Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Soil Sampling
2.2. Soil Physicochemical Analyses
2.3. Physical Fractionation of Soil Organic Matter
2.4. Soil Incubation for Potential C Mineralization
2.5. Measurement of Amino Sugar for Soil Microbial Residue C (MRC)
2.6. Statistical Analyses
3. Results
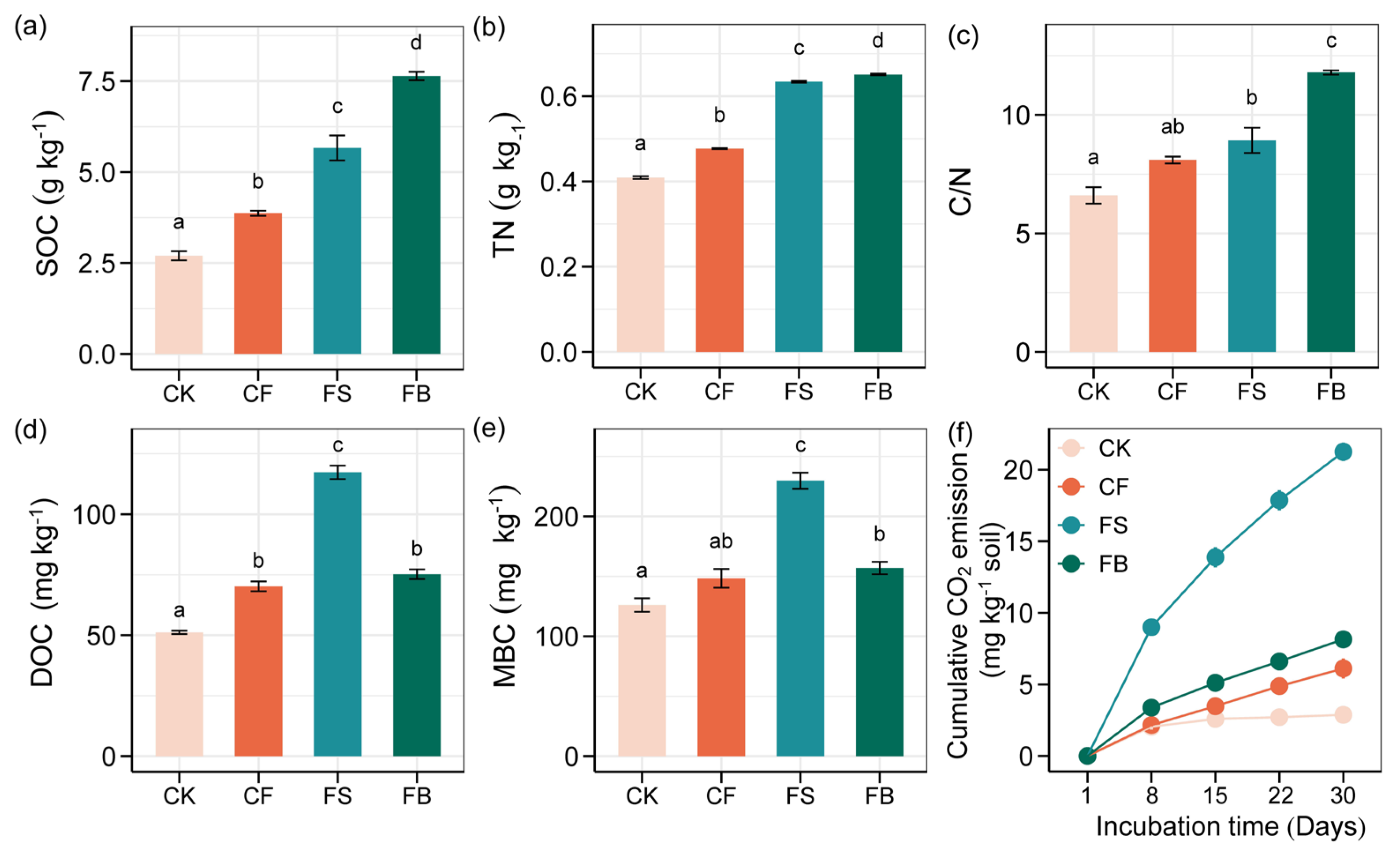
3.1. Soil Organic C Pools and Soil Properties
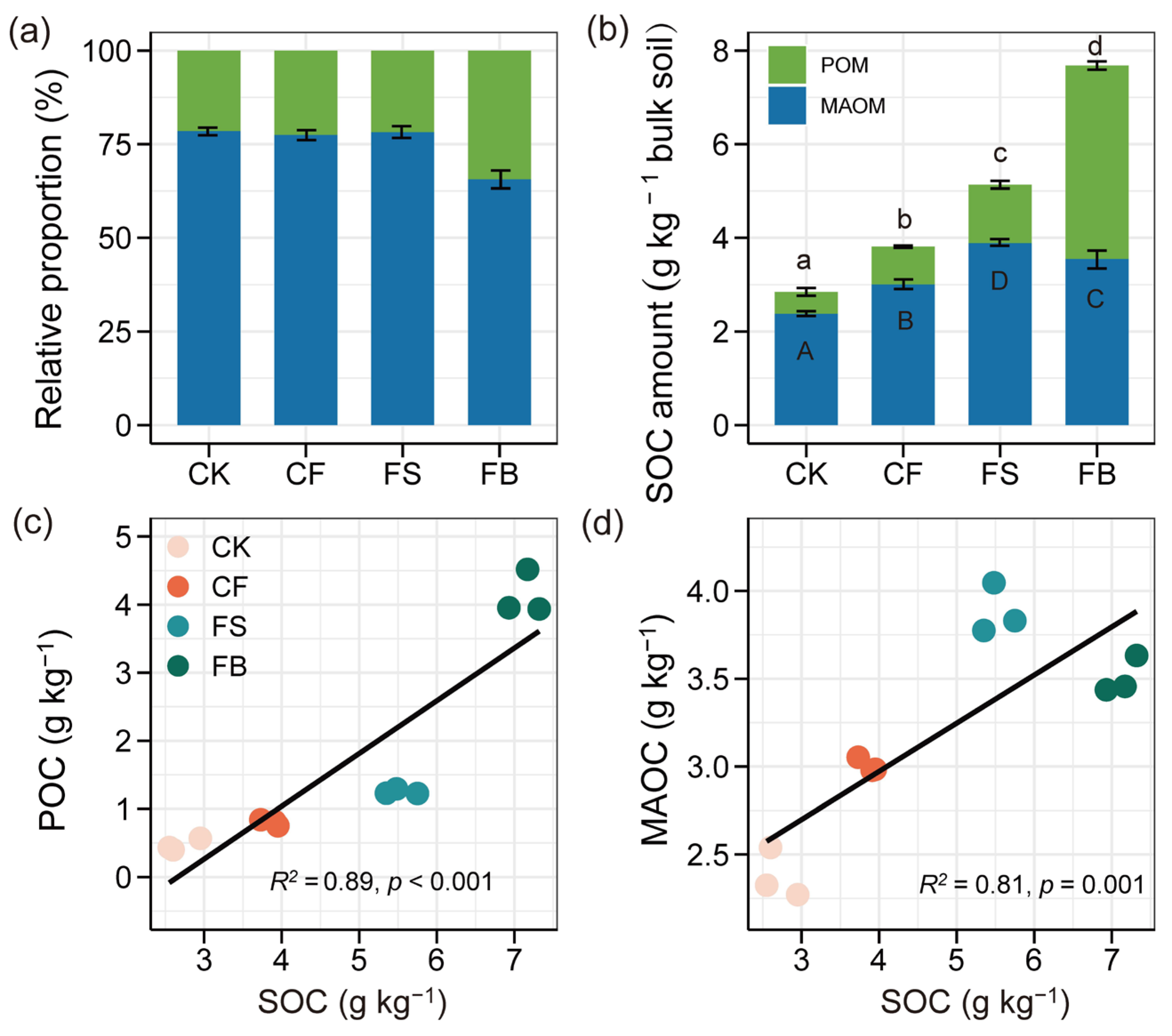
3.2. SOC Distribution in SOM Fractions
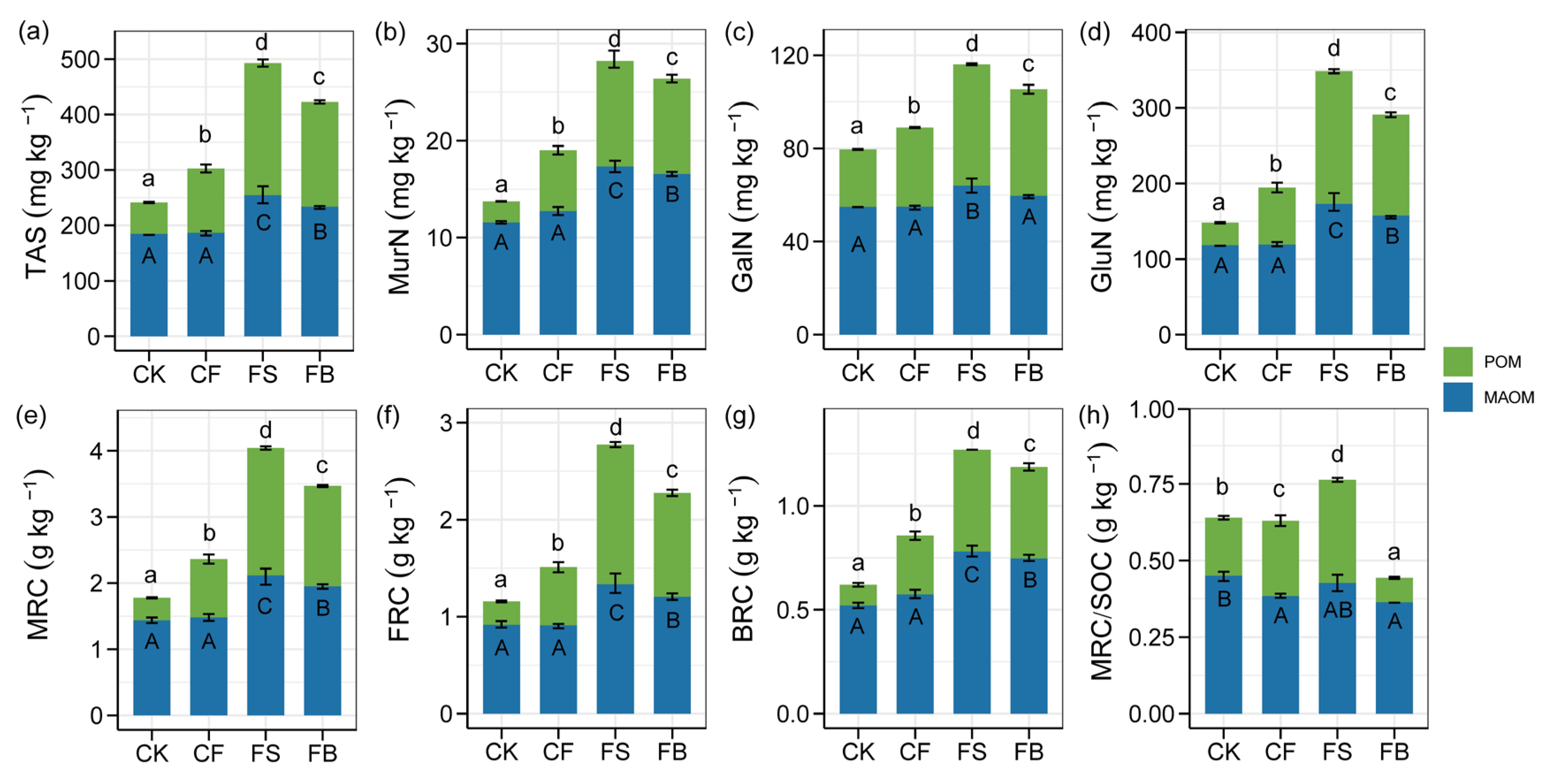
3.3. Amino Sugars and Microbial Necromass in the POM and MAOM Fractions
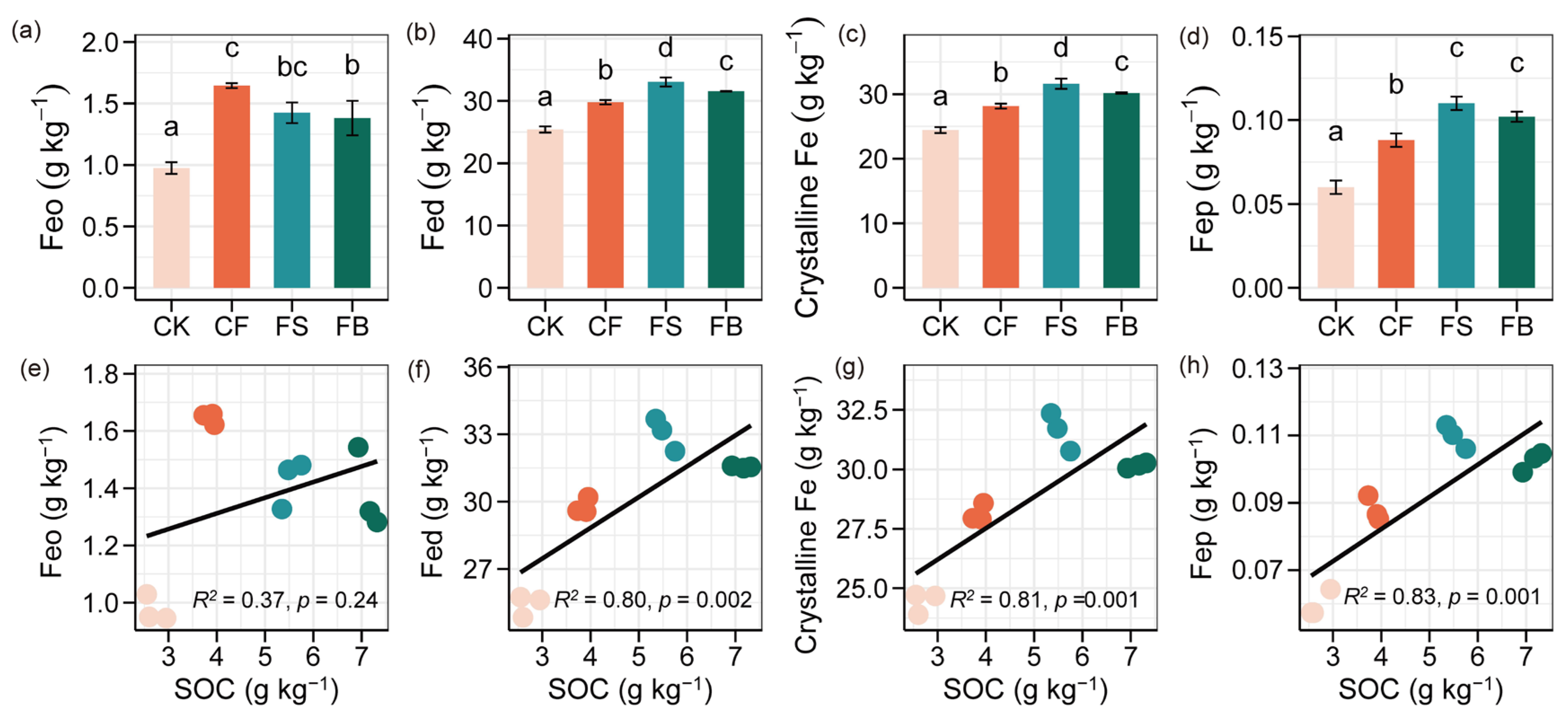
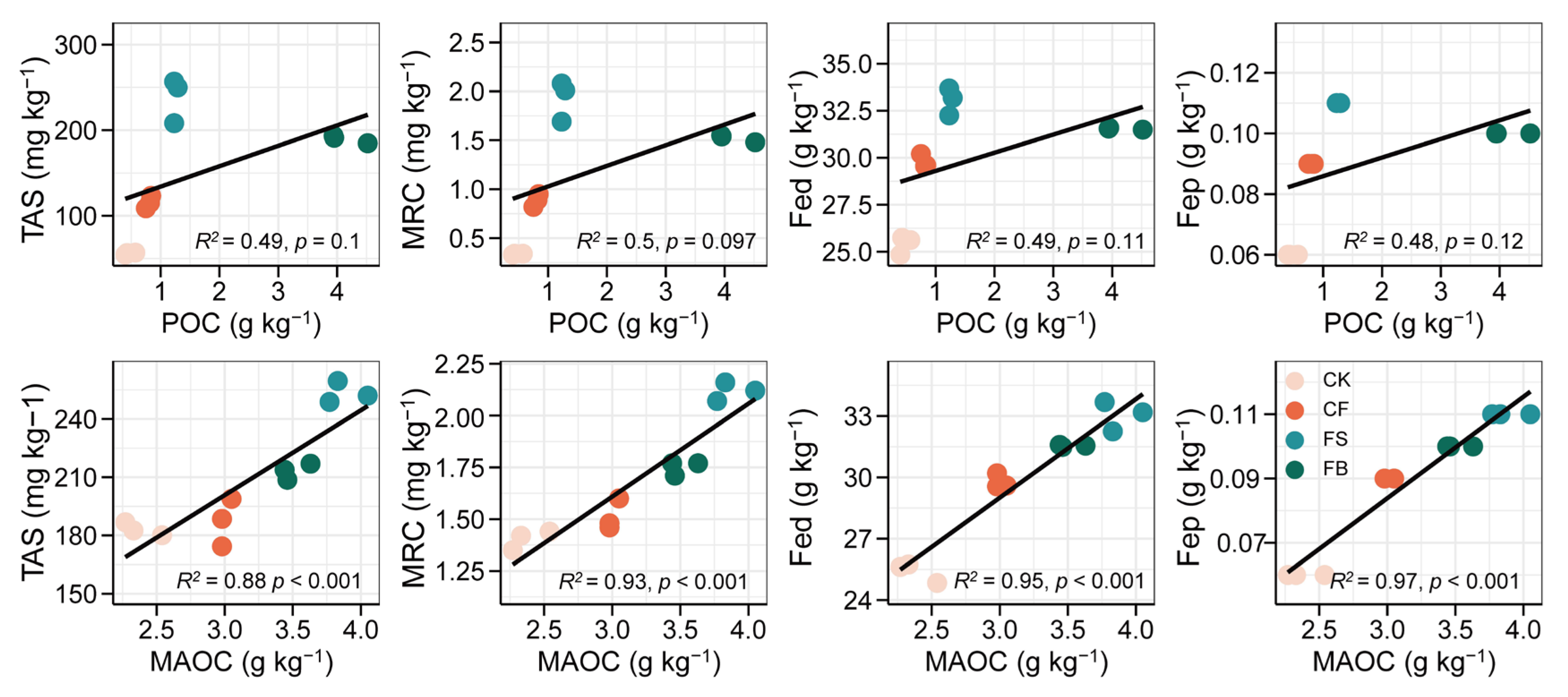
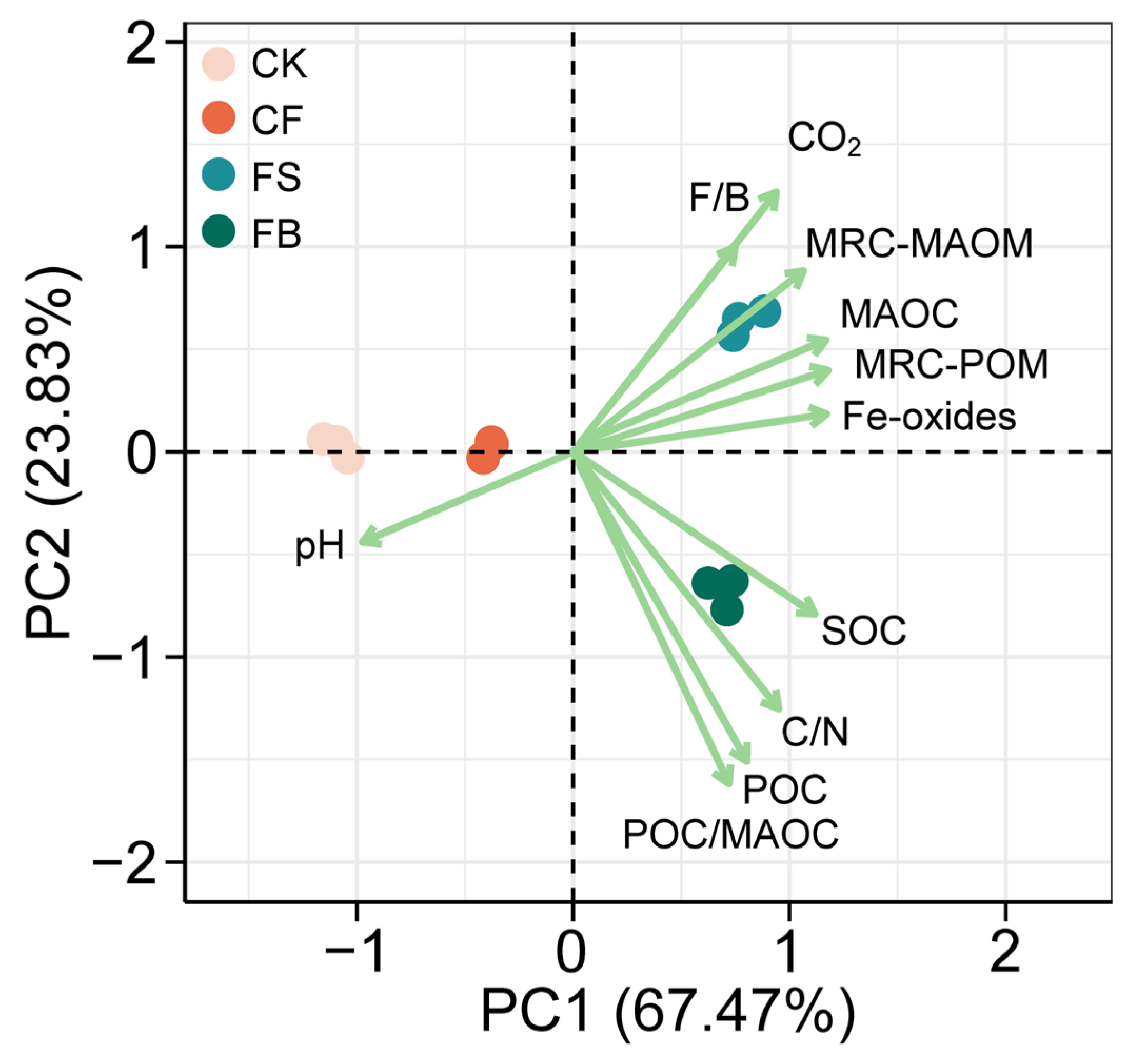
3.4. Relationships between Fe Oxides, Necromass, and SOC Fractions
4. Discussion
4.1. Long-Term Straw and Biochar Management Raises Soil Carbon Storage
4.2. Straw and Biochar Differently Affect Soil Organic Carbon Pool Composition and Functional Groups
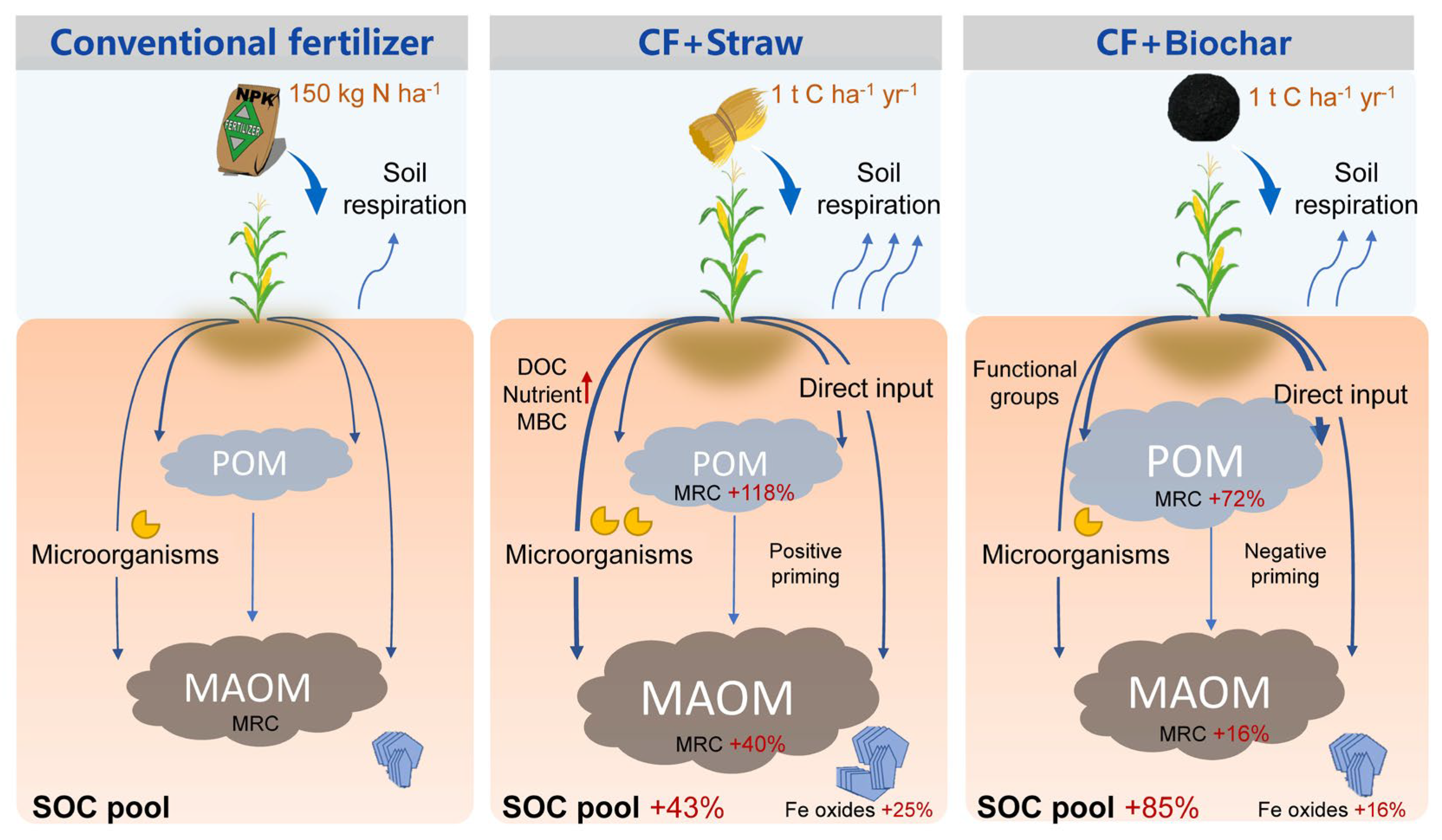
4.3. Soil Organic Carbon Stabilization Pathways under Straw and Biochar Amendments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Amelung, W.; Bossio, D.; de Vries, W.; Kögel-Knabner, I.; Lehmann, J.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 11, 5427. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Woolf, D.; Fan, M.; Qiao, L.; Li, R.; Lehmann, J. Global crop production increase by soil organic carbon. Nat. Geosci. 