Abstract
Fluorescence photography was used to evaluate the effects of bentazon, an herbicide that inhibits electron transport in photosystem II, on sunflower, taken as a model plant. We measured chlorophyll fluorescence to visualize the penetration, distribution, and effect of the herbicide on the plant. Our results showed that bentazon penetrates the leaf within 10–16 min, reaching full depth within 40 min. Also, we show that the herbicide is distributed along the conducting tissues of the leaf. The presence of necrotic spots, as well as the size of increased-fluorescence areas, were positively correlated to the concentration of herbicide. These findings suggest that fluorescence imaging may be a useful tool for observing the absorption and distribution of photosystem II-inhibiting herbicides in plants as an alternative to radioactive labeling in some cases.
1. Introduction
Herbicides are used to control weeds, and herbicide active ingredients account for nearly half the weight of all pesticide active ingredients used annually worldwide [1]. The action of different pesticides is mediated by different mechanisms, among them the inhibition of electron transfer from chlorophyll during photosynthesis [2,3].
Bentazon (3-isopropyl-1H-2,1,3-benzothiadiazin-4(3H)-one-2,2-dioxide) is an active ingredient of herbicides that are widely used for selective post-emergence control of broadleaf weeds in numerous summer crops, such as maize, beans, and soybean [1]. Bentazon is considered a binder to Histidine 215 of D1 protein of PSII [2,3], although molecular docking has not revealed a special role of this amino acid in bentazon binding to the QB plastoquinone binding site [4]. The mechanism of action of bentazon is associated with blocking electron transport in the photosynthetic apparatus of a plant cell. Penetrating the chloroplasts of photosynthetic cells, bentazon competes with quinone B (QB) for the binding site in the reaction center of photosystem II, thereby blocking the flow of electrons. The death of plant cells under the bentazon action is related not to energy starvation caused by the cessation of photosynthesis but to the formation of free radicals—triplet chlorophyll and singlet oxygen, which damage lipids, proteins, and pigments of photosynthetic cells [5]. The blockage of electron transport by bentazon causes dramatic changes in chlorophyll fluorescence parameters. Among them are the complete blockage of the normal fluorescence decay after the P-step in the Kautsky curve [6], the increase in chlorophyll fluorescence under constant light during the first minutes after application [7], the increase in fluorescence in J and decrease in P steps during the first day after application, the loss of variable fluorescence (FV) on the second and further days after application, and the increase in minimum (FO) and decrease in maximum fluorescence (FM) [1].
Previous studies of chlorophyll fluorescence changes after the treatment of plants with bentazon were carried out with significant time intervals between measurements or did not give a spatial picture. In our study, using a hyperspectral camera operating in the fluorescence shooting mode, we observed the appearance and spread of chlorophyll fluorescence in sunflower leaves beneath the drop of the applied bentazon preparation at intervals of 5-6 min. The study was conducted on sunflower because it is generally susceptible to bentazon, its seeds are commercially available, and it can appear as a weed in soybean fields if it comes before soybeans in crop rotation [8,9].
The observations of changes in chlorophyll fluorescence during the first minutes after the application of the photosynthetic electron transport-inhibiting herbicide should aid the process of developing formulations that ensure rapid and complete penetration of the active substance into the leaves of weeds.
2. Materials and Methods
We used sunflower plants of the cultivar Enisei (seeds purchased from JSC “Agroelita”, Moscow, Russia) at the 3rd pair of leaves stage (BBCH16) grown in a greenhouse in plastic pots with dimensions 9.5 × 9.5 × 10 cm on a peat neutralized substrate (Agrobalt S, JSC “Pindstrup”, Mytishchi, Russia) containing all essential plant nutrients.
The herbicide, a concentrated water solution of 480 g/L bentazon, was purchased from the “Shans” Group of Companies (Voronezh, Russia). The working solution of the herbicide was prepared by diluting the concentrate with distilled water to 4.8 g/L (around the normal concentration for application in the field), 0.96 g/L (5 times less), and 24 g/L (5 times more). The working solution of the herbicide was applied to the leaves in the form of a large drop, 10 µL in volume.
