Abstract
DNA methylation, an evolutionarily conserved epigenetic mechanism, is crucial for controlling gene activity and ensuring genomic integrity. Altered methylation patterns can profoundly affect plant development, often resulting in atypical phenotypes. The regulation of these methylation states relies on the coordinated actions of de novo methylation, maintenance, and active demethylation, orchestrated by specialized enzymes within distinct pathways. This review delves into the diverse roles of DNA methylation in plants, offering an in-depth analysis of the enzymes and regulatory factors involved. We explore how these elements function within the broader epigenetic framework, focusing on their contributions to silencing transposable elements, modulating gene expression, and shaping chromatin architecture. The review also examines the significance of DNA methylation in plant development, particularly its role in adapting to biotic and abiotic stresses. Lastly, we highlight its potential for driving innovations in crop breeding, emphasizing its applicability in advancing sustainable agriculture.
1. Introduction
Epigenetic modifications represent heritable chemical changes that influence phenotypic traits without altering the underlying DNA nucleotide sequence [1,2]. In plants, the epigenome’s dynamic and plastic nature is crucial for regulating development, environmental adaptation, and evolutionary processes [3]. Key epigenetic marks encompass DNA methylation and diverse post-translational modifications of histones [4,5,6]. Additionally, non-coding RNAs and chromatin remodeling factors play integral roles in modulating gene expression and genome accessibility, thereby facilitating phenotypic plasticity [7]. Collectively, these epigenetic mechanisms enable plants to rapidly adapt to fluctuating environmental conditions, underscoring their evolutionary significance.
DNA methylation is a fundamental and extensively studied epigenetic mechanism in eukaryotes. Often referred to as 5-methylcytosine (5mC), it plays pivotal roles in transposon silencing, transcriptional regulation, and the maintenance of normal growth and development in numerous organisms [8,9,10]. Notably, plant genomes consistently exhibit extensive DNA methylation, with 5mC constituting up to 50% of total cytosine residues in some species [11]. This methylation occurs across all sequence contexts (CG, CHG, and CHH, where H represents A, C, or T). Euchromatin refers to loosely packed chromatin associated with transcriptionally active genes. DNA methylation within euchromatin predominantly occurs at CpG islands, modulating gene expression by either promoting activation or repressing transcription. Methylation levels are typically low to moderate, serving a dynamic role in the precise regulation of gene activity. This modification is frequently associated with active histone marks, such as H3K4me3, which facilitate transcriptional processes. In contrast, heterochromatin is tightly packed and harbors transcriptionally silent regions, including repetitive DNA and centromeres. DNA methylation in heterochromatin is generally elevated, contributing to gene silencing and the maintenance of genomic stability. This form of methylation is often linked to repressive histone marks, such as H3K9me3, and plays a key role in silencing transposable elements (TEs) and other repetitive sequences. Furthermore, many genic regions in plant genomes are methylated at CG sites, although this methylation is not directly linked to transcriptional gene silencing [12]. Despite these insights, the broader functional implications of genomic methylation remain incompletely understood and warrant further investigation.
DNA methylation, a conserved epigenetic modification shared by plants and animals, plays a crucial role in regulating development by maintaining specific genomic patterns. This modification is facilitated by DNA methyltransferases, which are highly conserved across both kingdoms and utilize S-adenosyl-L-methionine as a methyl donor. Conversely, active demethylation occurs through the base excision repair pathway, reflecting the dynamic nature of methylation [13,14,15,16]. In Arabidopsis thaliana, five DNA methyltransferases—METHYLTRANSFERASE 1 (MET1), CHROMOMETHYLASE 2 (CMT2), CMT3, and DOMAINS REARRANGED METHYLTRANSFERASE 1 (DRM1) and DRM2—are essential for establishing and maintaining methylation patterns [17]. MET1 specifically regulates CG methylation by interacting with methyl-CG-binding proteins VIM1, VIM2, and VIM3, ensuring semi-conservative methylation during DNA replication [18]. CHG methylation is primarily maintained by CMT3 through a positive feedback loop involving histone H3 lysine 9 mono- and dimethylation (H3K9me1/2) [19]. Similarly, CMT2 facilitates CHH methylation, particularly in heterochromatic regions, using mechanisms akin to those of CMT3 [19]. DRM1 and DRM2, with DRM2 as the key enzyme, are responsible for CHH methylation via the RNA-directed DNA methylation (RdDM) pathway, which relies on non-coding RNAs for de novo methylation in plants [20]. Beyond its role in RdDM, DRM2 is the principal enzyme for de novo methylation across all sequence contexts, underscoring its central role in epigenetic regulation (Figure 1).

Figure 1.
Specific DNA methyltransferases and demethylases mediate cytosine methylation in different sequence contexts [20]. CG, CHG, and CHH methylation are carried out by MET1, CMT3, and CMT2, respectively. DRM2, involved in the RdDM pathway, regulates all sequence context methylation. ROS1, DME, DML2, and DML3 act as demethylases.
This review examines recent advances and current perspectives on the regulation and function of DNA methylation in plants. The mechanisms that establish specific DNA methylation patterns are most comprehensively characterized in the model plant Arabidopsis thaliana, where mutations in DNA methylation and demethylation pathways, as well as alterations in regulatory factor composition, typically do not lead to plant lethality. In contrast, for plants with more complex genomes, DNA methylation is increasingly recognized as a critical regulator of development and adaptation to environmental stress. Emerging research has uncovered pivotal mechanisms governing plant DNA methylation. Notable examples include the initiation of de novo DNA methylation mediated by non-coding RNAs (ncRNAs), the activity of the novel protein complex IDM, which targets active DNA demethylation and paradoxically enhances DNA methylation, and the regulatory role of methylation-sensing genetic elements that dynamically balance methylation and demethylation processes. Additionally, this review highlights the dynamic roles of DNA methylation in regulating various biological processes, including transposon silencing, gene expression, chromosomal interactions, plant development, and responses to both biotic and abiotic environmental stimuli. Finally, the potential applications of DNA methylation dynamics in crop improvement and breeding are discussed, emphasizing its transformative potential in agricultural innovation.
2. DNA Methylation Kinetics
2.1. Establishment of DNA Methylation
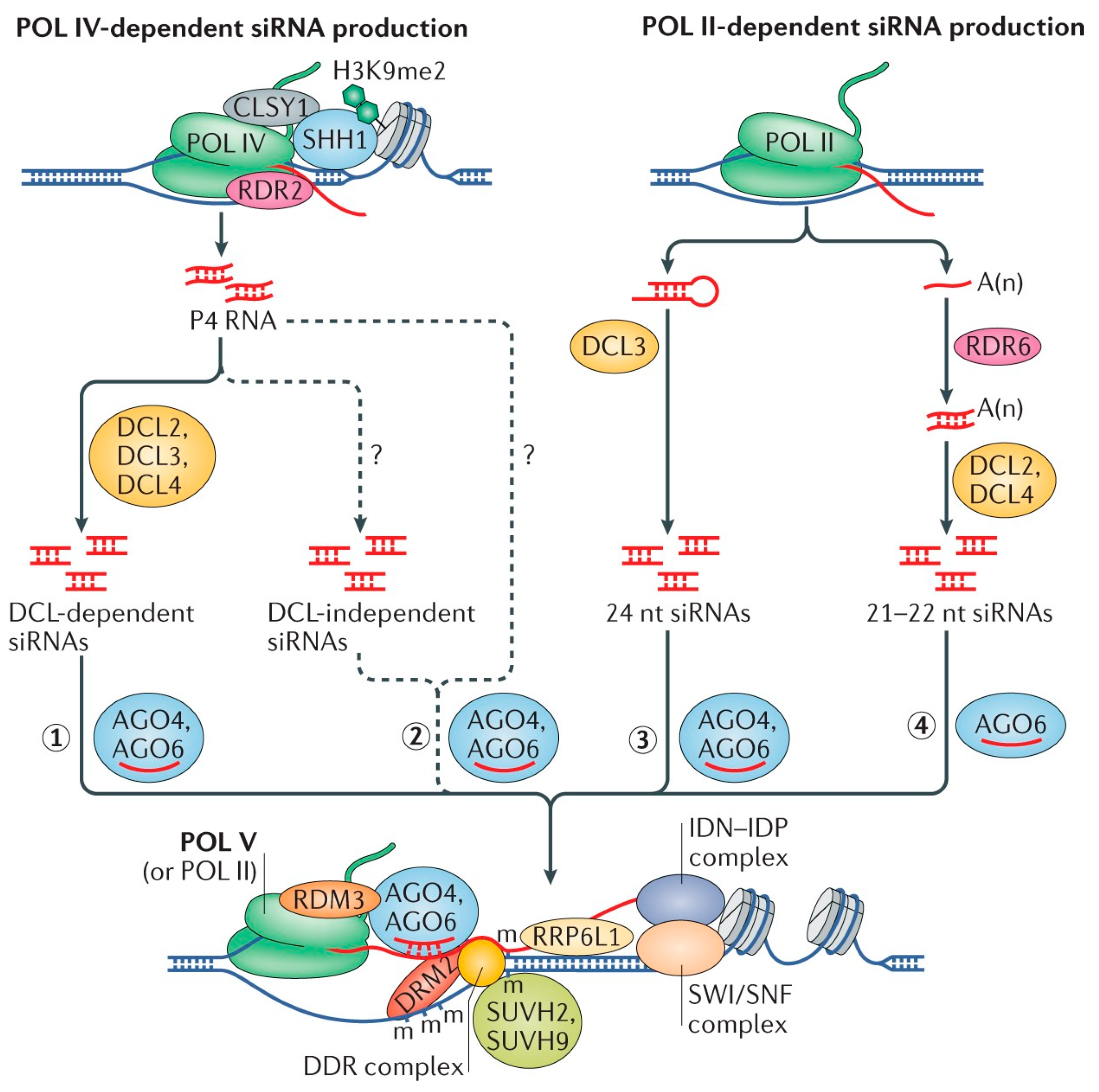
In plants, de novo DNA methylation is mediated by the RdDM pathway, which involves small interfering RNAs (siRNAs), scaffold RNAs, and associated proteins. In Arabidopsis thaliana, the canonical RdDM pathway begins with RNA polymerase IV (Pol IV), which transcribes a precursor RNA that is then converted into double-stranded RNA (dsRNA) by RNA-dependent RNA polymerase 2 (RDR2) [20,21,22]. Dicer-like protein 3 (DCL3) processes this dsRNA into 24-nucleotide siRNAs, which are incorporated into ARGONAUTE (AGO) proteins, primarily AGO4 [23,24]. These siRNAs guide the recognition of complementary scaffold RNAs transcribed by RNA polymerase V (Pol V). AGO4 interacts with the DNA methyltransferase DRM2 to catalyze de novo DNA methylation in a sequence-independent manner [25,26,27]. RdDM is also involved in the regulation of parasitic sequences, such as TEs and viruses, where foreign RNAs are converted into dsRNA and processed into siRNAs by RNA-dependent RNA polymerases like RDR6. These siRNAs guide DNA methylation through AGO proteins, although the mechanisms of non-canonical RdDM remain poorly understood [28,29,30].
