Traditional Strategies and Cutting-Edge Technologies Used for Plant Disease Management: A Comprehensive Overview
Abstract
:1. Introduction
1.1. Conventional Agricultural Practices
Limitations of Conventional Agricultural Practices
1.2. Chemical Pesticides
1.3. Biopesticides
1.4. Molecular Techniques/Approaches
1.4.1. Meganucleases (MegNs)
1.4.2. Zinc Finger Nucleases (ZFNs)
1.4.3. Transcription Activator-like Effector Nucleases (TALENs)
1.4.4. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
2. Nanotechnology Used for Crop Protection
2.1. Inorganic NPs
2.1.1. Metallic NPs Used for Plant Disease Management
2.1.2. Metal Oxide NPs
2.2. Organic NPs
2.3. Carbon-Based NPs
2.4. Polymeric NPs
| Polymeric NPs | NPs Type | Concentration | Disease & Pathogen Management | References |
|---|---|---|---|---|
| Chitosan | Moringa chitosan NPs | 200 mg/L | Rice blast (Magnoparthae oryzae) | [311] |
| Chitosan in acetic acid distilled water solution | 4 g/L | Dry rot and wilt (Fusarium sp.) | [312] | |
| Chitosan biopolymer | 2.5 mg/mL | Powdery mildew of cucumber | [313,314] | |
| Chitosan | 100 and 200 µg/mL | Bacterial wilt of potato and tomato | [315] | |
| Bioengineered chitosan iron nanocomposites | In vitro: 250 μg m/L In vivo: 250 μg m/L | Bacterial leaf blight of rice (Xanthomonas oryzae pv. oryzae) | [172] | |
| Chitosan | 300 mg/L and 400 mg/L | Bean yellow mosaic virus of faba bean | [316] | |
| Chitosan composite film having chitosan, calcium, auxiliaries, ferulic acid and dextrin | 0.71–1.42 g/L | Soft rot of kiwi (B. dothidea and Phomopsis sp.) | [317] | |
| Copper chitosan NPs | 0.10, 0.20, and 0.30 mg/mL | Fusarium wilt of banana (Fusarium oxysporum f. sp. cubense) | [318] | |
| Chitosan | 0.1–2.0 g/L | Root rot of fenugreek (Fusarium solani) | [319] | |
| Chitosan | 500 mg/L | Powdery mildew of Rosa roxburghii | [320] | |
| Chitosan | 0.2 g/L and 0.4 g/L | Blue mold of apple (Penicillium expansum) | [321] | |
| Chitosan/dextran NPs | 100 µg m/L | Alfalafa mosaic virus on Nicotiana glutinosa plant | [322] | |
| Nickle chitosan nanocojugate | 0.04 mg/mL | Fusarium rot of wheat | [323] | |
| Fluoroalkenyl-Grafted Chitosan Oligosaccharide Derivative | 1 mg/mL | Root knot nematode (Meloidogyne incongita) | [324] | |
| Chitosan along with botanicals (Argemone mexicana L., Achyranthes aspera L., and Ricinus communis L.) | 2500, 2000, 1500, 1000, and 500 ppm | Meloidogyne incongita in carrot | [325] | |
| Zein NPs | Natamycin-loaded zein-casein NPs (N-Z/C NPs) | 20 and 80 µg/m | Brown rot of peach (Monilinia fructicola) | [326] |
| Carvacrol-loaded zein NPs | 135 μg/mL and 270 μg/mL | Bacterial canker (Pseudomonas syringae) Fusarium wilt (Fusarium oxysporum) | [327] | |
| Natamycin-loaded zein NPs stabilized by carboxymethyl chitosan | 10 mg/L | Postharvest gray mold, rot and mildew of strawberry | [313] | |
| Satureja montana Essential Oil in combination with zein NPs | 1 mg/mL | Bacterial spot of tomato (Xanthomonas sp.) | [314] | |
| Rotenone loaded zein NPs | 16 μg m/L 48 μg m/L | Pseudomonas syringae Fusarium oxysporum | [328] | |
| PLGA NPs | CTAB-PLGA Curcumin NP | 52.57 μg/mL and 44.67 μg/mL and 15 μg/mL | Pythium ultimum var. ultimum | [329] |
| Poly (lactic-co-glycolic acid) NPs(PLGA NPs) | 1.25–0.07 μg mL | Gray mold disease (Botrytis cinerea) | [330] | |
| Alginate NPs | Alginate oligosaccharide (AOS) combined with Meyerozyma guilliermondii | 5 g/L | Blue mold decay (Penicillium expansum) | [331] |
| Alginate oligosaccharide (AOS) | 50 mg/L | Gray mold of kiwi fruit (Botrytis cinerea) | [332] | |
| Alginate polysaccharide | 1 g/L | Bayoud disease of date palm (Fusarium oxysporum f. sp. albedins) | [333] | |
| Alginate | 2 g/L | Verticillium wilt of olive (Verticillium dahliae) | [334] | |
| Nano Cu-Cu2O/Alginate | 17.8 mg Cu/L. | Rice blast (Pyricularia oryzae) | [335] |
2.5. Limitations of Nanotechnology
3. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elizabath, A.; Babychan, M.; Mathew, A.M.; Syriac, G.M. Application of nanotechnology in agriculture. Int. J. Pure Appl. Biosci. 2019, 7, 131–139. [Google Scholar] [CrossRef]
- Ma, W.; Hong, S.; Reed, W.R.; Duan, J.; Luu, P. Yield effects of agricultural cooperative membership in developing countries: A meta-analysis. Ann. Public Coop. Econ. 2023, 94, 761–780. [Google Scholar] [CrossRef]
- Asfaw, S.; Pallante, G.; Palma, A. Distributional impacts of soil erosion on agricultural productivity and welfare in Malawi. Ecol. Econ. 2020, 177, 106764. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Lachaud, M.A.; Bravo-Ureta, B.E.; Ludena, C.E. Economic effects of climate change on agricultural production and productivity in Latin America and the Caribbean (LAC). Agric. Econ. 2022, 53, 321–332. [Google Scholar] [CrossRef]
- Varma, V.; Bebber, D.P. Climate change impacts on banana yields around the world. Nat. Clim. Chang. 2019, 9, 752–757. [Google Scholar] [CrossRef]
- Moore, D.; Robson, G.D.; Trinci, A.P. 21st Century Guidebook to Fungi; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Crooks, J.A. Lag times and exotic species: The ecology and management of biological invasions in slow-motion1. Ecoscience 2005, 12, 316–329. [Google Scholar] [CrossRef]
- Goss, E.M.; Carbone, I.; Grünwald, N.J. Ancient isolation and independent evolution of the three clonal lineages of the exotic sudden oak death pathogen Phytophthora ramorum. Mol. Ecol. 2009, 18, 1161–1174. [Google Scholar] [CrossRef]
- Moralejo, E.P.; Pérez-Sierra, A.M.; Álvarez, L.A.; Belbahri, L.; Lefort, F.; Descals, E. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathol. 2009, 58, 100–110. [Google Scholar] [CrossRef]
- Katan, J. Diseases caused by soilborne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Yadav, B.; Gurjar, M.K.; Sheshma, R.; Chopdar, R. Management Strategies for Seed and Soil Borne Diseases in Vegetable Production. Agric. Mag. 2022, 1, 35–40. [Google Scholar]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Baysal-Gurel, F.; Gardener, B.M.; Miller, S.A. Soil Borne Disease Management in Organic Vegetable Production. 2012. Available online: https://eorganic.org/node/7581 (accessed on 27 August 2024).
- Baysal-Gurel, F.; Liyanapathiranage, P.; Mullican, J. Biofumigation: Opportunities and challenges for control of soilborne diseases in nursery production. Plant Health Prog. 2018, 19, 332–337. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W. Effects of different 3-year cropping systems on soil microbial communities and Rhizoctonia diseases of potato. Phytopathology 2006, 96, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Baysal-Gurel, F.; Liyanapathiranage, P.; Addesso, K.M. Effect of Brassica crop-based biofumigation on soilborne disease suppression in woody ornamentals. Can. J. Plant Pathol. 2020, 42, 94–106. [Google Scholar] [CrossRef]
- Tanaka, S.; Kobayashi, T.; Iwasaki, K.; Yamane, S.; Maeda, K.; Sakurai, K. Properties and metabolic diversity of microbial communities in soils treated with steam sterilization compared with methyl bromide and chloropicrin fumigations. Soil. Sci. Plant Nutr. 2003, 49, 603–610. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Butler, D.M.; Holzinger, J.; Rosskopf, E.N. Evaluation of steam for meloidogyne arenaria control in production of in-ground floriculture crops in florida. J. Nematol. 2016, 48, 183–192. [Google Scholar] [CrossRef]
- El-Sharouny, E.E. Effect of different soil amendments on the microbial count correlated with resistance of apple plants towards pathogenic Rhizoctonia solani AG-5. Biotechnol. Biotechnol. Equip. 2015, 29, 463–469. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M.; Wong, P.T.; Mead, A.; Howe, G.; Newell, M. Field studies on the biofumigation of take-all by Brassica break crops. Aust. J. Agric. Res. 2000, 51, 445–456. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Welke, S.E. The effect of compost extract on the yield of strawberries and the severity of Botrytis cinerea. J. Sustain. Agric. 2005, 25, 57–68. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Miller, R.O. Compost Quality Attributes, Measurements, and Variability. In Compost Utilization In Horticultural Cropping Systems; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Luvisi, A.; Panattoni, A.; Materazzi, A. Heat treatments for sustainable control of soil viruses. Agron. Sustain. Dev. 2015, 35, 657–666. [Google Scholar] [CrossRef]
- Kwerepe, B.; Labuschagne, N. Biofumigation and solarization as integrated pest management (IPM) components for control of root knot nematode (Meloidogyne incognita (Kofoid & White) Chitwoodi) on bambara groundnut (Vigna subterranea (L.) Verdc.). UNISWA J. Agric. 2003, 11, 56–63. [Google Scholar]
- Abeyratne, R. Regulation of Air Transport; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sarkar, S.; Gil, J.D.; Keeley, J.; Jansen, K. The Use of Pesticides in Developing Countries and Their Impact on Health and the Right to Food; European Union: Brussels, Belgium, 2021. [Google Scholar]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Lamichhane, J.R. Pesticide use and risk reduction in European farming systems with IPM: An introduction to the special issue. Crop Prot. 2017, 97, 1–6. [Google Scholar] [CrossRef]
- Khan, B.A.; Nadeem, M.A.; Nawaz, H.; Amin, M.M.; Abbasi, G.H.; Nadeem, M.; Ali, M.; Ameen, M.; Javaid, M.M.; Maqbool, R.; et al. Pesticides: Impacts on agriculture productivity, environment, and management strategies. In Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies; Springer: Berlin/Heidelberg, Germany, 2023; pp. 109–134. [Google Scholar]
- Ahemad, M.; Khan, M. Toxicological assessment of selective pesticides towards plant growth promoting activities of phosphate solubilizing Pseudomonas aeruginosa. Acta Microbiol. Et. Immunol. Hung. 2011, 58, 169–187. [Google Scholar] [CrossRef]
- Hussain, J.; Ullah, R.; Rehman, N.U.; Khan, A.L.; Muhammad, Z.; Khan, F.U.; Hussain, S.T.; Anwar, S. Endogenous transitional metal and proximate analysis of selected medicinal plants from Pakistan. J. Med. Plants Res. 2010, 4, 267–270. [Google Scholar]
- Sebiomo, A.; Ogundero, V.W.; Bankole, S.A. Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr. J. Biotechnol. 2011, 10, 770–778. [Google Scholar]
- Essiedu, J.A.; Adepoju, F.O.; Ivantsova, M.N. Benefits and limitations in using biopesticides: A review. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2020. [Google Scholar]
- Thakur, N.J.P. Increased soil-microbial-eco-physiological interactions and microbial food safety in tomato under organic strategies. In Probiotics and Plant Health; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–232. [Google Scholar]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Chang, Y.H.; Yan, H.Z.; Liou, R.F. A novel elicitor protein from P hytophthora parasitica induces plant basal immunity and systemic acquired resistance. Mol. Plant Pathol. 2015, 16, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef]
- Abdullah, S.; Zahoor, I. Biopesticides: A Green Substitute to Chemical Pesticide. Int. J. Chem. Biochem. Sci. 2023, 24, 141–156. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Munjanja, B.; Chaparadza, A.; Majoni, S. Biopesticide residue in foodstuffs. In Biopesticides Handbook; CRC Press: Boca Raton, FL, USA, 2015; pp. 71–92. [Google Scholar]
- Shiberu, E.G.T. Assessment of selected botanical extracts against Liriomyza species (Diptera: Agromyzidae) on tomato under glasshouse condition. Int. J. Fauna Biol. Stud. 2016, 3, 87–90. [Google Scholar]