The plants were imaged using a hyperspectral camera Muses9-HS (Spectricon, Chania, Greece) with a lens FL-CC1614-5M (Ricoh International, Amstelveen, Netherlands) mounted on a tripod along with the original two-branch light source and using a PC with software supplied with the camera. To track the changes caused by the herbicide in time, the series of images were taken at three wavelengths: around the peaks of chlorophyll fluorescence, 685 nm (photosystem II peak), 720 nm (saddle), and 740 nm (photosystem I peak), and combined into pseudo-color images. During imaging, the plants were lit with blue (450 nm wavelength) excitation light at a photon flux density of about 7 µM/m2s. As the original camera software does not allow automatic serial shooting, we wrote a program in python3 using the “pynput” library, which allows one to automate mouse control and keyboard input (https://github.com/MikhailBazhenov/fluorescence-imaging/ accessed on 1 August 2024). The surfaces of the leaves were placed 60 cm apart from the lens and the light sources. The shooting was made at intervals of 5 min 39 s until 6 h after treatment and one more time 18 h after treatment. In between shots, the plants were illuminated by wide-spectrum halogen lamps with a photon flux density of about 25 µM/m2s, as light is supposed to be needed for bentazon action as an herbicide. Light intensity measurements were carried out using a PG200N spectrometer (UPRtek, Zhunan Township, Taiwan). To measure the spectrum of chlorophyll fluorescence in different areas of the herbicide-treated leaves, a hyperspectral image (spectral cube) of the plants was taken, which comprises the series of images in the range of wavelengths from 500 nm to 1000 nm with a 5 nm interval. During hyperspectral imaging, the plants were lit with blue excitation light with the same parameters as mentioned above.
The area of leaves occupied by increased chlorophyll fluorescence occurred under herbicide droplets, and the intensity of this fluorescence was measured using a custom program (BIMP-F) based on the “cv2” python library. This program was intended to reveal only the areas containing pixels of higher than () intensity, thus giving the margins to the spots of increased fluorescence. The spectra of fluorescence of the treated and control leaves were extracted from the spectral cube images using the HYPER-S program developed by the authors, which is based on the “cv2” python library (https://github.com/MikhailBazhenov/hyperspectral-imaging/ accessed on 1 August 2024). The spectrum data were treated with the Savitsky–Golay procedure to smooth the curves. The cross-line profiles of fluorescence intensity in the spots of herbicide action were measured using ImageJ 1.54d software. The graphics were built in MS Excel 2019. The movie, composed of time-series images, was made using MS PowerPoint 2019.
3. Results
After applying single drops of bentazon solution, increased fluorescence of chlorophyll began to appear underneath them 10 to 16 min later, indicating the penetration of the substance into the mesophyll of the leaf (Figure 1). Until 32 min after application, the spot under the drop with the lowest concentration rapidly increased in size. That was the consequence of the occasional spread of the drop over the surface. The average fluorescence intensity of these spots continued to increase until 30 to 40 min after application and then stabilized. The spots kept sharp contours until 1.5–2 h after application. After that, the areas of increased fluorescence began to gradually expand (Video S1, Data S1). At the same time (2–3 h after application), we observed more intense fluorescence at the edges of the fluorescent spots along the frontline of the spreading herbicide (Figure 2c). An increase in the fluorescent area in size was most obvious under the drop containing the highest concentration of the herbicide. The distribution of the substance within the leaf occurred mainly along its veins, both towards the edge of the leaf and towards the midrib. After 3 to 4 h after application, the increased fluorescence of chlorophyll became detectable in the midrib.