Scaffold RNAs, produced by Pol V, are retained on chromatin through interactions with chromatin-remodeling factors such as ribosomal RNA processing 6-like 1 (RRP6L1), a homolog of yeast RRP6. These RNAs, along with siRNAs, are stabilized by the INVOLVED IN DE NOVO 2 (IDN2-IDN2) paralogue complex and the SWI/SNF chromatin remodeling complex, facilitating Pol V-mediated transcriptional silencing [31,32,33,34]. Furthermore, histone modifications also play a critical role in RdDM. Six histone H1 (SHH1), a histone-binding protein, recruits Pol IV to chromatin at specific loci, while zinc finger, mouse double-minute/switching complex B, Plus-3 protein (ZMP) regulates Pol IV binding to pericentromeric regions [35,36,37]. The CLASSY family of chromatin remodelers is involved in Pol IV-dependent siRNA production, and the DDR complex, which includes D(1A) dopamine receptor (DRD1) and DMS3, is essential for Pol V-mediated RNA transcription [38,39,40,41,42]. Pol V generates non-coding RNAs (ncRNAs), which lack polyadenylation and are typically ~200 nucleotides long. These ncRNAs can initiate transcription without a promoter and are capped with 7-methylguanosine [43,44,45,46,47,48]. The siRNAs produced in the canonical pathway can also be generated through non-canonical routes involving RNA polymerase II (Pol II), which produces 24-nt siRNAs and recruits Pol IV and Pol V for RdDM [49,50,51,52]. In some regions, RdDM relies on RDR6 and Pol II, with DCL2 and DCL4 generating shorter siRNAs (21–22 nt), suggesting an alternative, DCL-independent RdDM mechanisms [53,54].
Overall, while much is understood about the canonical RdDM pathway (Figure 2), alternative pathways and interactions with RNA processing factors continue to present challenges in fully delineating the molecular mechanisms of RdDM [55,56,57].

Figure 2.
RdDM pathways in Arabidopsis thaliana [57].
2.2. Maintenance of DNA Methylation
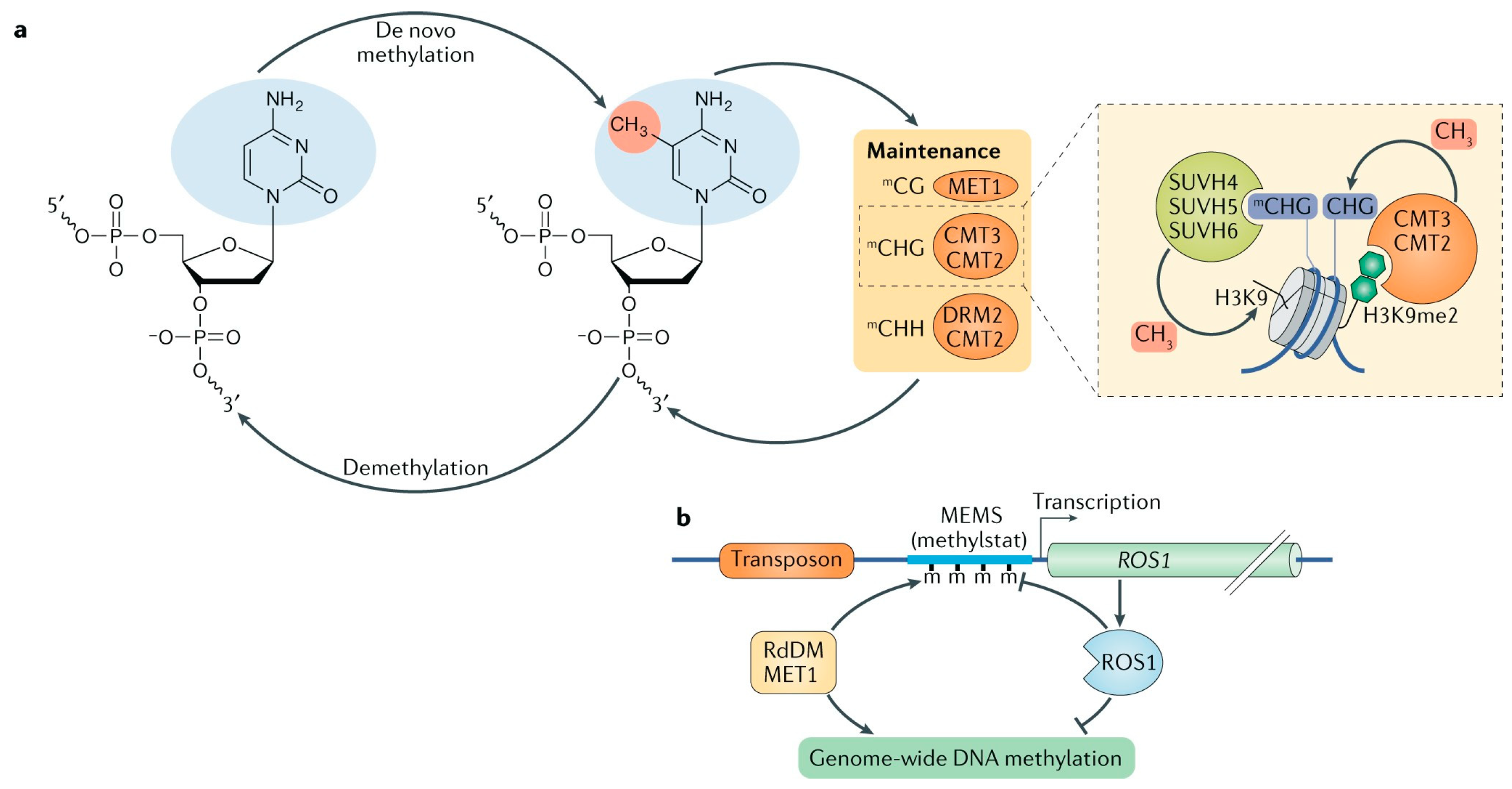
In Arabidopsis thaliana, maintaining DNA methylation patterns relies on the coordinated actions of five DNA methyltransferases. CHG and CHH methylation are preserved through self-reinforcing feedback systems that involve CMT3, CMT2, DRM1, DRM2, histone methyltransferases, and nucleosomes modified with repressive marks such as H3K9me1 and H3K9me2 [58,59]. CG methylation, however, is predominantly maintained by MET1, which is believed to methylate hemimethylated CG sites during DNA replication, ensuring methylation continuity on newly synthesized DNA strands [59,60]. Research on DNMT1, the mammalian homolog of MET1, shows a preference for hemimethylated DNA substrates both in vitro and in vivo, aligning with this semi-conservative methylation model [61,62,63].
During replication, DNMT1 and the E3 ubiquitin ligase UHRF1 are localized to replication foci, a process critical for preserving CG methylation in mammals [64,65]. In plants, UHRF1 homologs are encoded by the VIM gene family, whose role in CG methylation parallels their mammalian counterparts. Notably, CG methylation is abolished in Arabidopsis mutants lacking MET1 or in triple mutants of VIM1, VIM2, and VIM3 [66]. Both DNMT1 and UHRF1 are multi-domain proteins, with catalytic domains located at their C-terminus and regulatory domains at the N-terminus [67]. Interestingly, the N-terminal domains of MET1 and VIM proteins diverge structurally from those of DNMT1 and UHRF1, indicating that plants and mammals utilize distinct regulatory strategies (Figure 3).

