Resulting Key Physiological Changes in Triticum aestivum L. Plants Under Drought Conditions After Priming the Seeds with Conventional Fertilizer and Greenly Synthesized Zinc Oxide Nanoparticles from Corn Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. A Sustainable Method for Making Zinc Oxide Nanoparticles Using the Aqueous Extract from the Corn Husks
2.2. Investigation of Zinc Oxide Nanoparticles Produced via Eco-Friendly Method
2.3. Seed Priming
2.4. Design, Preparations, and Equipment for Wheat Planting
2.5. Calculation of Total Sugars That Can Be Hydrolyzed
2.6. Calculating Total Phenolic Content (TPC)
2.7. Calculating Total Flavonoid Content (TFC)
2.8. Determination of the Activities of Glutathione Reductase, Peroxidase, and Catalase Enzymes
2.8.1. Assessment of the Peroxidase (POX) (EC 1.11.1.7) Activity
2.8.2. Assessment of the Glutathione Reductase (GR) (EC 1.6.4.2) Activity
2.8.3. Assessment of Catalase (CAT) Activity (EC 1.11.1.6)
2.9. Statistical Analysis
3. Results
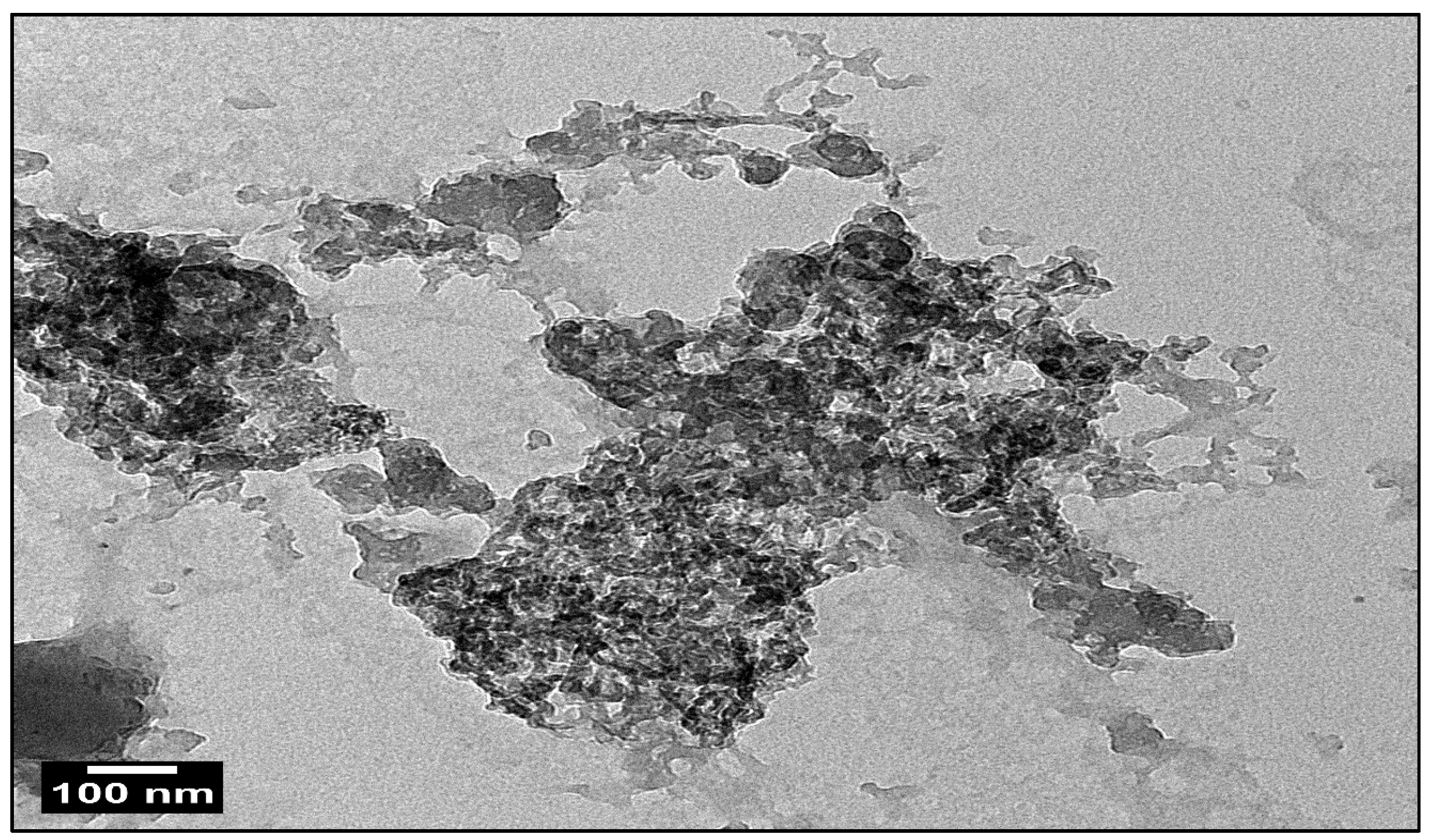
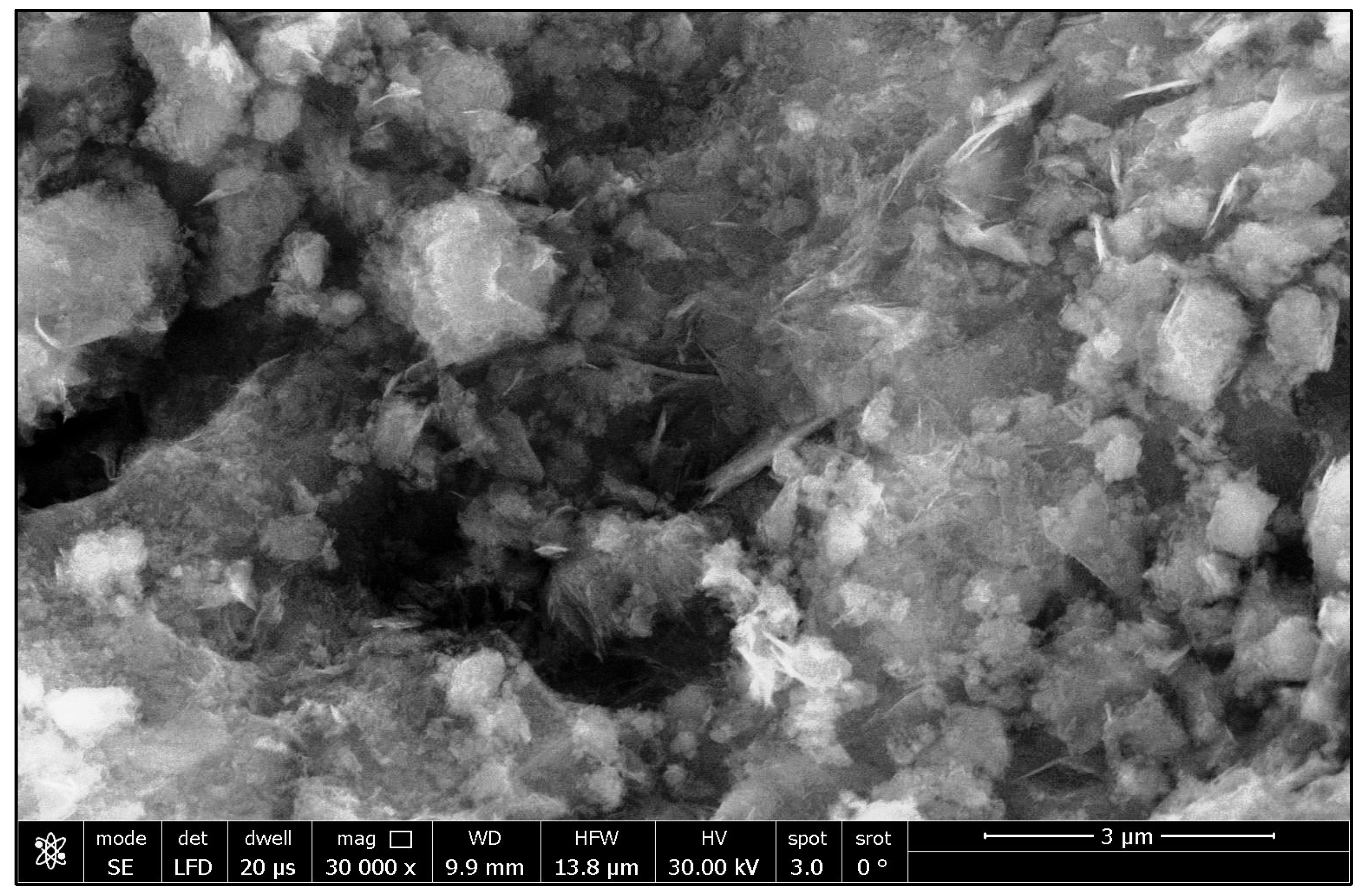
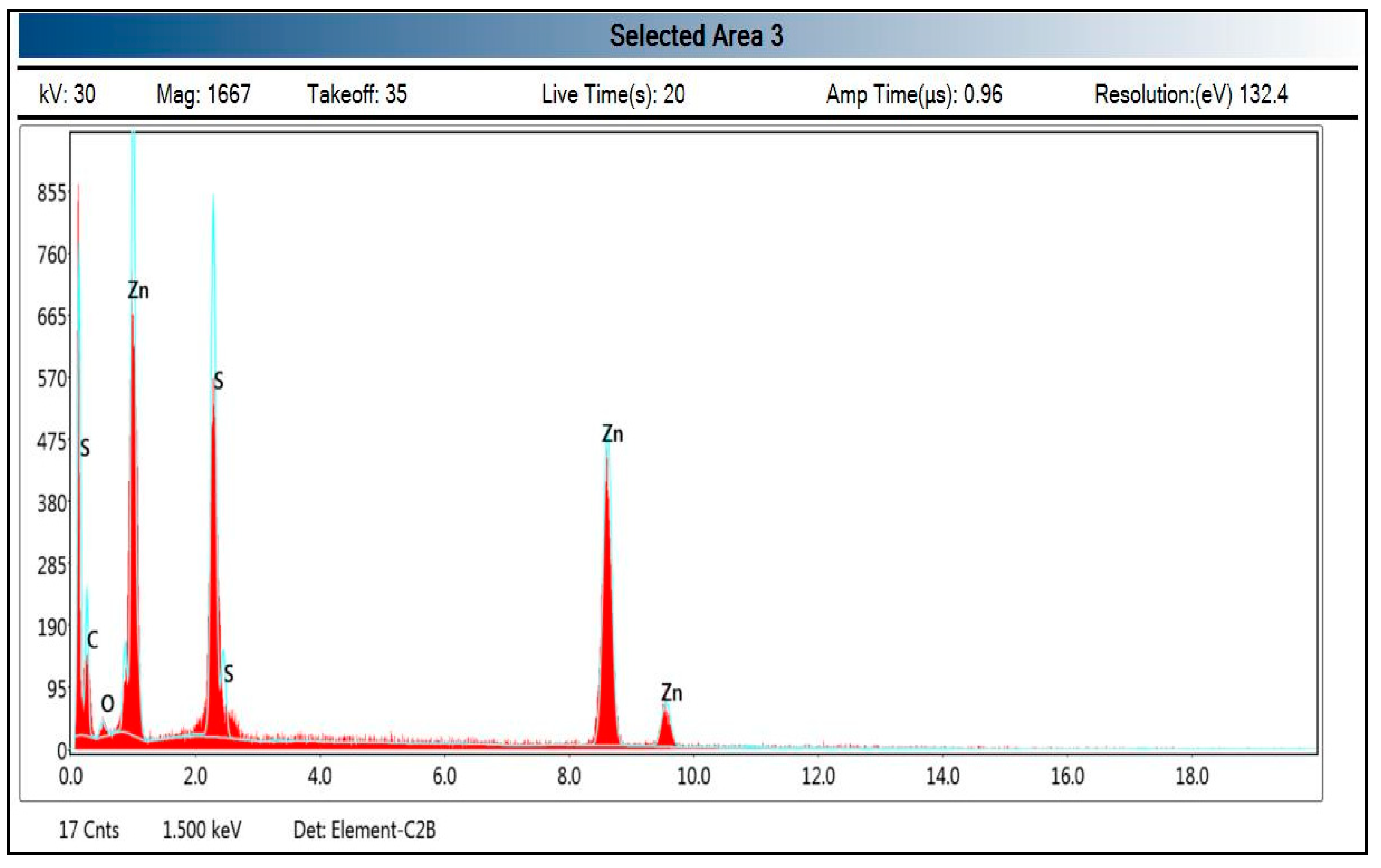
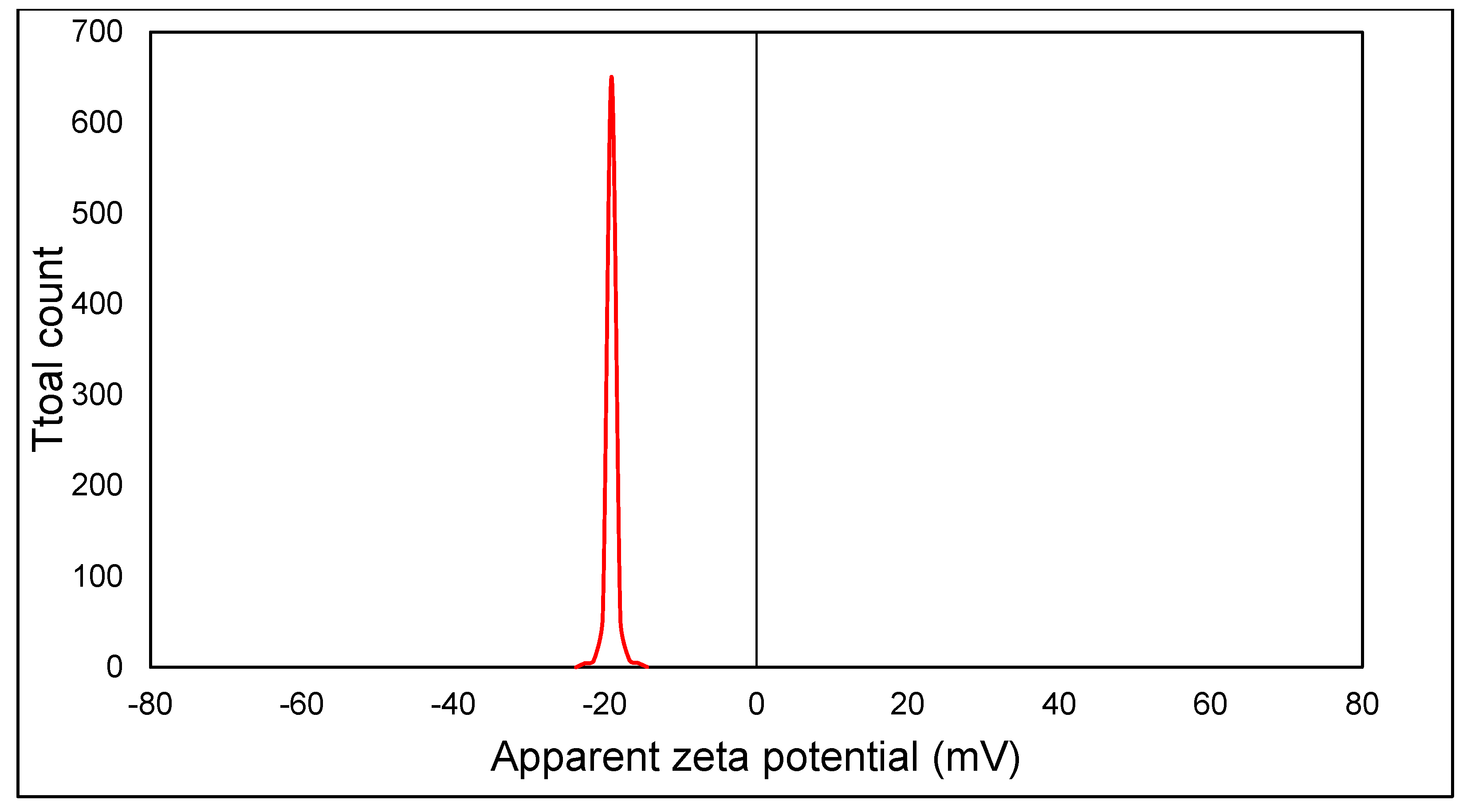
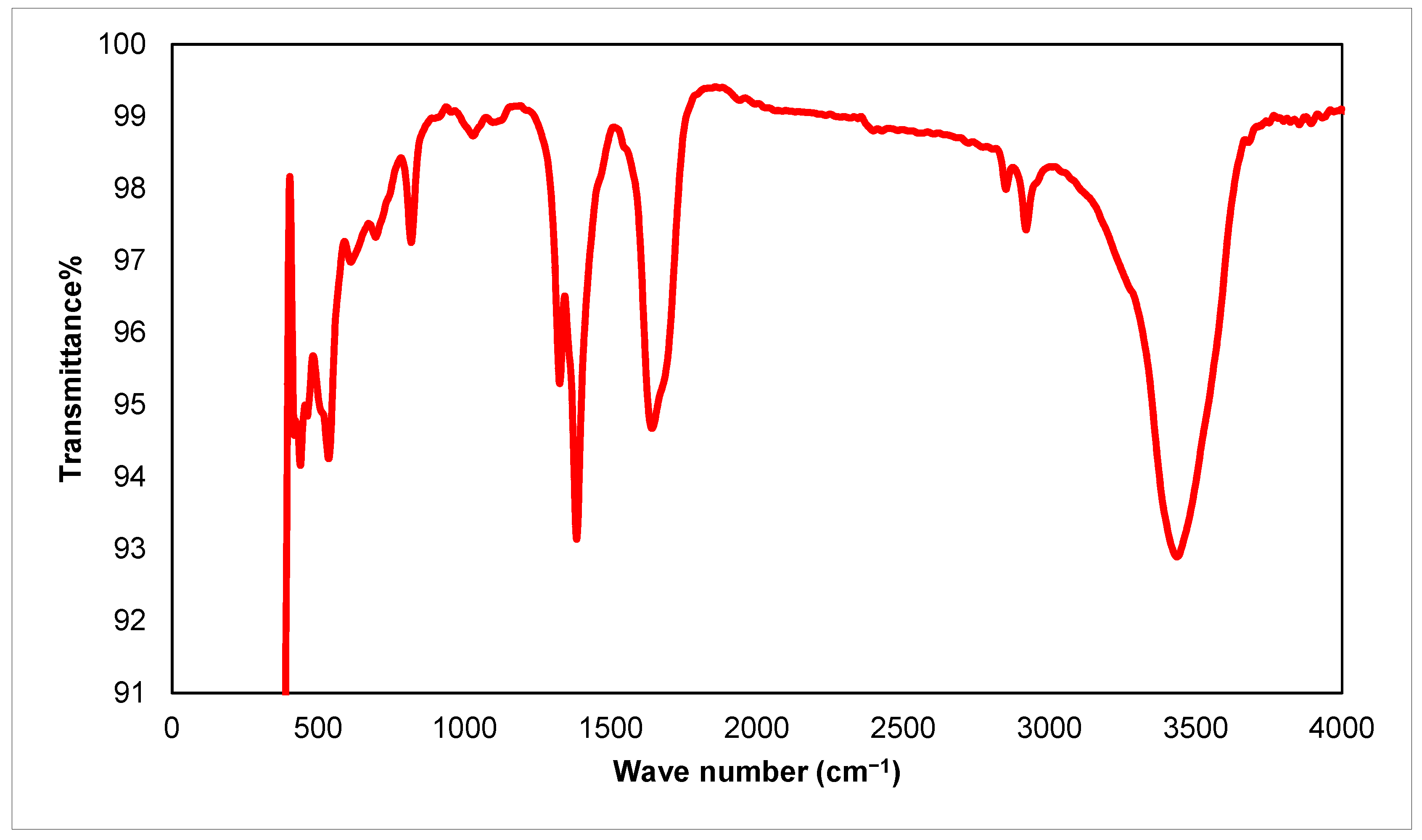
3.1. An Investigation of Green ZnO-NPs
3.2. Biochemical and Stress Marker Analysis of Wheat Leaves Subjected to Various Treatments
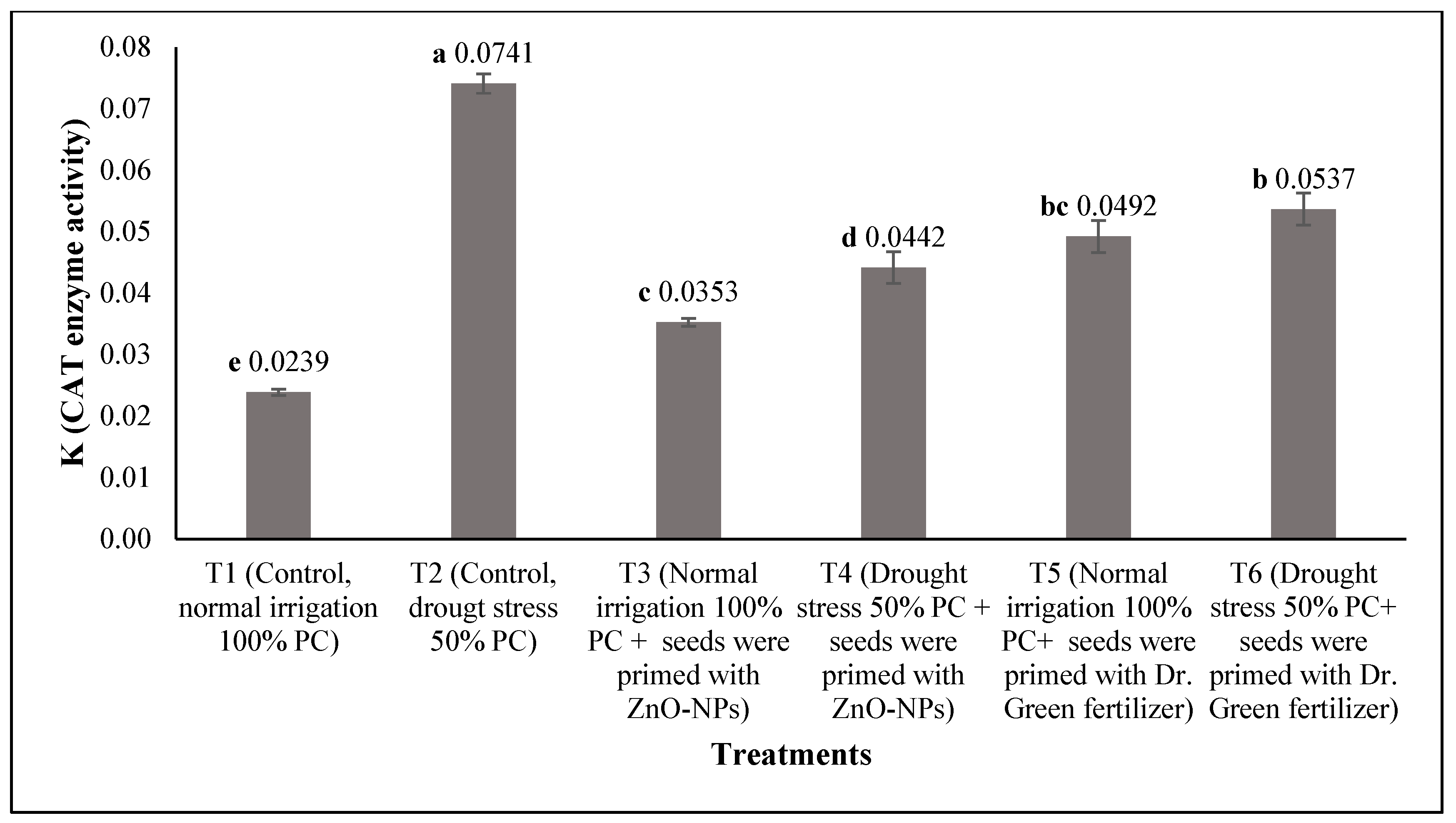