- Nawaz, M.; Mabubu, J.I.; Hua, H. Current status and advancement of biopesticides: Microbial and botanical pesticides. J. Entomol. Zool. Stud. 2016, 4, 241–246. [Google Scholar]
- Tadele, S.; Emana, G. Entomopathogenic effect of Beauveria bassiana (Bals.) and Metarrhizium anisopliae (Metschn.) on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) larvae under laboratory and glasshouse conditions in Ethiopia. J. Plant Pathol. Microbiol. 2017, 8, 411–414. [Google Scholar]
- Khalil, A.M. The genome editing revolution. J. Genet. Eng. Biotechnol. 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.K.; Ashiq, M.; Tahir, M. Potential of biological agents in decontamination of agricultural soil. Scientifica 2016, 2016, 1598325. [Google Scholar] [CrossRef] [PubMed]
- Gerson, U. Pest control by mites (Acari): Present and future. Acarologia 2014, 54, 371–394. [Google Scholar] [CrossRef]
- Stoneman, B. Challenges to commercialization of biopesticides. In Proceedings Microbial Biocontrol of Arthropods, Weeds and Plant Pathogens: Risks, Benefits and Challenges; National Conservation Training Center: Shepherdstown, WV, USA, 2010; pp. 123–127. [Google Scholar]
- Kumar, S.; Singh, A. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 2015, 6, 129. [Google Scholar] [CrossRef]
- Moose, S.P.; Mumm, R.H. Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiol. 2008, 147, 969–977. [Google Scholar] [CrossRef]
- Ramarasu, A.; Asokan, R.; Pavithra, B.S.; Sridhar, V. Innovative molecular approaches for pest management. In Genetic Methods and Tools for Managing Crop Pests; Springer: Berlin/Heidelberg, Germany, 2022; pp. 27–43. [Google Scholar]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Stoddard, B.L. Homing endonucleases: From microbial genetic invaders to reagents for targeted DNA modification. Structure 2011, 19, 7–15. [Google Scholar] [CrossRef]
- Gallagher, R.R.; Li, Z.; Lewis, A.O.; Isaacs, F.J. Rapid editing and evolution of bacterial genomes using libraries of synthetic DNA. Nat. Protoc. 2014, 9, 2301–2316. [Google Scholar] [CrossRef]
- Mandip, K.; Steer, C.J. A new era of gene editing for the treatment of human diseases. Swiss Med. Wkly. 2019, 149, w20021. [Google Scholar]
- Devarajan, A. Optically Controlled CRISPR-Cas9 and Cre Recombinase for Spatiotemporal Gene Editing: A Review. ACS Synth. Biol. 2023, 13, 25–44. [Google Scholar] [PubMed]
- Daboussi, F.; Stoddard, T.J.; Zhang, F. Engineering meganuclease for precise plant genome modification. In Advances in New Technology for Targeted Modification of Plant Genomes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 21–38. [Google Scholar]
- Viana, V.E.; Pegoraro, C.; Busanello, C.; Costa de Oliveira, A. Mutagenesis in rice: The basis for breeding a new super plant. Front. Plant Sci. 2019, 10, 1326. [Google Scholar]
- Zhu, C.; Bortesi, L.; Baysal, C.; Twyman, R.M.; Fischer, R.; Capell, T.; Schillberg, S.; Christou, P. Characteristics of genome editing mutations in cereal crops. Trends Plant Sci. 2017, 22, 38–52. [Google Scholar] [PubMed]
- Singh, A.; Srivastava, A.; Saidulu, D.; Gupta, A.K. Advancements of sequencing batch reactor for industrial wastewater treatment: Major focus on modifications, critical operational parameters, and future perspectives. J. Environ. Manag. 2022, 317, 115305. [Google Scholar] [CrossRef] [PubMed]
- D’Halluin, K.; Vanderstraeten, C.; Van Hulle, J.; Rosolowska, J.; Van Den Brande, I.; Pennewaert, A.; D’Hont, K.; Bossut, M.; Jantz, D.; Ruiter, R.; et al. Targeted molecular trait stacking in cotton through targeted double-strand break induction. Plant Biotechnol. J. 2013, 11, 933–941. [Google Scholar]
- Prieto, J.; Redondo, P.; Padro, D.; Arnould, S.; Epinat, J.C.; Pâques, F.; Blanco, F.J.; Montoya, G. The C-terminal loop of the homing endonuclease I-CreI is essential for site recognition, DNA binding and cleavage. Nucleic Acids Res. 2007, 35, 3262–3271. [Google Scholar]
- Majid, A.; Parray, G.A.; Wani, S.H.; Kordostami, M.; Sofi, N.R.; Waza, S.A.; Shikari, A.B.; Gulzar, S. Genome editing and its necessity in agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 5435–5443. [Google Scholar] [CrossRef]
- Yin, K.; Qiu, J.-L. Genome editing for plant disease resistance: Applications and perspectives. Philos. Trans. R. Soc. B 2019, 374, 20180322. [Google Scholar] [CrossRef]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef]
- Carroll, D. Genome engineering with zinc-finger nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef]
- Osakabe, K.; Osakabe, Y.; Toki, S. Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc. Natl. Acad. Sci. USA 2010, 107, 12034–12039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.J.; Zhang, F.; Sander, J.D.; Haun, W.J.; Starker, C.; Baltes, N.J.; Reyon, D.; Dahlborg, E.J.; Goodwin, M.J.; Coffman, A.P.; et al. Targeted mutagenesis of duplicated genes in soybean with zinc-finger nucleases. Plant Physiol. 2011, 156, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Even-Faitelson, L.; Samach, A.; Melamed-Bessudo, C.; Avivi-Ragolsky, N.; Levy, A.A. Localized egg-cell expression of effector proteins for targeted modification of the Arabidopsis genome. Plant J. 2011, 68, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Petolino, J.F.; Worden, A.; Curlee, K.; Connell, J.; Strange Moynahan, T.L.; Larsen, C.; Russell, S. Zinc finger nuclease-mediated transgene deletion. Plant Mol. Biol. 2010, 73, 617–628. [Google Scholar] [CrossRef]
- Qi, Y.; Li, X.; Zhang, Y.; Starker, C.G.; Baltes, N.J.; Zhang, F.; Sander, J.D.; Reyon, D.; Joung, J.K.; Voytas, D.F. Targeted deletion and inversion of tandemly arrayed genes in Arabidopsis thaliana using zinc finger nucleases. G3 Genes Genomes Genet. 2013, 3, 1707–1715. [Google Scholar] [CrossRef]
- Townsend, J.A.; Wright, D.A.; Winfrey, R.J.; Fu, F.; Maeder, M.L.; Joung, J.K.; Voytas, D.F. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 2009, 459, 442–445. [Google Scholar] [CrossRef]
- Wright, D.A.; Townsend, J.A.; Winfrey, R.J., Jr.; Irwin, P.A.; Rajagopal, J.; Lonosky, P.M.; Hall, B.D.; Jondle, M.D.; Voytas, D.F. High-frequency homologous recombination in plants mediated by zinc-finger nucleases. Plant J. 2005, 44, 693–705. [Google Scholar] [CrossRef]
- Ainley, W.M.; Sastry-Dent, L.; Welter, M.E.; Murray, M.G.; Zeitler, B.; Amora, R.; Corbin, D.R.; Miles, R.R.; Arnold, N.L.; Strange, T.L.; et al. Trait stacking via targeted genome editing. Plant Biotechnol. J. 2013, 11, 1126–1134. [Google Scholar] [CrossRef]
- Shukla, V.K.; Doyon, Y.; Miller, J.C.; DeKelver, R.C.; Moehle, E.A.; Worden, S.E.; Mitchell, J.C.; Arnold, N.L.; Gopalan, S.; Meng, X.; et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 2009, 459, 437–441. [Google Scholar] [CrossRef]
- de Galarreta, M.R.; Lujambio, A. Therapeutic editing of hepatocyte genome in vivo. J. Hepatol. 2017, 67, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Khandagale, K.; Nadaf, A. Genome editing for targeted improvement of plants. Plant Biotechnol. Rep. 2016, 10, 327–343. [Google Scholar] [CrossRef]
- Sera, T. Inhibition of virus DNA replication by artificial zinc finger proteins. J. Virol. 2005, 79, 2614–2619. [Google Scholar] [CrossRef]
- Takenaka, K.; Koshino-Kimura, Y.; Aoyama, Y.; Sera, T. Inhibition of tomato yellow leaf curl virus replication by artificial zinc-finger proteins. Nucleic Acids Symp. Ser. 2007, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qian, Y.; Wu, X.; Sun, Y.; Wu, X.; Cheng, X. Inhibiting replication of begomoviruses using artificial zinc finger nucleases that target viral-conserved nucleotide motif. Virus Genes 2014, 48, 494–501. [Google Scholar] [CrossRef]
- Nemudryi, A.A.; Valetdinova, K.R.; Medvedev, S.P.; Zakian, S.Á. TALEN and CRISPR/Cas genome editing systems: Tools of discovery. Acta Naturae (Англoязычная Версия) 2014, 6, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Thibodeau-Beganny, S.; Osiak, A.; Wright, D.A.; Anthony, R.M.; Eichtinger, M.; Jiang, T.; Foley, J.E.; Winfrey, R.J.; Townsend, J.A.; et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol. Cell 2008, 31, 294–301. [Google Scholar] [CrossRef]
- Boch, J.; Scholze, H.; Schornack, S.; Landgraf, A.; Hahn, S.; Kay, S.; Lahaye, T.; Nickstadt, A.; Bonas, U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 2009, 326, 1509–1512. [Google Scholar] [CrossRef]
- Moscou, M.J.; Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 2009, 326, 1501. [Google Scholar] [CrossRef]
- Christian, M.L.; Demorest, Z.L.; Starker, C.G.; Osborn, M.J.; Nyquist, M.D.; Zhang, Y.; Carlson, D.F.; Bradley, P.; Bogdanove, A.J.; Voytas, D.F. Targeting G with TAL effectors: A comparison of activities of TALENs constructed with NN and NK repeat variable di-residues. PLoS ONE 2012, 7, e45383. [Google Scholar] [CrossRef] [PubMed]
- de Lange, O.; Schreiber, T.; Schandry, N.; Radeck, J.; Braun, K.H.; Koszinowski, J.; Heuer, H.; Strauß, A.; Lahaye, T. Breaking the DNA-binding code of Ralstonia solanacearum TAL effectors provides new possibilities to generate plant resistance genes against bacterial wilt disease. New Phytol. 2013, 199, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Jankele, R.; Svoboda, P. TAL effectors: Tools for DNA targeting. Brief. Funct. Genom. 2014, 13, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T.; Holm, P.B.; Starker, C.G.; Christian, M.; Voytas, D.F.; Brinch-Pedersen, H.; Holme, I.B. TAL effector nucleases induce mutations at a pre-selected location in the genome of primary barley transformants. Plant Mol. Biol. 2013, 83, 279–285. [Google Scholar] [CrossRef]
- Char, S.N.; Unger-Wallace, E.; Frame, B.; Briggs, S.A.; Main, M.; Spalding, M.H.; Vollbrecht, E.; Wang, K.; Yang, B. Heritable site-specific mutagenesis using TALEN s in maize. Plant Biotechnol. J. 2015, 13, 1002–1010. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Haun, W.; Coffman, A.; Clasen, B.M.; Demorest, Z.L.; Lowy, A.; Ray, E.; Retterath, A.; Stoddard, T.; Juillerat, A.; Cedrone, F.; et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Shan, Q.; Zhang, Y.; Chen, K.; Zhang, K.; Gao, C. Creation of fragrant rice by targeted knockout of the Os BADH 2 gene using TALEN technology. Plant Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, F.; Li, Z.; Zhang, J.; Li, X.; Dong, J.; Wang, T. TALEN-based mutagenesis of lipoxygenase LOX3 enhances the storage tolerance of rice (Oryza sativa) seeds. PLoS ONE 2015, 10, e0143877. [Google Scholar] [CrossRef]
- Clasen, B.M.; Stoddard, T.J.; Luo, S.; Demorest, Z.L.; Li, J.; Cedrone, F.; Tibebu, R.; Davison, S.; Ray, E.E.; Daulhac, A.; et al. Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J. 2016, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Sirk, S.J.; Shui, S.L.; Liu, J. Genome-editing technologies: Principles and applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754. [Google Scholar] [CrossRef]
- Khan, S.H. Genome-editing technologies: Concept; pros, and cons of various genome-editing techniques and bioethical concerns for clinical application. Mol. Ther. Nucleic Acids 2019, 16, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Malzahn, A.; Lowder, L.; Qi, Y. Plant genome editing with TALEN and CRISPR. Cell Biosci. 2017, 7, 21. [Google Scholar] [CrossRef]
