The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Dendranthema morifolium Under Cd Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Plant Materials
2.2. Inoculation Assay
2.3. Determination of Morphological Indicators in Plants
2.4. Measurement of Physiological and Biochemical Indicators
2.5. Determination of Soil Factor Index
2.6. Active Ingredient Content and IAA
2.7. Statistical Analysis
3. Results
3.1. Fungal Colonization
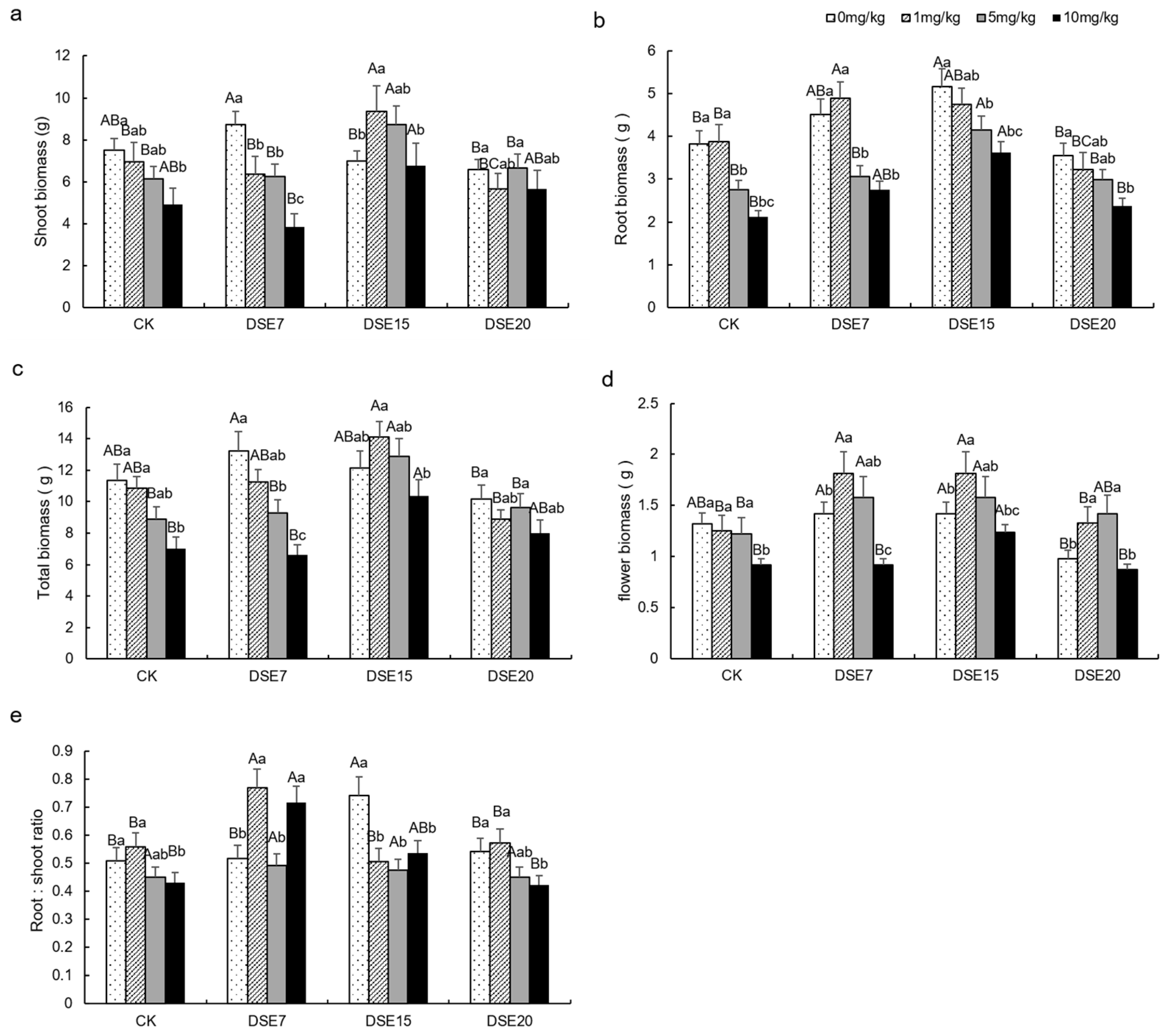
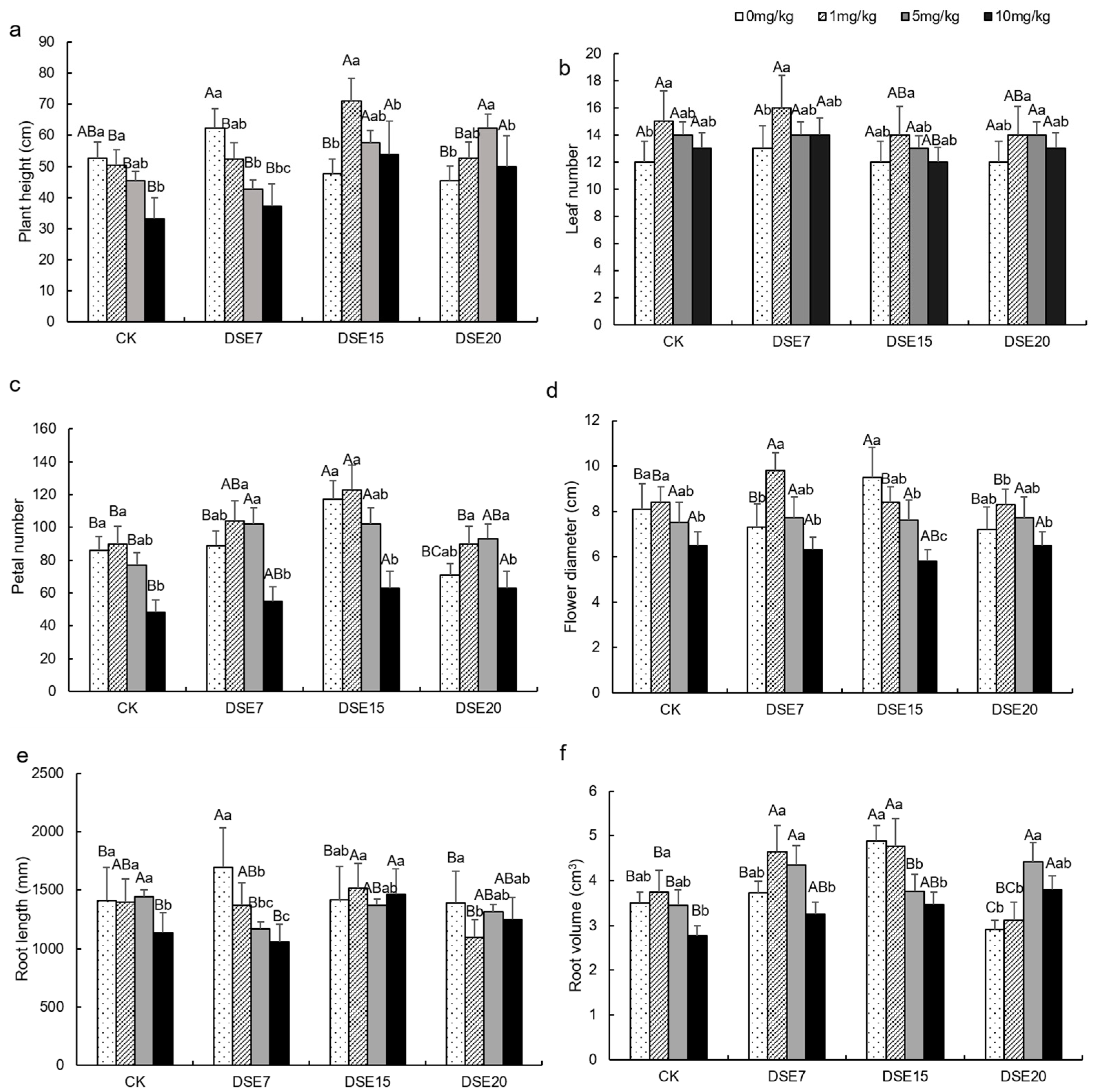
3.2. Plant Morphological Parameter
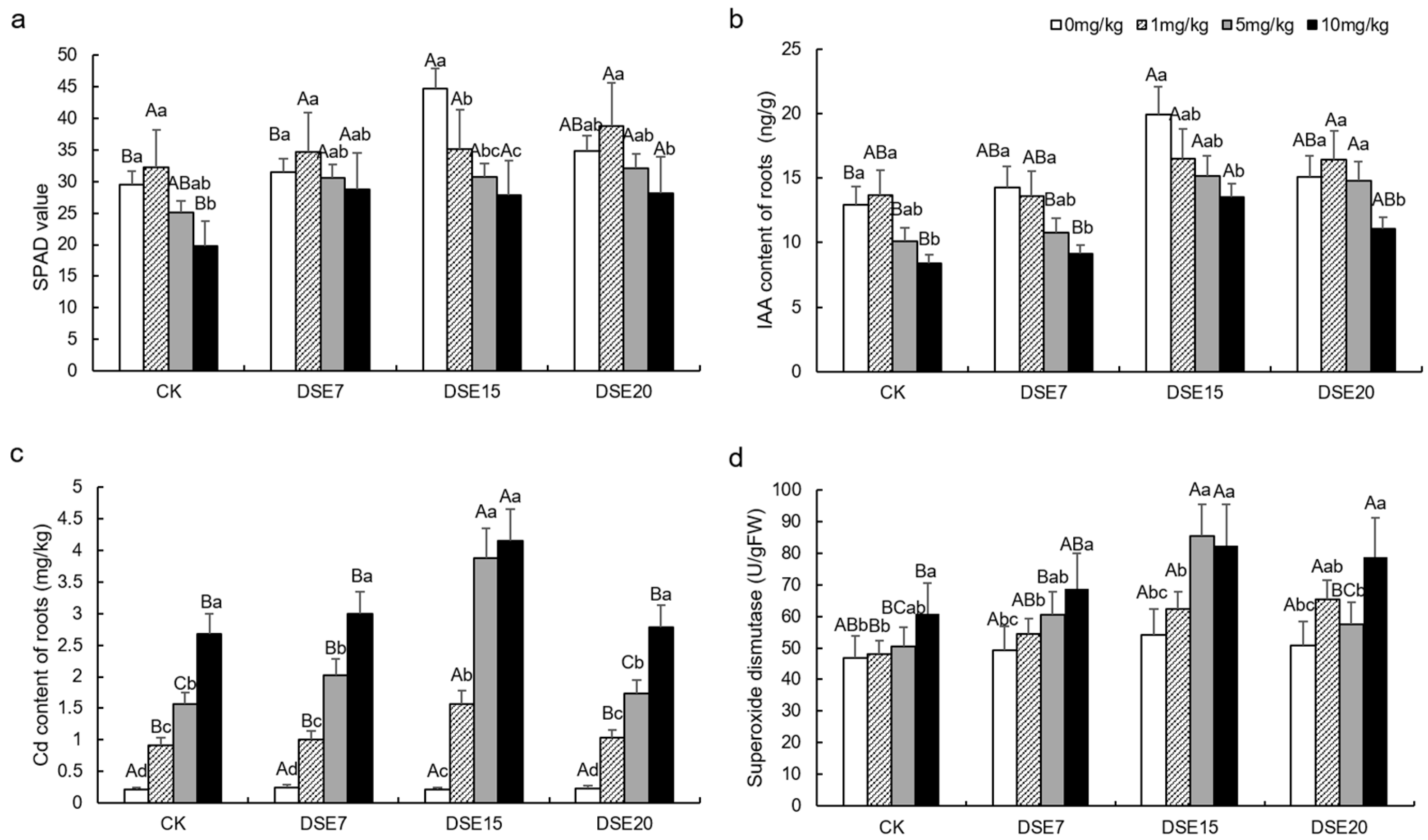
3.3. Plant Physiological Parameter and Active Ingredient
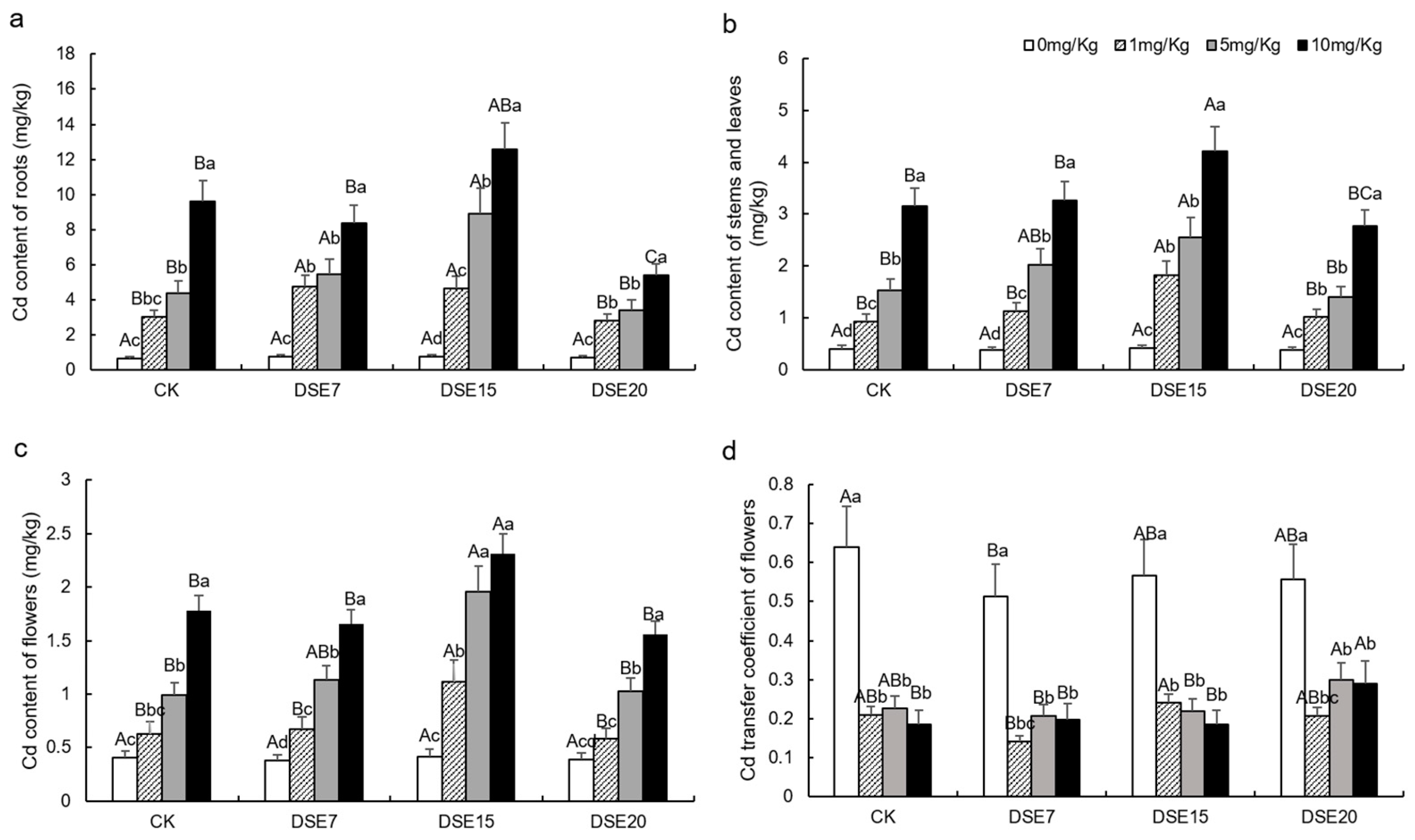
3.4. Soil Properties and Cd Concentrations
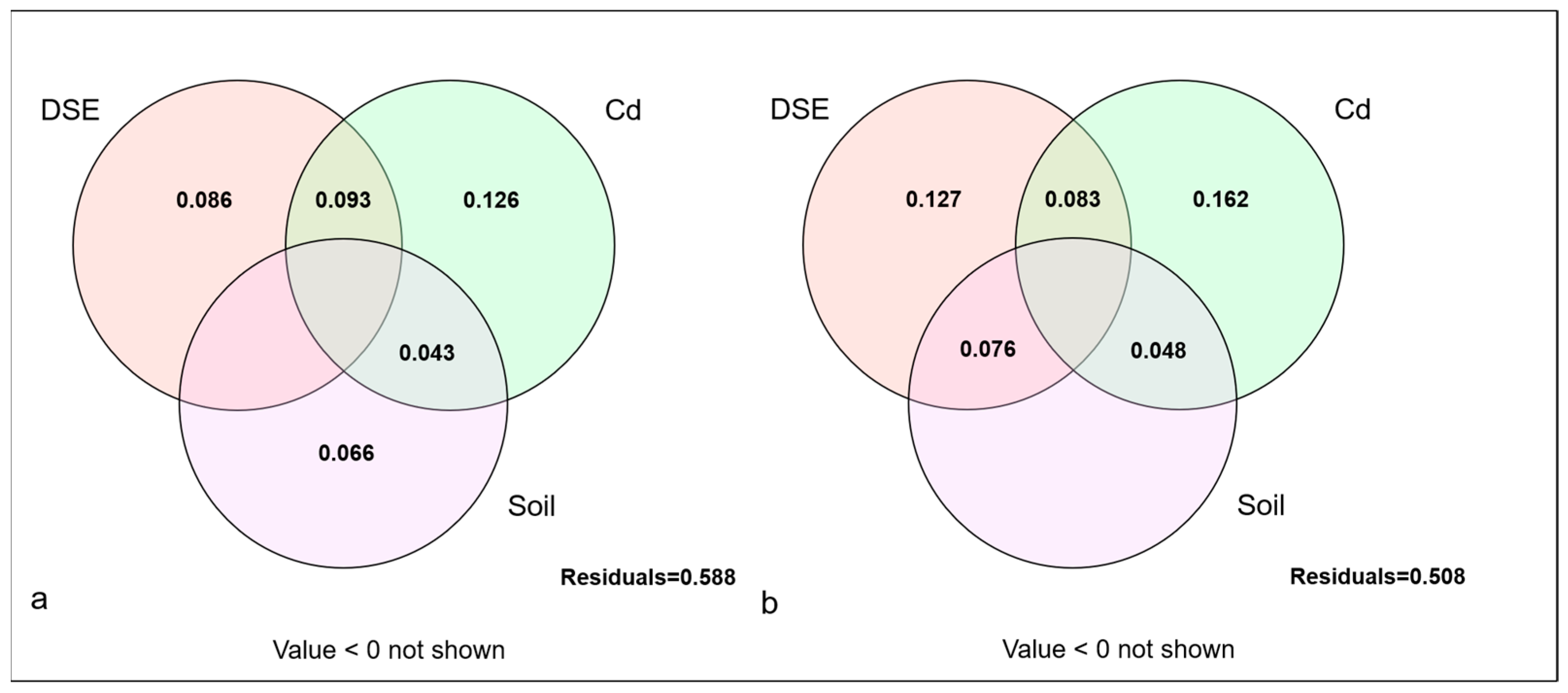
3.5. Variance Decomposition of Different Factors on the Growth of D. morifolium Under Non-Sterile Inoculation
4. Discussion
4.1. Effect of DSE Strains on Biomass
4.2. Effect of DSE Inoculation on Physiological Parameters and Active Ingredients
4.3. Effect of DSE Strains on Cd Absorption
4.4. Effect of DSE Strains on Soil Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugathas, S.; Neththasinghe, N.A.S.A.; Sirisena, D.N.; Thilakasiri, R.; Ariyarathna, M.; Kadupitiya, H.K.; Chandrajith, R.; Suriyagoda, L.D.B. Effects of agroclimatic zones, soil orders, and irrigation types on the exchangeable cadmium in paddy soils. Soil Environ. Health 2024, 2, 100078. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 1, 56120. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xu, Z.M.; Wang, J.F.; Zhang, K.; Wang, F.P.; Li, W.L.; Tian, P.; Li, Q.S. Inoculation of Escherichia coli enriched the key functional bacteria that intensified cadmium accumulation by halophyte Suaeda salsa in saline soils. J. Hazard. Mater. 2023, 458, 131922. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Piotrowska Seget, Z. Plant association with dark septate endophytes: When the going gets tough (and stressful), the tough fungi get going. Chemosphere 2022, 302, 134830. [Google Scholar] [CrossRef] [PubMed]