Figure 3.
Dynamic regulation of DNA methylation in plants [57]. (a) De novo DNA methylation can occur in all cytosine contexts. Following DNA replication, methylation in the symmetric CG context is maintained by METHYLTRANSFERASE 1 (MET1), whereas CHG (H represents A, T or C) methylation is maintained by CHROMOMETHYLASE 3 (CMT3) or CMT2. (b) The methylation (m) of DNA methylation moni-toring sequence (MEMS) within the promoter region of REPRESSOR OF SILENCING 1 (ROS1; a major DNA demethylase in Arabidopsis thaliana) is required for ROS1 gene expression.
2.3. Demethylation of Active DNA
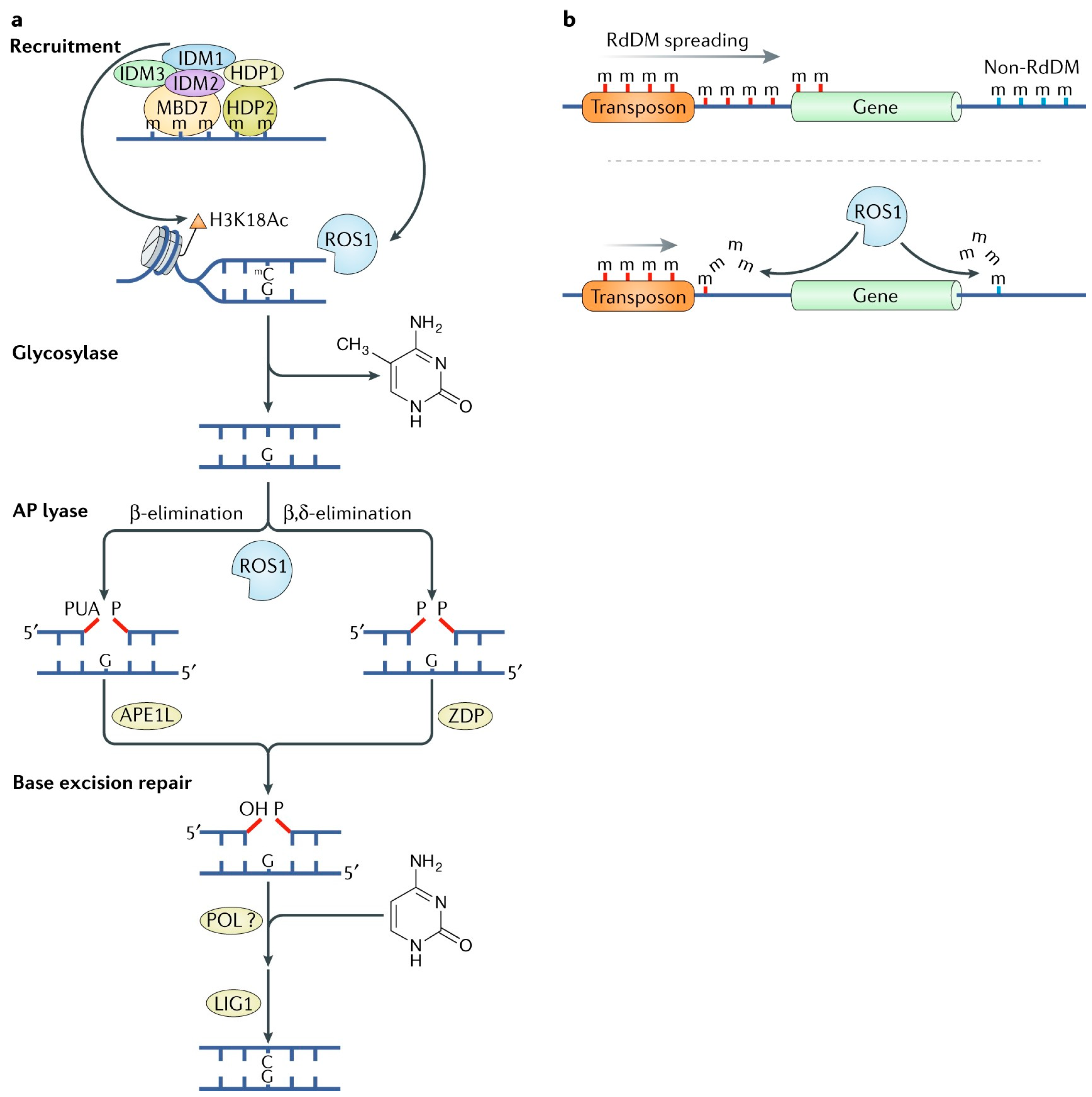
The failure to maintain DNA methylation following replication, due to the absence of either DNA methyltransferase activity or a methyl donor, results in passive DNA demethylation—a process distinct from enzymatic removal of methylation marks known as active DNA demethylation [68,69,70]. In plants, active DNA demethylation is initiated by a family of bifunctional 5-mC DNA glycosylase-purine/depyrimidine lyases, which excise 5-mC through the base excision repair (BER) pathway [71,72]. Similarly, active demethylation in mammals involves DNA glycosylases and the BER mechanism. However, a key distinction lies in substrate recognition: plant DNA glycosylases directly identify and excise 5-mC bases, whereas in mammals, 5-mC undergoes oxidation prior to glycosylase-mediated excision [73] (Figure 4).

Figure 4.
ROS1-mediated active DNA demethylation in Arabidopsis thaliana [57]. (a) REPRESSOR OF SILENCING 1 (ROS1) is a 5-methylcytosine (m) DNA glycosylase and apurinic/apyrimidinic lyase that is recruited to a subset of demethylation target loci by the INCREASED DNA METHYLATION (IDM) complex, in which IDM1 catalyses acetylation of histone H3 lysine 18 (H3K18Ac) to create a permissive chromatin environment for ROS1 function. (b) ROS1-mediated demethylation helps to establish boundaries between transposons and genes, thereby preventing the spreading of DNA methylation and transcriptional silencing from transposons to neighbouring genes.
Arabidopsis thaliana contains a group of four bifunctional 5-mC DNA glycosylases: REPRESSOR OF SILENCING 1 (ROS1), DEMETER (DME), and the DEMETER-LIKE PROTEINS DML2 and DML3 [71,73]. These glycosylases actively remove 5-mC from cytosines in all sequence contexts [72,74,75,76]. While ROS1, DML2, and DML3 are widely expressed in vegetative tissues, DME shows a more restricted expression profile, being primarily active in the companion cells of male and female gametophytes. Specifically, DME is expressed in the central cells of female gametophytes and in the trophic cells of male gametophytes [75,77].
During DNA demethylation, bifunctional enzymes initially function as DNA glycosylases, hydrolyzing the glycosyl bond between the base and deoxyribose. Subsequently, they act as apurinic/apyrimidinic (AP) lyases, cleaving the DNA backbone to generate abasic (base-free) sites. Following the excision of the 5-methylcytosine (5-mC) base, either a β-elimination or β, δ-elimination reaction occurs. These reactions create a gap in the DNA strand, terminating with either a 3′-phosphate-α, β-unsaturated aldehyde or a 3′-phosphate, respectively. In the downstream processing of these elimination reactions, AP endonuclease 1 (APE1) and the polynucleotide 3′-phosphatase ZDP play crucial roles. APE1 operates following β-elimination, while ZDP acts after β, δ-elimination, both facilitating the generation of a 3′-hydroxyl (3′-OH) group. This modification prepares the DNA strand for subsequent repair by DNA polymerases and ligases, which restore the integrity of the DNA [78,79,80].
3. Molecular Functions of Plant DNA Methylation
3.1. Regulation of Gene Expression
In plants, DNA methylation associated with genes typically occurs in promoter regions or transcribed areas of the genome (Figure 5A,B). Promoter methylation is generally linked to transcriptional suppression, though it can occasionally promote gene activation. This dual functionality has been documented in the regulation of the ROS1 gene in Arabidopsis and in numerous genes that inhibit tomato fruit ripening [18,81,82,83]. Transcriptional repression by promoter methylation involves both direct and indirect pathways. Directly, it may block the binding of transcriptional activators or enhance the interaction with repressors. Indirectly, it contributes to transcriptional silencing by promoting repressive histone modifications, such as H3K9me2, and reducing activating modifications like histone acetylation [84,85].

Figure 5.
Different roles of DNA methylation in plant cells. (A) The effect of DNA methylation on the transcription factor (TF) recognition motifs may directly regulate the binding activity of transcription factors. Most transcription factors, including transcriptional activators and suppressors, are highly sensitive to DNA methylation. When methylation on cis-elements affects the binding of transcriptional activators, it usually suppresses transcription. Similarly, methylation of cis-elements can also lead to transcriptional inhibition if it interferes with transcription suppressor binding. In the case of W-box motifs, any transcription factor binding motifs that contain cytosine (C) or guanine (G) may be affected by this mechanism. (B) DNA methylation in the coding region maintains transcription accuracy by forming inaccessible chromatin structures that inhibit the activation of abnormal transcription initiation sites. (C) DNA methylation plays a key role in maintaining genomic stability by silencing transposons (TEs) and other repeat sequences. (D) DNA methylation may also be involved in the production of circular RNA, suggesting a potential regulatory function in RNA biogenesis.