- Huo, Z.; Tu, J.; Xu, A.; Li, Y.; Wang, D.; Liu, M.; Zhou, R.; Zhu, D.; Lin, Y.; Gingold, J.A.; et al. Generation of a heterozygous p53 R249S mutant human embryonic stem cell line by TALEN-mediated genome editing. Stem Cell Res. 2019, 34, 101360. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The biology of CRISPR-Cas: Backward and forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a Bacterial Speck Resistant Tomato by CRISPR /Cas9-mediated Editing of Sl JAZ 2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Jia, H.; Sosso, D.; Li, T.; Frommer, W.B.; Yang, B.; White, F.F.; Wang, N.; Jones, J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA 2014, 111, E521–E529. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene Cs LOB 1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Viola, R.; Jung, M.H.; Koo, O.J.; Kim, S.; Kim, J.S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Kis, A.; Hamar, É.; Tholt, G.; Bán, R.; Havelda, Z. Creating highly efficient resistance against wheat dwarf virus in barley by employing CRISPR/Cas9 system. Plant Biotechnol. J. 2019, 17, 1004. [Google Scholar] [CrossRef]
- Bao, A.; Burritt, D.J.; Chen, H.; Zhou, X.; Cao, D.; Tran, L.S. The CRISPR/Cas9 system and its applications in crop genome editing. Crit. Rev. Biotechnol. 2019, 39, 321–336. [Google Scholar] [CrossRef]
- Hussain, B.; Lucas, S.J.; Budak, H. CRISPR/Cas9 in plants: At play in the genome and at work for crop improvement. Brief. Funct. Genom. 2018, 17, 319–328. [Google Scholar] [CrossRef]
- Chao, S.; Cai, Y.; Feng, B.; Jiao, G.; Sheng, Z.; Luo, J.; Tang, S.; Wang, J.; Hu, P.; Wei, X. Editing of rice isoamylase gene ISA1 provides insights into its function in starch formation. Rice Sci. 2019, 26, 77–87. [Google Scholar]
- Fiaz, S.; Ahmad, S.; Noor, M.A.; Wang, X.; Younas, A.; Riaz, A.; Riaz, A.; Ali, F. Applications of the CRISPR/Cas9 system for rice grain quality improvement: Perspectives and opportunities. Int. J. Mol. Sci. 2019, 20, 888. [Google Scholar] [CrossRef]
- Zafar, S.A.; Zaidi, S.S.; Gaba, Y.; Singla-Pareek, S.L.; Dhankher, O.P.; Li, X.; Mansoor, S.; Pareek, A. Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. J. Exp. Bot. 2020, 71, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Barman, H.N.; Sheng, Z.; Fiaz, S.; Zhong, M.; Wu, Y.; Cai, Y.; Wang, W.; Jiao, G.; Tang, S.; Wei, X.; et al. Generation of a new thermo-sensitive genic male sterile rice line by targeted mutagenesis of TMS5 gene through CRISPR/Cas9 system. BMC Plant Biol. 2019, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, L.; Hu, P.; Liu, Q.; Zhu, X.; Qian, Q.; Wang, K.; Wang, Y. Developing disease-resistant thermosensitive male sterile rice by multiplex gene editing. J. Integr. Plant Biol. 2019, 61, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hua, Y.; Fu, Y.; Li, J.; Liu, Q.; Jiao, X.; Xin, G.; Wang, J.; Wang, X.; Yan, C.; et al. Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice. Sci. China Life Sci. 2017, 60, 506–515. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.C.; Takken, F.L. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR crops: Plant genome editing toward disease resistance. Annu. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84–88. [Google Scholar] [CrossRef]
- Tycko, J.; Myer, V.E.; Hsu, P.D. Methods for optimizing CRISPR-Cas9 genome editing specificity. Mol. Cell 2016, 63, 355–370. [Google Scholar] [CrossRef]
- Scheben, A.; Wolter, F.; Batley, J.; Puchta, H.; Edwards, D. Towards CRISPR/Cas crops–bringing together genomics and genome editing. New Phytol. 2017, 216, 682–698. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Joung, J.K. Defining and improving the genome-wide specificities of CRISPR–Cas9 nucleases. Nat. Rev. Genet. 2016, 17, 300–312. [Google Scholar] [CrossRef]
- Yin, K.; Gao, C.; Qiu, J.L. Progress and prospects in plant genome editing. Nat. Plants 2017, 3, 17107. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Stürchler, A.; Anjanappa, R.B.; Zaidi, S.S.; Hirsch-Hoffmann, M.; Gruissem, W.; Vanderschuren, H. Linking CRISPR-Cas9 interference in cassava to the evolution of editing-resistant geminiviruses. Genome Biol. 2019, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Front. Microbiol. 2020, 11, 593700. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kumar, R.; Kumar, V.; Won, S.Y.; Shukla, P. Engineering disease resistant plants through CRISPR-Cas9 technology. GM Crop. Food 2021, 12, 125–144. [Google Scholar] [CrossRef]
- Delgado, J.A.; Short Jr, N.M.; Roberts, D.P.; Vandenberg, B. Big data analysis for sustainable agriculture on a geospatial cloud framework. Front. Sustain. Food Syst. 2019, 3, 54. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Ghidan, A.Y.; Al-Antary, T.M.; Salem, N.M.; Awwad, A.M. Facile green synthetic route to the zinc oxide (ZnONPs) nanoparticles: Effect on green peach aphid and antibacterial activity. J. Agric. Sci. 2017, 9, 131–138. [Google Scholar] [CrossRef]
- Sharon, M.; Choudhary, A.K.; Kumar, R. Nanotechnology in agricultural diseases and food safety. J. Phytol. 2010, 2, 83–92. [Google Scholar]
- Guleria, G.; Thakur, S.; Shandilya, M.; Sharma, S.; Thakur, S.; Kalia, S. Nanotechnology for sustainable agro-food systems: The need and role of nanoparticles in protecting plants and improving crop productivity. Plant Physiol. Biochem. 2023, 194, 533–549. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Al-Gheethi, A.; Iqbal, J. Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. J. Environ. Chem. Eng. 2021, 9, 106160. [Google Scholar] [CrossRef]
- Zhang, P.; Lynch, I.; Handy, R.D.; White, J.C. A brief history of nanotechnology in agriculture and current status. In Nano-Enabled Sustainable and Precision Agriculture; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–14. [Google Scholar]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Z.; Ullah, S.; Melagraki, G.; Afantitis, A.; Lynch, I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 2021, 7, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.; Laës-Huon, A.; Barus, C.; Beaton, A.D.; Blandfort, D.; Guigues, N.; Knockaert, M.; Munaron, D.; Salter, I.; Woodward, E.M.; et al. Toward a harmonization for using in situ nutrient sensors in the marine environment. Front. Mar. Sci. 2020, 6, 773. [Google Scholar] [CrossRef]
- Sargazi, S.; Fatima, I.; Kiani, M.H.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Díez-Pascual, A.M.; Behzadmehr, R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: A comprehensive review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J. Recent progress in micro- and nanotechnology-enabled sensors for biomedical and environmental challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef]
- Shawon, Z.B.; Hoque, M.E.; Chowdhury, S.R. Nanosensors and nanobiosensors: Agricultural and food technology aspects. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–161. [Google Scholar]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci. 2016, 29, 1–13. [Google Scholar]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of nanotechnology in agriculture. Environ. Nanotechnol. 2020, 4, 317–348. [Google Scholar]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A review on silver nanoparticles: Classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Pandiyaraj, V.; Murmu, A.; Pandy, S.K.; Sevanan, M.; Arjunan, S. Metal nanoparticles and its application on phenolic and heavy metal pollutants. Phys. Sci. Rev. 2023, 8, 2879–2897. [Google Scholar] [CrossRef]
- Amer, M.; Awwad, A. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem. Int. 2020, 7, 1–8. [Google Scholar]
- Acharya, A.; Pal, P.K. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact 2020, 19, 100232. [Google Scholar] [CrossRef]
- Das, A.; Dutta, P. Antifungal activity of biogenically synthesized silver and gold nanoparticles against sheath blight of rice. J. Nanosci. Nanotechnol. 2021, 21, 3547–3555. [Google Scholar] [CrossRef]
- Ponmurugan, P. Biosynthesis of silver and gold nanoparticles using Trichoderma atroviride for the biological control of Phomopsis canker disease in tea plants. IET Nanobiotechnology 2017, 11, 261–267. [Google Scholar] [CrossRef]
- Thakur, R.K.; Prasad, P. Synthesis of gold nanoparticles and assessment of in vitro toxicity against plant pathogens. Indian Phytopathol. 2022, 75, 101–108. [Google Scholar] [CrossRef]
- Kaman, P.; Dutta, P.; Bhattacharyya, A. Synthesis of gold nanoparticles from Metarhizium anisopliae for management of blast disease of rice and its effect on soil biological index and physicochemical properties. Res. Sq. 2022, 1–21. [Google Scholar] [CrossRef]
- Patra, P.; Mitra, S.; Debnath, N.; Goswami, A. Biochemical-, biophysical-, and microarray-based antifungal evaluation of the buffer-mediated synthesized nano zinc oxide: An in vivo and in vitro toxicity study. Langmuir 2012, 28, 16966–16978. [Google Scholar] [CrossRef]
- Wagner, G.; Korenkov, V.; Judy, J.D.; Bertsch, P.M. Nanoparticles composed of Zn and ZnO inhibit Peronospora tabacina spore germination in vitro and P. tabacina infectivity on tobacco leaves. Nanomaterials 2016, 6, 50. [Google Scholar] [CrossRef]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, B.R.; Singh, A.; Keswani, C.; Naqvi, A.H.; Singh, H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 2014, 9, e97881. [Google Scholar] [CrossRef]
- Jahan, Q.S.; Sultana, Z.; Ud-Daula, M.A.; Ashikuzzaman, M.; Reja, M.S.; Rahman, M.M.; Khaton, A.; Tang, M.A.; Rahman, M.S.; Faruquee, H.M.; et al. Optimization of green silver nanoparticles as nanofungicides for management of rice bakanae disease. Heliyon 2024, 10, e27579. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmed, S.; Khan, M.T.; Hamad, N.A.; Ali, H.M.; Abbasi, A.; Mubeen, I.; Intisar, A.; Hasan, M.E.; Jasim, I.K. Evaluation of in vitro and in vivo antifungal activity of green synthesized silver nanoparticles against early blight in tomato. Horticulturae 2023, 9, 369. [Google Scholar] [CrossRef]
- Bibi, S.; Raza, M.; Shahbaz, M.; Ajmal, M.; Mehak, A.; Fatima, N.; Abasi, F.; Seelan, J.S.; Raja, N.I.; Yongchao, B.; et al. Biosynthesized silver nanoparticles enhanced wheat resistance to Bipolaris sorokiniana. Plant Physiol. Biochem. 2023, 203, 108067. [Google Scholar] [CrossRef]
- Qureshi, A.K.; Farooq, U.; Shakeel, Q.; Ali, S.; Ashiq, S.; Shahzad, S.; Tariq, M.; Seleiman, M.F.; Jamal, A.; Saeed, M.F.; et al. The Green Synthesis of Silver Nanoparticles from Avena fatua Extract: Antifungal Activity against Fusarium oxysporum f. sp. lycopersici. Pathogens 2023, 12, 1247. [Google Scholar] [CrossRef]
- Ghasemi, S.; Harighi, B.; Ashengroph, M. Biosynthesis of silver nanoparticles using Pseudomonas canadensis, and its antivirulence effects against Pseudomonas tolaasii, mushroom brown blotch agent. Sci. Rep. 2023, 13, 3668. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Jiang, H.; Shahid, M.; Ma, C.; Wu, Z.; Nazir, M.M.; Ali, M.A.; White, J.C.; Chen, J.; et al. Bioengineered chitosan-iron nanocomposite controls bacterial leaf blight disease by modulating plant defense response and nutritional status of rice (Oryza sativa L.). Nano Today 2022, 45, 101547. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Khan, S.A.; Ali, A.; Hanif, A.; Hussain, M.; Anwar, T.; Qureshi, H.; Saeed, M.; Rauf, A.; et al. Evaluation of Green Synthesized Silver Nanoparticles against Bacterial Pathogenic Strains of Plants. Pak. J. Bot. 2023, 55, 1967–1972. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.; Almunqedhi, B.M. Biosynthesis of silver nanoparticles using Penicillium verrucosum and analysis of their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Namburi, K.R.; Kora, A.J.; Chetukuri, A.; Kota, V.S. Biogenic silver nanoparticles as an antibacterial agent against bacterial leaf blight causing rice phytopathogen Xanthomonas oryzae pv. oryzae. Bioprocess Biosyst. Eng. 2021, 44, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, S.; Ahmed, T.; Shahid, M.; Noman, M.; Shah, A.A.; Mehmood, M.A.; Abbas, A.; Cheema, A.I.; Iqbal, M.Z.; Li, B. Bioinspired green synthesis of silver nanoparticles by using a native Bacillus sp. strain AW1-2: Characterization and antifungal activity against Colletotrichum falcatum Went. Enzym. Microb. Technol. 2021, 144, 109745. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef]
