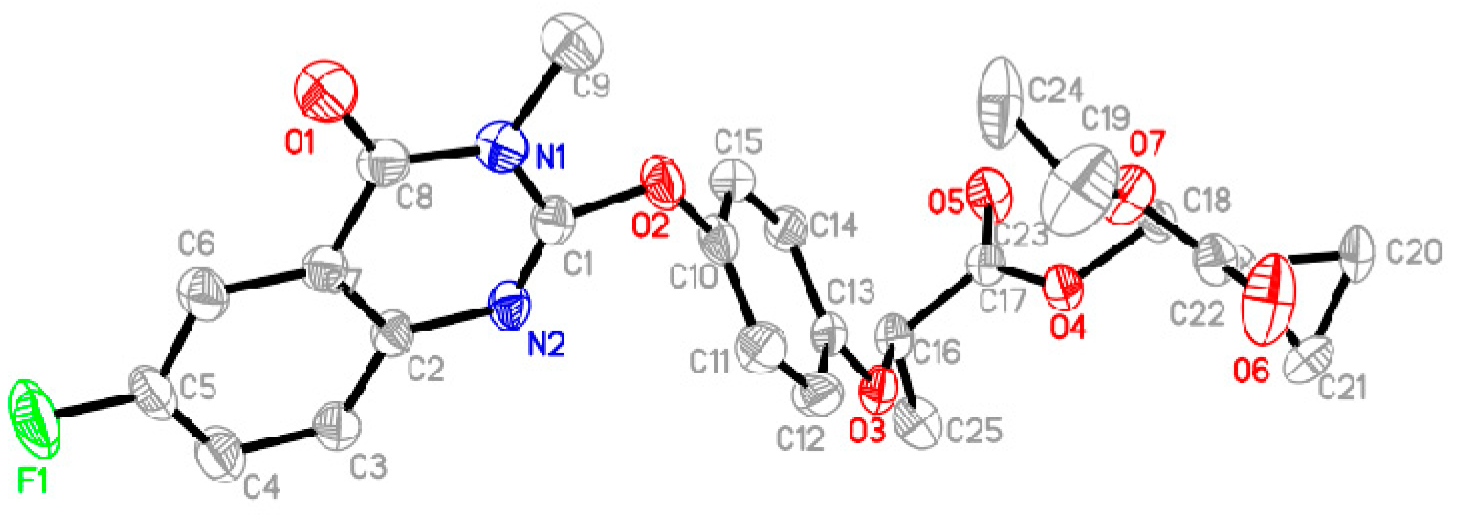
2.3. X-ray Diffraction Analysis of the Target Compound QPPE-I-7
2.4. Evaluation of Herbicidal Activity
2.5. Safety for Crops
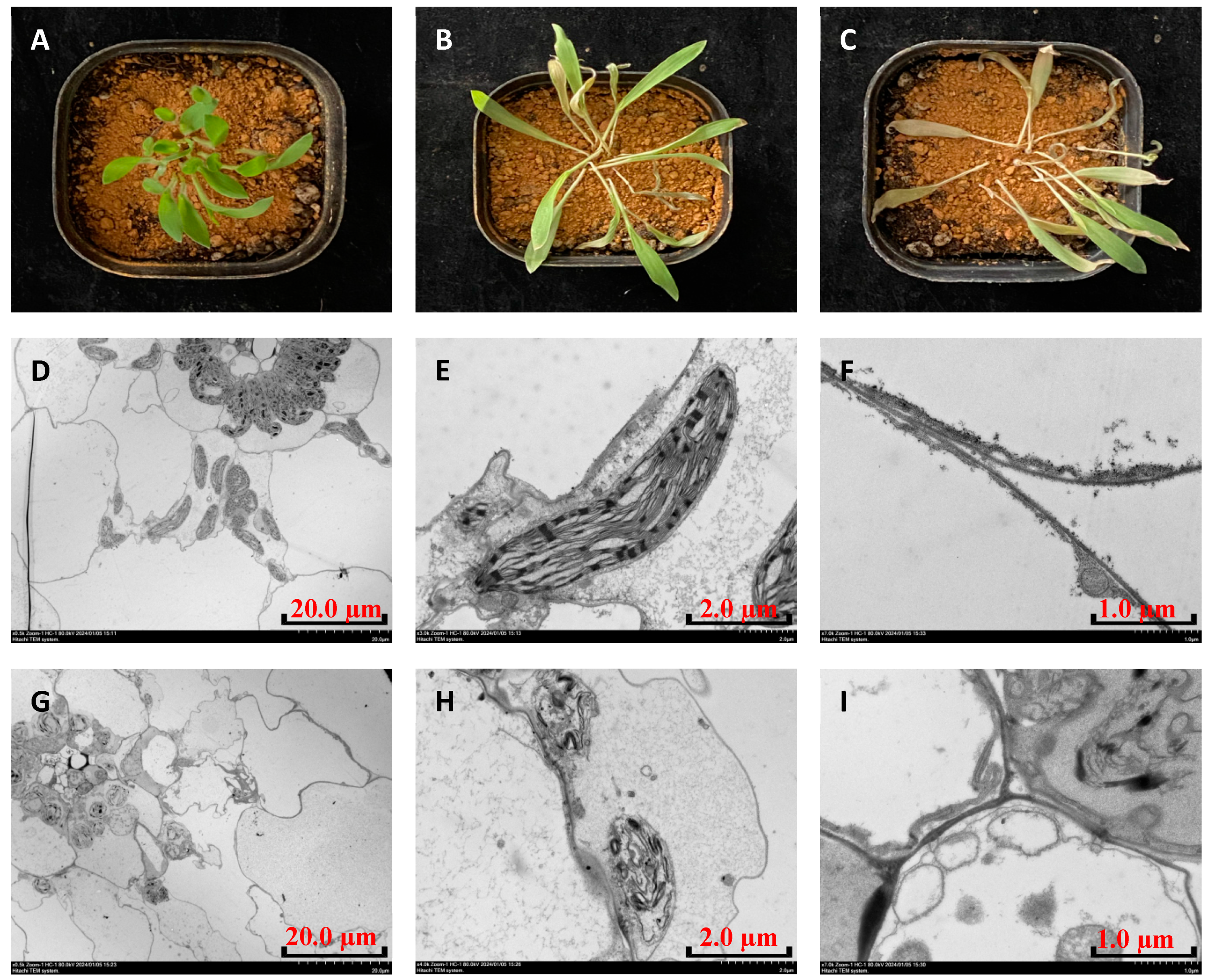
2.6. Phenotypic Study of P. miliaceum
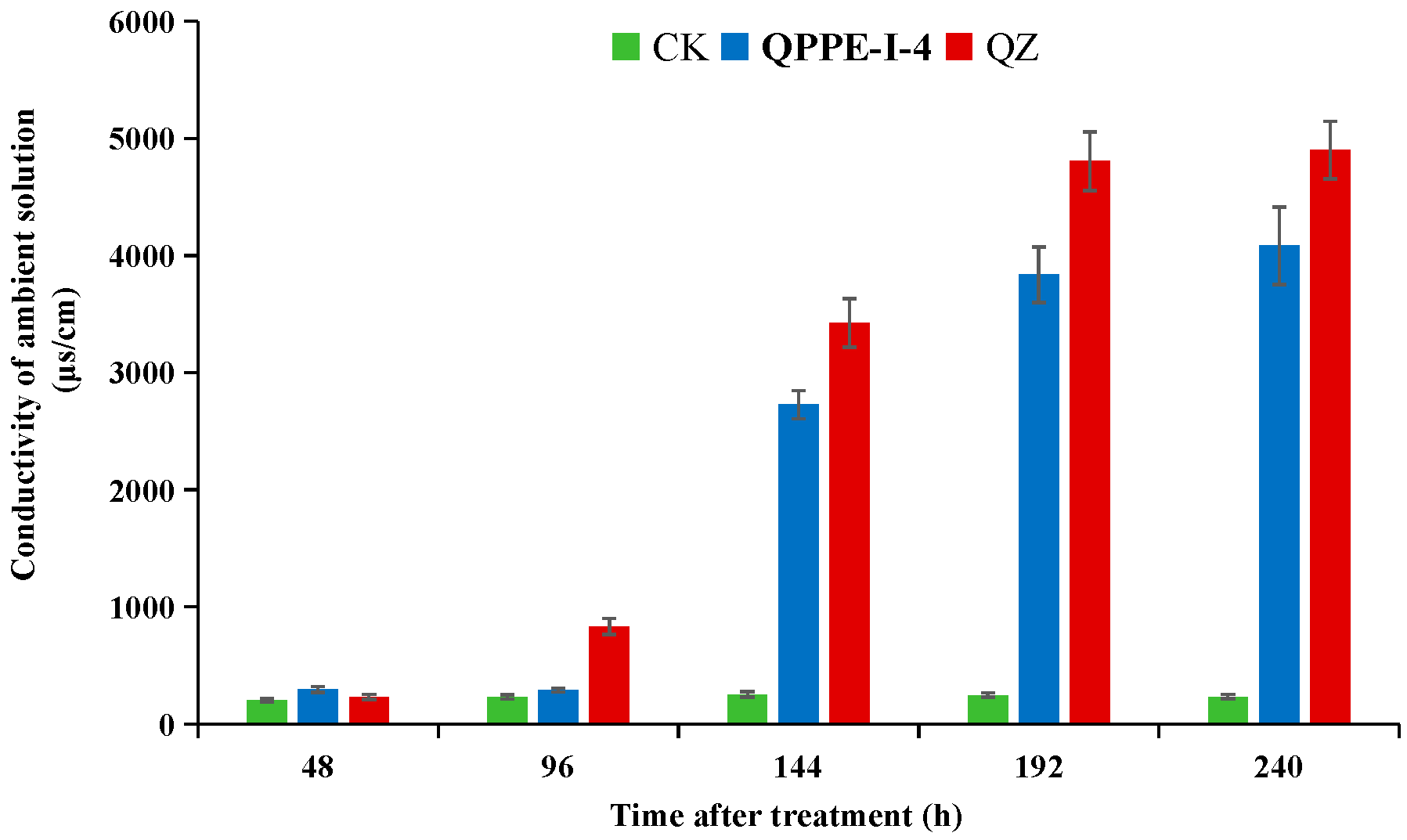
2.7. Determination of Cell Membrane Permeability
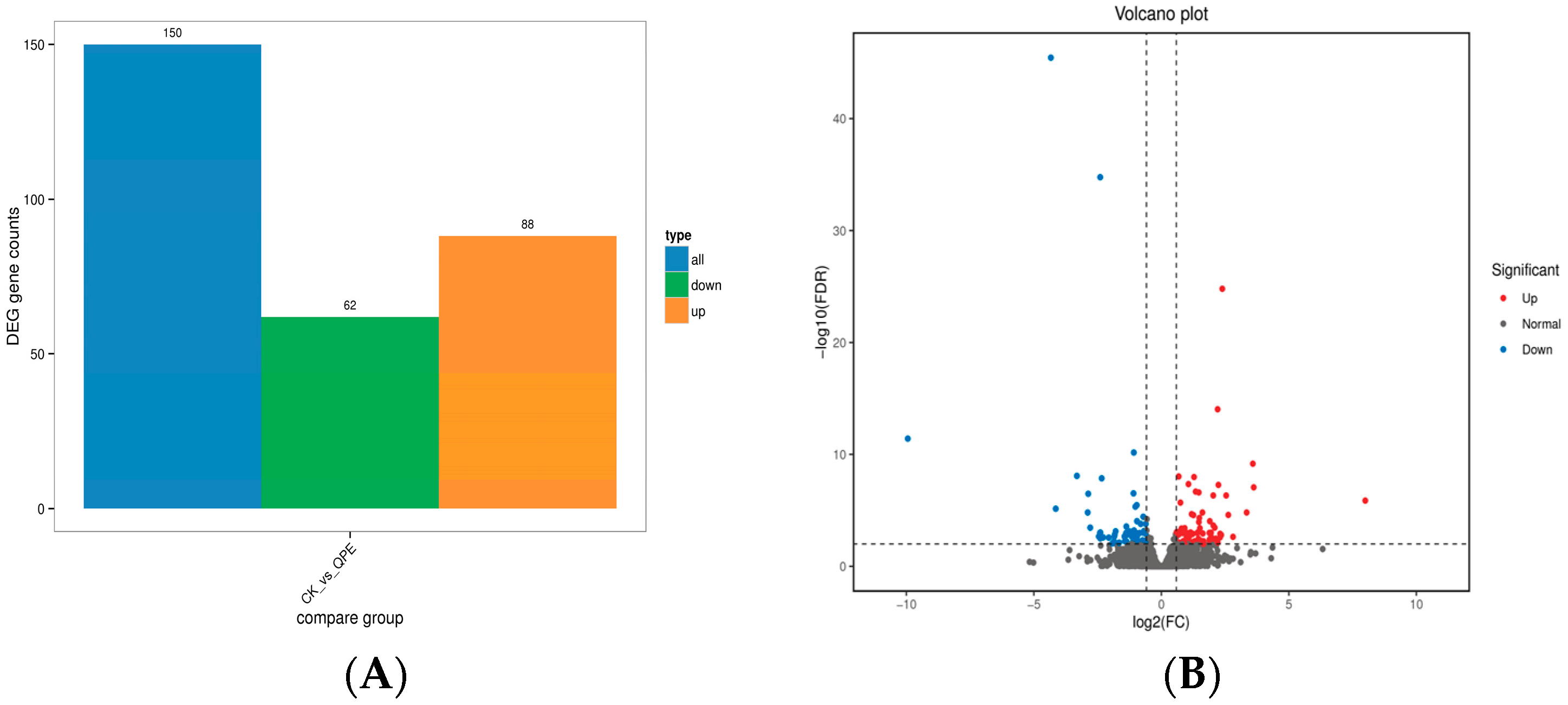
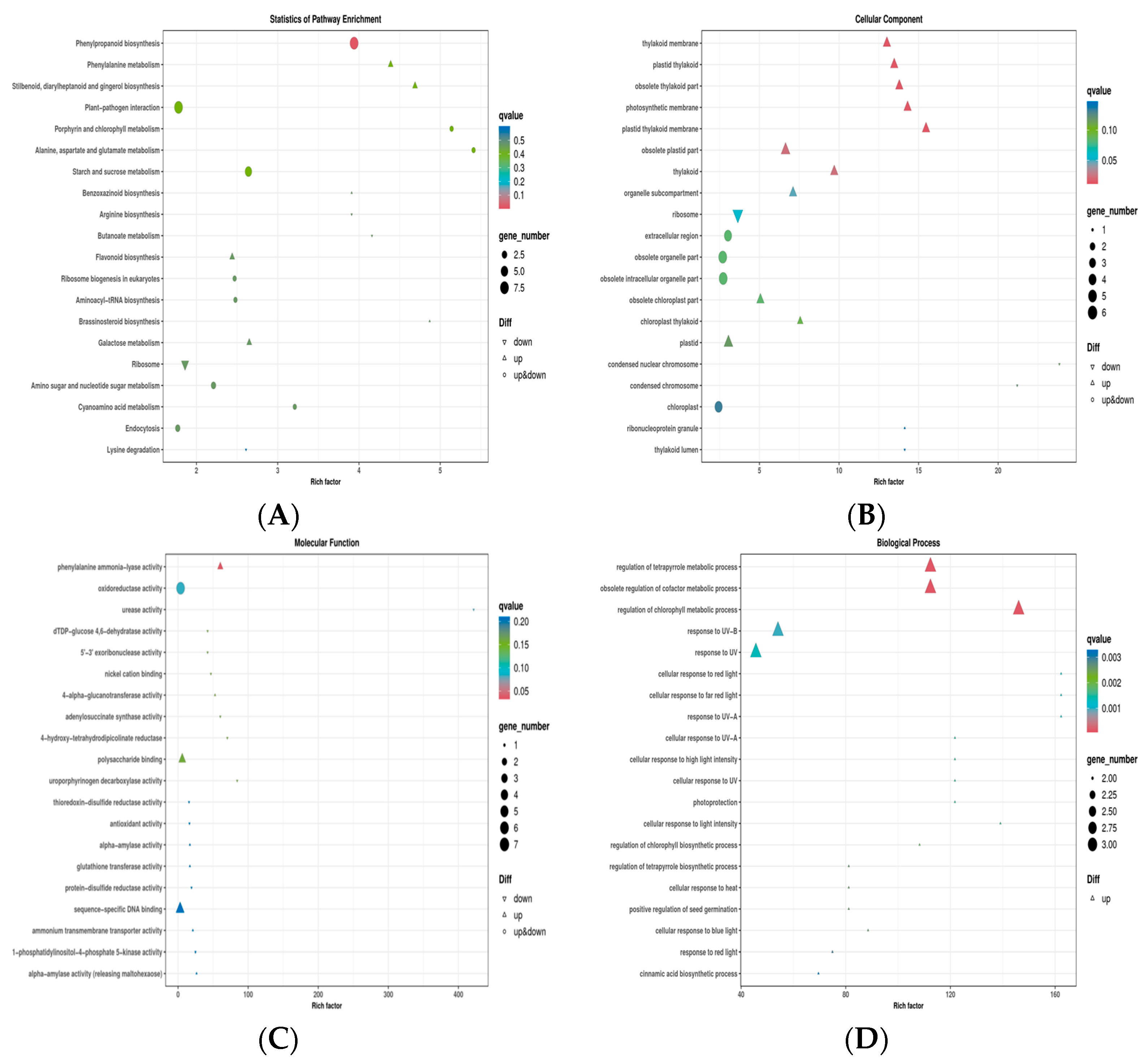
2.8. Transcriptome Study
2.9. ACCase Inhibition Activity Assay
3. Results and Discussion
3.1. Chemistry
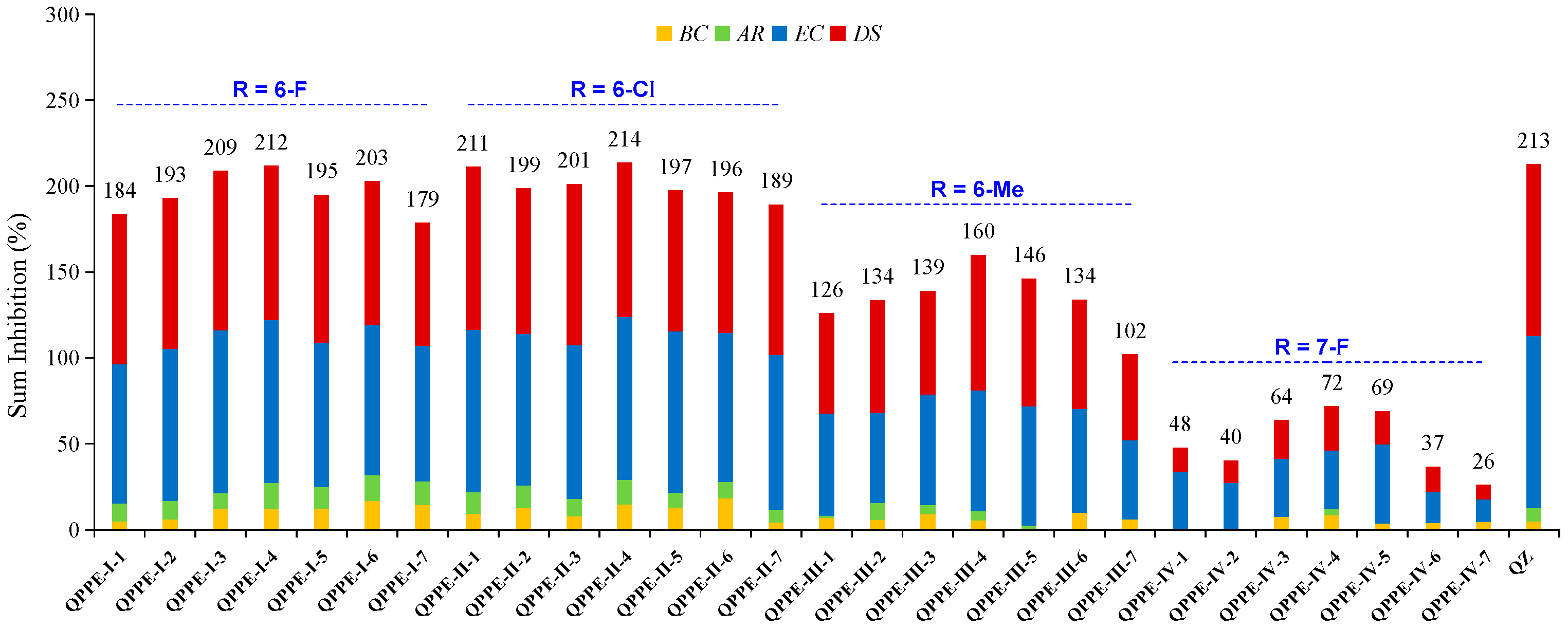
3.2. Herbicidal Activities and SAR
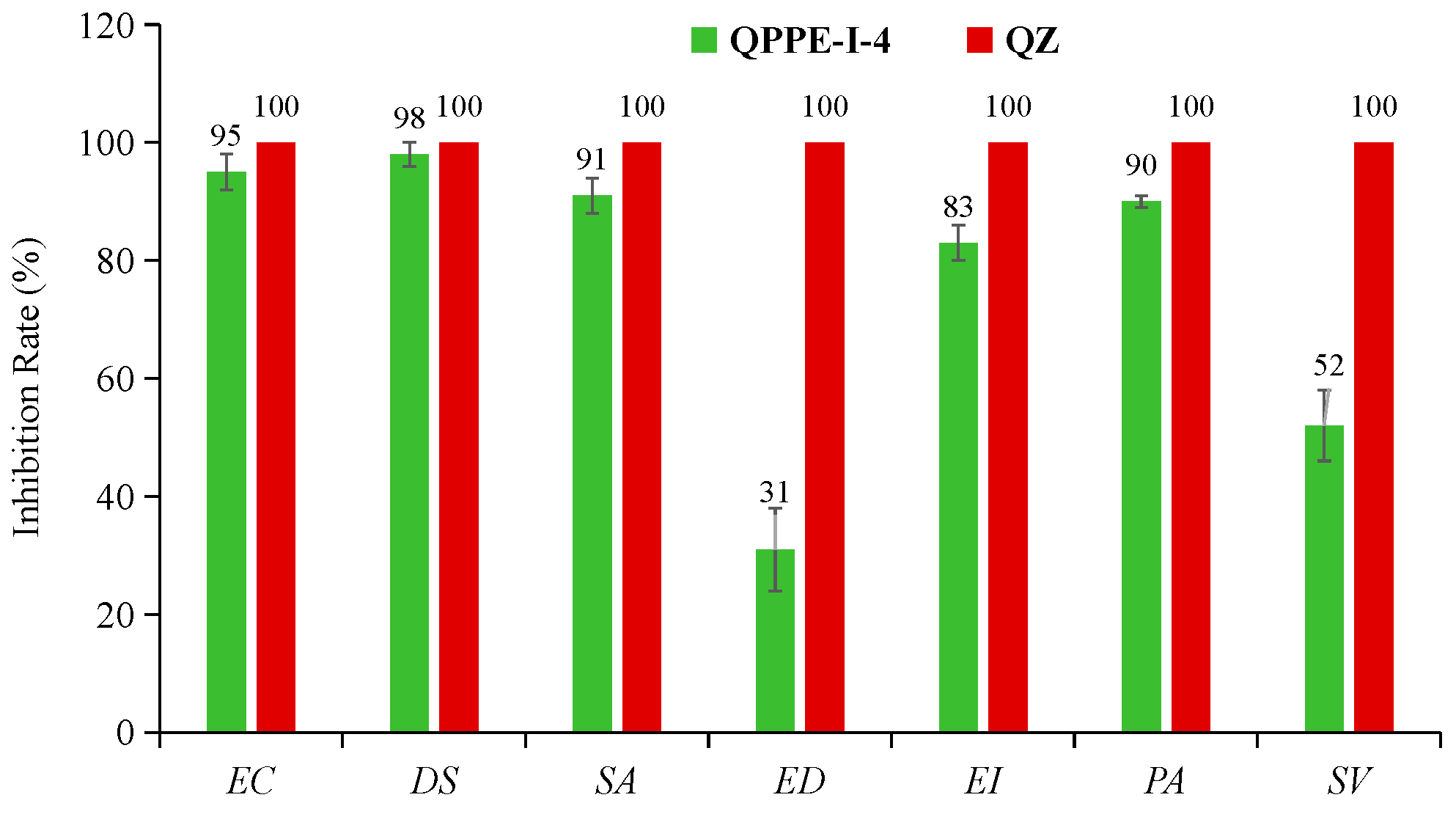
3.3. Herbicidal Spectrum and Crop Safety of Compound QPPE-I-4
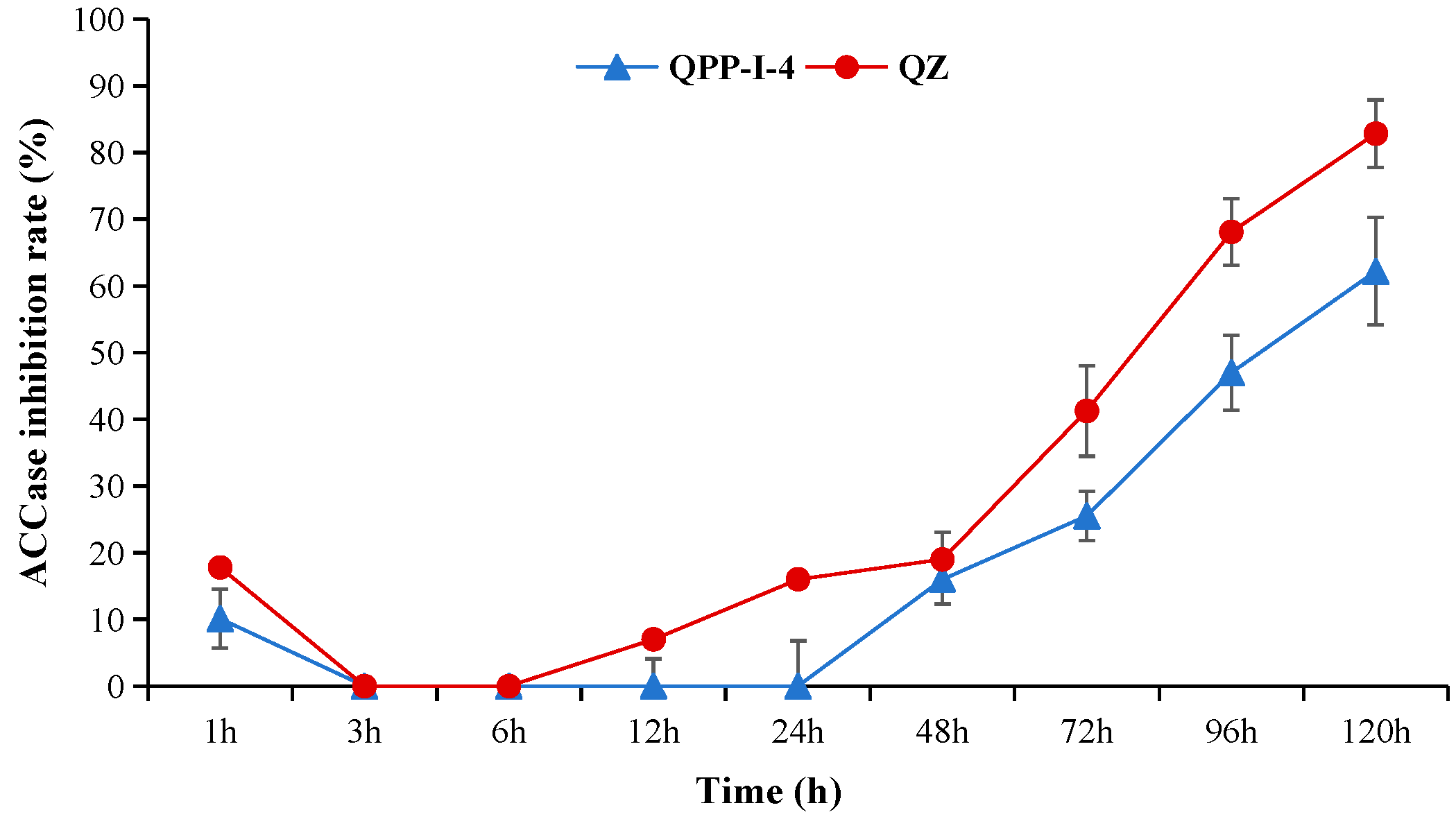
3.4. Molecular Mode of Action of the Compound QPPE-I-4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Duan, C.B.; Jin, B.; Ali, A.S.; Han, X.Y.; Zhang, H.F.; Zhang, M.Z.; Zhang, W.H.; Gu, Y.C. Recent advances in the natural products-based lead discovery for new agrochemicals. Adv. Agrochem. 2023, 2, 324–339. [Google Scholar] [CrossRef]
- Zagnitko, O.; Jelenska, J.; Tevzadze, G.; Haselkorn, R.; Gornicki, P. An isoleuciney leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 6617–6622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Yang, Z.R.; Shen, Y.; Tong, L. Crystal structure of the carboxyltransferase domain of Acetyl-Coenzyme A carboxylase. Science 2003, 299, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef]