3.3. Determination of the Activities of Glutathione Reductase, Peroxidase, and Catalase Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benito-Verdugo, P.; Martínez-Fernández, J.; González-Zamora, Á.; Almendra-Martín, L.; Gaona, J.; Herrero-Jiménez, C.M. Impact of Agricultural Drought on Barley and Wheat Yield: A Comparative Case Study of Spain and Germany. Agriculture 2023, 13, 2111. [Google Scholar] [CrossRef]
- Yu, L.; Gao, X.; Zhao, X. Global Synthesis of the Impact of Droughts on Crops’ Water-Use Efficiency (WUE): Towards Both High WUE and Productivity. Agric. Syst. 2020, 177, 102723. [Google Scholar] [CrossRef]
- Iqbal, M.; Singh, A.; Ansari, M.I. Effect of Drought Stress on Crop Production; Springer: Berlin/Heidelberg, Germany, 2020; pp. 35–47. ISBN 9789811513213. [Google Scholar]
- Khan, S.; Anwar, S.; Yu, S.; Sun, M.; Yang, Z.; Gao, Z. Development of Drought-Tolerant Transgenic Wheat: Achievements and Limitations. Int. J. Mol. Sci. 2019, 20, 3350. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Z.; Du, Q.; Duan, Z. Winter Wheat Drought Risk Assessment by Coupling Improved Moisture-Sensitive Crop Model and Gridded Vulnerability Curve. Remote Sens. 2023, 15, 3197. [Google Scholar] [CrossRef]
- Ferrari, E.; Elleby, C.; De, J.B.; M’barek, R.; Perez, D.I. Cumulative Economic Impact of Upcoming Trade Agreements on EU Agriculture. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC135540 (accessed on 13 June 2024).