2023, 16, 1159–1165. [Google Scholar] [CrossRef]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, Y.; Wang, J.; Hao, J.; Jiang, Y.; Shi, J.; Meng, X.; Tian, X. Effects of the combined application of livestock manure and plant residues on soil organic carbon sequestration in the southern Loess Plateau of China. Agric. Ecosyst. Environ. 2024, 368, 109011. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon Sequestration Strategies in Soil Using Biochar: Advances, Challenges, and Opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.-S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, Y.; Yi, F.; Li, J.; Wang, X.; Fu, Q.; Fu, X.; Yao, Z.; Dai, Z.; Qiu, Y.; et al. Biochar dose-dependent impacts on soil bacterial and fungal diversity across the globe. Sci. Total Environ. 2024, 930, 172509. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, X.; Fang, Y.; Sun, X.; Tavakkoli, E.; Li, Y.; Wu, D.; Du, Z. Biochar more than stubble management affected carbon allocation and persistence in soil matrix: A 9-year temperate cropland trial. J. Soils Sediments 2023, 23, 3018–3028. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Liao, X.; Hu, P.; Wang, W.; Ling, Q.; Xie, L.; Xiao, J.; Zhang, W.; Wang, K. Pathways of soil organic carbon accumulation are related to microbial life history strategies in fertilized agroecosystems. Sci. Total Environ. 2024, 927, 172191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, Y.; Dungait, J.A.J.; Kumar, A.; Wang, J.; Tiemann, L.K.; Zhang, F.; Kuzyakov, Y.; Tian, J. Microbial necromass in cropland soils: A global meta-analysis of management effects. Glob. Chang. Biol. 2023, 29, 1998–2014. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, S. Plant influences on soil microbial carbon pump efficiency. Glob. Chang. Biol. 2023, 29, 3854–3856. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.-A. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Li, B.; Guo, Y.; Liang, F.; Liu, W.; Wang, Y.; Cao, W.; Song, H.; Chen, J.; Guo, J. Global integrative meta-analysis of the responses in soil organic carbon stock to biochar amendment. J. Environ. Manag. 2024, 351, 119745. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Song, X.; Wang, P.; Van Zwieten, L.; Bolan, N.; Wang, H.; Li, X.; Cheng, K.; Yang, Y.; Wang, M.; Liu, T.; et al. Towards a better understanding of the role of Fe cycling in soil for carbon stabilization and degradation. Carbon Res. 2022, 1, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, X.; Qiu, Q.; Yu, R.; Yao, Y.; Li, H.; Shao, M.; Wei, X. Soil and water conservation measures reduce erosion but result in carbon and nitrogen accumulation of red soil in Southern China. Agric. Ecosyst. Environ. 2023, 346, 108346. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, Q. Initial exploration of red soil ecology. Acta Ecol. Sin. 2014, 34, 5173–5181. [Google Scholar]
- FAO. World Reference Base for Soil Resources, 2006: A Framework for International Classification, Correlation, and Communication; FAO: Rome, Italy, 2006. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. In Clays and Clay Minerals; Ingerson, E., Ed.; Pergamon: Washington, DC, USA, 1958; pp. 317–327. [Google Scholar]