The exact mechanism by which promoter methylation activates transcription remains unclear. One theory posits that methylation might enhance the binding of specific transcriptional activators or impede the attachment of repressors. Promoter methylation frequently originates from the expansion of methylation from adjacent transposable elements (TEs) or repetitive sequences. To protect neighboring genes from being silenced, these TEs and repeats are often subjected to active DNA demethylation [86]. Notably, for genes requiring promoter methylation for expression, active demethylation may unexpectedly result in transcriptional repression. This dynamic interplay between DNA methylation and gene expression underscores the complexity of transcriptional regulation [82,87].
Promoter methylation relies on various effectors, including methyl-DNA-binding proteins, histone modifiers, chromatin remodeling factors, and molecular chaperones, to establish a condensed chromatin structure that limits transcription factor access. In Arabidopsis, chaperones play a notable role in modulating chromatin states enriched in mCG sequences, particularly in promoter regions [88,89,90]. Proteins such as MBD7, MBD5, and MBD6, which recognize mCG sites, contain a C-terminal StkyC domain that interacts with ACD family chaperones [88,90]. ACD chaperones, including IDM2 and IDM3, facilitate the recruitment of histone acetyltransferase IDM1 and accessory proteins HDP1 and HDP2, forming IDM complexes essential for demethylating and activating transcription at the highly methylated viral 35S promoter [90,91].
At another 35S promoter locus, ACD proteins like RDS1, RDS2, and IDM3 recruit additional chaperones, such as DnaJ domain proteins SDJ4 and HSP70, to assemble molecular chaperone complexes. These complexes can modulate the transcriptional state of the locus depending on the genetic context, either silencing or activating it [88]. Despite these advances, the precise functions of chaperone proteins in methylated loci require further investigation.
Interestingly, MBD proteins possess intrinsically disordered regions, which may facilitate phase separation to form molecular aggregates. ACD proteins, known as “maintainers”, bind target proteins to prevent irreversible aggregation [92]. DnaJ domain proteins, often working with HSP70, contribute to ATP-dependent chaperone activity that supports substrate protein refolding or depolymerization [92]. This regulatory interplay between chaperone proteins and MBD proteins may influence the aggregation state of MBDs, thereby controlling chromatin accessibility at mCG-rich loci.
3.2. TEs Silence
By relocating transposons (TE) or inserting new retrotransposon copies, TE poses a significant threat to genomic stability. In Arabidopsis, the heterochromatin regions around the centromeres, as well as certain non-heterochromatin regions containing TE or repeat sequences, exhibit extensive methylation in all cytosine environments [93] (Figure 5C). Rna-directed DNA methylation (RdDM) plays a key role in maintaining asymmetric CHH methylation at the edges of short and long TE. In the internal region of long heterochromatin transposons, asymmetric methylation is regulated by chromatin remodeling factor DDM1 and catalyzed by CMT2 [19,94]. In the maize genome, active genes, and inactive TE are staggered, often separated by RDDM-dependent “islands” of CHH methylation. These methylated islands are short genomic regions with high levels of CHH methylation. Loss of these islands often results in transcriptional activation, accompanied by decreased methylation levels at CG and CHG sites in adjacent TE. This suggests that in maize, RdDM is critical for silencing transposons in costained regions containing adjacent active genes [95]. In sugar beets, CHH methylation appears to be more specifically involved in the silencing of DNA transposons. DNA transposons show higher levels of CHH methylation compared to retrotransposons and genes [96]. The CG and CWG (a subset of CHG) motifs are symmetric, while the CHH motifs are asymmetric. In partially symmetric CCG motifs, the methylation of the first cytosine is dependent on CG methylation (citation), so CCG methylation levels are generally lower than CWG methylation [97]. In addition, in monocotyledonous plants such as maize and rice, environment-specific CHH methylation is more evenly distributed on chromosomes, further indicating the diversity of its regulatory mechanisms [97].
3.3. Chromosome Interaction and Biogenesis of circRNAs
DNA methylation shapes the epigenetic state of chromatin and is pivotal in regulating chromosomal interactions. In the nucleus of Arabidopsis thaliana, all five chromosomes interact through a specialized structure called the KNOT [98]. This structure consists of interactive heterochromatin islands (IHIs), which are repressive chromatin regions embedded within euchromatin arms and enriched with transposable elements (TEs) [98,99]. Interestingly, the integrity of IHI interactions remains intact in met1 and ddm1 mutants, despite extensive DNA hypomethylation across all cytosine contexts. Similarly, these interactions are unaffected in the suvh4-suvh5-suvh6 triple mutant, which lacks functional H3K9 methylation [99]. These observations indicate that both DNA methylation and H3K9me2 are indispensable for preserving chromosomal interactions within IHIs (Figure 5D).
Interestingly, mutants deficient in RdDM display increased frequencies of chromosomal interactions in specific RdDM-targeted regions. This indicates that, in wild-type plants, RdDM acts to suppress chromosome interactions in certain genomic regions [100]. Moreover, enhanced chromosomal interactions are observed between POL V-dependent DNA methylation sites and distal genes that are repressed by RdDM. These findings suggest that chromosomal interactions may also play a regulatory role in gene expression [100]. Furthermore, some studies have suggested that there may be synergies between DNA methylation and long non-coding Rnas (lncrnas) or micrornas (mirnas) that co-regulate circRNA synthesis and degradation (Figure 5D) [100].
4. The Role of DNA Methylation in Plant Development
4.1. Genomic Imprinting and Seed Development
Arabidopsis utilizes a specialized double fertilization mechanism, which depends on the multicellular structure of both male and female gametophytes. In this process, the two sperm cells carried within a single pollen grain fertilize separate cells of the female gametophyte: one sperm cell fuses with the egg cell to form the embryo, while the other combines with the central cell to develop the endosperm. In both rice and Arabidopsis thaliana, the endosperm exhibits global DNA hypomethylation compared to the embryo [101,102,103]. In A. thaliana, this hypomethylation is attributed in part to active demethylation mediated by DEMETER (DME) in pre-fertilization central cells, which are the partner cells of female gametes [101,103]. Interestingly, although MET1 transcription is repressed during female gametogenesis, this repression does not appear to drive extensive demethylation. Consistent with this observation, genome-wide CG hypomethylation, which would be expected under reduced MET1 activity, is not detected in wild-type endosperm. Moreover, DNA methylation is almost entirely restored in the endosperm of dme mutants, underscoring the critical role of DME-dependent pathways in establishing endosperm-specific epigenetic patterns [102,104].
DME-mediated demethylation occurs in androtrophic cells and is accompanied by the downregulation of DDM1 [105]. This process triggers the production of small interfering RNAs (siRNAs) derived from demethylated TEs, which are subsequently transported to sperm cells, where they enhance RNA-directed DNA methylation (RdDM). These transposon-derived siRNAs in sperm cells may also contribute to transposon silencing in egg cells after fertilization [105,106,107]. The levels of CHH methylation dynamically fluctuate during seed development and germination. Specifically, CHH methylation increases during seed development but decreases during germination, primarily due to passive demethylation, suggesting its potential role in regulating seed dormancy [108]. Notably, while male reproductive lineages exhibit lower overall CHH methylation than somatic cells, certain hypermethylated CHH sites are essential for meiosis [109]. In the endosperm, methylation levels of the maternal genome are lower than those of the paternal genome, particularly at CG sites, which are closely associated with gene imprinting. Maternal expression genes (MEGs) typically exhibit hypomethylation of maternal alleles and hypermethylation of paternal alleles. For instance, certain MEGs, such as MEDEA, are silenced not through DNA methylation but via histone modification by H3K27me3 [109,110,111,112]. Disruptions in the maternal function of DME or the paternal function of MET1 interfere with the imprinting of MEGs, underscoring the crucial role of DNA methylation in allele-specific regulation [109,113,114,115]. Conversely, the maternal alleles of paternally expressed genes (PEGs) are typically marked by H3K27me3, while the paternal alleles are expressed via H3K36me3. This coordinated regulation by histone modifications and DNA methylation establishes the distinct expression patterns of MEGs and PEGs in the endosperm [116,117].