- Adel, S.; Carels, N. Plant Tolerance to Drought Stress with Emphasis on Wheat. Plants 2023, 12, 2170. [Google Scholar] [CrossRef]
- Ruf, F.; Schroth, G.; Doffangui, K. Climate Change, Cocoa Migrations and Deforestation in West Africa: What Does the Past Tell Us about the Future? Sustain. Sci. 2015, 10, 101–111. [Google Scholar] [CrossRef]
- Pradhan, G.P.; Prasad, P.V.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. Effects of Drought and High Temperature Stress on Synthetic Hexaploid Wheat. Funct. Plant Biol. 2012, 39, 190–198. [Google Scholar] [CrossRef]
- Dong, B.; Zheng, X.; Liu, H.; Able, J.A.; Yang, H.; Zhao, H.; Zhang, M.; Qiao, Y.; Wang, Y.; Liu, M. Effects of Drought Stress on Pollen Sterility, Grain Yield, Abscisic Acid and Protective Enzymes in Two Winter Wheat Cultivars. Front. Plant Sci. 2017, 8, 1008. [Google Scholar] [CrossRef]
- Senapati, N.; Halford, N.G.; Semenov, M.A. Vulnerability of European Wheat to Extreme Heat and Drought around Flowering under Future Climate. Environ. Res. Lett. 2021, 16, 024052. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 Synergistically Regulate TaP5CS1/TaP5CR1-Mediated Proline Biosynthesis to Enhance Drought Tolerance in Wheat. New Phytol. 2023, 237, 232–250. [Google Scholar] [CrossRef]
- de Carvalho, M.H.C. Drought Stress and Reactive Oxygen Species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, A.; Kafi, M.; Khazaei, H.R.; Davary, K. Effect of Drought Stress on Water Status, Elecrolyte Leakage and Enzymatic Antioxidants of Kochia (Kochia scoparia) under Saline Condition. Pak. J. Bot. 2010, 42, 3517–3524. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought Stress Responses and Inducing Tolerance by Seed Priming Approach in Plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Dynamics of the Antioxidant System during Seed Osmopriming, Post-Priming Germination, and Seedling Establishment in Spinach (Spinacia oleracea). Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 212–220. [Google Scholar] [CrossRef]
- Bose, B.; Kumar, M.; Singhal, R.K.; Mondal, S. Impact of Seed Priming on the Modulation of Physico-Chemical and Molecular Processes During Germination, Growth, and Development of Crops. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer Singapore: Singapore, 2018; pp. 23–40. ISBN 978-981-13-0032-5. [Google Scholar]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Nanoparticle-Based Amelioration of Drought Stress and Cadmium Toxicity in Rice via Triggering the Stress Responsive Genetic Mechanisms and Nutrient Acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles Potentially Mediate Salt Stress Tolerance in Plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- Ahmed, M.; Marrez, D.A.; Rizk, R.; Zedan, M.; Abdul-Hamid, D.; Decsi, K.; Kovács, G.P.; Tóth, Z. The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants. Plants 2024, 13, 1418. [Google Scholar] [CrossRef]
- Rehman, A.; Khan, S.; Sun, F.; Peng, Z.; Feng, K.; Wang, N.; Jia, Y.; Pan, Z.; He, S.; Wang, L.; et al. Exploring the Nano-Wonders: Unveiling the Role of Nanoparticles in Enhancing Salinity and Drought Tolerance in Plants. Front. Plant Sci. 2024, 14, 1324176. [Google Scholar] [CrossRef]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N.; Pabarja, A.; Abadi, M.F.S.; Tahroudi, M.H.M. Biosynthesis of Zinc Oxide Nanoparticles Using Hertia Intermedia and Evaluation of Its Cytotoxic and Antimicrobial Activities. BioNanoSci 2021, 11, 245–255. [Google Scholar] [CrossRef]
- Ahmed, M.; Decsi, K.; Tóth, Z. Different Tactics of Synthesized Zinc Oxide Nanoparticles, Homeostasis Ions, and Phytohormones as Regulators and Adaptatively Parameters to Alleviate the Adverse Effects of Salinity Stress on Plants. Life 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, R.; Kausar, A.; Hussain, S.; Javed, T.; Zafar, S.; Anwar, S.; Hussain, S.; Zahra, N.; Saqib, M. Zinc Oxide Nanoparticles as Potential Hallmarks for Enhancing Drought Stress Tolerance in Wheat Seedlings. Plant Physiol. Biochem. 2023, 195, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.; Meydan, I.; Seckin, H.; Bekmezci, M.; Sen, F. Green Synthesis, Characterization and Bioactivity of Biogenic Zinc Oxide Nanoparticles. Environ. Res. 2022, 204, 111897. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Hasan, M.; Mustafa, G.; Tariq, T.; Ahmed, M.M.; Golzari Dehno, R.; Ghorbanpour, M. Zinc Oxide Nano-Fertilizer Differentially Effect on Morphological and Physiological Identity of Redox-Enzymes and Biochemical Attributes in Wheat (Triticum aestivum L.). Sci. Rep. 2024, 14, 13091. [Google Scholar] [CrossRef]
- Bakayoko, M.; Fall, A.; Ngom, I.; Sackey, J.; Ngom, B.D.; Tall, P.D.; Maaza, M. Synthesis and Characterization of Zinc Oxide Nanoparticles (ZnO NPs) in Powder and in Thin Film Using Corn Husk Extract via Green Chemistry. MRS Adv. 2020, 5, 1083–1093. [Google Scholar] [CrossRef]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma betulina Natural Extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.A.; Darroudi, M. Synthesis and Characterization of a Narrow Size Distribution of Zinc Oxide Nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef]