- Schwertmann, U. Differenzierung der eisenoxide des bodens durch extraktion mit ammoniumoxalat-lösung. Z. Pflanzenernähr. Düng. Bodenkd. 1964, 105, 194–202. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; He, J.-S.; Feng, X. Iron-mediated soil carbon response to water-table decline in an alpine wetland. Nat. Commun. 2017, 8, 15972. [Google Scholar] [CrossRef]
- Marriott, E.E.; Wander, M.M. Total and Labile Soil Organic Matter in Organic and Conventional Farming Systems. Soil Sci. Soc. Am. J. 2006, 70, 950–959. [Google Scholar] [CrossRef]
- Tang, Q.; Li, W.; Dai, W.; Wang, J.; Zhang, F.; Daniell, T.J.; Cheng, Y.; Wang, S.; Yin, W.; Wang, X. Patterns and determinants of microbial- and plant-derived carbon contributions to soil organic carbon in tea plantation chronosequence. Plant Soil 2024. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y.; et al. Formation of soil organic carbon pool is regulated by the structure of dissolved organic matter and microbial carbon pump efficacy: A decadal study comparing different carbon management strategies. Glob. Chang. Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zhu, F.; Wang, X.; Guan, S.; Dou, S.; Ndzelu, B.S. Contrasting effects of straw and straw-derived biochar application on soil organic matter and corn yield in a Chinese Mollisol. J. Soils Sediments 2023, 23, 3843–3856. [Google Scholar] [CrossRef]
- Heinemann, H.; Hirte, J.; Seidel, F.; Don, A. Increasing root biomass derived carbon input to agricultural soils by genotype selection—A review. Plant Soil 2023, 490, 19–30. [Google Scholar] [CrossRef]
