Responses of Leaf Senescence for Stipa krylovii to Interactive Environmental Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Experimental Design
2.3. Phenological Observation
2.4. Statistical Analyses
3. Results
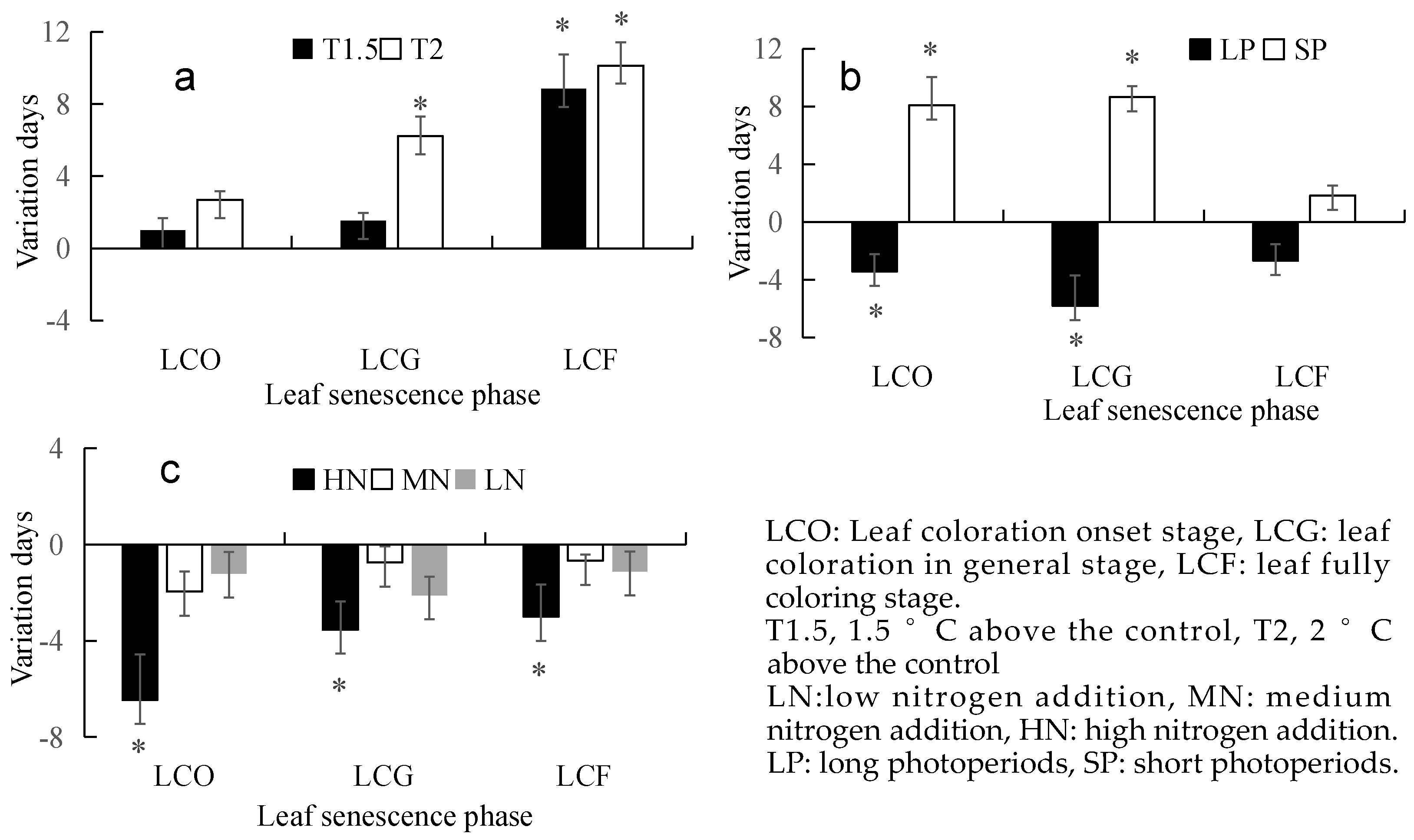
3.1. Effects of a Single Environmental Factor
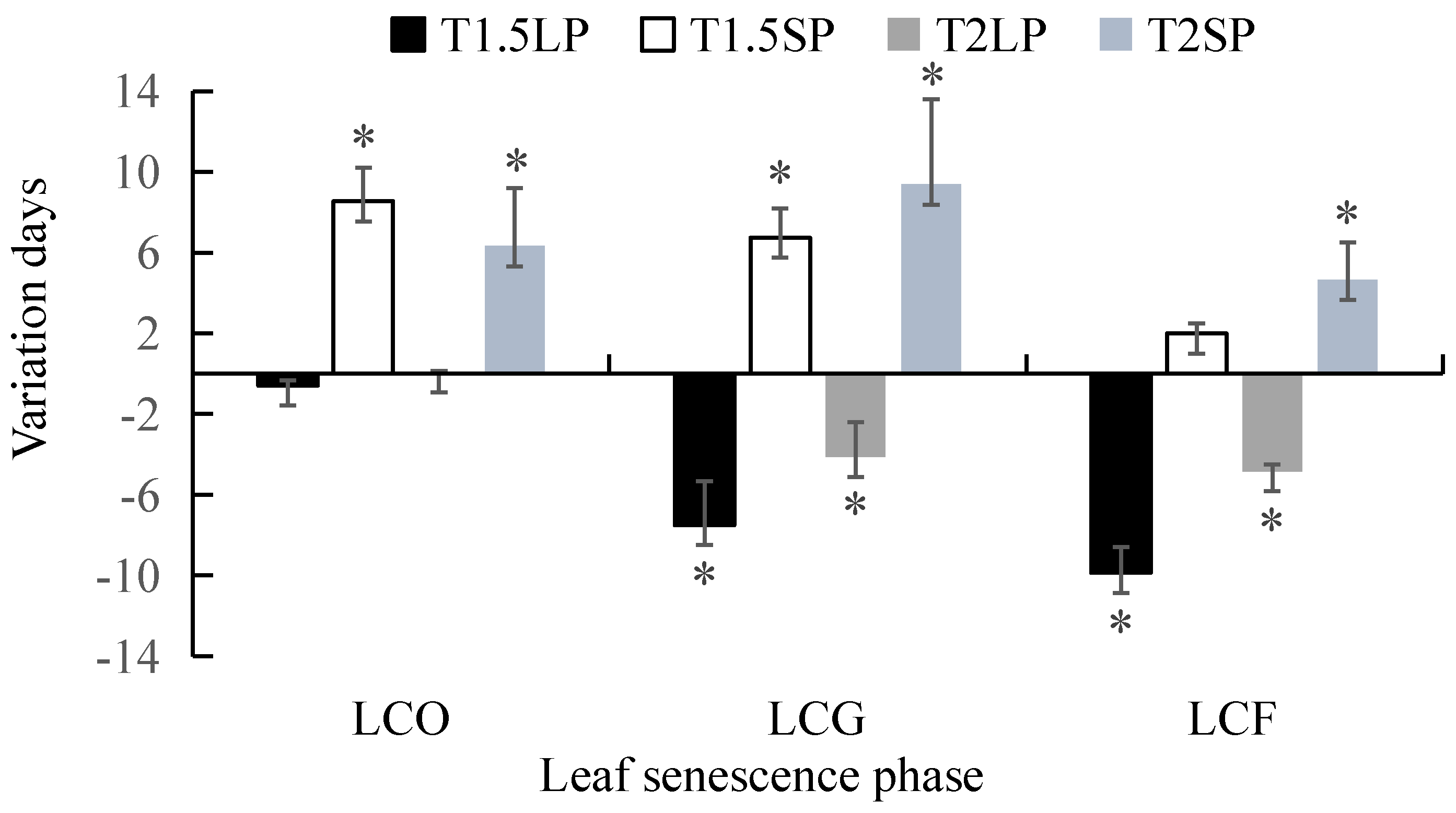
3.2. Effects of the Interaction between Temperature and Photoperiod
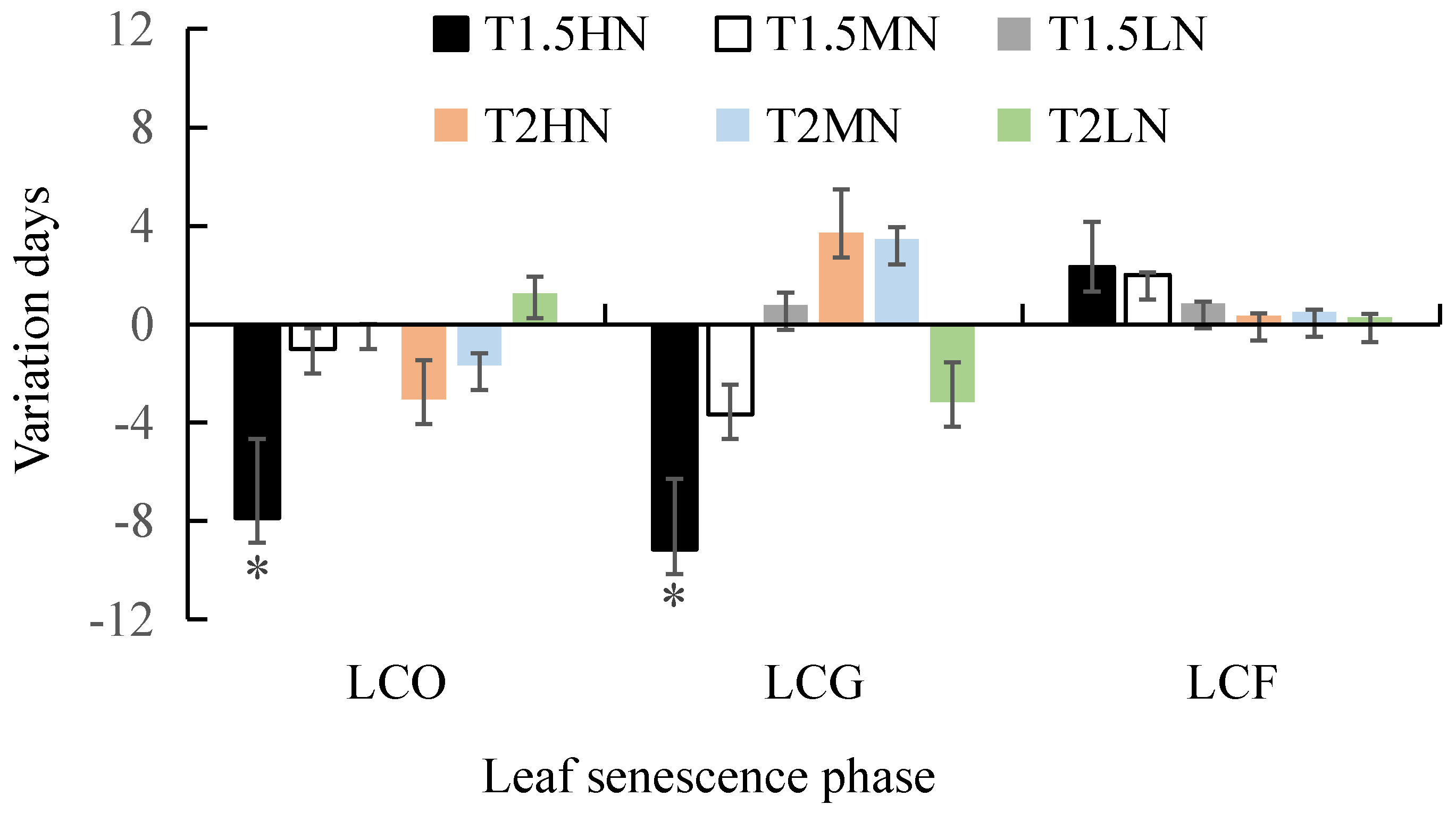
3.3. Effects of the Interaction between Temperature and Nitrogen Addition
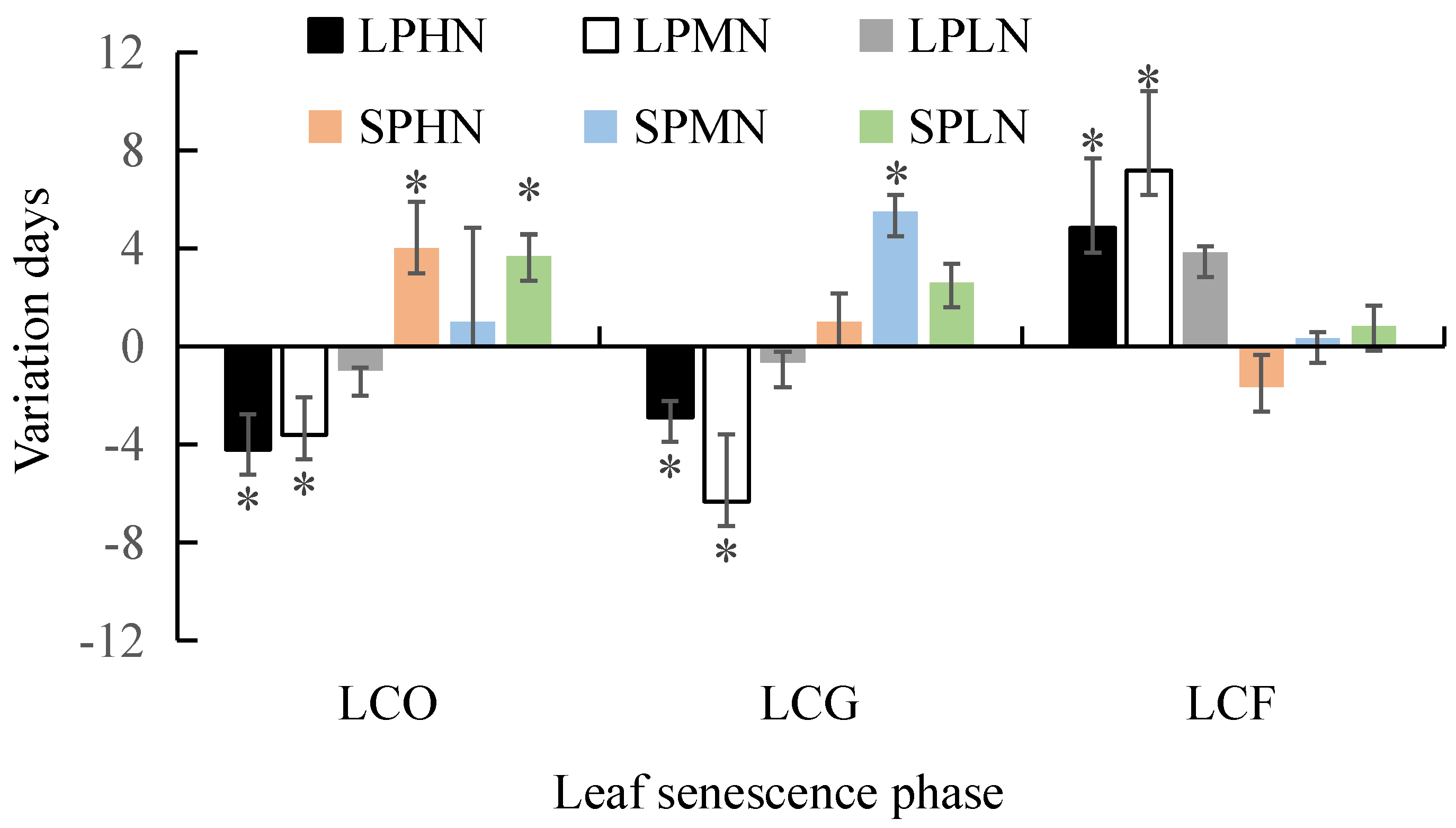
3.4. Effects of the Interaction between Photoperiod and Nitrogen Addition
3.5. Effects from the Interactions among Temperature, Photoperiod, and Nitrogen Addition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, X.; Shen, M.; Wu, C.; Peñuelas, J.; Ciais, P.; Zhang, J.; Freeman, C.; Palmer, P.I.; Liu, B.; Henderson, M.; et al. Critical role of water conditions in the responses of autumn phenology of marsh wetlands to climate change on the Tibetan Plateau. Glob. Change Biol. 2024, 30, e17097. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Zohner, C.M.; Renner, S.S. Common garden comparison of the leaf-out phenology of woody species from different native climates, combined with herbarium records, forecasts long-term change. Ecol. Lett. 2014, 17, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, C.; Ge, Q. Low temperature and short daylength interact to affect the leaf senescence of two temperate tree species. Tree Physiol. 2022, 42, 2252–2265. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Lin, S.; Wang, H.; Ge, Q.; Hu, Z. Effects of chilling on heat requirement of spring phenology vary between years. Agric. Forest Meteorol. 2022, 312, 108718. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, H.; Rutishauser, T.; Dai, J. Phenological response to climate change in China: A meta-analysis. Glob. Change Biol. 2015, 21, 265–274. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Jia, Q.; Zhou, G. Increasing temperature shortened the carbon uptake period and decreased the cumulative net ecosystem productivity in a maize cropland in Northeast China. Field Crop Res. 2021, 267, 108150. [Google Scholar] [CrossRef]