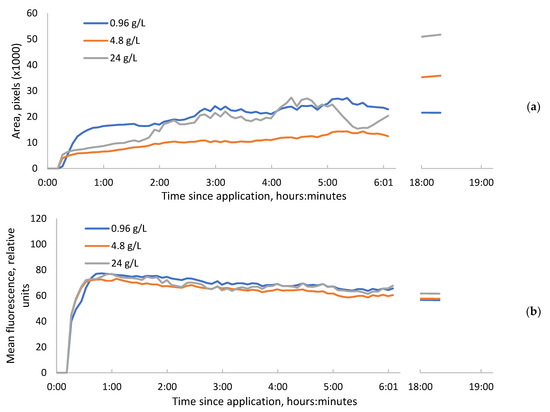
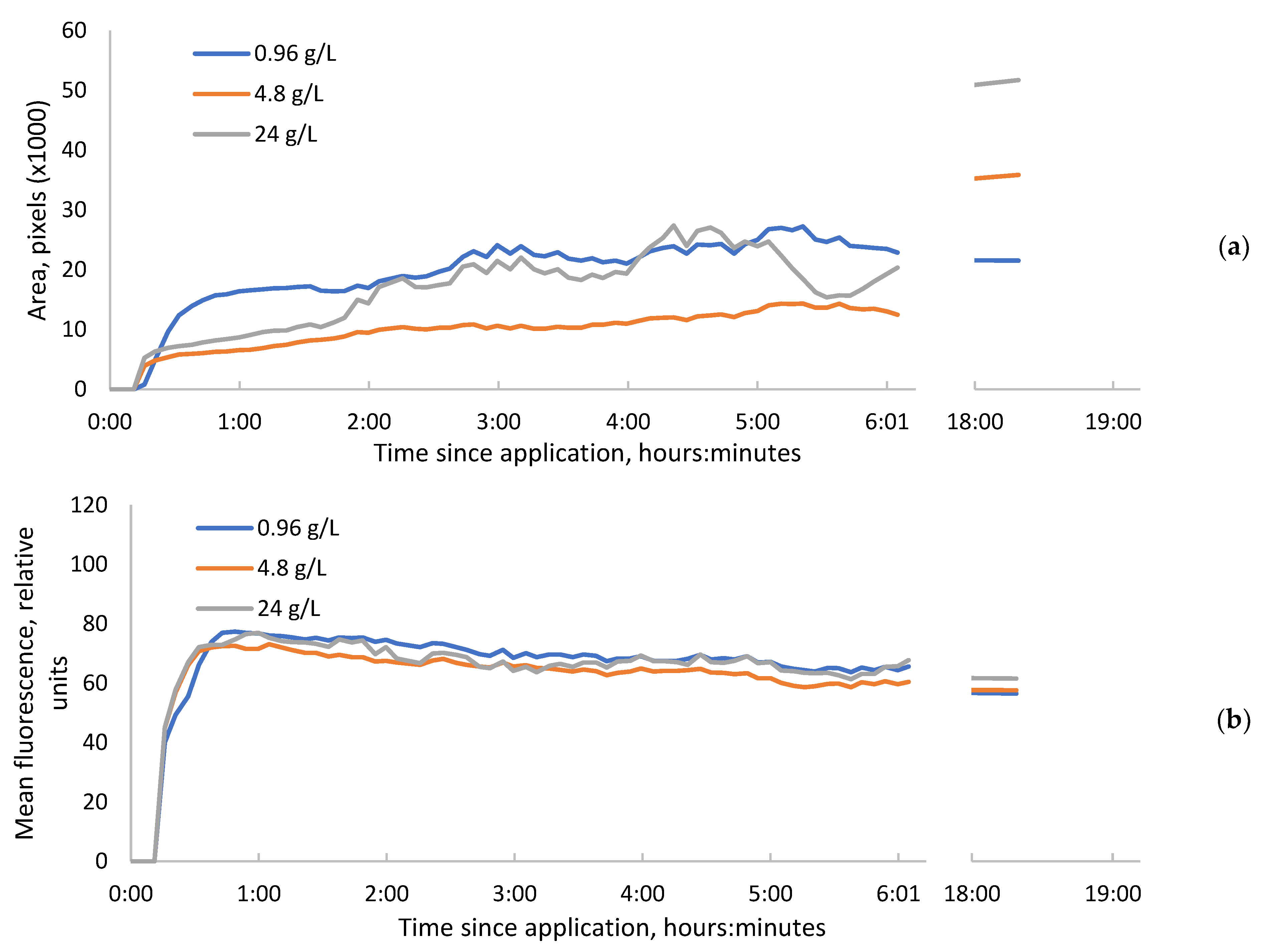
Figure 1.