4.2. Role of DNA Methylation in Plant Meristem and Leaf Epidermal Development
Plant meristem stem cells are fundamental to tissue and organ development, with RdDM factors exhibiting higher transcription levels in meristematic tissues compared to differentiated tissues. Notably, the highest DNA methylation levels are observed in the small columnar cells of the root meristem, potentially due to reduced exposure of pericentromeric chromatin to RdDM factors [118]. While RdDM mutants in Arabidopsis do not exhibit pronounced meristem defects, corresponding mutants in rice and maize display severe developmental abnormalities, underscoring the critical role of RdDM in meristem development [118,119]. In rice, SDG711-mediated H3K27me3 deposition represses the expression of leaf development genes following shoot apical meristem development. This repression coincides with DRM2-catalyzed non-CG DNA methylation, suggesting a coordinated regulatory mechanism. Furthermore, physical interactions between SDG711 and DRM2 imply a synergistic effect in gene silencing [120]. In maize, differential regulation of maintenance DNA methyltransferases establishes distinct DNA methylation patterns across the division, transition, extension, and maturation zones of leaves. These methylation changes predominantly occur near genes involved in growth and cell cycle regulation, where promoter methylation is inversely correlated with gene expression [121]. DNA methylation also plays a pivotal role in leaf epidermal patterning in Arabidopsis. Dysfunction of ROS1 leads to hypermethylation of the EPF2 promoter, suppressing the expression of stomatal inhibitory factors and resulting in excessive stomatal production [122]. Similarly, IBM1 dysfunction enhances H3K9me2 and CHG methylation, inhibiting EPF2 receptor gene expression and disrupting stomatal patterning. Interestingly, ROS1 mutation-induced abnormalities can be alleviated by RdDM factor mutations, whereas IBM1 mutation-induced defects are mitigated by SUVH4 or CMT3 mutations. These findings suggest that DNA methylation governs leaf epidermal development through distinct regulatory pathways [123].
4.3. Role of DNA Methylation in Flower Development
DNA methylation has been shown to play a pivotal role in various aspects of flower development, including anthocyanidin deposition and floral patterning. For instance, DNA methylation influences anthocyanin accumulation in the orchid Oncidium. In Oncidium Gower Ramsey (GR), the lips of the flowers appear bright yellow due to the accumulation of carotenoid pigments. The genetic pathway responsible for this pigmentation is well characterized, and its variation accounts for the white coloration observed in the Oncidium variety known as ‘White Jade’ (WJ) [124]. The distinct color patterns observed between these orchid varieties arise from differential methylation levels of carotenoid-related genes. Specifically, in GR, the OgCCD1 gene is methylated and silenced. In contrast, in WJ tissues, the absence of methylation in the OgCCD1 promoter results in its active expression, leading to the degradation of carotenoids and the development of white flowers [125]. Another notable feature of the pigmentation pattern in GR flowers is the presence of red stripes in the perianth, which are absent in the Oncidium Honey Dollp (HD) cultivar. In this case, the contrasting flower colors are determined by the methylation status of genes involved in anthocyanin biosynthesis. In the HD variety, methylation of the OgCHS gene promoter prevents anthocyanin accumulation, whereas, in GR, unmethylated genes are expressed, facilitating anthocyanin production [126]. Additionally, DNA methylation plays a significant role in floral color formation in many other ornamental plants. For example, in Malus halliana, the color of the petals changes from red to pale pink during development, which is linked to the downregulation of several genes. Notably, the promoter of the MhMYB10 gene, which is involved in anthocyanin biosynthesis, is highly methylated, leading to reduced expression of MhMYB10 and a consequent decrease in anthocyanin accumulation [127].
In addition, DNA methylation plays a crucial role in regulating floral production. In Chinese and Japanese Prunus mume, multiple genes involved in eight floral biosynthetic pathways exhibit different methylation levels during flower development [128]. These genes code for enzymes that are key regulators of floral production, including coniferol acetyltransferase (PmCFAT1a/1c) and benzyl acetyltransferase (PmBEAT36/3). CFAT protein belongs to the acyltransferase family and is an important component of eugenol synthesis, responsible for catalyzing the conversion of coniferol-to-coniferol acetate [116]. BEAT proteins produce benzyl acetate by transferring the acetyl group from acetyl-CoA to the carbonyl group of benzyl alcohol [117]. Genome-wide sulfite sequencing showed that most of the differentially methylated genes were involved in multiple links of the benzoylalanine biosynthesis pathway, which produces more than 90% of floral volatiles [128].
4.4. Role of DNA Methylation in Fruit Ripening
During the development of tomato fruit, about 1% of the DNA methylation groups in the peel are changed. Active DNA demethylation occurs on many genes associated with fruit ripening whose promoter regions contain binding sites for ripening inhibitors (Rins). Rins are a major mature transcription factor [129]. In most known mature genes, RIN binds to the target promoter, and the expression of these genes is negatively correlated with the level of DNA methylation in the promoter region. Treatment with chemical inhibitors of DNA methylation can induce hypomethylation of the promoter region and activate the expression of the encoding colorless immature gene (CNR). CNR is a key rin target gene during fruit ripening, and this treatment can also lead to the early ripening of tomato fruits [129]. In ripe fruit of tomato (Solanum lycopersicum), expression of DNA demethylase DME-LIKE 2 (DML2) is significantly increased, mediating progressive DNA demethylation that occurs during fruit ripening [130]. Tomato DML2 acts not only on ripening inducer genes, but also on ripening suppressor genes, indicating that both activation of ripening inducer genes and inhibition of ripening suppressor genes depend on active DNA demethylation [131]. Changes in DNA methylation may be related to growth and ripening processes in other fruits. In apple fruit, anthocyanin accumulation was negatively correlated with DNA methylation levels in the apple MYB10 gene promoter region [132,133]. At the genome-wide level, CHH hypermethylation was observed in developing apple fruits compared to leaves, and comparisons with transgenic fruits also showed an association between lower DNA methylation levels and smaller fruit size [134].
5. The Role of Plant DNA Methylation in Abiotic Stress
5.1. High Temperature Stress
High temperatures and drought are critical climate stressors that significantly impair global crop yields. Projections suggest that global temperatures may rise by approximately 4–5 °C by the end of the 21st century [135]. Prolonged heat stress coupled with insufficient rainfall often precipitates drought conditions. The synergistic effects of heat and drought typically result in more severe damage to crop production than either stressor alone [136]. Both environmental stresses have been shown to induce alterations in DNA methylation, including hypermethylation and demethylation, in both coding and intergenic regions. In response to heat stress, the heat-resistant rapeseed (Brassica napus) genotype Huyou2 exhibited predominantly hypomethylated DNA, in contrast to the more heat-sensitive genotype Fengyou1 [137]. Additionally, in the drought-tolerant crop millet (Setaria italica), methylation of CHG and CHH motifs in the upstream and coding regions of heat shock protein (HSP) genes was enhanced under environmental stress conditions. In heat-tolerant varieties, heat stress-induced hypomethylation of many SiHSP genes in CHG and CHH motifs, which in turn enhanced their expression [138]. A MethylRAD library constructed from heat-treated maize inbred line B73 identified 325 differentially methylated genes (DMGs) [139]. Notably, DMGs associated with RNA splicing exhibited low methylation levels and were highly expressed, suggesting that spliceosome activity is augmented under heat stress. A nucleoproteome analysis of Pinus radiata subjected to high-temperature stress revealed a decrease in the abundance of S-adenosylmethionine (SAM) synthase and S-adenosyl-L-homocysteine hydrolase (SAHH), resulting in altered DNA methylation profiles [140]. SAM is a crucial cofactor and methyl group donor, while SAHH is essential for the regeneration of SAM during methylation-mediated gene silencing. Moreover, it was observed that DNA methylation regulates the activity of plant transit factors under heat stress [140].
5.2. Drought Stress
Drought stress typically induces dynamic alterations in DNA methylation across the plant genome, which are negatively correlated with gene expression [141]. These modifications encompass methylation changes in promoter regions, genomic sequences, and TEs regions. For instance, research on Polygonum persicaria has demonstrated that drought-treated parents enhance the ability of their offspring to develop deeper and faster-growing roots in arid environments by altering their DNA methylation patterns. This cross-generational stress memory suggests that DNA methylation serves as a molecular link between genetic and environmental signals [142]. Moreover, plant hormone regulation plays a significant role in modulating DNA methylation during drought stress. Studies on poplar have shown that hormone-responsive genes exhibit hypomethylation during stress recovery, which facilitates gene reactivation. Additionally, drought-induced abscisic acid (ABA) signaling pathways are closely associated with DNA methylation modifications [143]. In maize, drought-induced differential methylation was detected via AMP-PCR, with changes predominantly occurring in regions within genes responsible for plant survival functions, such as protein synthesis, DNA repair, and amino acid metabolism [143]. These findings suggest that hormone signaling enhances stress tolerance by regulating dynamic DNA methylation changes. The cumulative impact of multiple drought events reflects stress memory in plants, which is intricately linked to DNA methylation. In alfalfa, a two-stage drought stress regime significantly enhanced drought tolerance compared to a single drought event, a phenomenon associated with methylation remodeling of relevant genes [144]. Stress memory enables plants to respond more efficiently to repeated stress, indicating that DNA methylation underpins long-term adaptation to abiotic stress [144].