- Zhu, W.; Tian, H.; Xu, X.; Pan, Y.; Chen, G.; Lin, W. Extension of the growing season due to delayed autumn over mid and high latitudes in North America during 1982–2006. Glob. Ecol. Biogeogr. 2012, 21, 260–271. [Google Scholar] [CrossRef]
- Garonna, I.; De Jong, R.; De Wit, A.J.W.; Mücher Caspar, A.; Schmid, B.; Schaepman, M.E. Strong contribution of autumn phenology to changes in satellite-derived growing season length estimates across Europe (1982–2011). Glob. Change Biol. 2015, 20, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Tuya, A.; Bayarsaikhan, S.; Dorjsuren, A.; Mandakh, U.; Bao, Y.; Li, C.; Vanchindorj, B. Variations and climate constraints of terrestrial net primary productivity over Mongolia. Quat. Int. 2022, 537, 112–125. [Google Scholar] [CrossRef]
- Wu, C.; Peng, J.; Ciais, P.; Peñuelas, J.; Wang, H.; Beguería, S.; Andrew Black, T.; Jassal, R.S.; Zhang, X.; Yuan, W.; et al. Increased drought effects on the phenology of autumn leaf senescence. Nat. Clim. Chang. 2022, 12, 943–949. [Google Scholar] [CrossRef]
- Maurya, J.P.; Bhalerao, R.P. Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: A molecular perspective. Ann. Bot. 2007, 120, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Chuine, I.; Bonhomme, M.; Legave, J.M.; García de Cortázar-Atauri, I.; Charrier, G.; Lacointe, A.; Améglio, T. Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Glob. Change Biol. 2016, 22, 3444–3460. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, W.; Liu, E.; Zhou, L.; Song, X.; Lv, X.; Ji, Y. Plant Phenology Simulation and Trigger Threshold Based on Total Climatic Production Factors—A Case Study of Stipa krylovii Phenology. Agronomy 2023, 13, 1768. [Google Scholar] [CrossRef]