- Solaiman, M.A.; Ali, M.A.; Abdel-Moein, N.M.; Mahmoud, E.A. Synthesis of Ag-NPs Developed by Green-Chemically Method and Evaluation of Antioxidant Activities and Anti-Inflammatory of Synthesized Nanoparticles against LPS-Induced NO in RAW 264.7 Macrophages. Biocatal. Agric. Biotechnol. 2020, 29, 101832. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of Nanomaterial Dispersion in Solution Prior to in Vitro Exposure Using Dynamic Light Scattering Technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef]
- Imakumbili, M.L.E.; Semu, E.; Semoka, J.M.R.; Abass, A.; Mkamilo, G. Managing Cassava Growth on Nutrient Poor Soils under Different Water Stress Conditions. Heliyon 2021, 7, 07331. [Google Scholar] [CrossRef]
- Prud’homme, M.-P.; Gonzalez, B.; Billard, J.-P.; Boucaud, J. Carbohydrate Content, Fructan and Sucrose Enzyme Activities in Roots, Stubble and Leaves of Ryegrass (Lolium perenne L.) as Affected by Source/Sink Modification after Cutting. J. Plant Physiol. 1992, 140, 282–291. [Google Scholar] [CrossRef]
- Ahmed, M.; Marrez, D.A.; Rizk, R.; Abdul-Hamid, D.; Tóth, Z.; Decsi, K. Interventional Effect of Zinc Oxide Nanoparticles with Zea mays L. Plants When Compensating Irrigation Using Saline Water. Nanomaterials 2024, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Venisse, J.-S.; Gullner, G.; Brisset, M.-N. Evidence for the Involvement of an Oxidative Stress in the Initiation of Infection of Pear by Erwinia amylovora. Plant Physiol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. [136] Assay of Catalases and Peroxidases. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; Volume 2, pp. 764–775. [Google Scholar]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Wiley: Hoboken, NJ, USA, 1954; pp. 357–424. [Google Scholar]
- Ahmed, M.; Marrez, D.A.; Mohamed Abdelmoeen, N.; Abdelmoneem Mahmoud, E.; Ali, M.A.-S.; Decsi, K.; Tóth, Z. Studying the Antioxidant and the Antimicrobial Activities of Leaf Successive Extracts Compared to the Green-Chemically Synthesized Silver Nanoparticles and the Crude Aqueous Extract from Azadirachta indica. Processes 2023, 11, 1644. [Google Scholar] [CrossRef]
- Jangam, A.K.; Thali, P. WASP-Web Agri Stat Package; ICAR Research Complex for Goa, Ela: Old Goa, Goa, India, 2004. [Google Scholar]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of Zinc Oxide Nanoparticles Using Plant Leaf Extract against Urinary Tract Infection Pathogen. Resour. Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Duru, C.E. Mineral and Phytochemical Evaluation of Zea mays Husk. Sci. Afr. 2020, 7, e00224. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; El-Naggar, H.A.; Abd-El Rahman, I.E.; Kalaba, M.H.; Moghannem, S.A.; Al-Otibi, F.; Alahmadi, R.M.; Abdelzaher, O.F.; Mabrouk, M.M.; Gewida, A.G.A.; et al. Biosynthesized ZnO-NPs Using Sea Cucumber (Holothuria impatiens): Antimicrobial Potential, Insecticidal Activity and In Vivo Toxicity in Nile Tilapia Fish, Oreochromis niloticus. Separations 2023, 10, 173. [Google Scholar] [CrossRef]
- Kalaba, M.H.; El-Sherbiny, G.M.; Ewais, E.A.; Darwesh, O.M.; Moghannem, S.A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Streptomyces baarnensis and Its Active Metabolite (Ka): A Promising Combination against Multidrug-Resistant ESKAPE Pathogens and Cytotoxicity. BMC Microbiol. 2024, 24, 254. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Calotropis gigantea: Characterization and Its Evaluation on Tree Seedling Growth in Nursery Stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V. Eco-Friendly Synthesis of Zinc Oxide Nanoparticles Using Cinnamomum tamala Leaf Extract and Its Promising Effect towards the Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Elbahnasawy, M.A.; Hasaballah, A.I. Green Phytosynthesis of Silver Nanoparticles Using Echinochloa stagnina Extract with Reference to Their Antibacterial, Cytotoxic, and Larvicidal Activities. BioNanoScience 2021, 11, 526–538. [Google Scholar] [CrossRef]
- Kumari, B.; Rao, K.V. Germination and Growth Characteristics of Mungbean Seeds (Vigna radiata L.) Affected by Synthesized Zinc Oxide Nanoparticles. Int. J. Curr. Eng. Technol. 2014, 4, 3411–3416. [Google Scholar]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root Uptake and Phytotoxicity of ZnO Nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.-C.; Braam, J.; Alvarez, P.J.J. Developmental Phytotoxicity of Metal Oxide Nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Salehi, H.; Cheheregani Rad, A.; Raza, A.; Djalovic, I.; Prasad, P.V.V. The Comparative Effects of Manganese Nanoparticles and Their Counterparts (Bulk and Ionic) in Artemisia annua Plants via Seed Priming and Foliar Application. Front. Plant Sci. 2023, 13, 1098772. [Google Scholar] [CrossRef]
- Mahendra, S.; Zhu, H.; Colvin, V.L.; Alvarez, P.J. Quantum Dot Weathering Results in Microbial Toxicity. Environ. Sci. Technol. 2008, 42, 9424–9430. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar]
- GhiassiTarzi, B.; Gharachorloo, M.; Baharinia, M.; Mortazavi, S.A. The Effect of Germination on Phenolic Content and Antioxidant Activity of Chickpea. Iran. J. Pharm. Res. 2012, 11, 1137–1143. [Google Scholar]
- Javed, R.; Mohamed, A.; Yücesan, B.; Gürel, E.; Kausar, R.; Zia, M. CuO Nanoparticles Significantly Influence in Vitro Culture, Steviol Glycosides, and Antioxidant Activities of Stevia rebaudiana Bertoni. Plant Cell Tissue Organ Cult. 2017, 131, 611–620. [Google Scholar] [CrossRef]