The leaf area occupied by increased fluorescence, induced by bentazon 10 µL droplets (a) and mean relative intensity of fluorescence within these spots (b). The saw-like fluctuations of the curves are connected with the nutation movements of the plants.
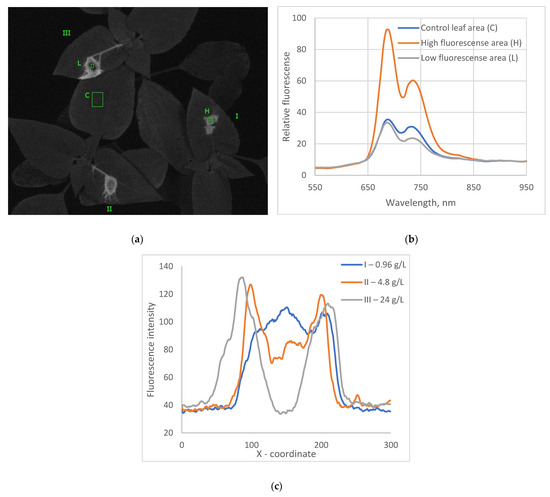
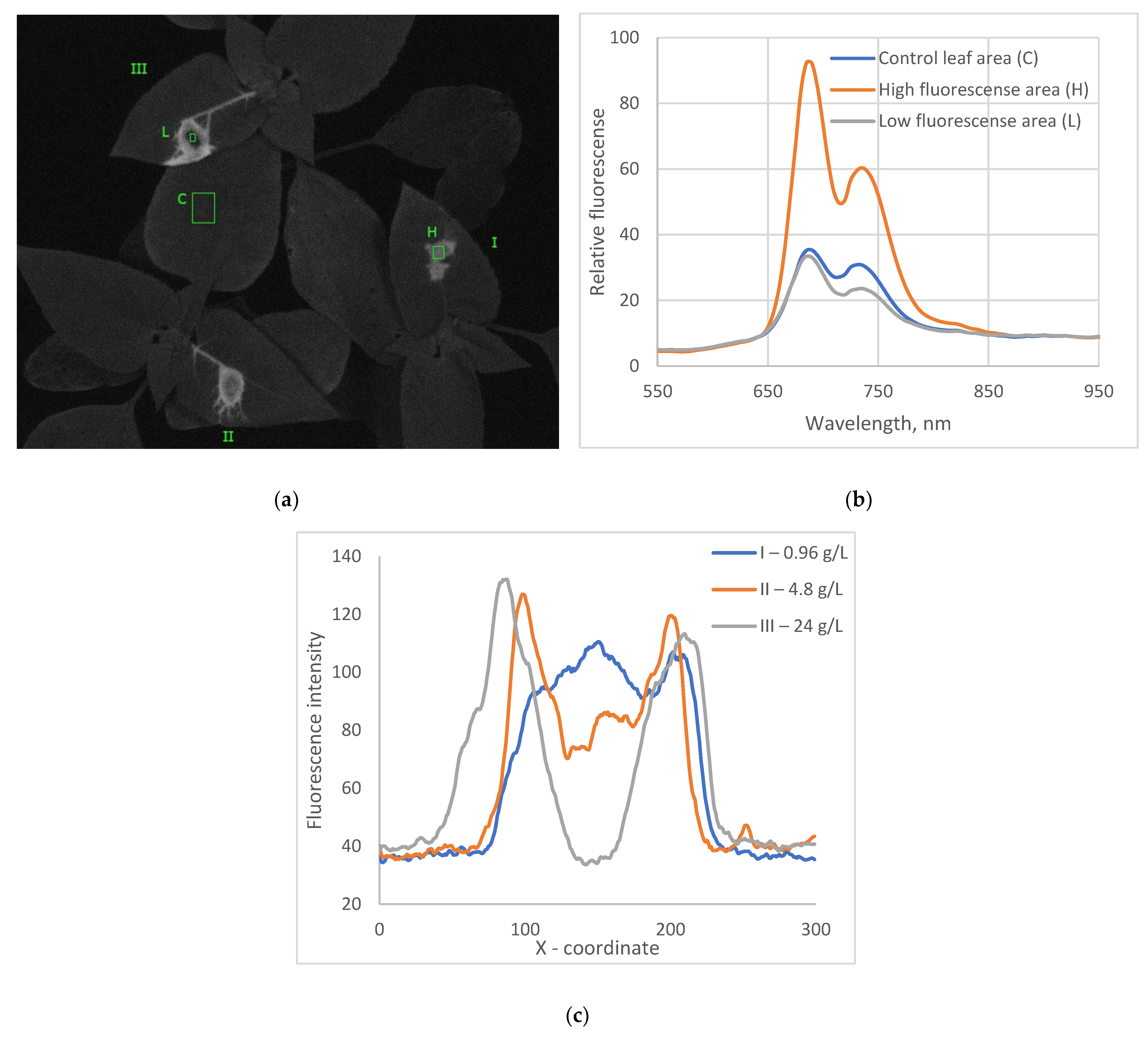
Figure 2.