- Lesica, P.; Kittelson, P.M. Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J. Arid. Environ. 2010, 74, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, J.; Zhang, D. Phenological observations on Larix principis-rupprechtii Mayr. in primary seed orchard. J. For. Res. 2001, 12, 201–204. [Google Scholar] [CrossRef]
- Ren, S.; Li, Y.; Peichl, M. Diverse effects of climate at different times on grassland phenology in mid-latitude of the Northern Hemisphere. Ecol. Indic. 2020, 113, 106260. [Google Scholar] [CrossRef]
- Heide, O.M. Temperature rather than photoperiod controls growth cessation and dormancy in Sorbus species. J. Exp. Bot. 2011, 62, 5397–5404. [Google Scholar] [CrossRef]
- Lang, W.; Chen, X.; Qian, S.; Liu, G.; Piao, S. A new process-based model for predicting autumn phenology: How is leaf senescence controlled by photoperiod and temperature coupling? Agric. For. Meteorol. 2019, 268, 124–135. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Cleland, E.E. Warming experiments underpredict plant phenological responses to climate change. Nature 2011, 485, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, T.; Luterbacher, J.; Defla, C.; Frank, D.; Wanner, H. Swiss spring plant phenology 2007: Extremes, a multi-century perspective, and changes in temperature sensitivity. Geophys. Res. Lett. 2008, 35, L05703. [Google Scholar] [CrossRef]
- Yu, H.; Luedeling, E.; Xu, J. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2010, 107, 22151–22156. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, J.; Li, X.; Cheng, G.; Ma, M.; Zhu, G.; Arain, M.A.; TA Black, T.A.; Jassal, R.S. No trends in spring and autumn phenology during the global warming hiatus. Nat. Commun. 2019, 10, 2389. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Uelas, J.P. Declining global warming efects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Monaco, T.A.; Sun, F.; Liu, W.; Gan, Y.; Sun, G. Altered precipitation patterns and simulated nitrogen deposition effects on phenology of common plant species in a Tibetan Plateau alpine meadow. Agric. For. Meteorol. 2017, 236, 36–47. [Google Scholar] [CrossRef]