- Délye, C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: An update. Weed Sci. 2005, 53, 728–746. [Google Scholar] [CrossRef]
- Tong, L.; Harwood, H.J. Acetyl-coenzyme A carboxylases: Versatile targets for drug discovery. J. Cell. Biochem. 2006, 99, 1476–1488. [Google Scholar] [CrossRef]
- Jia, L.; Gao, S.; Zhang, Y.Y.; Zhao, L.X.; Fu, Y.; Ye, F. Fragmenlt recombination design, synthesis, and safener activity of novel ester-substituted pyrazole derivatives. J. Agric. Food Chem. 2021, 69, 8366–8379. [Google Scholar] [CrossRef]
- Takano, H.K.; Ovejero, R.F.L.; Belchior, G.G.; Maymone, G.P.L.; Dayan, F.E. ACCase-inhibiting herbicides: Mechanism of action, resistance evolution and stewardship. Sci. Agric. 2021, 78, e20190102. [Google Scholar] [CrossRef]
- Stoltenberg, D.E.; Wiederholt, R.J. Giant foxtail (Setaria faberi) resistance to aryloxyphenoxypropionate and cyclohexanedione herbicides. Weed Sci. 1995, 43, 527–535. [Google Scholar] [CrossRef]
- Bakkali, Y. Late watergrass (Echinochloa phyllopogon): Mechanism involved in the resistance to fenoxaprop-P-ethyl. J. Agric. Food Chem. 2007, 55, 4052–4058. [Google Scholar] [CrossRef]