- Dorjee, L.; Gogoi, R.; Kamil, D.; Kumar, R.; Verma, A. Copper nanoparticles hold promise in the effective management of maize diseases without impairing environmental health. Phytoparasitica 2023, 51, 593–619. [Google Scholar] [CrossRef]
- Truong, H.T.; Nguyen, L.C.; Le, L.Q. Synthesis and antifungal activity of copper nanoparticles against Fusarium oxysporum pathogen of plants. Mater. Res. Express 2023, 10, 065001. [Google Scholar] [CrossRef]
- Iliger, K.S.; Sofi, T.A.; Bhat, N.A.; Ahanger, F.A.; Sekhar, J.C.; Elhendi, A.Z.; Al-Huqail, A.A.; Khan, F. Copper nanoparticles: Green synthesis and managing fruit rot disease of chilli caused by Colletotrichum capsici. Saudi J. Biol. Sci. 2021, 28, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Natesan, K.; Ponmurugan, P.; Gnanamangai, B.M.; Manigandan, V.; Joy, S.P.J.; Jayakumar, C.; Amsaveni, G. Biosynthesis of silica and copper nanoparticles from Trichoderma, Streptomyces and Pseudomonas spp. evaluated against collar canker and red root-rot disease of tea plants. Arch. Phytopathol. Plant Prot. 2021, 54, 56–85. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; White, J.C.; Nazir, M.M.; Azizullah Li, D.; Song, F. Bacillus altitudinis-Stabilized Multifarious Copper Nanoparticles Prevent Bacterial Fruit Blotch in Watermelon (Citrullus lanatus L.): Direct Pathogen Inhibition, In Planta Particles Accumulation, and Host Stomatal Immunity Modulation. Small 2023, 19, 2207136. [Google Scholar] [CrossRef]
- Majumdar, T.D.; Singh, M.; Thapa, M.; Dutta, M.; Mukherjee, A.; Ghosh, C.K. Size-dependent antibacterial activity of copper nanoparticles against Xanthomonas oryzae pv. oryzae–A synthetic and mechanistic approach. Colloid Interface Sci. Commun. 2019, 32, 100190. [Google Scholar] [CrossRef]
- Soliman, A.M.M.; Abdallah, E.A.M.; Hafez, E.E.; Kadous, E.A.; Kassem, F.A. Nematicidal Activity of Chemical and Green Biosynthesis of Copper Nanoparticles Against Root-Knot Nematode, Meloidogyne Incognita. Alex. Sci. Exch. J. 2022, 43, 583–591. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal-and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Maity, D.; Gupta, U.; Saha, S. Biosynthesized metal oxide nanoparticles for sustainable agriculture: Next-generation nanotechnology for crop production, protection and management. Nanoscale 2022, 14, 13950–13989. [Google Scholar] [CrossRef]
- Cheira, M.F. Performance of poly sulfonamide/nano-silica composite for adsorption of thorium ions from sulfate solution. SN Appl. Sci. 2020, 2, 398. [Google Scholar] [CrossRef]
- Sharma, S.; Virk, K.; Sharma, K.; Bose, S.K.; Kumar, V.; Sharma, V.; Focarete, M.L.; Kalia, S. Preparation of gum acacia-poly (acrylamide-IPN-acrylic acid) based nanocomposite hydrogels via polymerization methods for antimicrobial applications. J. Mol. Struct. 2020, 1215, 128298. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef] [PubMed]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K. Ecofriendly synthesis of zinc oxide nanoparticles by Carica papaya leaf extract and their applications. J. Clust. Sci. 2022, 33, 603–617. [Google Scholar] [CrossRef]
- Helmy, K.G.; Partila, A.M.; Salah, M. Gamma radiation and polyvinyl pyrrolidone mediated synthesis of zinc oxide/zinc sulfide nanoparticles and evaluation of their antifungal effect on pre and post harvested orange and pomegranate fruits. Biocatal. Agric. Biotechnol. 2020, 29, 101728. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, C.; Ren, Y.; Lu, Y.; Song, Y.; Ji, Y.; Han, C.; He, J. Green synthesis of zinc oxide nanoparticles using Cinnamomum camphora (L.) Presl leaf extracts and its antifungal activity. J. Environ. Chem. Eng. 2021, 9, 106659. [Google Scholar] [CrossRef]
- Khan, R.A.; Tang, Y.; Naz, I.; Alam, S.S.; Wang, W.; Ahmad, M.; Najeeb, S.; Rao, C.; Li, Y.; Xie, B.; et al. Management of Ralstonia solanacearum in tomato using ZnO nanoparticles synthesized through Matricaria chamomilla. Plant Dis. 2021, 105, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Wang, X.; Haroon, U.; Chaudhary, H.J.; Kamal, A.; Ali, Q.; Saleem, M.H.; Usman, K.; Alatawi, A.; Ali, S.; et al. Antifungal activity of Zinc nitrate derived nano Zno fungicide synthesized from Trachyspermum ammi to control fruit rot disease of grapefruit. Ecotoxicol. Environ. Saf. 2022, 233, 113311. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Chaithanya, G.; Kumar, A.; Vijay, D.; Singh, P.K.; Hussain, Z.; Basu, S.; Lal, S.K. Efficacy of nanoparticles against purple blotch (Alternaria porri) of onion. Indian Phytopathol. 2023, 76, 845–852. [Google Scholar] [CrossRef]
- Rehman, F.U.; Paker, N.P.; Khan, M.; Naeem, M.; Munis, M.F.H.; Rehman, S.U.; Chaudhary, H.J. Bio-fabrication of zinc oxide nanoparticles from Picea smithiana and their potential antimicrobial activities against Xanthomonas campestris pv. Vesicatoria and Ralstonia solanacearum causing bacterial leaf spot and bacterial wilt in tomato. World J. Microbiol. Biotechnol. 2023, 39, 176. [Google Scholar] [CrossRef]
- Jomeyazdian, A.; Pirnia, M.; Alaei, H.; Taheri, A.; Sarani, S. Control of Fusarium wilt disease of tomato and improvement of some growth factors through green synthesized zinc oxide nanoparticles. Eur. J. Plant Pathol. 2024, 169, 333–345. [Google Scholar] [CrossRef]
- Karmous, I.; Vaidya, S.; Dimkpa, C.; Zuverza-Mena, N.; da Silva, W.; Barroso, K.A.; Milagres, J.; Bharadwaj, A.; Abdelraheem, W.; White, J.C.; et al. Biologically synthesized zinc and copper oxide nanoparticles using Cannabis sativa L. enhance soybean (Glycine max) defense against Fusarium virguliforme. Pestic. Biochem. Physiol. 2023, 194, 105486. [Google Scholar] [CrossRef]
- Sardar, M.; Ahmed, W.; Al Ayoubi, S.; Nisa, S.; Bibi, Y.; Sabir, M.; Khan, M.M.; Ahmed, W.; Qayyum, A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J. Biol. Sci. 2022, 29, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Siddiqui, Z.A. Effect of silver oxide nanoparticles on growth, activities of defense enzymes and fungal and bacterial diseases of tomato. Gesunde Pflanz. 2023, 75, 405–414. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Luo, J.; Abdallah, Y.; Yu, S.; Wang, X.; Alkhalifah, D.H.; Hozzein, W.N.; Wang, F.; Bi, J.A.; Yan, C.; et al. Copper oxide nanoparticles: An effective suppression tool against bacterial leaf blight of rice and its impacts on plants. Pest. Manag. Sci. 2024, 80, 1279–1288. [Google Scholar] [CrossRef]
- Tiwari, V.; Bambharoliya, K.S.; Bhatt, M.D.; Nath, M.; Arora, S.; Dobriyal, A.K.; Bhatt, D. Application of green synthesized copper oxide nanoparticles for effective mitigation of Fusarium wilt disease in roots of Cicer arietinum. Physiol. Mol. Plant Pathol. 2024, 131, 102244. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Sawake, M.M.; Moharil, M.P.; Ingle, Y.V.; Jadhav, P.V.; Ingle, A.P.; Khelurkar, V.C.; Paithankar, D.H.; Bathe, G.A.; Gade, A.K. Management of Phytophthora parasitica causing gummosis in citrus using biogenic copper oxide nanoparticles. J. Appl. Microbiol. 2022, 132, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Anjum, T.; Riaz, S.; Batool, T.; Naseem, S.; Ahmad, I.S. Sustainable synthesis of microwave assisted IONPs by using spinacia oleracea: Enhances resistance against fungal wilt infection by inducing ROS and modulating defense system in tomato plants. J. Nanobiotechnol. 2021, 20, 8. [Google Scholar]
- Gaba, S.; Rai, A.K.; Varma, A.; Prasad, R.; Goel, A. Biocontrol potential of mycogenic copper oxide nanoparticles against Alternaria brassicae. Front. Chem. 2022, 10, 966396. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, M.; Khan, A.A.; Parveen, A.; Ansari, S.; Alam, M. Effect of Phyto-Assisted Synthesis of Magnesium Oxide Nanoparticles (MgO-NPs) on Bacteria and the Root-Knot Nematode. Bioinorg. Chem. Appl. 2022, 2022, 3973841. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Zaheer, S.; Shehzad, J.; Chaudhari, S.K.; Hasan, M.; Mustafa, G. Morphological and biochemical responses of Vigna radiata L. seedlings towards green synthesized SiO2 NPs. Silicon 2023, 15, 5925–5936. [Google Scholar] [CrossRef]
- Abdallah, Y.; Nehela, Y.; Ogunyemi, S.O.; Ijaz, M.; Ahmed, T.; Elashmony, R.; Alkhalifah, D.H.; Hozzein, W.N.; Xu, L.; Yan, C.; et al. Bio-functionalized nickel-silica nanoparticles suppress bacterial leaf blight disease in rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1216782. [Google Scholar] [CrossRef]
- Goswami, P.; Sharma, M.; Srivastava, N.; Mathur, J. Assessment of the fungicidal efficacy of biogenic SiO2 NPs in Eruca sativa against fusarium wilt. J. Nat. Pestic. Res. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Awad-Allah, E.F.; Shams, A.H.; Helaly, A.A. Suppression of bacterial leaf spot by green synthesized silica nanoparticles and antagonistic yeast improves growth, productivity and quality of sweet pepper. Plants 2021, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Abdelrhim, A.S.; Mazrou, Y.S.; Nehela, Y.; Atallah, O.O.; El-Ashmony, R.M.; Dawood, M.F. Silicon dioxide nanoparticles induce innate immune responses and activate antioxidant machinery in wheat against Rhizoctonia solani. Plants 2021, 10, 2758. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Tahoon, A.M.; Elsharnoby, D.E.; El-Shafey, R.A. Bio-production of silica nanoparticles from rice husk and their impact on rice bakanae disease and grain yield. Arch. Phytopathol. Plant Prot. 2020, 53, 459–478. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, Z.A. Efficacy of titanium dioxide nanoparticles in the management of disease complex of beetroot (Beta vulgaris L.) caused by Pectobacterium betavasculorum, Rhizoctonia solani, and Meloidogyne incognita. Gesunde Pflanz. 2021, 73, 445–464. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.U.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Siddiqui, Z.A.; Parveen, A.; Khan, A.A.; Moon, I.S.; Alam, M. Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita. Nanotechnol. Rev. 2022, 11, 1606–1619. [Google Scholar] [CrossRef]
- Hossain, A.; Abdallah, Y.; Ali, M.A.; Masum, M.M.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeya dadantii. Biomolecules 2019, 9, 863. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Ismail, A.M. The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Ikram, M.; Oraby, H.F.; Mashwani, Z.U.; Mohamed, A.H.; Singh, A.; Omar, A.A. Plant-based titanium dioxide nanoparticles trigger biochemical and proteome modifications in Triticum aestivum L. under biotic stress of Puccinia striiformis. Molecules 2022, 27, 4274. [Google Scholar] [CrossRef]
- Acuña-Fuentes, N.L.; Vargas-Hernandez, M.; Rivero-Montejo, S.D.; Rivas-Ramirez, L.K.; Macias-Bobadilla, I.; Palos-Barba, V.; Rivera-Muñoz, E.M.; Guevara-Gonzalez, R.G.; Torres-Pacheco, I. Antiviral activity of TiO2 NPs against tobacco mosaic virus in chili pepper (Capsicum annuum L.). Agriculture 2022, 12, 2101. [Google Scholar] [CrossRef]
- Monclou-Salcedo, S.A.; Correa-Torres, S.N.; Kopytko, M.I.; Santoyo-Muñóz, C.; Vesga-Guzmán, D.M.; Castellares-Lozano, R.; López-Amaris, M.; Saavedra-Mancera, A.D.; Herrera-Barros, A.P. Evaluación antifúngica de nanopartículas de TiO2 para inhibición de Fusarium solani en Palma Africana. Int. J. Agric. Nat. Resour. 2020, 47, 126–133. [Google Scholar]