- Meshram, V.; Elazar, M.; Maymon, M.; Sharma, G.; Shawahna, R.; Belausov, E.; Charuvi, D.; Freeman, S. Endophytic Fusarium clavum confers growth and salt tolerance in Cucumis melo. Environ. Exp. Bot. 2023, 206, 105153. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Jin, H.Q.; Liu, H.B.; Xie, Y.Y.; Zhang, Y.G.; Xu, Q.Q.; Mao, L.J.; Li, X.J.; Chen, J.; Lin, F.C.; Zhang, C.L. Effect of the dark septate endophytic fungus Acrocalymma vagum on heavy metal content in tobacco leaves. Symbiosis 2017, 74, 89–95. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, M.H.; Ye, Q.N.; Gao, H.L.; He, X.L. Improved tolerance of Artemisia ordosica to drought stress via dark septate endophyte (DSE) symbiosis. J. Fungi 2022, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Leyval, C.; Foulon, J.; Chalot, M.; Blaudez, D. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal polluted poplar phytomanagement sites. FEMS Microbiol. Ecol. 2016, 92, fiw144. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Dai, M.D.; Zhu, J.N.; Liu, X.H.; Li, L.; Zhu, X.M.; Wang, J.Y.; Yuan, Z.L.; Lin, F.C. Dark septate endophyte Falciphora oryzae-assisted alleviation of cadmium in rice. J. Hazard. Mater. 2021, 419, 126435. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.H.; Li, X.; Ye, Q.N.; Gong, F.; He, X.L. Occurrence of dark septate endophytes in Phragmites australis in Baiyang Lake and their resistance to Cd stress. Pedosphere 2023, 34, 484–496. [Google Scholar] [CrossRef]
- Li, X.M.; Ma, L.J.; Li, Y.Y.; Wang, L.L.; Zhang, L.H. Endophyte infection enhances accumulation of organic acids and minerals in rice under Pb2+ stress conditions. Ecotoxicol. Environ. Saf. 2019, 174, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Sanhueza, T.; Ortiz, J.; Luz Mora, M.D.L.; Garcia Romera, I.; Arriagada, C. Plant growth-promotion and abiotic stress tolerance of dark septate endophyte fungi isolated from roots of Native Andean Ericaceae plants colonizing volcanic deposits in southern Chile. J. Soil Sci. Plant Nutr. 2024, 24, 5144–5153. [Google Scholar] [CrossRef]
- Salducci, M.D.; Folzer, H.; Issartel, J.; Rabier, J.; Masotti, V.; Prudent, P.; Affre, L.; Hardion, L.; Tatoni, T.; Laffont Schwob, I. How can a rare protected plant cope with the metal and metalloid soil pollution resulting from past industrial activities? Phytometabolites, antioxidant activities and root symbiosis involved in the metal tolerance of Astragalus tragacantha. Chemosphere 2019, 217, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.F.; Yu, J.; Zhao, L.L.; He, X.L. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, G.Q.; Liang, X.R.; Hu, L.Y.; Li, Y.; He, Y.M.; Zhan, F.D. Dark septate endophyte Exophiala pisciphila promotes maize growth and alleviates cadmium toxicity. Front. Microbiol. 2023, 14, 1165131. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.Q.; Liang, X.R.; Wang, L.; Li, Z.R.; He, Y.M.; Li, B.; Zhan, F.D. A dark septate endophyte improves cadmium tolerance of maize by modifying root morphology and promoting cadmium binding to the cell wall and phosphate. J. Fungi 2023, 9, 531. [Google Scholar] [CrossRef]