- Kaundun, S.S.; Hutchings, S.J.; Dale, R.P.; McIndoe, E. Role of a novel I1781T mutation and other mechanisms in conferring resistance to acetyl-CoA carboxylase inhibiting herbicides in a black-grass population. PLoS ONE 2013, 8, e69568. [Google Scholar] [CrossRef] [PubMed]
- Kaundun, S.S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 2014, 70, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, L.; Wang, H.; Li, Q.; Liu, W.; Wang, J.X. A rare Ile-2041-Thr mutation in the ACCase gene confers resistance to ACCase inhibiting herbicides in Shortawn Foxtail (Alopecurus aequalis). Weed Sci. 2017, 65, 239–246. [Google Scholar] [CrossRef]
- Colbach, N.; Chauvel, B.; Darmency, H.; Délye, C.; Le Corre, V. Choosing the best cropping systems to target pleiotropic effects when managing single-gene herbicide resistance in grass weeds: A blackgrass simulation study. Pest Manag. Sci. 2016, 72, 1910–1925. [Google Scholar] [CrossRef]
- Fang, J.P.; He, Z.Z.; Liu, T.T.; Li, J.; Dong, L.Y. A novel mutation Asp-2078-Glu in ACCase confers resistance to ACCase herbicides in barnyardgrass (Echinochloa crus-galli). Pestic. Biochem. Physiol. 2020, 168, 104634. [Google Scholar] [CrossRef]