- Adisa, I.O.; Reddy Pullagurala, V.L.; Rawat, S.; Hernandez-Viezcas, J.A.; Dimkpa, C.O.; Elmer, W.H.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Role of cerium compounds in Fusarium wilt suppression and growth enhancement in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2018, 66, 5959–5970. [Google Scholar] [CrossRef]
- Shahbaz, M.; Fatima, N.; Mashwani, Z.U.; Akram, A.; Haq, E.U.; Mehak, A.; Abasi, F.; Ajmal, M.; Yousaf, T.; Raja, N.I.; et al. Effect of phytosynthesized selenium and cerium oxide nanoparticles on wheat (Triticum aestivum L.) against stripe rust disease. Molecules 2022, 27, 8149. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.O.; Alotaibi, N.M.; Ghoneim, A.M.; ul Ain, N.; Irshad, M.A.; Nawaz, R.; Abbas, T.; Abbas, A.; Rizwan, M.; Ali, S. Effect of green synthesized cerium oxide nanoparticles on fungal disease of wheat plants: A field study. Chemosphere 2023, 339, 139731. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Abdelaziz, A.M.; Rizk, S.H.; Tolba, M.M.; Attia, M.S. Stable Colloidal Iron Oxide Nanoparticles: A New Green Nanofertilizer and Therapeutic Nutrient for Eggplant Immune Response against Fusarium Wilt Disease. J. Clust. Sci. 2024, 35, 983–997. [Google Scholar] [CrossRef]
- Niazi, F.; Ali, M.; Haroon, U.; Farhana Kamal, A.; Rashid, T.; Anwar, F.; Nawab, R.; Chaudhary, H.J.; Munis, M.F. Effect of green Fe2O3 nanoparticles in controlling Fusarium fruit rot disease of loquat in Pakistan. Braz. J. Microbiol. 2023, 54, 1341–1350. [Google Scholar] [CrossRef]
- Akbar, M.; Haroon, U.; Ali, M.; Tahir, K.; Chaudhary, H.J.; Munis, M.F. Mycosynthesized Fe2O3 nanoparticles diminish brown rot of apple whilst maintaining composition and pertinent organoleptic properties. J. Appl. Microbiol. 2022, 132, 3735–3745. [Google Scholar] [CrossRef]
- Umar, H.; Aliyu, M.R.; Ozsahin, D.U. Iron oxide nanoparticles synthesized using Mentha spicata extract and evaluation of its antibacterial, cytotoxicity and antimigratory potential on highly metastatic human breast cells. Biomed. Phys. Eng. Express 2024, 10, 035019. [Google Scholar] [CrossRef]
- Alam, T.; Khan, R.A.; Ali, A.; Sher, H.; Ullah, Z.; Ali, M. Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater. Sci. Eng. C 2019, 98, 101–108. [Google Scholar] [CrossRef]
- Ali, M.; Haroon, U.; Khizar, M.; Chaudhary, H.J.; Hussain Munis, M.F. Scanning electron microscopy of bio-fabricated Fe2O3 nanoparticles and their application to control brown rot of citrus. Microsc. Res. Tech. 2021, 84, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mogazy, A.M.; Mohamed, H.I.; El-Mahdy, O.M. Calcium and iron nanoparticles: A positive modulator of innate immune responses in strawberry against Botrytis cinerea. Process Biochem. 2022, 115, 128–145. [Google Scholar] [CrossRef]
- Anwaar, S.; Ijaz, D.E.; Anwar, T.; Qureshi, H.; Nazish, M.; Alrefaei, A.F.; Almutairi, M.H.; Alharbi, S.N. Boosting Solanum tuberosum resistance to Alternaria solani through green synthesized ferric oxide (Fe2O3) nanoparticles. Sci. Rep. 2024, 14, 2375. [Google Scholar] [CrossRef]
- Zubair, M.S.; Munis, M.F.; Alsudays, I.M.; Alamer, K.H.; Haroon, U.; Kamal, A.; Ali, M.; Ahmed, J.; Ahmad, Z.; Attia, H. First report of fruit rot of cherry and its control using Fe2O3 nanoparticles synthesized in Calotropis procera. Molecules 2022, 27, 4461. [Google Scholar] [CrossRef]
- Ismail, A.; Kabary, H.; Samy, A. Synthesis of α-Al2O3 Nanoparticles from Pepsi Cans Wastes and Its Fungicidal Effect on Some Mycotoxins Producing Fungal Isolates. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.-mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT Food Sci. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Xu, X.; Xu, L.; Abdallah, Y.; Rizwan, M.; Lv, L.; Ahmed, T.; Ali, H.M.; Khan, F.; Yan, C.; et al. Cobalt oxide nanoparticles: An effective growth promoter of Arabidopsis plants and nano-pesticide against bacterial leaf blight pathogen in rice. Ecotoxicol. Environ. Saf. 2023, 257, 114935. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Batool, T.; Anjum, T.; Illyas, A.; Li, G.; Naseem, S.; Riaz, S. Antifungal Potential of Green Synthesized Magnetite Nanoparticles Black Coffee–Magnetite Nanoparticles against Wilt Infection by Ameliorating Enzymatic Activity and Gene Expression in Solanum lycopersicum L. Front. Microbiol. 2022, 13, 754292. [Google Scholar]
- Elenany, A.M.; Atia, M.M.; Abbas, E.E.; Moustafa, M.; Alshaharni, M.O.; Negm, S.; Elnahal, A.S. Nanoparticles and Chemical Inducers: A Sustainable Shield against Onion White Rot. Biology 2024, 13, 219. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; El-Bakery, A.M.; Hafez, H.M.; Ismail, A.M.; El-Abdeen, A.Z.; Ata, A.A.; Elraheem, O.A.; El Kady, Y.M.; Hamouda, A.F.; El-Beltagi, H.S.; et al. Humic acid-coated Fe3O4 nanoparticles confer resistance to Acremonium wilt disease and improve physiological and morphological attributes of grain Sorghum. Polymers 2022, 14, 3099. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Luo, J.; Muhammad, S.; Shahid, M.; Ali, M.A.; Zhang, M.; Li, B. Bioengineered chitosan-magnesium nanocomposite: A novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int. J. Biol. Macromol. 2021, 168, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L.(Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae. BioMed Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Zhang, M.; Abdallah, Y.; Ahmed, T.; Qiu, W.; Ali, M.A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. The bio-synthesis of three metal oxide nanoparticles (ZnO, MnO2, and MgO) and their antibacterial activity against the bacterial leaf blight pathogen. Front. Microbiol. 2020, 11, 588326. [Google Scholar] [CrossRef]
- Rabea, A.; Naeem, E.; Balabel, N.M.; Daigham, G.E. Management of potato brown rot disease using chemically synthesized CuO-NPs and MgO-NPs. Bot. Stud. 2023, 64, 20. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, L.; Azmat, A.L.; Masudulla, K.H.; Farid, O.; Mahboob, A.L. Exploring the nano-fungicidal efficacy of green synthesized magnesium oxide nanoparticles (MgO NPs) on the development, physiology, and infection of carrot (Daucus carota L.) with Alternaria leaf blight (ALB): Molecular docking. J. Integr. Agric. 2023, 22, 3069–3080. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Emam, T.M.; Elsherbiny, E.A. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater. Sci. Eng. C 2020, 109, 110617. [Google Scholar] [CrossRef]
- Abdelfattah, N.A.; Yousef, M.A.; Badawy, A.A.; Salem, S.S. Influence of biosynthesized magnesium oxide nanoparticles on growth and physiological aspects of cowpea (Vigna unguiculata L.) plant, cowpea beetle, and cytotoxicity. Biotechnol. J. 2023, 18, 2300301. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zhang, X.; Fan, G.J.; Que, Y.; Xue, F.; Liu, Y.H. Toxicity effects and mechanisms of MgO nanoparticles on the oomycete pathogen Phytophthora infestans and its host Solanum tuberosum. Toxics 2022, 10, 553. [Google Scholar] [CrossRef]
- Ismail, A.M. Efficacy of copper oxide and magnesium oxide nanoparticles on controlling black scurf disease on potato. Egypt. J. Phytopathol. 2021, 49, 116–130. [Google Scholar] [CrossRef]
- Ismail, A.M.; El-Gawad, A.; Mona, E. Antifungal activity of MgO and ZnO nanoparticles against powdery mildew of pepper under greenhouse conditions. Egypt. J. Agric. Res. 2021, 99, 421–434. [Google Scholar]
- Liao, J.; Yuan, Z.; Wang, X.; Chen, T.; Qian, K.; Cui, Y.; Rong, A.; Zheng, C.; Liu, Y.; Wang, D.; et al. Magnesium oxide nanoparticles reduce clubroot by regulating plant defense response and rhizosphere microbial community of tumorous stem mustard (Brassica juncea var. tumida). Front. Microbiol. 2024, 15, 1370427. [Google Scholar] [CrossRef] [PubMed]
- Hantoosh, M.N.; Hussein, H. Bioactivity of Magnesium Oxide Nanoparticles Synthesized by Alcoholic Extract of Walnut Tree Bark Juglans regia against Thielaviopsis paradoxa and Thielaviopsis punctulata in vitro. IOP Conf. Ser. Earth Environ. Sci. 2023, 1252, 012021. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Huang, Y.; Carvalho, R.; Choudhary, M.; Da Silva, S.; Colee, J.; Huerta, A.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Magnesium oxide nanomaterial, an alternative for commercial copper bactericides: Field-scale tomato bacterial spot disease management and total and bioavailable metal accumulation in soil. Environ. Sci. Technol. 2021, 55, 13561–13570. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, J.; Zhang, L.; Chen, Q.; Yue, W.; Ke, N.; Xie, H. Coumarin-containing light-responsive carboxymethyl chitosan micelles as nanocarriers for controlled release of pesticide. Polymers 2020, 12, 2268. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z.; Idris, A.S.; Hilmi, N.H.; Ramli, N.R.; Fakurazi, S. The effect of surfactant type on the physico-chemical properties of hexaconazole/dazomet-micelle nanodelivery system and its biofungicidal activity against Ganoderma boninense. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128402. [Google Scholar] [CrossRef]
- Su, W.; Qin, Y.; Wu, J.; Meng, G.; Yang, S.; Cui, L.; Liu, Z.; Guo, X. Linear Supramolecular Block Copolymer Micelles for ROS-Responsive Release of Antimicrobial Pesticides. ACS Appl. Nano Mater. 2023, 6, 12736–12743. [Google Scholar] [CrossRef]
- Luong, J.H.; Tran, C.; Ton-That, D. A paradox over electric vehicles, mining of lithium for car batteries. Energies 2022, 15, 7997. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Cifuentes, Z.; Beckstead, J.A.; Sillero, J.C.; Ávila, C.; Rubio, J.; Ryan, R.O. Effect of amphotericin B nanodisks on plant fungal diseases. Pest. Manag. Sci. 2012, 68, 67–74. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Preparation and characterization of tea tree oil solid liposomes to control brown rot and improve quality in peach fruit. Lwt 2022, 162, 113442. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Liu, Q.; He, Y. Preparation of an environmentally friendly nano-insecticide through encapsulation in polymeric liposomes and its insecticidal activities against the fall armyworm, Spodoptera frugiperda. Insects 2022, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Meng, Y.; Wang, Y.; Gao, M.; Dong, J.; Cao, Z.; Zhang, L.; Ma, S. Preparation of nanoliposomes containing the extracts of Eleocharis dulcis corm-peels and ascertaining their aphidicidal activity against Megoura crassicauda and Acyrthosiphon pisum. Ind. Crop. Prod. 2024, 207, 117746. [Google Scholar] [CrossRef]
- Liu, X.; He, B.; Xu, Z.; Yin, M.; Yang, W.; Zhang, H.; Cao, J.; Shen, J. A functionalized fluorescent dendrimer as a pesticide nanocarrier: Application in pest control. Nanoscale 2015, 7, 445–449. [Google Scholar] [CrossRef]
- Thanh, V.M.; Bui, L.M.; Bach, L.G.; Nguyen, N.T.; Thi, H.L.; Hoang Thi, T.T. Origanum majorana L. essential oil-associated polymeric nano dendrimer for antifungal activity against Phytophthora infestans. Materials 2019, 12, 1446. [Google Scholar] [CrossRef]
- Labidi, S.; Sandhu, R.K.; Beaulieu, C.; Beaudoin, N. Increased ferritin and iron accumulation in tubers of thaxtomin A-habituated potato var. Yukon Gold somaclones with enhanced resistance to common scab. J. Plant Pathol. 2023, 105, 107–119. [Google Scholar] [CrossRef]