- Xiao, Y.; Dai, M.X.; Zhang, G.Q.; Yang, Z.X.; He, Y.M.; Zhan, F.D. Effects of the dark septate endophyte (DSE) Exophiala pisciphila on the growth of root cell wall polysaccharides and the cadmium content of Zea mays L. under cadmium stress. J. Fungi 2021, 7, 1035. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.H.; He, S.; Chen, D.; Li, T.; Zhao, Z.W. EpABC genes in the adaptive responses of Exophiala pisciphila to metal stress: Functional importance and relation to metal tolerance. Appl. Environ. Microbiol. 2019, 85, e01844-19. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Fan, X.M.; Zhang, G.Q.; Li, B.; Li, T.G.; Zu, Y.Q.; Zhan, F.D. Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. S. Afr. J. Bot. 2020, 134, 415–423. [Google Scholar] [CrossRef]
- He, C.; Cui, J.; Chen, X.Y.; Wang, W.Q.; Hou, J.L. Effects of enhancement of liquorice plants with dark septate endophytes on the root growth, glycyrrhizic acid and glycyrrhizin accumulation amended with organic residues. Curr. Plant Biol. 2020, 23, 100154. [Google Scholar] [CrossRef]
- Flores Duarte, N.J.; Pajuelo, E.; Mateos Naranjo, E.; Navarro Torre, S.; Rodríguez Llorente, I.D.; Redondo Gómez, S.; Carrasco López, J.A. A culturomics-based bacterial synthetic community for improving resilience towards arsenic and heavy metals in the nutraceutical plant Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2023, 24, 7003. [Google Scholar] [CrossRef]
- Li, M.; Ren, Y.F.; He, C.; Yao, J.J.; Wei, M.; He, X.L. Complementary effects of dark septate endophytes and Trichoderma strains on growth and active ingredient accumulation of Astragalus mongholicus under drought stress. J. Fungi 2022, 8, 920. [Google Scholar] [CrossRef] [PubMed]
- El Shafey, N.M.; Marzouk, M.A.; Yasser, M.M.; Shaban, S.A.; Beemster, G.T.S.; AbdElgawad, H. Harnessing endophytic fungi for enhancing growth, tolerance and quality of rose-scented geranium (Pelargonium graveolens (L’Hér) Thunb.) plants under cadmium stress: A biochemical study. J. Fungi 2021, 7, 1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hao, L.J.; Zhu, J.J.; Wang, Z.M.; Zhang, X.; Song, X.M. Comparative evaluation of Chrysanthemum flos from different origins by HPLC-DAD-MS n and relative response factors. Food Anal. Methods 2014, 8, 40–51. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Sun, Y.M.; Cai, C.Y.; He, Y.; Nie, P.C. Rapid determination of chlorogenic acid, luteoloside and 3,5-O-dicaffeoylquinic acid in Chrysanthemum using near-infrared spectroscopy. Sensors 2019, 19, 1981. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Jang, H.J.; Ryu, J.S.; Ko, J.H.; Ahn, K.S.; Oh, S.R.; Oh, J.H.; Chung, J.H.; Oh, J.Y. 1,5-Dicaffeoylquinic acid from Pseudognaphalium affine ameliorates dry eye disease via suppression of inflammation and protection of the ocular surface. Ocul. Surf. 2023, 29, 469–479. [Google Scholar] [CrossRef]
- Zeng, L.; Xiang, R.; Fu, C.Y.; Qu, Z.H.; Liu, C.W. The regulatory effect of chlorogenic acid on gut-brain function and its mechanism: A systematic review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef] [PubMed]
- Lal, K.; Minhas, P.S.; Shipra; Chaturvedi, R.K.; Yadav, R.K. Extraction of cadmium and tolerance of three annual cut flowers on Cd-contaminated soils. Bioresour. Technol. 2008, 99, 1006–1011. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, T.; Guo, Q.S.; Dong, X.N.; Qin, M.J.; Xiao, N.W.; Han, Z.Z.; Wei, M. Effect of chrysanthemum indicum on absorption characteristics of Cd and its effect on quality of medicinal materials. China J. Chin. Medica 2019, 44, 641–647. [Google Scholar]
- Zuo, T.T.; Jin, H.Y.; Zhang, L.; Liu, Y.L.; Nie, J.; Chen, B.L.; Fang, C.F.; Xue, J.; Bi, X.Y.; Zhou, L.; et al. Innovative health risk assessment of heavy metals in Chinese herbal medicines based on extensive data. Pharmacol. Res. 2020, 159, 104987. [Google Scholar] [CrossRef]
- Li, M.; Han, L.; He, C.; Li, X.; He, X.L. The promotion of dark septate endophytes on the performance and active ingredients accumulation of Astragalus mongholicus under cadmium stress. Agronomy 2024, 14, 1801. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi Pearson, V. Mesure du taux de mycorhization VAd’un système radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–281. [Google Scholar]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Bever, J.D.; Morton, J.B.; Antonovics, J.; Schultz, P.A. Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. J. Ecol. 1996, 84, 71–82. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil; Chinese Agricultural: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wang, Y.P. Chinese Pharmacopoeia (English); China Medical Science and Technology Press: Beijing, China, 2015; Volume 4, pp. 132–135. [Google Scholar]