- Wang, J.Z.; Peng, Y.J.; Chen, W.; Yu, Q.; Bai, L.Y.; Pan, L. The Ile-2041-Val mutation in the ACCase gene confers resistance to clodinafop-propargyl in American sloughgrass (Beckmannia syzigachne Steud). Pest Manag. Sci. 2021, 77, 2425–2432. [Google Scholar] [CrossRef]
- Li, N.; Chen, K.; Han, S.B.; Wang, S.M.; He, Y.Q.; Wang, X.K.; Li, P.; Ji, L.S.; Liu, R.; Lei, K. Synthesis, herbicidal activity, and molecular mode of action evaluation of novel aryloxyphenoxypropionate/amide derivatives containing a quinazolinone moiety. J. Agric. Food Chem. 2024, 72, 9445–9456. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, K.; Li, N.; Fu, S.Y.; Li, P.; Ji, L.S.; Liu, G.Y.; Wang, X.K.; Lei, K. Design, synthesis, mode of action and herbicidal evaluation of quinazolin-4(3H)-one derivatives based of aryloxyphenoxypropionate motif. Agronomy 2022, 12, 1840. [Google Scholar] [CrossRef]
- Liu, Q.X.; Huang, M.Z.; Liu, A.P.; Nie, S.Q.; Lei, M.X.; Ren, Y.G.; Pei, H.; He, L.Y.; Hu, L.; Hu, A.X. Synthesis and herbicidal activity of novel N-hetrocyclo containing nitrogen methoxy-O-(4-aryloxy-phenyl) lactamide derivatives. Chin. J. Org. Chem. 2014, 34, 118–125. [Google Scholar] [CrossRef]
- Liu, Q.X.; Huang, M.Z.; Liu, A.P.; Hu, A.X.; Lei, M.X.; Ren, Y.G.; Huang, L. Synthesis and herbicidal activity of novel N-allyloxy/propargyloxy aryloxyphenoxy propionamide derivatives. Chem. Res. Chin. Univ. 2016, 32, 188–194. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.W.; Yang, Z.H.; Li, B.B.; Hu, A.X.; Ye, J. Synthesis and herbicidal activity of N-(2, 2-dimethyl-7-alkoxy-2, 3-dihydrobenzofuran-5-yl)-2-(4-arylxoyphenoxy) propionamides. Chem. Res. Chin. Univ. 2017, 33, 74–79. [Google Scholar] [CrossRef]
- Yan, Z.Z.; Yang, Z.H.; Deng, X.L.; Lin, D.; Wu, M.F.; Li, J.M.; Chen, A.Y.; Ye, J.; Hu, A.X.; Liao, H.D. Novel aryloxyphenoxypropionate derivates containing benzofuran moiety: Design, synthesis, herbicidal activity, docking study and theoretical calculation. Pestic. Biochem. Physiol. 2019, 154, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.T.; Zheng, H.W.; Zhang, P.; Yu, J.X.; Li, J.; Dong, L.Y. Weed control, rice safety, and mechanism of the novel paddy field herbicide glyamifop. Agronomy 2022, 12, 3026. [Google Scholar] [CrossRef]
- Li, B.; Xu, J.D.; Xu, L.H.; Zhang, Z.J. Herbicides of Benzoyloxy Unsaturated Carboxylate Type. Patent C.N1325624, 22 July 2001. [Google Scholar]
- Yu, H.B.; Yang, H.B.; Cui, D.L.; Lv, L.; Li, B. Synthesis and herbicidal activity of diphenyl ether derivatives containing unsaturated carboxylates. J. Agric. Food Chem. 2011, 59, 11718–11726. [Google Scholar] [CrossRef]
- Frederick, G.; Steven, P.; Sidney, R. Process of Preparation of Substituted Diphenyl Ethers. U.S. Patent 4,424,393, 3 January 1984. [Google Scholar]