- Maleki, R.; Abdollahi, H.; Piri, S. Variation of active iron and ferritin content in pear cultivars with different levels of pathogen resistance following inoculation with Erwinia amylovora. J. Plant Pathol. 2022, 104, 281–293. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Khare, P.; Talreja, N.; Deva, D.; Sharma, A.; Verma, N. Carbon nanofibers containing metal-doped porous carbon beads for environmental remediation applications. Chem. Eng. J. 2013, 229, 72–81. [Google Scholar] [CrossRef]
- Kumar, D.; Talreja, N. Nickel nanoparticles-doped rhodamine grafted carbon nanofibers as colorimetric probe: Naked eye detection and highly sensitive measurement of aqueous Cr3+ and Pb2+. Korean J. Chem. Eng. 2019, 36, 126–135. [Google Scholar] [CrossRef]
- Saraswat, R.; Talreja, N.; Deva, D.; Sankararamakrishnan, N.; Sharma, A.; Verma, N. Development of novel in situ nickel-doped, phenolic resin-based micro–nano-activated carbon adsorbents for the removal of vitamin B-12. Chem. Eng. J. 2012, 197, 250–260. [Google Scholar] [CrossRef]
- Talreja, N.; Jung, S.; Kim, T. Phenol-formaldehyde-resin-based activated carbons with controlled pore size distribution for high-performance supercapacitors. Chem. Eng. J. 2020, 379, 122332. [Google Scholar] [CrossRef]
- Talreja, N.; Kumar, D.; Verma, N. Removal of hexavalent chromium from water using Fe-grown carbon nanofibers containing porous carbon microbeads. J. Water Process Eng. 2014, 3, 34–45. [Google Scholar] [CrossRef]
- Talreja, N.; Verma, N.; Kumar, D. Carbon bead-supported ethylene diamine-functionalized carbon nanofibers: An efficient adsorbent for salicylic acid. CLEAN–Soil. Air Water 2016, 44, 1461–1470. [Google Scholar] [CrossRef]
- Omar, R.A.; Talreja, N.; Chauhan, D.; Mangalaraja, R.V.; Ashfaq, M. Nano metal-carbon–based materials: Emerging platform for the growth and protection of crops. In Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 341–354. [Google Scholar]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Cabrera, R.I.; Juárez-Maldonado, A. Carbon nanotubes decrease the negative impact of Alternaria solani in tomato crop. Nanomaterials 2021, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Fang, P.; Ma, C.; White, J.C.; Xiang, Z.; Wang, H.; Zhang, Z.; Rui, Y.; Xing, B. Engineered nanomaterials inhibit Podosphaera pannosa infection on rose leaves by regulating phytohormones. Environ. Res. 2019, 170, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lipșa, F.D.; Ursu, E.L.; Ursu, C.; Ulea, E.; Cazacu, A. Evaluation of the antifungal activity of gold–chitosan and carbon nanoparticles on Fusarium oxysporum. Agronomy 2020, 10, 1143. [Google Scholar] [CrossRef]
- Adeel, M.; Farooq, T.; White, J.C.; Hao, Y.; He, Z.; Rui, Y. Carbon-based nanomaterials suppress tobacco mosaic virus (TMV) infection and induce resistance in Nicotiana benthamiana. J. Hazard. Mater. 2021, 404, 124167. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Mosa, M.A.; Ismail, A.M.; Khalil, A.E. Lignin-loaded carbon nanoparticles as a promising control agent against Fusarium verticillioides in maize: Physiological and biochemical analyses. Polymers 2023, 15, 1193. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Alhag, S.K.; Dablool, A.S.; Ahmed, A.E.; Alghamdi, S.; Ali, B.; Al-Saeed, F.A.; Saleem, M.H.; Poczai, P. Manufactured nano-objects confer viral protection against cucurbit chlorotic yellows virus (CCYV) infecting nicotiana benthamiana. Microorganisms 2022, 10, 1837. [Google Scholar] [CrossRef]
- Baka, Z.A.; El-Zahed, M.M. Biocontrol of chocolate spot disease of broad bean (Vicia faba L.) caused by Botrytis fabae using biosynthesized reduced graphene oxide/silver nanocomposite. Physiol. Mol. Plant Pathol. 2023, 127, 102116. [Google Scholar] [CrossRef]
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.A.; Mohamed, M.A. Reduced graphene oxide nanosheet-decorated copper oxide nanoparticles: A potent antifungal nanocomposite against fusarium root rot and wilt diseases of tomato and pepper plants. Nanomaterials 2020, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Bytešníková, Z.; Pečenka, J.; Tekielska, D.; Kiss, T.; Švec, P.; Ridošková, A.; Bezdička, P.; Pekárková, J.; Eichmeier, A.; Pokluda, R.; et al. Reduced graphene oxide-based nanometal-composite containing copper and silver nanoparticles protect tomato and pepper against Xanthomonas euvesicatoria infection. Chem. Biol. Technol. Agric. 2022, 9, 84. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Qu, J.; Wang, X.; Huang, F.; Jiao, Y.; Zhang, Y. Bi2O3/TiO2@ reduced graphene oxide with enzyme-like properties efficiently inactivates Pseudomonas syringae pv. tomato DC3000 and enhances abiotic stress tolerance in tomato. Environ. Sci. Nano 2022, 9, 118–132. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N. Fullerenol increases effectiveness of foliar iron fertilization in iron-deficient cucumber. PLoS ONE 2020, 15, e0232765. [Google Scholar] [CrossRef]
- Gyawali, B.; Rahimi, R.; Alizadeh, H.; Mohammadi, M. Graphene Quantum Dots (GQD)-Mediated dsRNA Delivery for the Control of Fusarium Head Blight Disease in Wheat. ACS Appl. Bio Mater. 2024, 7, 1526–1535. [Google Scholar] [CrossRef]
- Wang, X.; Cai, A.; Wen, X.; Jing, D.; Qi, H.; Yuan, H. Graphene oxide-Fe3O4 nanocomposites as high-performance antifungal agents against Plasmopara viticola. Sci. China Mater. 2017, 60, 258–268. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Z.; Li, X.; Yu, Y. Effects of modified nanoscale carbon black on plant growth, root cellular morphogenesis, and microbial community in cadmium-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 18423–18433. [Google Scholar] [CrossRef] [PubMed]
- Gahoi, P.; Omar, R.A.; Verma, N.; Gupta, G.S. Rhizobacteria and Acylated homoserine lactone-based nanobiofertilizer to improve growth and pathogen defense in Cicer arietinum and Triticum aestivum Plants. ACS Agric. Sci. Technol. 2021, 1, 240–252. [Google Scholar] [CrossRef]
- Joshi, A.; Sharma, A.; Nayyar, H.; Verma, G.; Dharamvir, K. Carbon nanofibers suppress fungal inhibition of seed germination of maize (Zea mays) and barley (Hordeum vulgare L.) crop. AIP Conf. Proc. 2015, 1675, 030034. [Google Scholar]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M.; Watanabe, F.; Biris, A. Ca2+ cross-linked alginic acid nanoparticles for solubilization of lipophilic natural colorants. J. Agric. Food Chem. 2009, 57, 7505–7512. [Google Scholar] [CrossRef]
- Goh, K.L.; Manikam, J.; Qua, C.S. High-dose rabeprazole–amoxicillin dual therapy and rabeprazole triple therapy with amoxicillin and levofloxacin for 2 weeks as first and second line rescue therapies for H elicobacter pylori treatment failures. Aliment. Pharmacol. Ther. 2012, 35, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Ye, F.; Astete, C.E.; Sabliov, C.M. Entrapment and delivery of α-tocopherol by a self-assembled, alginate-conjugated prodrug nanostructure. Food Hydrocoll. 2017, 72, 62–72. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef]
- Murugeshu, A.; Astete, C.; Leonardi, C.; Morgan, T.; Sabliov, C.M. Chitosan/PLGA particles for controlled release of α-tocopherol in the GI tract via oral administration. Nanomedicine 2011, 6, 1513–1528. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef]
- Prasad, A.; Astete, C.E.; Bodoki, A.E.; Windham, M.; Bodoki, E.; Sabliov, C.M. Zein nanoparticles uptake and translocation in hydroponically grown sugar cane plants. J. Agric. Food Chem. 2017, 66, 6544–6551. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Astete, C.E.; Bodoki, E.; Sabliov, C.M. Zein nanoparticles uptake by hydroponically grown soybean plants. Environ. Sci. Technol. 2017, 51, 14065–14071. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Ying, J.; Shen, J.; Dou, D.; Yin, M.; Whisson, S.C.; Birch, P.R.; Yan, S.; Wang, X. High-efficiency green management of potato late blight by a self-assembled multicomponent nano-bioprotectant. Nat. Commun. 2023, 14, 5622. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Huang, Y.; Zhang, X.; Tang, G.; Gao, Y.; Zhou, Z.; Li, Y.; Wang, H.; Yu, X.; Li, X.; et al. Self-assembled nanoparticles of a prodrug conjugate based on pyrimethanil for efficient plant disease management. J. Agric. Food Chem. 2022, 70, 11901–11910. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Meng, J.; Shao, W.; Zeng, D.; Ji, J.; Wang, P.; Zhou, X.; Qi, P.; Liu, L.; Yang, S. Plant protein-based self-assembling core–shell nanocarrier for effectively controlling plant viruses: Evidence for nanoparticle delivery behavior, plant growth promotion, and plant resistance induction. Chem. Eng. J. 2023, 464, 142432. [Google Scholar] [CrossRef]
- Dong, B.R.; Jiang, R.; Chen, J.F.; Xiao, Y.; Lv, Z.Y.; Chen, W.S. Strategic nanoparticle-mediated plant disease resistance. Crit. Rev. Biotechnol. 2023, 43, 22–37. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Ahmad, A.; Khan, Z.; Khan, S.H.; Ahmed, F.K.; Faiz, S.; Nepovimova, E.; Kuča, K.; Abd-Elsalam, K.A. Exosome/liposome-like nanoparticles: New carriers for CRISPR genome editing in plants. Int. J. Mol. Sci. 2021, 22, 7456. [Google Scholar] [CrossRef]
- Hafeez, R.; Guo, J.; Ahmed, T.; Jiang, H.; Raza, M.; Shahid, M.; Ibrahim, E.; Wang, Y.; Wang, J.; Yan, C.; et al. Bio-formulated chitosan nanoparticles enhance disease resistance against rice blast by physiomorphic, transcriptional, and microbiome modulation of rice (Oryza sativa L.). Carbohydr. Polym. 2024, 334, 122023. [Google Scholar] [CrossRef]
- Mejdoub-Trabelsi, B.; Touihri, S.; Ammar, N.; Riahi, A.; Daami-Remadi, M. Effect of chitosan for the control of potato diseases caused by Fusarium species. J. Phytopathol. 2020, 168, 18–27. [Google Scholar] [CrossRef]
- Lin, M.; Fang, S.; Zhao, X.; Liang, X.; Wu, D. Natamycin-loaded zein nanoparticles stabilized by carboxymethyl chitosan: Evaluation of colloidal/chemical performance and application in postharvest treatments. Food Hydrocoll. 2020, 106, 105871. [Google Scholar] [CrossRef]
- Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Sousa, R.M.; Torres, A.; Tavares, F.; Ribeiro, A.; Cavaco-Paulo, A.; Fernandes-Ferreira, M.; Santos, C. Satureja montana essential oil, zein nanoparticles and their combination as a biocontrol strategy to reduce bacterial spot disease on tomato plants. Horticulturae 2021, 7, 584. [Google Scholar] [CrossRef]
- Khairy, A.M.; Tohamy, M.R.; Zayed, M.A.; Mahmoud, S.F.; El-Tahan, A.M.; El-Saadony, M.T.; Mesiha, P.K. Eco-friendly application of nano-chitosan for controlling potato and tomato bacterial wilt. Saudi J. Biol. Sci. 2022, 29, 2199–2209. [Google Scholar] [CrossRef]
- El Gamal, A.Y.; Atia, M.M.; Sayed, T.E.; Abou-Zaid, M.I.; Tohamy, M.R. Antiviral activity of chitosan nanoparticles for controlling plant-infecting viruses. S. Afr. J. Sci. 2022, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Long, Y.; Li, J.; Li, M.; Xing, D.; An, H.; Wu, X.; Wu, Y. A chitosan composite film sprayed before pathogen infection effectively controls postharvest soft rot in kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- Kumar, N.V.; Basavegowda, V.R.; Murthy, A.N. Synthesis and characterization of copper-chitosan based nanofungicide and its induced defense responses in Fusarium wilt of banana. Inorg. Nano Met. Chem. 2022, 1–9. [Google Scholar] [CrossRef]