- Ghorbanpour, M. Major Essential Oil Constituents, Total Phenolics and Flavonoids Content and Antioxidant Activity of Salvia Officinalis Plant in Response to Nano-Titanium Dioxide. Ind. J. Plant Physiol. 2015, 20, 249–256. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO Nanoparticles on Brassica nigra Seedlings and Stem Explants: Growth Dynamics and Antioxidative Response. Front. Plant Sci. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of Flavonols in Tomatoes and Tomato-Based Products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic Compounds and Related Enzymes as Determinants of Quality in Fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Sgherri, C.; Navari-Izzo, F.; Pardossi, A.; Soressi, G.P.; Izzo, R. The Influence of Diluted Seawater and Ripening Stage on the Content of Antioxidants in Fruits of Different Tomato Genotypes. J. Agric. Food Chem. 2007, 55, 2452–2458. [Google Scholar] [CrossRef]
- Hernández-Fuentes, A.D.; López-Vargas, E.R.; Pinedo-Espinoza, J.M.; Campos-Montiel, R.G.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest Behavior of Bioactive Compounds in Tomato Fruits Treated with Cu Nanoparticles and NaCl Stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Kubota, C.; Kroggel, M.; Choi, J.-H. Quality of Fresh-Cut Tomatoes as Affected by Salt Content in Irrigation Water and Post-Processing Ultraviolet-C Treatment. J. Sci. Food Agric. 2008, 88, 1969–1974. [Google Scholar] [CrossRef]
- Moles, T.M.; de Brito Francisco, R.; Mariotti, L.; Pompeiano, A.; Lupini, A.; Incrocci, L.; Carmassi, G.; Scartazza, A.; Pistelli, L.; Guglielminetti, L.; et al. Salinity in Autumn-Winter Season and Fruit Quality of Tomato Landraces. Front. Plant Sci. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. Mol Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants. Ukr. Bot. J. 2003, 49, 97–98. [Google Scholar]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.-J.; Alghamdi, S.S.; Siddique, K.H.M. Seed Priming Improves Chilling Tolerance in Chickpea by Modulating Germination Metabolism, Trehalose Accumulation and Carbon Assimilation. Plant Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Montagu, M.V.; Inzé, D.; Camp, W.V. Catalase Is a Sink for H2O2 and Is Indispensable for Stress Defence in C3 Plants. EMBO J. 1997, 16, 4806. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants–Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in Antioxidant Enzymes Activity and Plant Performance by Salinity Stress and Zinc Application in Soybean (Glycine max L.). Plant Omics 2012, 5, 60–67. [Google Scholar]
- Jahantab, E.; Farzadmehr, J.; Matinkhah, S.; Mohammad Abadi, N.T.; Shafeiyan, E.; Yazdanshenas, H. Effect of Metal Oxide Nanoparticles on the Activity of Glutathione Reductase, Catalase, Peroxidase and Superoxide Dismutase in Plants under Drought. Irrig. Drain. 2022, 71, 1351–1362. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of Glutathione Reductase in Plant Abiotic Stress. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer New York: New York, NY, USA, 2012; pp. 149–158. ISBN 978-1-4614-0634-1. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric Oxide Alleviates Silver Nanoparticles (AgNps)-Induced Phytotoxicity in Pisum sativum Seedlings. Plant Physiol. Biochem. PPB 2017, 110, 167–177. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Rai-Kalal, P.; Jajoo, A. Priming with Zinc Oxide Nanoparticles Improve Germination and Photosynthetic Performance in Wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Ali, N.; Farooq, M.; Hassan, M.A.; Arshad, M.S.; Saleem, M.K.; Faran, M.; Bouis, H.E.; Welch, R.M. Micronutrient Seed Priming Improves Stand Establishment, Grain Yield and Biofortification of Bread Wheat. Crop Pasture Sci. 2018, 69, 479–487. [Google Scholar] [CrossRef]
- Baltazar, M.; Oppolzer, D.; Carvalho, A.; Gouvinhas, I.; Ferreira, L.; Barros, A.; Lima-Brito, J. Hydropriming and Nutripriming of Bread Wheat Seeds Improved the Flour’s Nutritional Value of the First Unprimed Offspring. Plants 2023, 12, 240. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A New Tool to Reduce Micronutrient Malnutrition. Food Nutr. Bull. 2011, 32, 31–40. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification-A Sustainable Agricultural Strategy for Reducing Micronutrient Malnutrition in the Global South. Crop Sci. 2010, 50, 20–32. [Google Scholar] [CrossRef]
- Meenakshi, J.; Johnson, N.L.; Manyong, V.M.; Groote, H.; Javelosa, J.; Yanggen, D.R.; Naher, F.; Gonzalez, C.; García, J.; Meng, E. How Cost-Effective Is Biofortification in Combating Micronutrient Malnutrition? An Ex Ante Assessment. World Dev. 2009, 38, 64–75. [Google Scholar] [CrossRef]
- Saltzman, A.; Birol, E.; Bouis, H.E.; Boy, E.; Moura, F.F.; Islam, Y.; Pfeiffer, W.H. Biofortification: Progress toward a more nourishing future. Glob Food Secur 2013, 2, 9–17. [Google Scholar] [CrossRef]
- Carvalho, A.; Reis, S.; Pavia, I.; Lima-Brito, J.E. Influence of Seed Priming with Iron and/or Zinc in the Nucleolar Activity and Protein Content of Bread Wheat. Protoplasma 2018, 256, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Siddique, K.H.M. Micronutrient Application through Seed Treatments: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 125–142. [Google Scholar] [CrossRef]