- Zheng, J.; Tao, L.; Dini-Andreote, F.; Luan, L.; Kong, P.; Xue, J.; Zhu, G.; Xu, Q.; Jiang, Y. Dynamic Responses of Ammonia-Oxidizing Archaea and Bacteria Populations to Organic Material Amendments Affect Soil Nitrification and Nitrogen Use Efficiency. Front. Microbiol. 2022, 13, 911799. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Bolan, N.; Rehman, A.; Farooq, M. Enhancing crop productivity for recarbonizing soil. Soil Tillage Res. 2024, 235, 105863. [Google Scholar] [CrossRef]
- Bi, Y.; Kuzyakov, Y.; Cai, S.; Zhao, X. Accumulation of organic compounds in paddy soils after biochar application is controlled by iron hydroxides. Sci. Total Environ. 2021, 764, 144300. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Xu, Q.; Jiang, Y.; Sun, B. Biochar induced negative priming effect on soil organic carbon mineralisation by changing the microbial community structure across plant growth stages. J. Soils Sediments 2020, 20, 3340–3350. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.; Wang, Z.; Xing, B. Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 610–611, 951–960. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Lv, J.; Tang, C.; Zhang, H.; Fang, Y.; Tavakkoli, E.; Ge, T.; Luo, Y.; Cai, Y.; et al. Maize straw increases while its biochar decreases native organic carbon mineralization in a subtropical forest soil. Sci. Total Environ. 2024, 939, 173606. [Google Scholar] [CrossRef]
- Giannetta, B.; Plaza, C.; Galluzzi, G.; Benavente-Ferraces, I.; García-Gil, J.C.; Panettieri, M.; Gascó, G.; Zaccone, C. Distribution of soil organic carbon between particulate and mineral-associated fractions as affected by biochar and its co-application with other amendments. Agric. Ecosyst. Environ. 2024, 360, 108777. [Google Scholar] [CrossRef]