- Torelli, A.; Trotta, A.; Acerbi, A.; Arcidiaconol, G.; Berta, G.; Branca, C. IAA and ZR content in leek (Allium porrum L.), as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant Soil 2000, 226, 29–35. [Google Scholar] [CrossRef]
- Wu, G.X.; Chen, M.X.; Fu, L.; Jiang, J.M. Analysis of cytokinins in new apple roots by high performance liquid chromatography. J. Shandong Agric. Univ. 1996, 03, 341–345. [Google Scholar]
- Wang, Y.Z.; Peng, X.Y.; Lai, L.Y.; Li, H.; Zhang, X.Y.; Chen, H.X.; Xie, L.T. Phosphorus fertilization regimes and rates alter Cd extractability in rhizospheric soils and uptake in maize (Zea mays L.). Chemosphere 2022, 298, 134288. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.G.; Kovács, G.M.; Zajta, E.; Groenewald, J.Z.; Crous, P.W. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, F.N.; Tobar, N.E.; Fernández Di Pardo, A.; Chiocchio, V.M.; Lavado, R.S. Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl. Soil Ecol. 2017, 111, 25–32. [Google Scholar] [CrossRef]
- Wu, L.Q.; Guo, S.X. Interaction between an isolate of dark-septate fungi and its host plant Saussurea involucrata. Mycorrhiza 2008, 18, 79–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, Y.Y.; Li, P.W.; Xu, L.J.; Fu, Q. Ectomycorrhizal fungi and dark septate endophyte inoculation improve growth and tolerance of Pinus tabulaeformis under cadmium stress. Pedosphere 2024, 34, 473–483. [Google Scholar] [CrossRef]
- Li, M.; Hou, L.F.; Liu, J.Q.; Yang, J.Y.; Zuo, Y.L.; Zhao, L.L.; He, X.L. Growth-promoting effects of dark septate endophytes on the non-mycorrhizal plant Isatis indigotica under different water conditions. Symbiosis 2021, 85, 291–303. [Google Scholar] [CrossRef]
- Zhai, X.; Luo, D.; Li, X.Q.; Han, T.; Jia, M.; Kong, Z.Y.; Ji, J.C.; Rahman, K.; Qin, L.P.; Zheng, C.J. Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front. Microbiol. 2018, 8, 2694. [Google Scholar] [CrossRef]
- Milan, B. Response of Salix alba L. to heavy metals and diesel fuel contamination. Afr. J. Biotechnol. 2012, 11, 14313–14319. [Google Scholar] [CrossRef]