- Smith, J.; Sconiers, W.; Spasojevic, M.; Ashton, I.; Suding, K. Phenological changes in alpine plants in response to increased snowpack, temperature, and nitrogen. Arct. Antarct. Alp. Res. 2012, 44, 135–142. [Google Scholar] [CrossRef]
- Petraglia, A.; Tomaselli, M.; Mondoni, A.; Brancaleoni, L.; Carbognani, M. Effects of nitrogen and phosphorus supply on growth and flowering phenology of the snowbed forb Gnaphalium supinum L. Flora 2014, 209, 271–278. [Google Scholar] [CrossRef]
- Matson, P.; Lohse, K.A.; Hall, S.J. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio 2002, 31, 113–119. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xu, L.; Liu, L.; Ding, D. Modeling greenup date of dominant grass species in the Inner Mongolian Grassland using air temperature and precipitation data. Int. J. Biometeorol. 2013, 58, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhou, G.; Wang, Y.; Han, X.; Wang, Y. Simulating phenological characteristics of two dominant grass species in a semi-arid steppe ecosystem. Ecol. Res. 2007, 22, 784–791. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Lv, X.; He, Q.; Zhou, M. Effects of Phenological Changes on Plant Production-From the View of Stipa krylovii. Agronomy 2022, 12, 3208. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Ge, Q.; Dai, J. The Interactive Effects of Chilling, Photoperiod, and Forcing Temperature on Flowering Phenology of Temperate Woody Plants. Front. Plant Sci. 2020, 11, 443. [Google Scholar] [CrossRef]
- Pletsers, A.; Caffarra, A.; Kelleher, C.T.; Donnelly, A. Chilling temperature and photoperiod influence the timing of bud burst in juvenile Betula pubescens Ehrh. and Populus tremula L. trees. Ann. For. Sci. 2015, 72, 941–953. [Google Scholar] [CrossRef]