5.3. Salt Stress
Salt stress can cause changes in DNA methylation levels across the plant genome. This dynamic change usually involves cytosine methylation in all motifs (CG, CHG, and CHH). Studies have shown that in the initial stage of salt stress, demethylation occurs in the promoter region of many genes, which promotes the expression of related stress-resistance genes [145]. For example, Arabidopsis thaliana demethylates the promoter region of some salt-resistant genes (such as SOS1) under salt stress, thereby enhancing their expression levels and improving the plant’s ion elimination ability. On the other hand, the level of methylation in genomic regions is often increased, which may be related to gene silencing and maintenance of genomic stability [145]. In rice, genome-wide methylation analysis showed that salt stress induced differential methylation of a large number of genes involved in osmoregulation, antioxidant response, and signal transduction [146]. It has also been found that the demethylation of transposition factors induced by salt stress may activate the mobility of these elements, thereby increasing genomic instability [147]. However, through the RdDM pathway, these transposable factors are usually re-silenced to maintain genome integrity. Plant hormones and signaling molecules are closely related to DNA methylation regulation in response to salt stress. Abscisic acid (ABA) is an important signaling molecule in salt stress, and several genes involved in its signal transduction pathway (such as NCED3 and PYR1) exhibit significant changes in methylation levels under stress conditions [148]. For example, demethylation of genes associated with ABA synthesis under salt stress can promote ABA synthesis, thereby enhancing stomatal closure and antioxidant capacity [149]. In addition, the ABA signaling pathway also participates in DNA methylation regulation by regulating transcription factors (such as ABI5), forming a positive feedback mechanism. Studies have also shown that there is an interaction between methylation regulation and ion homeostasis maintenance [145,149]. For example, some genes in the SOS signaling pathway are regulated by methylation states that help maintain intracellular Na/K ion balance under salt stress.
The epigenetic memory function of plants enables them to show higher resilience when subjected to repeated salt stress. Studies have shown that DNA methylation is one of the important molecular bases of salt stress memory. For example, in Arabidopsis thaliana, gene demethylation induced by initial salt stress can be partially left over after stress relief and rapidly activate related gene expression during subsequent stress [142,144,145]. Cross-generational genetic studies have shown that salt stress can transmit salt-resistant characteristics to the next generation by affecting the methylation patterns of gametes and embryos [146]. For example, in barley and rice, the offspring of salt-stressed parents showed greater salt tolerance, which was closely associated with changes in methylation patterns of some salt-resistant genes. The function of many salt-resistant genes is directly regulated by DNA methylation [149]. For example, the level of methylation in the promoter region of the OsDREB2A gene, which is involved in osmoregulation in rice, is reduced under salt stress, promoting gene expression. This demethylation may be achieved by activating binding sites of transcription factors such as MYB or bZIP. In addition, genes encoding antioxidant enzymes (such as Superoxide dismutase, SOD, and Catalase, CAT) in plants are also regulated by DNA methylation, and their expression is enhanced by demethylation under salt stress, thus alleviating stress-induced oxidative damage [150]. In addition, the methylation regulation of transposable factors and their adjacent genes also plays an important role in salinity resistance. The increased activity of transposable factors under salt stress may provide the potential for resistance to mutation by influencing the expression of neighboring genes. However, this instability is usually inhibited by the RdDM pathway to avoid unwanted genomic disturbances [151].
6. The Role of Plant DNA Methylation in Biological Stress
6.1. Dynamic Changes of DNA Methylation and Response to Biotic Stress
In plants, DNA methylation patterns within the genome exhibit dynamic changes in response to biotic stress. The methylation levels at CG, CHG, and CHH sites can be rapidly reconfigured to meet stress-related demands. For instance, infection of Arabidopsis thaliana with Plasmodiophora brassicae induces genome-wide DNA demethylation, thereby promoting the expression of genes involved in resistance responses [152]. Specifically, demethylation of promoter regions of genes associated with the biosynthesis and signaling of defense hormones, such as salicylic acid (SA), enhances plant immune responses. Similarly, in rice (Oryza sativa) infected by Xanthomonas campestris, promoter demethylation of numerous defense-related genes facilitates the expression of disease-resistance proteins. Insect herbivory also drives the reprogramming of DNA methylation patterns [153]. For example, in tomato plants (Solanum lycopersicum) subjected to bollworm (Helicoverpa armigera) feeding, demethylation of promoters of insect-responsive genes, including those encoding protease inhibitors, enhances the synthesis of insect-resistance proteins, thereby strengthening the plant’s defense against herbivory [154,155,156,157,158].
Recent advancements in DNA methylation analysis and extensive research on Arabidopsis thaliana mutants deficient in various stages of DNA methylation, maintenance, and demethylation have significantly expanded our understanding of how these processes regulate plant responses to fungal infections [159] (Table 1). For example, the triple DNA demethylase mutant rdd (ros1dml2dml3) in A. thaliana, which exhibits high levels of DNA methylation, shows significantly increased susceptibility to Fusarium oxysporum [160]. In this mutant, many downregulated genes are involved in defense-related functions, and their promoter regions are enriched in transposable elements (TEs). Effective resistance to F. oxysporum relies on DNA demethylase activity, which targets TE sequences within defense gene promoters, thereby boosting gene expression.

Table 1.
The defense responses of the Arabidopsis mutants with hypomethylated and hypermethylated DNA to different bacterial, viral, and fungal pathogens.
Interestingly, studies of Arabidopsis mutants impaired in various stages of the RNA-directed DNA methylation (RdDM) pathway have shown contrasting effects on defense responses against biotrophic and necrotrophic pathogens. In general, RdDM-deficient mutants are more sensitive to necrotrophic pathogens but exhibit enhanced resistance to biotrophic ones. For instance, hypomethylated Arabidopsis mutants demonstrate increased susceptibility to the necrotrophic pathogen Botrytis cinerea and the bacterial pathogen Plectosphaerella cucumerina [161]. Mutants like rdr6 and dcl2/3/4 are particularly vulnerable to B. cinerea due to impaired small interfering RNA (siRNA) synthesis, which is essential for silencing fungal virulence genes [146]. In contrast, knockout of RdDM components such as DRM2 homologs in the wheat ancestor Aegilops tauschii leads to enhanced resistance against wheat powdery mildew caused by the biotrophic pathogen Blumeria graminis f. sp. tritici. Furthermore, the Arabidopsis trimutant ddc (drm1drm2cmt3), which lacks proper methylation maintenance, exhibits extreme sensitivity to the necrotrophic fungus Alternaria brassicicola [157,158,159].
6.2. DNA Methylation Regulation of Disease Resistance Genes
Resistance (R) genes are central to plant defense against pathogen invasion. DNA methylation plays a pivotal role in modulating plant resistance by regulating the expression of these genes. Under normal growth conditions, most R genes remain in a methylation-mediated silenced state, thereby conserving energy and minimizing the risk of autoimmune responses caused by their overexpression. For instance, in Arabidopsis thaliana, hypermethylation of the SUPPRESSOR OF npr1-1 CONSTITUTIVE 1 (SNC1) gene maintains its transcriptional silence [155]. However, during pathogen infection, DNA demethylation reactivates SNC1, bolstering the immune response. In addition to R gene regulation, TEs in the vicinity of these genes are often controlled by DNA methylation. Pathogen infection can suppress TE activity via the RdDM pathway, thereby preventing their insertion into R genes and the subsequent disruption of gene function. For example, in rice (Oryza sativa), many TEs located near R genes exhibit significantly increased methylation levels following infection with Magnaporthe oryzae (the causal agent of rice blast disease), contributing to genomic stability [152,155].
6.3. Synergistic Effect of DNA Methylation with Plant Hormone Signaling
Plant hormones play pivotal roles in mediating plant responses to biotic stress, with DNA methylation forming an intricate network that modulates plant defense mechanisms by regulating the expression of genes involved in hormone biosynthesis and signal transduction. Salicylic acid (SA), a crucial signaling molecule in plant immunity, undergoes significant alterations in the methylation status of genes involved in its biosynthesis and signaling in response to biotic stress. For instance, the demethylation of ICS1 (a gene encoding an SA biosynthetic enzyme) and NPR1 (a central regulator of SA signaling) in Arabidopsis thaliana facilitates SA-mediated systemic acquired resistance (SAR) [154]. Jasmonic acid (JA), predominantly associated with defense against herbivores and necrotrophic pathogens, is similarly regulated. Demethylation of key genes in the JA signaling pathway, such as COI1 and MYC2, has been shown to activate these pathways following the herbivore attack, thereby bolstering plant defenses. Ethylene also plays a critical role in coordinating broad-spectrum plant defenses against biotic stress. In rice (Oryza sativa) challenged by blast fungus, significant reductions in methylation levels within the promoter regions of ethylene signaling genes, such as ERF1, enhance the expression of defense-related genes. Conversely, the role of abscisic acid (ABA) in biotic stress responses appears more nuanced, with several genes in the ABA signaling pathway undergoing adaptive regulation via DNA methylation [150,152,157].
7. Prospects of DNA Methylation in Crop Breeding
Natural variations in DNA methylation are prevalent in plants and present significant opportunities for crop improvement. With advancements in gene-editing technologies, these opportunities are increasingly becoming achievable. Numerous instances of natural epigenetic variation have been identified in plants. One well-known example is the radially symmetrical flower mutant of Linaria vulgaris, first described by Linnaeus, which is caused by a hypermethylated allele of the transcription factor CYCLOIDEA [158]. In Arabidopsis, studies on epigenetic variation have revealed essential mechanisms involved in regulating DNA methylation [159]. Beyond model organisms, epialleles in various crops have been shown to control important agronomic traits, such as plant architecture in rice [160], fruit ripening in tomatoes [161], photoperiodic sensitivity in cotton [162], and grain quality in maize [163]. Moreover, DNA methylation plays a role in hybridization dynamics in Arabidopsis [164,165].