- Likar, M.; Regvar, M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 2013, 370, 593–604. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Hou, L.F.; He, X.L.; Li, X.; Wang, S.J.; Zhao, L.L. Species composition and colonization of dark septate endophytes are affected by host plant species and soil depth in the Mu Us sandland, northwest China. Fungal Ecol. 2019, 39, 276–284. [Google Scholar] [CrossRef]
- Collin Hansen, C.; Andersen, R.A.; Steinnes, E. Molecular defense systems are expressed in the king bolete (Boletus edulis) growing near metal smelters. Mycologia 2005, 97, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, K.B.; Chet, R. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. Abiotic Biot. Stress Plants 2018, 7, 1–10. [Google Scholar]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol 2012, 12, 3. [Google Scholar] [CrossRef]
- Khan, A.L.; Lee, I.J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, P.; Legué, V.; Vayssières, A.; Martin, F.; Tuskan, G.A.; Kalluri, U.C. Involvement of auxin pathways in modulating root architecture during beneficial plant-microorganism interactions. Plant Cell Environ. 2013, 36, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, M.; El Nahrawy, S.; Omara, A.E. Synergistic interaction between symbiotic N2 fixing bacteria and bacillus strains to improve growth, physiological parameters, antioxidant enzymes and Ni accumulation in Faba Bean Plants (Vicia faba) under Nickel Stress. Plants 2022, 11, 1812. [Google Scholar] [CrossRef]
- Karunamoorthi, K.; Jegajeevanram, K.; Vijayalakshmi, J.; Mengistie, E. Traditional medicinal plants. J. Evid. Based Complement. Altern. Med. 2012, 18, 67–74. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Fan, J.Y.; Guo, Q.S.; Liu, Z.Y.; Zhu, G.S. The growth and medicinal quality of Epimedium wushanenseare improved by an isolate of dark septate fungus. Pharm. Biol. 2015, 53, 1344–1351. [Google Scholar] [CrossRef]
- Xing, B.C.; Liang, L.J.; Liu, L.; Hou, Z.N.; Yang, D.F.; Yan, K.J.; Zhang, X.M.; Liang, Z.S. Overexpression of SmbHLH148 induced biosynthesis of tanshinones as well as phenolic acids in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2018, 37, 1681–1692. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Dai, C.C.; Chen, L.Q. Regulation and accumulation of secondary metabolites in plant fungus symbiotic system. Afr. J. Biotechnol. 2007, 6, 1266–1271. [Google Scholar]