- Ghule, M.R.; Ramteke, P.K.; Ramteke, S.D.; Kodre, P.S.; Langote, A.; Gaikwad, A.V.; Holkar, S.K.; Jambhekar, H. Impact of chitosan seed treatment of fenugreek for management of root rot disease caused by Fusarium solani under in vitro and in vivo conditions. 3 Biotech 2021, 11, 290. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Li, J.; Su, Y.; Wu, X. Chitosan as an adjuvant to enhance the control efficacy of low-dosage pyraclostrobin against powdery mildew of Rosa roxburghii and improve its photosynthesis, yield, and quality. Biomolecules 2022, 12, 1304. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Monir, G.A.; Hassan, M.S.; Ahmed, Y.; Refaat, M.H.; Ismail, I.A.; El-Garhy, H.A. Exogenously applied chitosan and chitosan nanoparticles improved apple fruit resistance to blue mold, upregulated defense-related genes expression, and maintained fruit quality. Horticulturae 2021, 7, 224. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan nanoparticles inactivate alfalfa mosaic virus replication and boost innate immunity in Nicotiana glutinosa plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef]
- Chouhan, D.; Dutta, A.; Kumar, A.; Mandal, P.; Choudhuri, C. Application of nickel chitosan nanoconjugate as an antifungal agent for combating Fusarium rot of wheat. Sci. Rep. 2022, 12, 14518. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Fluoroalkenyl-grafted chitosan oligosaccharide derivative: An exploration for control nematode Meloidogyne incognita. Int. J. Mol. Sci. 2022, 23, 2080. [Google Scholar] [CrossRef]
- Khan, A.; Tariq, M.; Ahmad, F.; Mennan, S.; Khan, F.; Asif, M.; Nadeem, H.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Assessment of nematicidal efficacy of chitosan in combination with botanicals against Meloidogyne incognita on carrot. Acta Agric. Scand. Sect. B Soil. Plant Sci. 2021, 71, 225–236. [Google Scholar] [CrossRef]
- Xu, X.; Peng, X.; Huan, C.; Chen, J.; Meng, Y.; Fang, S. Development of natamycin-loaded zein-casein composite nanoparticles by a pH-driven method and application to postharvest fungal control on peach against Monilinia fructicola. Food Chem. 2023, 404, 134659. [Google Scholar] [CrossRef] [PubMed]
- Bidyarani, N.; Srivastav, A.K.; Gupta, S.K.; Kumar, U. Synthesis and physicochemical characterization of rhamnolipid-stabilized carvacrol-loaded zein nanoparticles for antimicrobial application supported by molecular docking. J. Nanoparticle Res. 2020, 22, 307. [Google Scholar] [CrossRef]
- Bidyarani, N.; Kumar, U. Synthesis of rotenone loaded zein nano-formulation for plant protection against pathogenic microbes. RSC Adv. 2019, 9, 40819–40826. [Google Scholar] [CrossRef] [PubMed]
- Khatua, A.; Prasad, A.; Priyadarshini, E.; Virmani, I.; Ghosh, L.; Paul, B.; Meena, R.; Barabadi, H.; Patel, A.K.; Saravanan, M. CTAB-PLGA Curcumin Nanoparticles: Preparation, Biophysical Characterization and Their Enhanced Antifungal Activity against Phytopathogenic Fungus Pythium ultimum. ChemistrySelect 2020, 5, 10574–10580. [Google Scholar] [CrossRef]
- De Angelis, G.; Simonetti, G.; Chronopoulou, L.; Orekhova, A.; Badiali, C.; Petruccelli, V.; Portoghesi, F.; D’Angeli, S.; Brasili, E.; Pasqua, G.; et al. A novel approach to control Botrytis cinerea fungal infections: Uptake and biological activity of antifungals encapsulated in nanoparticle based vectors. Sci. Rep. 2022, 12, 7989. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, L.; Zhu, H.; Dhanasekaran, S.; Zhang, X.; Zhang, H. Study on the effect of alginate oligosaccharide combined with Meyerozyma guilliermondii against Penicillium expansum in pears and the possible mechanisms involved. Physiol. Mol. Plant Pathol. 2021, 115, 101654. [Google Scholar] [CrossRef]
- Zhuo, R.; Li, B.; Tian, S. Alginate oligosaccharide improves resistance to postharvest decay and quality in kiwifruit (Actinidia deliciosa cv. Bruno). Hortic. Plant J. 2022, 8, 44–52. [Google Scholar] [CrossRef]
- Bouissil, S.; Guérin, C.; Roche, J.; Dubessay, P.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; Mouzeyar, S.; Delattre, C.; El Modafar, C. Induction Induction of defense gene expression and the resistance of date palm to Fusarium oxysporum f. sp. albedinis in response to alginate extracted from Bifurcaria bifurcata. Mar. Drugs 2022, 20, 88. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Ngoc, D.T.; Du, B.D.; Tuan, L.N.; Thach, B.D.; Kien, C.T.; Phu, D.V.; Hien, N.Q. Study on antifungal activity and ability against rice leaf blast disease of nano Cu-Cu2O/alginate. Indian J. Agric. Res. 2020, 54, 802–806. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging frontiers in nanotechnology for precision agriculture: Advancements, hurdles and prospects. Agrochemicals 2023, 2, 220–256. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Ke, W.J.; Hsieh, C.T.; Lin, K.S.; Tzou, D.Y.; Chiang, C.L. ZnO nanoparticles affect Bacillus subtilis cell growth and biofilm formation. PLoS ONE 2015, 10, e0128457. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, D.; Luo, Y.; He, D.; Zhang, H.; El-Naggar, A.; Palansooriya, K.N.; Chen, K.; Yan, Y.; Lu, X.; et al. Nanoplastics induce molecular toxicity in earthworm: Integrated multi-omics, morphological, and intestinal microorganism analyses. J. Hazard. Mater. 2023, 442, 130034. [Google Scholar] [CrossRef]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1513. [Google Scholar] [CrossRef] [PubMed]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Jafari, S.M.; Katouzian, I.; Akhavan, S. Safety and regulatory issues of nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; pp. 545–590. [Google Scholar]



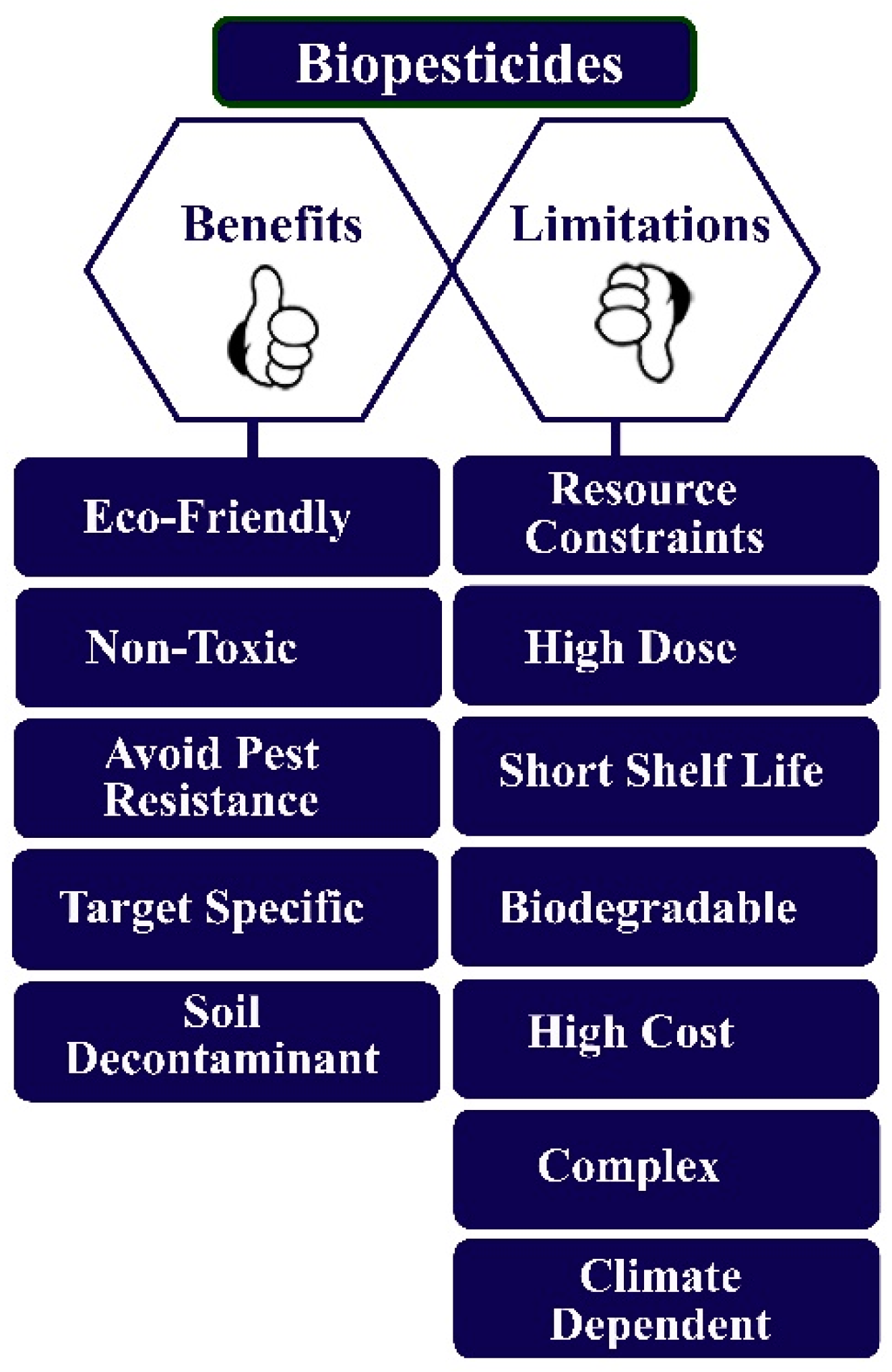
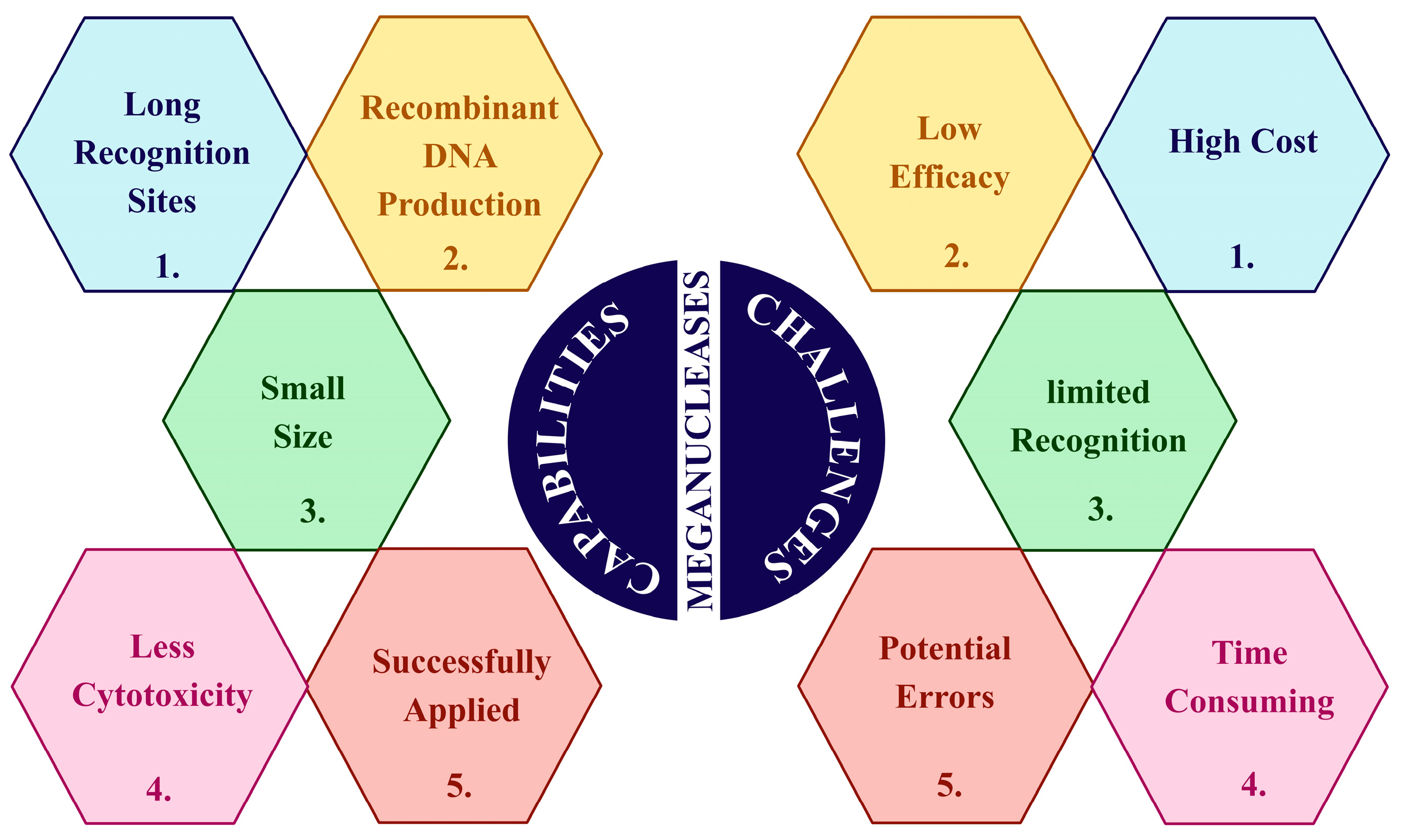
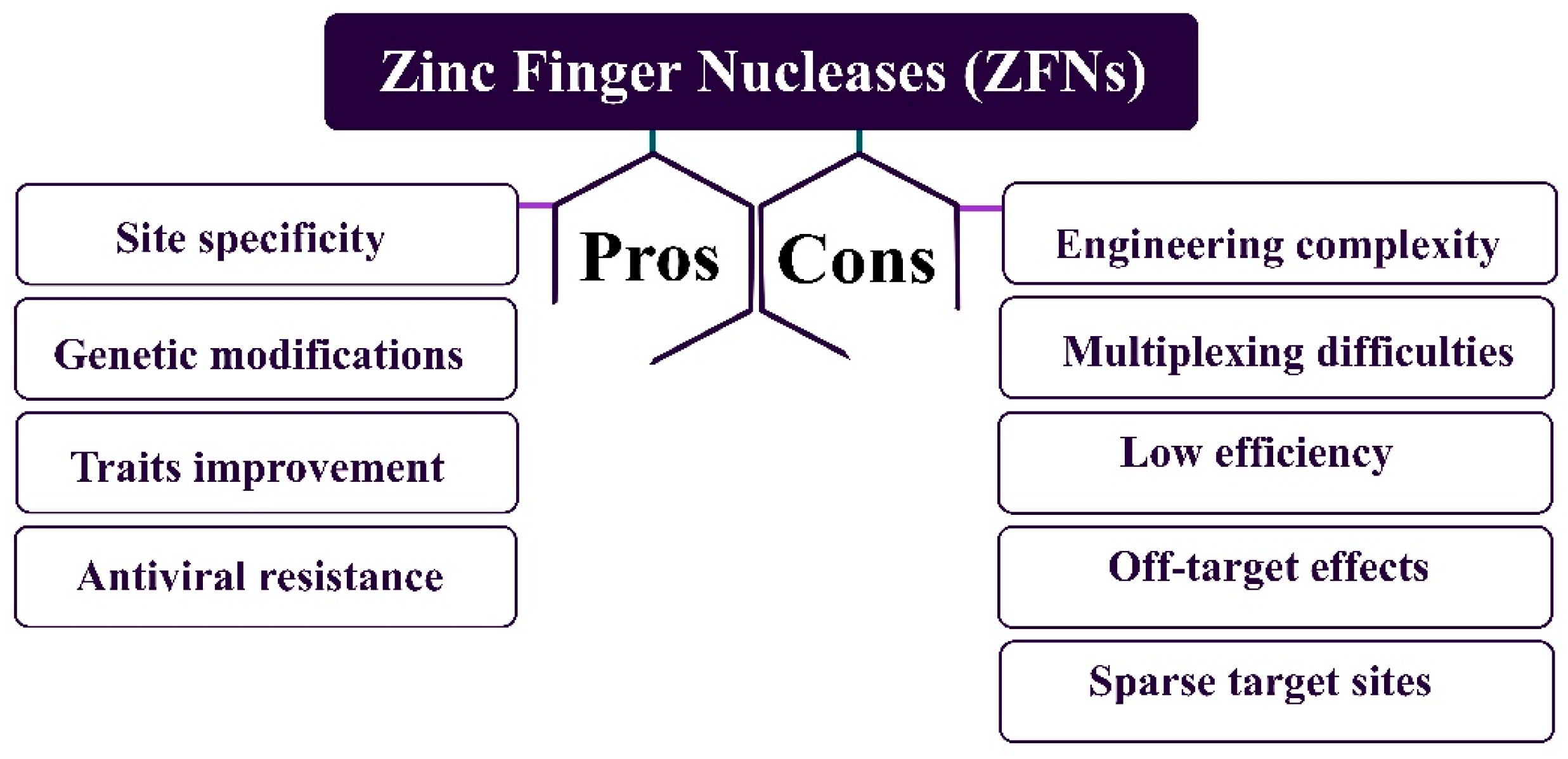

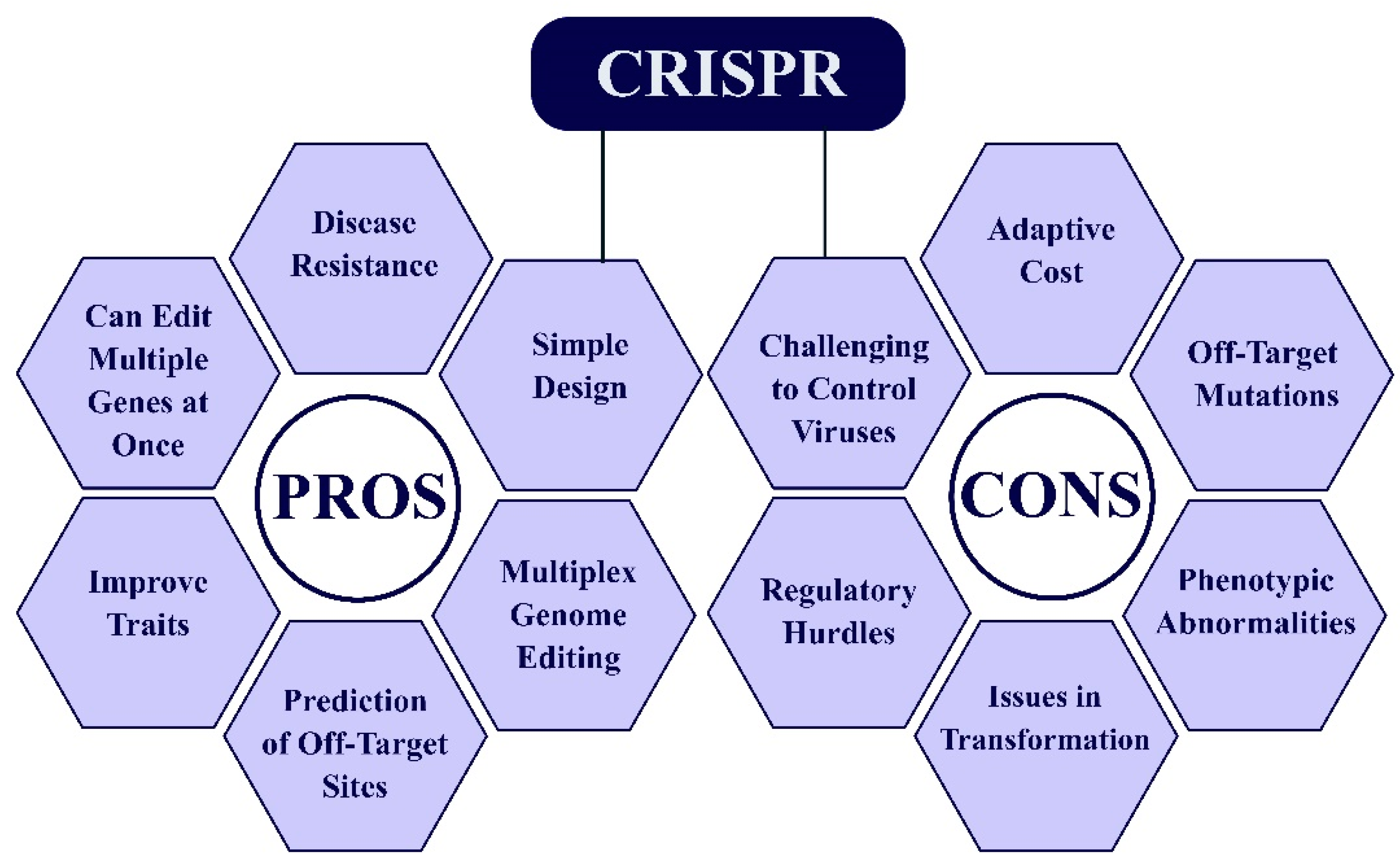

| MNPs | Concentration | Source of Synthesis | Size of NPs (nm) | Disease & Pathogen Management | Reference |
|---|---|---|---|---|---|
| Au | 200 ppm | Biosynthesis | N/A | Sheath blight of rice caused by Rhizoctonia solani | [159] |
| 2 ppm | Trichoderma atroviride | 50–75 | Phomopsis canker in tea pant | [160] | |
| 0, 20, 40, 60, and 80 ppm | Bacillus sonorensis | 10–35 | Dematophora necatrix, Fusarium oxysporum, Alternaria aternata, Alternaria mali, Sclerotium rolfsii and Colletotrichum capsici | [161] | |
| 150 ppm | Metarhizium anisopliae | 9–54 | Rice blast disease | [162] | |
| Zn | 50, 100, 250, and 500 ppm | N/A | DLS: 30–40 TEM: 15–20 | Aspergillus niger | [163] |
| 0–65 mg/L | N/A | >50 | Peronospora tabacina | [164] | |
| Ag | 25 ppm, 37.5 ppm and 50 ppm | N/A | 3 to 10 | Fusarium oxysporum f. sp. radicis-lycopersici | [165] |
| 2, 4 and 10 µg/mL | Serratia sp | 10 to 20 | Spot blotch of wheat (Bipolaris sorokiniana) | [166] | |
| 17.24 μg/mL | Azadirachta indica | 15 | Bakanae of rice (F. fujikuroi and F. proliferatum) | [167] | |
| 50 ppm | Azadirachta indica | N/A | Early blight of tomato (Alternaria solani) | [168] | |
| 40 mg/L | Allium sativum bulb | N/A | Spot blotch of wheat (B. sorokiniana) | [169] | |
| 40 ppm | Avena fatua | 5 to 25 | Fusarium oxysporum f. sp. lycopersici | [170] | |
| 40 mg/L | Allium sativum bulb extract | N/A | Black leg and soft rot of potato | [169] | |
| 7.8 μg/mL | Pseudomonas canadensis | 21 and 52 | Brown blotch of mushroom (Pseudomonas. tolaasii.) | [171] | |
| 100 ppm | Pleurotus ostreatus | N/A | Fusarium oxysporum | [172] | |
| 0.35 mg/100 uL | Hyppophae rhamnoides | N/A | Ralstonia solanacearum and Pseudomonas syringae | [173] | |
| N/A | Malva parviflora L. | 50.6 | Helminthosporium rostratum, Fusarium solani, Fusarium oxysporum, and Alternaria alternata. | [174] | |
| 150 ppm | Penicillium verrucosum | 10–12 | F. chlamydosporum and Aspergillus flavus | [175] | |
| 15 μg/mL | leaf extract of rice | 16.5 | Xanthomonas oryzae pv. oryzae | [176] | |
| 20 μg mL | Bacillus sp. | 22–41 | Red rot of sugarcane (Colletotrichum falcatum) | [177] | |
| N/A | Pseudomonas poae | 19.8–44.9, | Head blight of wheat (Fusarium graminearum) | [178] | |
| Cu | 20 ppm | CuSO4 precursor | 35–70 | Macrophomina phaseolina, Bipolaris maydis, and Fusarium verticillioides In maize | [179] |
| 50 ppm | CuSO4 precursor | 35–70 | Rhizoctonia solani in maize | [179] | |
| 30 ppm | CuSO4 precursor | 35–70 | Erwinia carotovora and Ralstonia solanacearum in maize | [179] | |
| ≥80 ppm | Chemical reduction of Cu2+ with reductive agent of NaHB4 | 26.5 | Fusarium oxysporum | [180] | |
| 500 ppm and 1000 ppm | Eucalyptus and Mint leaves | 10–130 23–39 | Colletotrichum capsici of chilli | [181] | |
| N/A | Pseudomonas fluorescens, Trichoderma atroviride and Streptomyces griseus | N/A | Red root-rot disease in tea (Poria hypolateritia), collar canker (Phomopsis theae) | [182] | |
| 20 ppm 50 ppm 30 ppm | CuSO4 precursor | 35–70 | Macrophomina phaseolina, Bipolaris maydis, and Fusarium verticillioides. Rhizoctonia solani. Erwinia carotovora and Ralstonia solanacearum. | [179] | |
| 100 µg/mL | Bacillus altitudinis strain WM-2/2 | 29.11–78.56 | Bacterial fruit blotch (BLB) of watermelon (Acidovorax citrulli) | [183] | |
| N/A | Chemical synthesis | 28 | Bacterial leaf blight (BLB) of rice (Xanthomonas oryzae pv. Oryzae) | [184] | |
| 100, 150 and 200 ppm | Chemical synthesis and green synthesis | 126 and 85 | Root knot nematode (Meloidogyne Incongita) | [185] |
| MONPs | Concentration | Source of Synthesis | Size of NPs (nm) | Disease & Pathogen Management | Reference |
|---|---|---|---|---|---|
| ZnO | 130.1 and 104.9 µg/mL | Carica papaya leaf extract | N/A | S. sclerotiorum | [192] |
| 10% of 3 mL/L | PVP/ZnSO4 irradiated to 30 kGy. | 38 | Black mould of pomegranate (Aspergillus niger) | [193] | |
| 5% of 9 mL/L. | PVP/ZnSO4 irradiated to 30 kGy. | 38 | Green mould of orange (Penicillium digitatum) | [193] | |
| 20 mg/L | Cinnamomum camphora | 13.92, 15.19 and 21.13 | Early blight of tomato (Alternaria solani) | [194] | |
| 18.0 µg/mL | Matricaria chamomilla flower extract | 8.9 to 32.6 | Bacterial wilt of tomato (Ralstonia solanacearum) | [195] | |
| 1.0 mg/mL | Trachyspermum ammi | 48.52 | Fruit rot (Rhizoctonia solani) | [196] | |
| 100 ppm | Eucalyptus globules | 52–70 | Alteraria blotch (Alternaria mali), Botryosphaeria canker of apple (Botryosphaeria dothidea) | [197] | |
| 250 ppm | N/A | N/A | Purple Blotch disease in onion (Alternaria porri) | [198] | |
| 100 µg/mL | Picea smithiana extract | 25 | Bacterial leaf spot of tomato Bacterial wilt of tomato | [199] | |
| 100 µg/mL | Trichoderma harzianum | 25–60 | Fusarium wilt of tomato (F. oxysporum) | [200] | |
| 200 μg/mL | Cannabis sativa L. | 13.51 | Fusarium virguliforme in soybean | [201] | |
| 100 mg/mL−1 | lemon peels | 16.8 | Citrus black rot (Alternaria citri) | [202] | |
| Ag2O | 0.10 and 0.20 g/L | solid homogeneous solution of silver oxide material | 38.23 | Pseudomonas syringae pv. tomato, Xanthomonas campestris pv. vesicatoria, Pectobacterium carotovorum subsp. carotovorum, Ralstonia solanacearum, Fusarium oxysporum f. sp. lycopersici and Alternaria solani in tomato | [203] |
| CuO | N/A | Hibiscus rosa-sinensis L. flower extract | 28.1 | Xanthomonas oryzae pv. oryzae | [204] |
| 10 ppm | coffee powder | 85–100 | Fusarium wilt in chickpea | [205] | |
| 200 μg/mL | Cannabis sativa L. | 7.36 | Fusarium virguliforme in soybean | [201] | |
| N/A | Chemical synthesis | 25.54 and 25.83 | Root rot disease in cucumber (Fusarium solani) | [206] | |
| 10, 15, 30, 50, 70, 100, and 150 mg/L | Pseudomonas fluorescens and Trichoderma viride | 40–100 and 20–80 | Gummosis of citrus (Phytophthora parasitica) | [207] | |
| 5–350 μg/mL | Cassia fistula | 12–38 | Fusarium wilt of tomato (Fusarium oxysporum f. sp. lycopersici) | [208] | |
| 200 ppm | Trichoderma asperellum | 22 | Alternaria brassicae | [209] | |
| 200.0 μg/mL | Hibiscus rosa-sinensis L. | 28.1 | Bacterial leaf blight of rice (Xanthomonas oryzae pv. Oryzae) | [204] | |