The fluorescence image at 690 nm wavelength (emission) of sunflower leaves, each treated with a 10 µL droplet of bentazon herbicide solution: leaf I—0.96 g/L, leaf II—4.8 g/L (normally used in agriculture), leaf III—24 g/L, 18 h after treatment. The green rectangles represent the area of low chlorophyll fluorescence within the necrosis spot (L), the area of high chlorophyll fluorescence induced by the herbicide (H), and non-treated control leaf area (C) (a). Fluorescence spectra in the areas H, L, and C are shown on the diagram. The measured local maxima are at 690 nm and around 730 ± 5 nm for any curve. (b) The cross-line profiles of fluorescence intensity in the spots on leaves I, II, and III in the same image (c). Excitation light with a maximum wavelength of 450 nm was used.
When we observed the leaves 18 h after the application of herbicide, the dark spot of reduced fluorescence occurred within the area of increased fluorescence, just under the drop that had the highest concentrations of bentazon (Figure 2a). This may be ascribed to necrosis. At the same time, weak fluorescence that is characteristic of chlorophyll remained within this dark area (Figure 2b). The final sizes of the increased fluorescence areas were proportional to the amount of active substance applied at each drop (Figure 1a).
Within these necrosis spots, the ratio between the peaks at 690 and 735 nanometers was the highest (1.5), while it was a bit lower (1.4) in the area of increased chlorophyll fluorescence. In the leaves non-treated with herbicide, this ratio was the lowest (1.3). Under the lowest concentration drop, the necrosis or area of decreased fluorescence did not occur.
4. Discussion
The traditional method of assessing the effectiveness of a new herbicide requires a considerable amount of time. This assessment is carried out by measuring the percentage of weed death or growth inhibition a few days after laboratory treatment or after several weeks in the field [10,11]. In addition, an assessment of changes in crop yield can be conducted. Faster methods for evaluating the effectiveness of herbicides would be beneficial for both researchers developing pesticide formulations and farmers, as it would allow them to quickly identify resistant forms of weeds and take effective control measures against them in a timely manner.
Many researchers suggested the measurement of chlorophyll fluorescence parameters as an effective and non-invasive method for assessing the efficacy of herbicides, allowing for the early detection of herbicide mode of action and the sensitivity of plants before visible damage appears [6,7,12,13,14]. The measurement of chlorophyll fluorescence allows for the early detection of herbicide-resistant weeds. The quantum yield of the photosystem II (Fv/Fm) increases significantly both under the action of herbicides targeting electron transport in photosystem II and under the action of a number of herbicides with other intracellular targets. Thus, acetolactate synthase (ALS) or acetyl CoA carboxylase (ACCase) inhibitors increase the Fv/Fm. For herbicides of these groups, by measurements of the quantum yield of the photosystem II, herbicide-sensitive and resistant weeds were identified with 90% accuracy in just 5 days after treatment. Even higher accuracy could be achieved if occasional abiotic stresses could be excluded for the plants tested.