- Grujcic, D.; Hansen, T.H.; Husted, S.; Drinic, M.; Singh, B.R. Effect of Nitrogen and Zinc Fertilization on Zinc and Iron Bioavailability and Chemical Speciation in Maize Silage. J. Trace Elem. Med. Biol. 2018, 49, 269–275. [Google Scholar] [CrossRef]
- Prom-U-Thai, C.; Rerkasem, B.; Yazici, A.; Cakmak, I. Zinc priming promotes seed germination and seedling vigor of rice. J. Plant Nutr. Soil Sci. 2012, 175, 482–488. [Google Scholar] [CrossRef]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; Lima-Brito, J. Seed Priming with Iron and Zinc in Bread Wheat: Effects in Germination, Mitosis and Grain Yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef]
- Iqbal, S.; Farooq, M.; Nawaz, A.; Atique-Ur-Rehman, A. Optimizing Boron Seed Priming Treatments for Improving the Germination and Early Seedling Growth of Wheat. J. Agric. Soc. Sci. 2012, 8, 57–61. [Google Scholar]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) Seed Priming Induced Growth and Biochemical Changes in Wheat Under Water Deficit Conditions. Biol. Trace Elem. Res. 2012, 151, 284–293. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ahmad, R.; Basra, S.M.A. Seed Priming with Zinc Improves the Germination and Early Seedling Growth of Wheat. Seed Sci. Technol. 2015, 43, 262–268. [Google Scholar] [CrossRef]
- Anand, M.; Kumar, B.; Sheel, R. Effect of Heavy Metals on Biochemical Profile of Azolla Filiculoides. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3629–3653. [Google Scholar] [CrossRef]
- Alam, M.U.; Fujita, M.; Nahar, K.; Rahman, A.; Anee, T.I.; Masud, A.A.C.; Amin, A.K.M.R.; Hasanuzzaman, M. Seed Priming Upregulates Antioxidant Defense and Glyoxalase Systems to Conferring Simulated Drought Tolerance in Wheat Seedlings. Plant Stress 2022, 6, 100120. [Google Scholar] [CrossRef]





| Treatments | TPC (mg/g) |
|---|---|
| T1 (Control, normal irrigation 100% PC) | 19.24 ± 0.04 a |
| T2 (Control, drought stress 50% PC) | 17.64 ± 0.20 b |
| T3 (Normal irrigation 100% PC+ seeds were primed with ZnO-NPs) | 17.76 ± 0.23 b |
| T4 (Drought stress 50% PC + seeds were primed with ZnO-NPs) | 16.21 ± 0.20 d |
| T5 (Normal irrigation 100% PC+ seeds were primed with DR GREEN fertilizer) | 16.97 ± 0.13 c |
| T6 (Drought stress 50% PC+ seeds were primed with DR GREEN fertilizer) | 17.36 ± 0.21 bc |
| LSD (0.01) | 0.784 |
| LSD (0.05) | 0.559 |
| Coefficient of variation | 1.793 |
| Treatments | TFC (mg/g) |
|---|---|
| T1 (Control, normal irrigation 100% PC) | 40.26 ± 1.29 ns |
| T2 (Control, drought stress 50% PC) | 35.72 ± 3.45 ns |
| T3 (Normal irrigation 100% PC+ seeds were primed with ZnO-NPs) | 34.95 ± 4.07 ns |
| T4 (Drought stress 50% PC + seeds were primed with ZnO-NPs) | 28.29 ± 1.90 ns |
| T5 (Normal irrigation 100% PC+ seeds were primed with DR GREEN fertilizer) | 30.95 ± 2.55 ns |
| T6 (Drought stress 50% PC+ seeds were primed with DR GREEN fertilizer) | 29.92 ± 0.56 ns |
| LSD (0.01) | ND |
| LSD (0.05) | ND |
| Coefficient of variation | 13.503 |
| Treatments | Total Sugars (mg/g) |
|---|---|
| T1 (Control, normal irrigation 100% PC) | 191.32 ± 5.09 b |
| T2 (Control, drought stress 50% PC) | 143.06 ± 6.66 c |
| T3 (Normal irrigation 100% PC+ seeds were primed with ZnO-NPs) | 140.83 ± 1.04 c |
| T4 (Drought stress 50% PC + seeds were primed with ZnO-NPs) | 152.43 ± 6.54 c |
| T5 (Normal irrigation 100% PC+ seeds were primed with DR GREEN fertilizer) | 156.32 ± 3.33 c |
| T6 (Drought stress 50% PC+ seeds were primed with DR GREEN fertilizer) | 211.67 ± 7.62 a |
| LSD (0.01) | 23.868 |
| LSD (0.05) | 17.024 |
| Coefficient of variation | 5.767 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizk, R.; Ahmed, M.; Abdul-Hamid, D.; Zedan, M.; Tóth, Z.; Decsi, K. Resulting Key Physiological Changes in Triticum aestivum L. Plants Under Drought Conditions After Priming the Seeds with Conventional Fertilizer and Greenly Synthesized Zinc Oxide Nanoparticles from Corn Wastes. Agronomy 2025, 15, 211. https://doi.org/10.3390/agronomy15010211
Rizk R, Ahmed M, Abdul-Hamid D, Zedan M, Tóth Z, Decsi K. Resulting Key Physiological Changes in Triticum aestivum L. Plants Under Drought Conditions After Priming the Seeds with Conventional Fertilizer and Greenly Synthesized Zinc Oxide Nanoparticles from Corn Wastes. Agronomy. 2025; 15(1):211. https://doi.org/10.3390/agronomy15010211
Chicago/Turabian StyleRizk, Roquia, Mostafa Ahmed, Donia Abdul-Hamid, Mostafa Zedan, Zoltán Tóth, and Kincső Decsi. 2025. "Resulting Key Physiological Changes in Triticum aestivum L. Plants Under Drought Conditions After Priming the Seeds with Conventional Fertilizer and Greenly Synthesized Zinc Oxide Nanoparticles from Corn Wastes" Agronomy 15, no. 1: 211. https://doi.org/10.3390/agronomy15010211
APA StyleRizk, R., Ahmed, M., Abdul-Hamid, D., Zedan, M., Tóth, Z., & Decsi, K. (2025). Resulting Key Physiological Changes in Triticum aestivum L. Plants Under Drought Conditions After Priming the Seeds with Conventional Fertilizer and Greenly Synthesized Zinc Oxide Nanoparticles from Corn Wastes. Agronomy, 15(1), 211. https://doi.org/10.3390/agronomy15010211