- Wu, L.Q.; Lv, Y.L.; Meng, Z.X.; Chen, J.; Guo, S.X. The promoting role of an isolate of dark septate fungus on its host plant Saussurea involucrata Kar. et Kir. Mycorrhiza 2010, 20, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Li, Y.; Tian, W.; Sun, Y.; Chen, F.; Zhang, Y.; Zhai, Y.; Zhang, J.; Su, H.; Wang, L.; et al. A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol. 2020, 40, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Bi, Y.L.; Quan, W.Z.; Christie, P. Growth and metabolism of dark septate endophytes and their stimulatory effects on plant growth. Fungal Biol. 2022, 126, 674–686. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Yang, Z.X.; Li, M.R.; Jiang, M.; Zhan, F.D.; Zu, Y.Q.; Li, T.; Zhao, Z.W. Effects of a dark septate endophyte (DSE) on growth, cadmium content, and physiology in maize under cadmium stress. Environ. Sci. Pollut. Res. 2017, 24, 18494–18504. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Huang, J.; Gao, Y.Z. Arbuscular mycorrhizal colonization alters subcellular distribution and chemical forms of cadmium in Medicago sativa L. and resists cadmium toxicity. PLoS ONE 2012, 7, e48669. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Meng, Q.; Wang, Q.; Luo, S.; Chen, B.; Khan, K.Y.; Yang, X.; Feng, Y. Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere 2016, 154, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Surono; Narisawa, K. The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Urquiaga, S.; Schultz, N.; Balieiro, F.D.C.; Medeiros, P.S.; Santos, L.A.; Xavier, G.R.; Zilli, J.E. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front. Microbiol. 2017, 8, 2437. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.L.; Su, F.; He, X.L.; Li, M. Colonization by dark septate endophytes improves the growth of Hedysarum scoparium under multiple inoculum levels. Symbiosis 2020, 82, 201–214. [Google Scholar] [CrossRef]
- Pu, Z.T.; Wang, D.D.; Song, W.X.; Wang, C.; Li, Z.Y.; Chen, Y.L.; Shimozono, T.; Yang, Z.M.; Tian, Y.Q.; Xie, Z.H. The impact of arbuscular mycorrhizal fungi and endophytic bacteria on peanuts under the combined pollution of cadmium and microplastics. J. Hazard. Mater. 2024, 469, 133934. [Google Scholar] [CrossRef] [PubMed]


| Species | Different Treatment | Plant Height (cm) | Leaf Number | Branches Number | Root Length (mm) | Root Volume (cm3) |
|---|---|---|---|---|---|---|
| CK | 0 mg/kg | 20.33 ± 3.07 Aa | 30.33 ± 2.34 Aa | 1.00 ± 0.00 Aab | 673.22 ± 63.03 Aa | 0.37 ± 0.02 Ba |
| 1 mg/kg | 20.33 ± 3.43 Ba | 30.00 ± 6.04 ABa | 1.00 ± 0.00 Aab | 593.37 ± 28.99 ABab | 0.36 ± 0.02 Ba | |