The more sensitive detection of plant response to herbicides affecting chlorophyll fluorescence compared to measuring quantum yield can be achieved by recording the Kautsky curve. This curve reflects the changes in chlorophyll fluorescence of a darkness-adapted plant leaf after the beginning of illumination. That includes the rapid stepwise transient increase in chlorophyll fluorescence occurring during 1 s of a high-intensity (saturating) light pulse. The rapid transient curve normally consists of four steps, each one higher than the previous, designated by letters O, J, I, and P. After the highest step, P, a gradual decrease in fluorescence for several minutes occurs until it reaches some stationary level [15]. From the parameters of the Kautsky curve, the relative change at the J step was a common response parameter for different herbicides and yielded consistent dose–response relationships [6]. For photosynthesis-inhibiting herbicides, changes in chlorophyll fluorescence were seen on the day of treatment [1,16]; however, the differences between resistant and susceptible genotypes become significant only some days later [17].
Various approaches to monitoring chlorophyll fluorescence provide opportunities for accelerated assessment of the effectiveness of herbicides, either acting directly on the biophysical processes of photosynthesis or indirectly [6,14]. In our study, we used fluorescent photography to register chlorophyll fluorescence. It was done using a hyperspectral camera with an external source of blue light; however, this can be done with more affordable equipment. We evaluated the effects of bentazon, an herbicide acting through inhibition of electron transport in photosystem II, thus increasing chlorophyll fluorescence on sunflower, a common model plant for herbicide testing. Using fluorescence imaging, we recorded the penetration time of the active substance into the leaf mesophyll, the dynamics of its distribution in tissues, and the formation of leaf necrosis.
We believe that the dynamics of fluorescence intensity rising during the early time after herbicide application reflects the dynamics of active substance penetration in photosynthetically active tissues. According to our results, the penetration of bentazon into sunflower leaves begins within 10–16 min. It seems that its penetration to the full depth of the mesophyll occurs within 40 min—the time it takes for the fluorescence level to reach a plateau. After that, the substance slowly spreads within the plane of the leaf and partially penetrates into the conducting system.
In our experiment, we did not get any dependence of the mean chlorophyll fluorescence intensity within treated areas and the concentration of herbicide at any time later than 40 min after application. The area of the fluorescent spot and the presence of the necrotic spot about one day after the application of bentazon were positively correlated with concentration, while fluorescence intensity in the centers of the spots was negatively correlated with the concentration in our study. The stronger chlorophyll fluorescence around the perimeter of the bentazon application area compared to the central part is due to the longer fluorescence emission of chlorophyll in the central zone, which leads to the bleaching of this chromophore. Therefore, the area of the fluorescent spot and fluorescence intensity in the centers of the spots could be useful in further studies, for example, for evaluating the sensitivity of crops and weeds to bentazon or similar inhibitors of photosynthesis or comparisons of different herbicide formulations. However, we have not yet detected a good predictor for plant death that could be used for the optimization of concentrations and that is readily visible in fluorescence images in early terms.
Considering other applications, fluorescence imaging may also prove useful for studying the absorption, transport, and distribution of active ingredients for herbicides affecting chlorophyll fluorescence, thus substituting, at least in part, radioactive labeling or labor-intensive analytical methods of detection. Overall, the fluorescence imaging of plants treated with PSII-affecting reagents provides a non-invasive assessment of the whole plant state.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14081748/s1, Video S1: The development of chlorophyll fluorescence spots on sunflower leaves under the action of bentazon droplets; Data S1: The spectra and dynamics of increased fluorescence development.
Author Contributions
Conceptualization, M.B., M.D. and A.K.; methodology, D.L. and G.K.; software, M.B.; formal analysis, M.B.; investigation, D.L.; resources, A.K.; writing—original draft preparation, M.B.; writing—review and editing, M.D., G.K., D.L. and A.K.; visualization, M.B.; supervision, G.K.; project administration, A.K.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, state assignment FGUM-2022-0008.