- Heckman, K.; Hicks Pries, C.E.; Lawrence, C.R.; Rasmussen, C.; Crow, S.E.; Hoyt, A.M.; von Fromm, S.F.; Shi, Z.; Stoner, S.; McGrath, C.; et al. Beyond bulk: Density fractions explain heterogeneity in global soil carbon abundance and persistence. Glob. Chang. Biol. 2022, 28, 1178–1196. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, Q.; Duan, C.; Huang, G.; Dong, K.; Wang, C. Biochar addition promotes soil organic carbon sequestration dominantly contributed by macro-aggregates in agricultural ecosystems of China. J. Environ. Manag. 2024, 359, 121042. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Haddix, M.L.; Kroeger, M.E.; Stewart, C.E. The role of plant input physical-chemical properties, and microbial and soil chemical diversity on the formation of particulate and mineral-associated organic matter. Soil Biol. Biochem. 2022, 168, 108648. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, F.; Zhang, H.; Wang, X.; Li, H.; Song, J.; Kan, Z.; Du, Z.; Zhou, J.; Chen, J.; et al. Divergent responses of particulate and mineral-associated organic carbon with soil depth under straw interlayer in saline-alkali soil. Agric. Ecosyst. Environ. 2024, 371, 109073. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Leite, M.F.A.; Momesso, L.; ten Berge, H.; Bloem, J.; Kuramae, E.E. Nitrogen input on organic amendments alters the pattern of soil–microbe-plant co-dependence. Sci. Total Environ. 2023, 890, 164347. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef]