- Jozsef, B.; Balint, H.; Imer, T.; Bela, E.; Istvan, G.; Ferenc, B.; Anna, D.P.; Gyula, E.; Jenoe, K.; Eva, K.N.H.; et al. Novel Herbicide Composition. U.S. Patent 5072022A, 10 December 1991. [Google Scholar]
- Johnson, W.O. Nitrodiphenyl Ether Herbicides. U.S. Patent 5304531A, 19 April 1994. [Google Scholar]
- Yan, G.; Zekarias, B.L.; Li, X.Y.; Jaffett, V.A.; Guzei, I.A.; Golden, J.E. Divergent 2-chloroquinazolin-4(3H)-one rearrangement: Twistedcyclic guanidine formation or ring-fused N-acylguanidines via a domino process. Chem. Eur. J. 2020, 26, 2486–2492. [Google Scholar] [CrossRef]
- Lei, K.; Li, P.; Yang, X.F.; Wang, S.B.; Wang, X.K.; Hua, X.W.; Sun, B.; Ji, L.S.; Xu, X.H. Design and synthesis of novel 4-hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one derivatives for use as herbicides and evaluation of their mode of action. J. Agric. Food Chem. 2019, 67, 10489–10497. [Google Scholar]
- Wang, C.C.; Liu, H.; Zhao, W.; Li, P.; Ji, L.S.; Liu, R.M.; Lei, K.; Xu, X.H. Synthesis and herbicidal activity of 5-(1-amino-2-phenoxyethylidene)barbituric acid derivatives. Chin. J. Org. Chem. 2021, 41, 2063–2073. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, K.; Li, N.; Wang, X.K.; Wang, S.B.; Li, P.; Hua, X.W.; Lei, K.; Ji, L.S. Discovery of 3-(1-amino-2-phenoxyethylidene)-6-methyl-2H-pyran-2, 4(3H)-dione derivatives as novel herbicidal leads. Agronomy 2023, 13, 202. [Google Scholar] [CrossRef]