- Caffarra, A.; Donnelly, A.; Chuine, I.; Jones, M.B. Modelling the timing of Betula pubescens budburst. I. Temperature and photoperiod: A conceptual model. Clim. Res. 2011, 46, 147–157. [Google Scholar] [CrossRef]
- Ettinger, A.K.; Chamberlain, C.J.; Morales-Castilla, I.; Buonaiuto, D.M.; Flynn, D.F.B.; Savas, T.; Samaha, J.A.; Wolkovich, E.M. Winter temperatures predominate in spring phenological responses to warming. Nat. Clim. Chang. 2020, 10, 1137–1142. [Google Scholar] [CrossRef]
- China Meteological Administration (Ed.) Observation Criterion of Agicultural Meteorology; China Meterological Press: Beijing, China, 1993; pp. 131–164. (In Chinese) [Google Scholar]
- Tao, Z.; Wang, H.; Dai, J.; Alatalo, J.; Ge, Q. Modeling spatiotemporal variations in leaf coloring date of three tree species across China. Agric. For. Meteorol. 2018, 249, 310–318. [Google Scholar] [CrossRef]
- Doi, H.; Takahashi, M. Latitudinal patterns in the phenological responses of leaf colouring and leaf fall to climate change in Japan. Glob. Ecol. Biogeogr. 2008, 17, 556–561. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, H.; Shen, M.; Liang, W.; Jiang, L. Changes in autumn vegetation dormancy onset date and the climate controls across temperate ecosystems in China from 1982 to 2010. Glob. Chang. Biol. 2015, 21, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hnninen, H.; Liu, Y.; Sun, W.; Janssens, I.A.; Campioli, M. Larger temperature response of autumn leaf senescence than spring leaf-out phenology. Glob. Chang. Biol. 2018, 24, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Cong, N.; Shen, M.; Piao, S. Spatial variations in responses of vegetation autumn phenology to climate change on the Tibetan Plateau. J. Plant Ecol. 2016, 10, 744–752. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Zhou, X.; Geng, X.; Guo, Y.; Zhang, Y. Progress in plant phenology modeling under global climate change. Sci. China Earth Sci. 2020, 63, 1237–1247. [Google Scholar] [CrossRef]