Beyond its impact on qualitative traits, DNA methylation variation is also thought to play a significant role in shaping quantitative traits. Epigenetic variations that occur naturally are believed to contribute to the phenomenon of “missing heritability” observed in both animal and plant studies [166]. In Arabidopsis thaliana, variations in DNA methylation are closely associated with environmental factors, particularly in genes related to immunity [167]. Furthermore, scans for positive selection have revealed methylated CG sites (mCGs) in the promoter regions of genes involved in the production of specific metabolites, suggesting that CG methylation may play a role in the adaptive evolution of Arabidopsis [168].
Comparative methylation analyses in crops like maize [169,170], soybean [171], rice [172], and wheat [173] have identified a broad array of differentially methylated regions (DMRs) across diverse population varieties, with some regions linked to domestication or environmental adaptation. Genome-wide association studies (GWAS) of DNA methylation profiles in 263 maize inbred lines revealed that over 60% of these DMRs did not overlap with single nucleotide polymorphisms (SNPs). These studies also found DMRs associated with 156 metabolic traits, suggesting that variations in DNA methylation, rather than traditional genetic differences, may play a crucial role in regulating these traits [168]. While genetic variation can influence DNA methylation patterns [174,175], current research indicates that the impact of DNA methylation on phenotypic variation may be underappreciated and requires further exploration. In tomato and maize, hybridization-induced epialleles behave similarly to somatic mutations, with changes that can persist through several generations of backcrossing and self-pollination [156,176,177]. This suggests that epigenetic modifications resulting from hybridization could contribute to phenotypic diversity in traditional breeding programs. Furthermore, epigenetic recombinant inbred lines (epiRILs) provide additional support for the role of epigenetic variation in crop improvement. In Arabidopsis, epiRILs exhibit pleiotropic phenotypic variation, some of which is comparable to the phenotypic differences observed in recombinant inbred lines (RILs) [178,179]. Likewise, artificial selection of traits such as energy efficiency in genetically uniform populations of rapeseed and rice has produced epigenetic lines that significantly outperform conventional varieties in the field, highlighting the practical value of epigenetic selection for enhancing crop yields [180,181,182].
The introduction of epigenetic variation into traditional breeding programs is often constrained by extended breeding cycles and the phenomenon of epigenetic drag, wherein undesirable epigenetic mutations are inadvertently propagated. When specific target genes are identified, epigenome editing emerges as a precise and controllable strategy for enhancing agronomic traits. Numerous epigenome editing tools have been developed in plants, facilitating the study of DNA methylation regulation [183]. These tools generally comprise a targeting module that directs proteins to specific genomic loci, a modification module that alters epigenetic states, and, optionally, an auxiliary module to modulate the activity of the modification process.
Among the various epigenetic editing systems, CRISPR-dCas9 has gained significant attention due to its ease of design and construction, particularly when compared to traditional systems such as TALE and artificial zinc finger proteins [165]. In addition to the standard use of dCas9, the nuclease activity of Cas9 can be inhibited by shortening the spacer region of guide RNAs [184]. The modifier domains, responsible for inducing epigenetic changes, are highly versatile. These range from the catalytic domain of the DRM gene to prokaryotic DNA methyltransferase MQ1, mammalian DNA demethylase TET1, and components of the RNA-directed DNA methylation (RdDM) pathway, all of which have been successfully applied for DNA methylation editing in Arabidopsis [62,185,186,187]. Advanced systems like SunTag and MS2 can be integrated into the targeting module to increase the local concentration of effector domains. Although still in the early stages of development, precise epigenome editing shows great potential for efficiently modulating the epigenetic states of specific genes in crops. However, realizing its full potential in breeding programs requires addressing several challenges: minimizing off-target effects, improving the cross-generational stability of edits, and accurately predicting optimal guide RNA target sites. In mammalian cells lacking DNMT activity, the dCas9-Dnmt3a modifier exhibits widespread activity, although ectopic methylation primarily occurs at sites that were already methylated, with minimal impact on gene expression [188]. Off-target effects can be reduced by lowering overall effector levels while enhancing their local concentration [189]. These findings highlight the need for more precise regulation of DNA methyltransferase or demethylase activity, as compared to the endonuclease activity of Cas9. Moreover, targeted de novo methylation of thousands of promoters in human cells has shown environmentally contingent stability and transcriptional responses [190]. Given that chromatin state maintenance relies on self-reinforcing feedback loops involving multiple components, disrupting these loops and creating new ones may necessitate simultaneous editing of several epigenetic marks [191]. Achieving precise transcriptional regulation of target loci also requires a thorough understanding of their cis-regulatory elements and trans-regulatory factors.
8. Conclusions and Prospect
The escalating frequency and severity of extreme weather events driven by global climate change pose mounting challenges to agricultural production. Stresses such as elevated temperatures, drought, salinization, and pest infestations critically undermine crop yield and quality, highlighting the pressing need to develop crop varieties with enhanced adaptability and robust stress resistance. Recent advances have unveiled the pivotal role of DNA methylation in mediating plant responses to environmental stresses. Its inherent plasticity and heritable characteristics offer a promising theoretical foundation and a valuable tool for advancing crop breeding strategies.
DNA methylation plays a pivotal role in regulating plant responses to environmental stress and disease resistance by modulating gene expression. Leveraging this mechanism, modern molecular breeding techniques can be employed to enhance crop resilience through targeted adjustments of methylation patterns in specific genes. Traditional agriculture heavily relies on fertilizers and pesticides to sustain high yields, a practice that significantly pollutes the environment and poses risks to food safety. Investigations into the role of DNA methylation in nutrient uptake and stress responses have revealed its potential to enhance nutrient use efficiency in crops, thereby reducing reliance on chemical inputs. For instance, under nitrogen-deficient conditions, certain plants improve nitrogen absorption by modifying the methylation status of key genes, offering a novel strategy for breeding nutrient-efficient crop varieties. Moreover, regulating DNA methylation can bolster the natural disease resistance of crops, thereby decreasing pesticide requirements. This dual benefit—sustaining high yields while mitigating environmental harm—positions DNA methylation as a cornerstone in the development of sustainable green agriculture. Recent advancements in genomics, transcriptomics, and epigenetics have further enriched our understanding of crop trait improvement. By integrating multi-omics datasets, researchers can unravel the complex roles of DNA methylation in gene regulatory networks with greater precision. For example, analyses of stress-resistance traits frequently link dynamic methylation changes to the upregulation or downregulation of specific genes. Multi-omics approaches thus enable the identification of key regulatory genes and pathways, providing a robust scientific foundation for precision breeding.
The dynamic and multifaceted nature of DNA methylation offers immense potential for advancing crop breeding, yet it also presents significant challenges. Environmental factors influence DNA methylation patterns, raising critical questions about their stability and heritability that warrant further investigation. Moreover, the regulatory mechanisms of DNA methylation vary across species and genomic contexts, necessitating careful consideration when developing universal breeding strategies. The precision and safety of epigenetic editing technologies likewise require further refinement to enable their broad application in agriculture. Looking ahead, deeper insights into the role of DNA methylation in plant growth, development, and stress responses, combined with cutting-edge approaches such as genome and epigenetic editing, could revolutionize the breeding of high-yield, high-quality, and stress-resilient crops. International collaboration and interdisciplinary integration will be crucial for building comprehensive epigenetic data repositories spanning diverse crop species, thereby providing robust datasets and technical resources to advance breeding efforts.
In conclusion, harnessing DNA methylation in crop breeding has the potential to address pressing challenges in global agriculture while fostering the development of efficient, sustainable, and environmentally friendly farming practices. This field of research is poised to drive a transformative revolution in crop breeding, enhancing global food security and agricultural productivity.