- Houot, V.; Etienne, P.; Petitot, A.; Barbier, S.; Blein, J.; Suty, L. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 2001, 52, 1721–1730. [Google Scholar]
- Lee, H.J.; Duke, M.V.; Birk, J.H.; Yamamoto, M.; Duke, S.O. Biochemical and physiological effects of benzheterocycles and related compounds. J. Agric. Food Chem. 1995, 43, 2722–2727. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current contributions of organofluorine compounds to the agrochemical industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. Exploration of fluorine chemistry at the multidisciplinary interface of chemistry and biology. J. Org. Chem. 2013, 78, 6358–6383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.B.; Yu, S.Y.; Liang, L.; Ismail, I.; Wang, D.W.; Li, Y.H.; Xu, H.; Wen, X.; Xi, Z. Design, synthesis, and molecular mechanism studies of N-phenylisoxazoline-thiadiazolo[3,4-a] pyridazine hybrids as protoporphyrinogen IX oxidase inhibitors. J. Agric. Food Chem. 2020, 68, 13672–13684. [Google Scholar] [PubMed]
- Pascual, M.B.; El-Azaz, J.; Torre, F.N.; Cañas, R.A.; Avila, C.; Cánovas, F.M. Biosynthesis and metabolic fate of phenylalanine in conifers. Front. Plant Sci. 2016, 7, 1030. [Google Scholar] [CrossRef] [PubMed]


| Comp. | % Injury | ||||||
|---|---|---|---|---|---|---|---|
| O. sativa | Z. mays | T. aestivum | P. miliaceum | G. hirsutum | G. max | A. hypogaea | |
| QPPE-I-4 | 74 ± 3 | 79 ± 5 | 81 ± 3 | 92 ± 4 | 0 | 0 | 0 |
| QZ | 100 | 88 ± 2 | 91 ± 4 | 100 | 19 ± 3 | 14 ± 4 | 17 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, N.; Han, S.; Fu, S.; Chen, K.; Cheng, W.; Lei, K. Synthesis, Herbicidal Activity, and Molecular Mode of Action Evaluation of Novel Quinazolinone—Phenoxypropionate Hybrids Containing a Diester Moiety. Agronomy 2024, 14, 2124. https://doi.org/10.3390/agronomy14092124
Wang S, Li N, Han S, Fu S, Chen K, Cheng W, Lei K. Synthesis, Herbicidal Activity, and Molecular Mode of Action Evaluation of Novel Quinazolinone—Phenoxypropionate Hybrids Containing a Diester Moiety. Agronomy. 2024; 14(9):2124. https://doi.org/10.3390/agronomy14092124
Chicago/Turabian StyleWang, Shumin, Na Li, Shibo Han, Shuyue Fu, Ke Chen, Wenjing Cheng, and Kang Lei. 2024. "Synthesis, Herbicidal Activity, and Molecular Mode of Action Evaluation of Novel Quinazolinone—Phenoxypropionate Hybrids Containing a Diester Moiety" Agronomy 14, no. 9: 2124. https://doi.org/10.3390/agronomy14092124