Data Availability Statement
Data are contained within the article or Supplementary Material. The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author. The program code used in this study is available at https://github.com/MikhailBazhenov/ (accessed on 1 August 2024).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hassannejad, S.; Porheidar Ghafarbi, S.; Lotfi, R. The Effect of Nicosulfuron and Bentazon on Photosynthetic Performance of Common Cocklebur (Xanthium strumarium L.). Environ. Sustain. Indic. 2020, 6, 100026. [Google Scholar] [CrossRef]
- 2024 HRAC Global Herbicide MoA Classification. Available online: https://hracglobal.com/tools/2024-hrac-global-herbicide-moa-classification (accessed on 6 August 2024).
- C, T.; Ta, G.; A, K.; P, L.; Fe, D. The Nexus between Reactive Oxygen Species and the Mechanism of Action of Herbicides. J. Biol. Chem. 2023, 299. [Google Scholar] [CrossRef]
- Battaglino, B.; Grinzato, A.; Pagliano, C. Binding Properties of Photosynthetic Herbicides with the QB Site of the D1 Protein in Plant Photosystem II: A Combined Functional and Molecular Docking Study. Plants 2021, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Czékus, Z.; Farkas, M.; Bakacsy, L.; Ördög, A.; Gallé, Á.; Poór, P. Time-Dependent Effects of Bentazon Application on the Key Antioxidant Enzymes of Soybean and Common Ragweed. Sustainability 2020, 12, 3872. [Google Scholar] [CrossRef]
- Christensen, M.G.; Teicher, H.B.; Streibig, J.C. Linking Fluorescence Induction Curve and Biomass in Herbicide Screening. Pest Manag. Sci. 2003, 59, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, G.; Hilton, J.L.; St. John, J.B. Inhibition of Photosynthesis by Bentazon in Intact Plants and Isolated Cells in Relation to the pH. Z. Für Naturforschung C 1979, 34, 944–947. [Google Scholar] [CrossRef]
- Irons, S.M.; Burnside, O.C. Selective Control of Sunflower in Soybeans. Agron. J. 1982, 74, 291–296. [Google Scholar] [CrossRef]
- Allen, J.R.; Johnson, W.G.; Smeda, R.J.; Wiebold, W.J.; Massey, R.E. Management of Acetolactate Synthase (ALS)-Resistant Common Sunflower (Helianthus Annuus L.) in Soybean (Glycine max). Weed Technol. 2001, 15, 571–575. [Google Scholar] [CrossRef]
- Chen, X.X.; Yang, H.F.; Qu, B.; Pang, Q.M.; Jiang, N.; He, L.L.; Zhao, F.J.; Shao, M.N. Evaluation of the Indoor Efficacy of Various Foliar-Applied Herbicides on Sicyos Angulatus Seedlings. J. Exp. Agric. Int. 2024, 46, 888–897. [Google Scholar] [CrossRef]
- Kousta, A.; Katsis, C.; Tsekoura, A.; Chachalis, D. Effectiveness and Selectivity of Pre- and Post-Emergence Herbicides for Weed Control in Grain Legumes. Plants 2024, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Peteinatos, G.; Li, H.; Gerhards, R. Rapid In-Season Detection of Herbicide Resistant Alopecurus Myosuroides Using a Mobile Fluorescence Imaging Sensor. Crop Prot. 2016, 89, 170–177. [Google Scholar] [CrossRef]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Camlin, M.S. Chlorophyll Fluorescence as a Rapid Test for Reaction to Urea Herbicides in Winter Wheat. J. Agric. Sci. 1988, 110, 627–632. [Google Scholar] [CrossRef]
- Strasserf, R.J.; Srivastava, A.; Govindjee. Polyphasic Chlorophyll a fluorescence transient in plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Kocurek, V.; Smutný, V.; Filová, J. Chlorophyll Fluorescence as an Instrument for the Assessment of Herbicide Efficacy. Cereal Res. Commun. 2009, 37, 289–292. [Google Scholar]
- Lichtenthaler, H.K.; Meier, D.; Retzlaff, G.; Hamm, R. Distribution and Effects of Bentazon in Crop Plants and Weeds. Z. Für Naturforschung C 1982, 37, 889–897. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).