- Estiarte, M.; Penuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Change Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- Soolanayakanahally, R.Y.; Guy, R.D.; Silim, S.N.; Song, M. Timing of photoperiodic competency causes phenological mismatch in balsam poplar (Populus balsamifera L.). Plant Cell Environ. 2013, 36, 116–127. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, D.; Shi, P.; Singh, V.P.; Sun, P. Vegetation phenology on the Qinghai-Tibetan Plateau and its response to climate change (1982–2013). Agric. For. Meteorol. 2018, 248, 408–417. [Google Scholar] [CrossRef]
- Zu, J.; Zhang, Y.; Huang, K.; Liu, Y.; Chen, N.; Cong, N. Biological and climate factors co-regulated spatial-temporal dynamics of vegetation autumn phenology on the Tibetan Plateau. Int. J. Appl. Earth Obs. 2018, 69, 198–205. [Google Scholar] [CrossRef]
- Basler, D.; Korner, C. Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agric. For. Meteorol. 2012, 165, 73–81. [Google Scholar] [CrossRef]
- Laube, J.; Sparks, T.H.; Estrella, N.; Höfler, J.; Ankerst, D.P.; Menzel, A. Chilling outweighs photoperiod in preventing precocious spring development. Glob. Chang. Biol. 2013, 20, 170–182. [Google Scholar] [CrossRef]
- Liu, Q.; Delpierre, N.; Campioli, M. Photoperiod alone does not explain the variations of leaf senescence onset across Europe. Agric. For. Meteorol. 2024, 355, 110134. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Wolkovich, E.M. Temperature and photoperiod drive spring phenology across all species in a temperate forest community. New Phytol. 2018, 219, 1353–1362. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chiariello, N.R.; Loarie, S.R.; Mooney, H.A.; Field, C.B. Diverse responses of phenology to global changes in a grassland ecosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 13740–13744. [Google Scholar] [CrossRef] [PubMed]