| 5 mg/kg | 19.30 ± 3.83 Ba | 34.33 ± 3.47 ABa | 1.33 ± 0.13 ABa | 503.28 ± 55.79 ABb | 0.32 ± 0.01 Bab | |
| 10 mg/kg | 16.00 ± 2.39 ABab | 22.00 ± 5.79 Bb | 1.00 ± 0.00 Aab | 493.25 ± 39.28 ABb | 0.28 ± 0.06 Bb | |
| DSE7 | 0 mg/kg | 22.32 ± 2.01 Aab | 27.67 ± 3.11 Aa | 1.00 ± 0.00 Aa | 637.44 ± 24.38 ABb | 0.56 ± 0.03 Aa |
| 1 mg/kg | 26.50 ± 1.25 Aa | 30.33 ± 3.74 ABa | 1.00 ± 0.00 Aa | 783.27 ± 39.53 Aa | 0.39 ± 0.08 Bab | |
| 5 mg/kg | 23.11 ± 3.47 ABab | 29.33 ± 2.07 Ba | 1.00 ± 0.00 ABa | 624.50 ± 35.02 Ab | 0.37 ± 0.04 Bab | |
| 10 mg/kg | 20.33 ± 4.91 Ab | 29.03 ± 3.77 Aa | 1.00 ± 0.00 Aa | 572.60 ± 39.47 Ab | 0.33 ± 0.06 Bb | |
| DSE15 | 0 mg/kg | 21.43 ± 2.19 Aab | 33.33 ± 4.21 Ab | 1.00 ± 0.00 Aab | 703.70 ± 58.34 Aa | 0.41 ± 0.07 Bb |
| 1 mg/kg | 25.89 ± 2.21 Aa | 37.66 ± 4.72 Aa | 1.00 ± 0.00 Aab | 634.44 ± 45.57 ABab | 0.89 ± 0.03 Aa | |
| 5 mg/kg | 28.20 ± 5.53 Aa | 42.00 ± 3.01 Aa | 1.33 ± 0.13 ABa | 535.50 ± 78.45 ABb | 0.79 ± 0.10 Aa | |
| 10 mg/kg | 19.49 ± 3.45 Ab | 20.00 ± 1.89 Bc | 1.00 ± 0.00 Aab | 504.84 ± 56.44 ABb | 0.57 ± 0.12 Ab | |
| DSE20 | 0 mg/kg | 22.67 ± 3.53 Aab | 29.33 ± 4.22 Aa | 1.00 ± 0.00 Aa | 654.33 ± 48.33 Aa | 0.45 ± 0.04 ABb |
| 1 mg/kg | 26.77 ± 3.88 Aa | 29.00 ± 5.90 Ba | 1.00 ± 0.00 Aa | 509.33 ± 59.33 Bb | 0.81 ± 0.11 Aa | |
| 5 mg/kg | 22.80 ± 4.94 Bab | 30.33 ± 4.45 Ba | 1.00 ± 0.00 ABa | 612.89 ± 58.34 Aab | 0.28 ± 0.06 Bc | |
| 10 mg/kg | 19.30 ± 2.19 Ab | 25.67 ± 4.33 ABab | 1.00 ± 0.00 Aa | 572.54 ± 44.33 Aab | 0.23 ± 0.04 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Li, G.; Wang, S.; Wang, Z.; Li, L.; Han, L. The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Dendranthema morifolium Under Cd Stress. Agronomy 2025, 15, 208. https://doi.org/10.3390/agronomy15010208
Wu M, Li G, Wang S, Wang Z, Li L, Han L. The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Dendranthema morifolium Under Cd Stress. Agronomy. 2025; 15(1):208. https://doi.org/10.3390/agronomy15010208
Chicago/Turabian StyleWu, Meiling, Gen Li, Simiao Wang, Ziteng Wang, Longfei Li, and Li Han. 2025. "The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Dendranthema morifolium Under Cd Stress" Agronomy 15, no. 1: 208. https://doi.org/10.3390/agronomy15010208
APA StyleWu, M., Li, G., Wang, S., Wang, Z., Li, L., & Han, L. (2025). The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Dendranthema morifolium Under Cd Stress. Agronomy, 15(1), 208. https://doi.org/10.3390/agronomy15010208