- Li, Z.; Duan, X.; Guo, X.; Gao, W.; Li, Y.; Zhou, P.; Zhu, Q.; O’Donnell, A.G.; Dai, K.; Wu, J. Microbial metabolic capacity regulates the accrual of mineral-associated organic carbon in subtropical paddy soils. Soil Biol. Biochem. 2024, 195, 109457. [Google Scholar] [CrossRef]
- Liu, X.; Bol, R.; An, T.; Liu, Y.; Xu, Y.; Li, S.; Wang, J. Divergent accumulation of microbial necromass and plant lignin phenol induced by adding maize straw to fertilized soils. Soil Tillage Res. 2024, 243, 106177. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of necromass-derived soil organic carbon determined by microbial death pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Brabcová, V.; Štursová, M.; Davidová, A.; Jansa, J.; Cajthaml, T.; Baldrian, P. Decomposer food web in a deciduous forest shows high share of generalist microorganisms and importance of microbial biomass recycling. ISME J. 2018, 12, 1768–1778. [Google Scholar] [CrossRef]
- Veloso, M.G.; Angers, D.A.; Chantigny, M.H.; Bayer, C. Carbon accumulation and aggregation are mediated by fungi in a subtropical soil under conservation agriculture. Geoderma 2020, 363, 114159. [Google Scholar] [CrossRef]
- Gow Neil, A.R.; Latge, J.-P.; Munro Carol, A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 10-1128. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Kirsten, M.; Mikutta, R.; Vogel, C.; Thompson, A.; Mueller, C.W.; Kimaro, D.N.; Bergsma, H.L.T.; Feger, K.-H.; Kalbitz, K. Iron oxides and aluminous clays selectively control soil carbon storage and stability in the humid tropics. Sci. Rep. 2021, 11, 5076. [Google Scholar]