- Fracheboud, Y.; Luquez, V.; Bjorken, L.; Sjodin, A.; Tuominen, H.; Jansson, S. The control of autumn senescence in european Aspen. Plant Physiol. 2009, 149, 1982–1991. [Google Scholar] [CrossRef]
- Xia, J.; Wan, S. Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Ann. Bot. 2013, 111, 1207–1217. [Google Scholar] [CrossRef] [PubMed]


| Month | T (°C) | T1.5 (°C) | T2 (°C) | P (h) | LP (h) | SP (h) | RH (%) | Rainfall (mm) | IM (mL) |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 5.8 | 7.3 | 7.8 | 14 | 18 | 10 | 38.2 | 7.5 | 23.5 |
| 5 | 13.7 | 15.2 | 15.8 | 14 | 18 | 10 | 38.2 | 25.4 | 77.1 |
| 6 | 19.2 | 20.7 | 21.2 | 14 | 18 | 10 | 48.9 | 51.7 | 162.2 |
| 7 | 21.9 | 23.4 | 23.9 | 14 | 18 | 10 | 58.5 | 77.6 | 235.8 |
| 8 | 20.1 | 21.6 | 22.1 | 14 | 18 | 10 | 57.7 | 53.0 | 161.2 |
| 9 | 13.4 | 14.9 | 15.4 | 14 | 18 | 10 | 52.0 | 23.8 | 74.6 |
| 10 | 4.3 | 5.8 | 6.3 | 14 | 18 | 10 | 52.5 | 12.8 | 38.8 |
| Variables | LCO | LCG | LCF |
|---|---|---|---|
| T | 11.9 ** | 180.5 ** | 511.5 ** |
| P | 436.9 ** | 800.7 ** | 113.5 ** |
| N | 57.4 ** | 35.5 ** | 12.6 ** |
| T × P | 54.7 ** | 6.9 ** | 163.4 ** |
| T × N | 12.5 ** | 56.5 ** | 28.1 ** |
| P × N | 24.8 ** | 81.5 ** | 44.7 ** |
| T × P × N | 84.8 ** | 69.9 ** | 25.8 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhou, G. Responses of Leaf Senescence for Stipa krylovii to Interactive Environmental Factors. Agronomy 2024, 14, 2145. https://doi.org/10.3390/agronomy14092145
Song X, Zhou G. Responses of Leaf Senescence for Stipa krylovii to Interactive Environmental Factors. Agronomy. 2024; 14(9):2145. https://doi.org/10.3390/agronomy14092145
Chicago/Turabian StyleSong, Xingyang, and Guangsheng Zhou. 2024. "Responses of Leaf Senescence for Stipa krylovii to Interactive Environmental Factors" Agronomy 14, no. 9: 2145. https://doi.org/10.3390/agronomy14092145
APA StyleSong, X., & Zhou, G. (2024). Responses of Leaf Senescence for Stipa krylovii to Interactive Environmental Factors. Agronomy, 14(9), 2145. https://doi.org/10.3390/agronomy14092145