| 200 ppm | Jatropha curcas | 5 to 15 | Root-knot nematode in chickpea (Meloidogyne incognita) | [210] | |
| 100 mg/mL | lemon peels | 18 | Citrus black rot (Alternaria citri) | [202] | |
| SiO2 | 100 mg/L | N/A | 54–76 | P. syringae | [211] |
| 50, 100, 150, 200 and 250 ppm | bioleaching of sand | 22.5 | Meloidogyne javanica | [36] | |
| 2, 20, 200 and 2000 ppm. | Green synthesis | 58.6 | Vigna radiata L. | [212] | |
| 200 µg/mL | Crocus sativus L. | N/A | Bacterial leaf blight of rice (Xanthomonas oryzae pv. Oryzae) | [213] | |
| 250 to 1000 mg/kg | agro-waste | N/A | Fusarium oxysporum (Fusarium wilt in Eruca sativa) | [214] | |
| 150 ppm | Green synthesis | N/A | Pepper bacterial leaf spot (Xanthomonas vesicatoria) | [215] | |
| 25, 50, and 100 µg/mL | saffron extract | 9.92 and 19.8 | Rhizoctonia solani | [216] | |
| 50 mg/L | Milled/acid leaching rice husk | 15 | Bakanae of rice (F. fujikuroi) | [217] | |
| TiO2 | 100 and 200 mg/L | N/A | 21 | Pectobacterium betavasculorum, Rhizoctonia solani, and Meloidogyne incognita in beetroot | [218] |
| 40 mg/L | Moringa oleifera Lam | 10–100 | Spot blotch of wheat (Bipolaris sorokiniana) | [219] | |
| 0.20 mg/mL | N/A | 5–15 | Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode (Meloidogyne incognita) in egg plant | [220] | |
| 50 µg/mL | mixing of TiO2 solution with a lemon fruit extract at room temperature | 41.5 | Soft rot of sweet potato (Dickeya dadantii) | [221] | |
| 100 ppm | Aspergillus versicolor | 47.15 | Leaf blight of tomato (Alternaria alternata) | [222] | |
| 25, 50 and 75 μL | Trianthema portulacastrum, Chenopodium quinoa and by chemical conventional (sol-gel) method | <15 | Wheat rust (Ustillago tritici) | [223] | |
| 40 mg/L | Moringa oleifera | <100 | Stripe rust (Puccinia striiformis f. sp. tritici) | [224] | |
| 150 µg/mL | Chemical (sol gel) synthesis | 20 | Tobacco mosaic virus in chili pepper | [225] | |
| 400 mg/L | Green synthesis by African palm oil and Chemical synthesis by sol gel method | 14.60 ± 0.44 and 12.30 ± 0.54 | Fusarium solani | [226] | |
| CeO2 | 50 and 250 mg/L | N/A | 8 ± 1 | Fusarium wilt (Fusarium oxysporum f. sp. lycopersici) | [227] |
| 30 mg/L | Acorus calamusas rhizomes | 42 | Wheat stripe rust (Puccinia striformis) | [228] | |
| 100 mg/L | Chenopodium quinoa L. | 7–10 | Ustilago tritici in wheat | [229] | |
| Fe2O3 | 20 µg/mL | Green synthesis by hydrothermal process | HRTEM: 5 ± 1.0 DLS: 7.5 XRD: 5.95 | Fusarium wilt (F. oxysporum) | [230] |
| 1.0 mg/mL | Green synthesis | 49 | Fusarium fruit rot (Fusarium oxysporum) | [231] | |
| 1.0 mg/mL | Trichoderma harzianum | 17.78 | brown rot of apple (Fusarium oxysporum) | [232] | |
| 0, 10, 50, 250 and 500 6.2 μg/mL | Mentha spicata | 21–26 | E. coli and B. cereus | [233] | |
| 6 mg/mL | Skimmia laureola leaf extract | 56–350 | Bacterial wilt (Ralstonia solanacearum) | [234] | |
| 1.0 mg/mL | Azadirachta indica | 24 | brown rot of sweet oranges (Fusarium oxysporum) | [235] | |
| 100 and 200 ppm | Thyme plant | N/A | Gray mold of strawberry (Botrytis cinerea) | [236] | |
| 1.0 mg/mL | Calotropis procera | 49 | Fusarium fruit rot of loquat | [231] | |
| 15, 10 and 5 mg/L | Dried Guava | 29–41 | Alternaria solani | [237] | |
| 1.0 mg/mL | Calotropis procera | 32 | Fruit rot of cherry (Aspergillus flavus) | [238] | |
| Al2O3 | 1, 6 and 50 mg/100 mL | Pepsi cans | 4–10 | A. flavus, Fusarium sp. and Alternaria sp. | [239] |
| 150 mg/mL | Colletotrichum sp. | 39 ± 35 | F. oxysporum | [240] | |
| CoO | 200 µg/mL | Hibiscus rosa sinensis flower | 34.9 | Bacterial leaf blight of rice (Xanthomonas oryzae pv. oryzae) | [241] |
| Fe3O4 | 0.01–15 μg/mL | Spinach | 20 | Fusarium Wilt of tomato (Fusarium oxysporum) | [242] |
| 10 µg/mL | Spinacia oleracea | 4 | Fusarium wilt of tomato | [208] | |
| 1000–1400 ppm | N/A | 50 ± 5 | Onion white rot (Sclerotium cepivorum) | [243] | |
| 10, 40 and 80 mg/L | Chemical co-precipitation synthesis | 60 to 72 | Acremonium Wilt of sorghum (Acremonium striticum) | [244] | |
| MgO | 100 μg/mL | Green synthesis | 29–60 | Rhizoctonia solani, Acidovorax oryzae | [245] |
| 4 μg/mL | Aqueous Rosemary extract | <20 | Xanthomonas oryzae pv. oryzae | [246] | |
| 16.0 μg/mL | Paenibacillus polymyxa | 10.9 | Xanthomonas oryzae oryzae | [247] | |
| 75, 150, 300, and 500 μg/mL | Strawberry | 100 | Root-knot nematode (Meloidogynidae) | [233] | |
| 3 mg/mL | Chemical synthesis | N/A | Brown rot of potato (R. solanacearum) | [248] | |
| 50 and 100 mg/L | Lemon fruit extracts | N/A | Alternaria leaf blight of carrot | [249] | |
| 20 mg/mL | Acinetobacter johnsonii strain RTN1 | 18 to 45 | Acidovorax oryzae | [245] | |
| 15.36 μg/mL | Burkholderia rinojensis | 26.70 | Fusarium oxysporum f. sp. lycopersici | [250] | |
| 200 ppm | S. cerevisiae | 27 | Callosobruchus maculatus | [251] | |
| 50 μg/mL | Green synthesis | N/A | Phytophthora infestans | [252] | |
| 75, 150 and 200 mg/L | N/A | 52.5 to 57.3 | Black scurf of potato (Rhizoctonia solani) | [253] | |
| 74.81, 82.94, and 91.19 mg/g | Magnesium nitrate hexahydrates precursor | 52.97 ± 1.43 | Powdery mildew of peppers (Oidiopsis sicula) | [254] | |
| 500, 1500, 2500 mg/L | Magnesium nitrate hexahydrate precursor | 21.8 | Clubroot caused by Plasmodiophora brassica | [255] | |
| 79.43 ppm | Alcoholic extract of the bark of the walnut tree | 28.55 | Thielaviopsis paradoxa and Thielaviopsis punctulata | [256] | |
| 100, 200, and/or 1000 μg/mL | N/A | 20 | Bacterial spot of tomato (Xanthomonas perforans) | [257] |
| Organic NPs | Concentration | Nanocomposite | Disease & Pathogen Management | Rerefernces |
|---|---|---|---|---|
| Micelles | 0.013 to 0.042 mg/mL | Carboxymethylchitosan (CMCS) micelles | Smart delivery of agrochemicals | [259] |
| N/A | Hexaconazole/dazomet-micelle | Bio-fungicidal activity against Ganoderma boninense | [260] | |
| 30 mg/L | Linear Supramolecular Block Copolymer Micelles | Rhizoctonia solani | [261] | |
| 10, 20, 40, and 80 μL/L | Humidity-Responsive Cinnamon Essential Oil Nano micelles | High antifungal activity against Botrytis cinerea and nano-vesicles for preservation of fruit or vegetable | [262] | |
| Liposomes | 10 µg/mL | Liposomes bounded amphotericin | F. oxysporum f. sp. ciceris in chickpea | [263] |
| 0, 1 and 3 g/L | Tea tree oil solid liposomes (TTO-SLPs) | Brown rot of peach fruit caused by Monilinia fructicola | [264] | |
| 0.046 mg/L | Nano-Insecticide through Encapsulation of insecticides in Polymeric Liposomes | Fall armyworm Spodoptera frugiperda | [265] | |
| 136.59 and 83.99 mg/L, 315.78 and 154.34 mg/L | Eleocharis dulcis peel extract (EDPE) nanoliposomes | Megoura crassicauda and Acyrthosiphon pisum | [266] |
| Organic NPs | Concentration | Size (nm) | Disease & Pathogen Management | Reference |
|---|---|---|---|---|
| Dendrimers | 24 μg | 1.1, 1.8, and 3.2 | Cotton bollworm cells and larvae (H. armigera) | [267] |
| 500, 1000, 2000 and 5000 ppm | 20 to 30 | Phytophthora infestans | [268] | |
| Ferritin | N/A | N/A | Changes in the regulation of iron homeostasis are involved in increasing resistance to Common scab caused by Streptomyces scabies | [269] |
| N/A | N/A | Enhanced resistance against fire blight of pear caused by Erwinia amylovora | [270] |
| Concentration | Source | Size | Disease & Pathogen Management | Reference | |
|---|---|---|---|---|---|
| Carbon nanotubes | 100 mg/L | N/A | 30–50 nm | Stem and fruit rot and leaf blight of tomato caused by Alternaria solani | [279] |
| 200 mg/L | N/A | 20–30 nm | Powdery mildew of roses caused by Podosphaera pannosa | [280] | |
| 19 and 23 mg/mL. | Pulsed laser ablation in liquid (PLAL) | 23 nm | Fusarium oxysporum | [281] | |
| 100, 200 and 500 mg/L | N/A | 20–30 nm | Tobacco mosaic virus in Nicotiana benthamiana | [282] | |
| 100 and 500 mg/L | Chemical synthesis | 52 ± 1.2 nm | Stalk rot caused by Fusarium verticillioides in Maize | [283] | |
| Fullerenes | 100, 200 and 500 mg/L | N/A | 50 nm | Tobacco mosaic virus in Nicotiana benthamiana | [282,284] |
| 100 mg/L | N/A | 50 ± 5 nm | Cucurbit Chlorotic Yellows Virus (CCYV) Infecting Nicotiana benthamiana | [284] |
| Concentration | Nanocomposite | Size | Disease & Pathogen Management | Reference | |
|---|---|---|---|---|---|
| Graphene | 150 μg/mL | Reduced graphene oxide/silver nanocomposite (rGO-Ag) | 7–26 nm | Chocolate spot disease of broad bean caused by Botrytis fabae | [285] |
| 200 mg/L | Reduced graphene oxide copper oxide (rGO-CuO) | 0.55 to 3.74 nm | Powdery mildew of roses caused by Podosphaera pannosa | [280] | |
| 1 mg/L | Reduced Graphene Oxide Nanosheet-Decorated Copper Oxide (rGO-CuO) | 5, 20 and 50 nm | Fusarium wilt and root rot | [286] | |
| 50 and 500 µg/mL | Reduced graphene oxide based Cu and Ag NPs (rGO-Cu/Ag) | SEM: 2.4 nm EDS: 40 nm | Bacterial spot of tomato and pepper caused by Xanthomonas euvesicatoria | [287] | |
| 1280 μg/mL | Bi2O3/TiO2@reduced graphene oxide (rGO) | N/A | Pseudomonas syringae tomato | [288] | |
| N/A | Graphene quantum dots (GQD) | 2–5 nm | Fusariusm head blight of wheat caused by Fusarium graminearum | [289,290] | |
| 50 and 250 μg/mL | Graphene oxide-Fe3O4 nanocomposites (GO- Fe3O4) | 30–36nm | Downy mildew of grapevine (Plasmopara viticola) | [291] |
| NP Type | Crop | Effect | Reference | |
|---|---|---|---|---|
| Carbon Black | Modified nanoscale carbon black (MCB) | Ryegrass and chard | Reduction of heavy metals, increased plant growth and enhanced microbial communities | [292] |
| Carbon nanofibers | Acylated homoserine-coated iron-carbon nanofibers | Chickpea | Suppression of Fusarium oxyssporum f. sp. ciceris | [292] |
| Carbon nanofiber CNF-Cu | Chicory | Improved water absorption, germination rate, shoot and root ratio and protein content | [289] | |
| Acylated homoserine lactone coated-iron carbon nanofiber (AHL/Fe-CNF) | Cicer arietinum and Triticum aestivum | Fusarium wilt of chickpea and root rot of wheat caused by Fusarium oxysporum f. sp. ciceris and Cochliobolus sativus | [293] | |
| Carbon nanofibers (CNFs) | Maize and barley | Resistance against fungal diseases and enhanced seed germination | [294] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, H.; Usman, M.; Binyamin, R.; Hameed, A.; Arshad, S.F.; Aslam, H.M.U.; Khan, I.A.; Abbas, M.; Zaki, H.E.M.; Ondrasek, G.; et al. Traditional Strategies and Cutting-Edge Technologies Used for Plant Disease Management: A Comprehensive Overview. Agronomy 2024, 14, 2175. https://doi.org/10.3390/agronomy14092175
Akhtar H, Usman M, Binyamin R, Hameed A, Arshad SF, Aslam HMU, Khan IA, Abbas M, Zaki HEM, Ondrasek G, et al. Traditional Strategies and Cutting-Edge Technologies Used for Plant Disease Management: A Comprehensive Overview. Agronomy. 2024; 14(9):2175. https://doi.org/10.3390/agronomy14092175
Chicago/Turabian StyleAkhtar, Hira, Muhammad Usman, Rana Binyamin, Akhtar Hameed, Sarmad Frogh Arshad, Hafiz Muhammad Usman Aslam, Imran Ahmad Khan, Manzar Abbas, Haitham E. M. Zaki, Gabrijel Ondrasek, and et al. 2024. "Traditional Strategies and Cutting-Edge Technologies Used for Plant Disease Management: A Comprehensive Overview" Agronomy 14, no. 9: 2175. https://doi.org/10.3390/agronomy14092175
APA StyleAkhtar, H., Usman, M., Binyamin, R., Hameed, A., Arshad, S. F., Aslam, H. M. U., Khan, I. A., Abbas, M., Zaki, H. E. M., Ondrasek, G., & Shahid, M. S. (2024). Traditional Strategies and Cutting-Edge Technologies Used for Plant Disease Management: A Comprehensive Overview. Agronomy, 14(9), 2175. https://doi.org/10.3390/agronomy14092175