- Wang, P.; Wang, J.; Zhang, H.; Dong, Y.; Zhang, Y. The role of iron oxides in the preservation of soil organic matter under long-term fertilization. J. Soils Sediments 2019, 19, 588–598. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Du, H.; Lin, X.; Hu, W.; Li, Y. Soil conditioners promote the formation of Fe-bound organic carbon and its stability. J. Environ. Manag. 2024, 349, 119480. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, K.; Dai, W.; Wang, J.; Li, Z.; Cheng, Y.; Jiang, Y.; Yin, W.; Wang, X.; Song, X.; Tang, Q. Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways. Agronomy 2024, 14, 2176. https://doi.org/10.3390/agronomy14092176
Lei K, Dai W, Wang J, Li Z, Cheng Y, Jiang Y, Yin W, Wang X, Song X, Tang Q. Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways. Agronomy. 2024; 14(9):2176. https://doi.org/10.3390/agronomy14092176
Chicago/Turabian StyleLei, Kunjia, Wenxia Dai, Jing Wang, Zhenwang Li, Yi Cheng, Yuji Jiang, Weiqin Yin, Xiaozhi Wang, Xiaodong Song, and Quan Tang. 2024. "Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways" Agronomy 14, no. 9: 2176. https://doi.org/10.3390/agronomy14092176
APA StyleLei, K., Dai, W., Wang, J., Li, Z., Cheng, Y., Jiang, Y., Yin, W., Wang, X., Song, X., & Tang, Q. (2024). Biochar and Straw Amendments over a Decade Divergently Alter Soil Organic Carbon Accumulation Pathways. Agronomy, 14(9), 2176. https://doi.org/10.3390/agronomy14092176