Author Contributions
Conceptualization, S.Q. and W.S.; methodology, S.Q. and W.S.; software, S.Y.; validation, A.L.; formal analysis, F.W. and A.L.; data curation, W.T. and F.W.; writing—original draft preparation, S.Y. and W.H.; writing—review, and editing, S.Y., S.Q., and W.H; project administration, S.Y. and W.T.; funding acquisition, S.Y. and W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the earmarked fund for the China Agriculture Research System, funded by the Ministry of Finance of China and the Ministry of Agriculture and Rural Affairs of China, grant number CARS-10-Sweetpotato. This research was also supported by the Sichuan Science and Technology Program, funded by the Sichuan Science and Technology Department, grant numbers 2021YFYZ0019, 2021YFYZ0020, and 2024YFHZ0255.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.R.; Jacobsen, S.E. Epigenetic inheritance in plants. Nature 2007, 447, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Maeji, H.; Nishimura, T. Epigenetic Mechanisms in Plants. Adv. Bot. Res. 2018, 88, 21–47. [Google Scholar]
- Hari Sundar, G.V.; Swetha, C.; Basu, D.; Pachamuthu, K.; Raju, S.; Chakraborty, T.; Mosher, R.A.; Shivaprasad, P.V. Plant polymerase IV sensitizes chromatin through histone modifications to preclude spread of silencing into protein-coding domains. Genome Res. 2023, 33, 715–728. [Google Scholar] [CrossRef]
- Fan, X.; Peng, L.; Zhang, Y. Plant DNA Methylation Responds to Nutrient Stress. Genes 2022, 13, 992. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Zhou, K.; Wu, T.; Zhao, B.S.; Sun, M.; Chen, Z.; Deng, X.; Xiao, G.; Auer, F.; et al. Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally. Nature 2019, 567, 414–419. [Google Scholar] [CrossRef]
- Lee, C.H.; Carroll, B.J. Evolution and diversification of small RNA pathways in flowering plants. Plant Cell Physiol. 2018, 59, 2169–2187. [Google Scholar] [CrossRef]
- Andrews, S.; Krueger, C.; Mellado-Lopez, M.; Hemberger, M.; Dean, W.; Perez-Garcia, V.; Hanna, C.W. Mechanisms and function of de novo DNA methylation in placental development reveals an essential role for DNMT3B. Nat. Commun. 2023, 14, 371. [Google Scholar] [CrossRef]
- Yaari, R.; Katz, A.; Domb, K.; Harris, K.D.; Zemach, A.; Ohad, N. Publisher Correction: RdDM-independent de novo and heterochromatin DNA methylation by plant CMT and DNMT3 orthologs. Nat. Commun. 2019, 6, 2552. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, L.; Zhang, S.; Yao, S.; Ong, J.; Li, Y.; Wu, H.; Du, P. A plant immune protein enables broad antitumor response by rescuing microRNA deficiency. Cell 2022, 185, 1888–1904.e24. [Google Scholar] [CrossRef]
- Liu, Y.; Siejka-Zielińska, P.; Velikova, G.; Bi, Y.; Yuan, F.; Tomkova, M.; Bai, C.; Chen, L.; Schuster-Böckler, B.; Song, C.X. Bisulfite-free direct detection of 5-methylcytosine and 5-hydroxymethylcytosine at base resolution. Nat. Biotechnol. 2019, 37, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Vidalis, A.; Živković, D.; Wardenaar, R.; Roquis, D.; Tellier, A.; Johannes, F. Methylome evolution in plants. Genome Biol. 2016, 17, 264. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Arai, Y.; Umehara, H.; Masuhara, M.; Kimura, T.; Taniguchi, H.; Sekimoto, T.; Ikawa, M.; Yoneda, Y.; Okabe, M.; et al. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat. Cell Biol. 2007, 9, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, W.; Callen, E.; Pavani, R.; Zolnerowich, N.; Kodali, S.; Zong, D.; Wong, N.; Noriega, S.; Nathan, W.J.; et al. Active DNA demethylation promotes cell fate specification and the DNA damage response. Science 2022, 378, 983–989. [Google Scholar] [CrossRef]
- Lucibelli, F.; Valoroso, M.C.; Aceto, S. Plant DNA Methylation: An Epigenetic Mark in Development, Environmental Interactions, and Evolution. Int. J. Mol. Sci. 2022, 23, 8299. [Google Scholar] [CrossRef]
- He, X.J.; Chen, T.; Zhu, J.K. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef]
- Huang, S.C.; Ecker, J.R. Piecing together cis-regulatory networks: Insights from epigenomics studies in plants. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1411. [Google Scholar] [CrossRef]
- Stroud, H.; Greenberg, M.V.; Feng, S.; Bernatavichute, Y.V.; Jacobsen, S.E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 2013, 152, 352–364. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, S.; Ma, H.; Luo, S.; He, H.; Zhang, Y. Hyper non-CG methylation of expanded plant disease resistance NLR genes. Plant Cell Rep. 2023, 42, 1251–1254. [Google Scholar] [CrossRef]
- Arora, H.; Singh, R.K.; Sharma, S.; Sharma, N.; Panchal, A.; Das, T.; Prasad, A.; Prasad, M. DNA methylation dynamics in response to abiotic and pathogen stress in plants. Plant Cell Rep. 2022, 41, 1931–1944. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.K. RNA-directed DNA methylation. Curr. Opin. Plant Biol. 2011, 14, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Pikaard, C.S.; Haag, J.R.; Pontes, O.M.; Blevins, T.; Cocklin, R. A transcription fork model for Pol IV and Pol V-dependent RNA-directed DNA methylation. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hoareau, M.; Rincheval-Arnold, A.; Gaumer, S.; Guénal, I. DREAM a little dREAM of DRM: Model organisms and conservation of DREAM-like complexes: Model organisms uncover the mechanisms of DREAM-mediated transcription regulation. BioEssays 2024, 46, e2300125. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, H.-L.; Daxinger, L.; Pontes, O.; He, X.; Qian, W.; Lin, H.; Xie, M.; Lorkovic, Z.J.; Zhang, S.; et al. An RNA polymerase II- and AGO4-associated protein acts in RNA-directed DNA methylation. Nature 2010, 465, 106–109. [Google Scholar] [CrossRef]
- Vaucheret, H.; Voinnet, O. The plant siRNA landscape. Plant Cell 2023, 36, 246–275. [Google Scholar] [CrossRef]
- Creasey, K.M.; Zhai, J.; Borges, F.; Van Ex, F.; Regulski, M.; Meyers, B.C.; Martienssen, R.A. miRNAs trigger widespread epigenetically activated siRNAs from transposons in Arabidopsis. Nature 2014, 508, 411–415. [Google Scholar] [CrossRef]
- Cuerda-Gil, D.; Slotkin, R.K. Non-canonical RNA-directed DNA methylation. Nat. Plants 2016, 2, 16163. [Google Scholar] [CrossRef]
- Dunker, F.; Lederer, B.; Weiberg, A. Plant ARGONAUTE Protein Immunopurification for Pathogen Cross Kingdom Small RNA Analysis. Bio-Protoc. 2021, 11, e3911. [Google Scholar]
- Bies-Etheve, N.; Pontier, D.; Lahmy, S.; Picart, C.; Vega, D.; Cooke, R.; Lagrange, T. RNA-directed DNA methylation requires an AGO4-interacting member of the SPT5 elongation factor family. EMBO Rep. 2009, 10, 649–654. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Qian, W.; Duan, C.G.; Wang, B.; Zhang, H.; Wang, P.; Zhu, X.; Lang, Z.; Yang, Y.; et al. An Rrp6-like protein positively regulates noncoding RNA levels and DNA methylation in Arabidopsis. Mol. Cell 2014, 54, 418–430. [Google Scholar] [CrossRef]
- Ausin, I.; Mockler, T.C.; Chory, J.; Jacobsen, S.E. IDN1 and IDN2 are required for de novo DNA methylation in Arabidopsis thaliana. Nat. Struct. Mol. Biol. 2009, 16, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA Methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef] [PubMed]
- Ausin, I.; Greenberg, M.V.C.; Simanshu, D.K.; Hale, C.J.; Vashisht, A.A.; Simon, S.A.; Lee, T.-F.; Feng, S.; Española, S.D.; Meyers, B.C.; et al. INVOLVED IN DE NOVO 2-containing complex involved in RNA-directed DNA methylation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 8374–8381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ou, X.; Han, D.; He, Z.; Liu, S.; Mao, N.; Zhang, Z.; Peng, C.L.; Lai, J.; Yang, C. A diRNA-protein scaffold module mediates SMC5/6 recruitment in plant DNA repair. Plant Cell 2022, 34, 3899–3914. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.-Y.; Zeng, L.; Tanaka, K.; Zhang, C.-J.; Ma, J.; Bai, G.; Wang, P.; Zhang, S.-W.; Liu, Z.-W.; et al. DTF1 is a core component of RNA-directed DNA methylation may assist in the recruitment of Pol IV. Proc. Natl. Acad. Sci. USA 2013, 110, 8290–8295. [Google Scholar] [CrossRef]
- Fang, C.; Huang, K.; Wu, X.; Zhang, H.; Gu, Z.; Wang, J.; Zhang, Y. Transcription elongation of the plant RNA polymerase IV is prone to backtracking. Sci Adv. 2024, 10, eadq3087. [Google Scholar] [CrossRef]
- Wang, Y.; Le, B.H.; Wang, J.; You, C.; Zhao, Y.; Galli, M.; Xu, Y.; Gallavotti, A.; Eulgem, T.; Mo, B.; et al. ZMP recruits and excludes Pol IV-mediated DNA methylation in a site-specific manner. Sci. Adv. 2022, 8, eadc9454. [Google Scholar] [CrossRef]
- Zhou, M.; Coruh, C.; Xu, G.; Martins, L.M.; Bourbousse, C.; Lambolez, A.; Law, J.A. The CLASSY family controls tissue-specific DNA methylation patterns in Arabidopsis. Nat. Commun. 2022, 13, 244. [Google Scholar] [CrossRef]
- Zhou, M.; Palanca, A.M.S.; Law, J.A. Locus-specific control of the de novo DNA methylation pathway in Arabidopsis by the CLASSY family. Nat. Genet. 2018, 50, 865–873. [Google Scholar] [CrossRef]
- Kanno, T.; Mette, M.F.; Kreil, D.P.; Aufsatz, W.; Matzke, M.; Matzke, A.J. Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr. Biol. 2004, 14, 801–805. [Google Scholar] [CrossRef]
- Kanno, T.; Bucher, E.; Daxinger, L.; Huettel, B.; Böhmdorfer, G.; Gregor, W.; Kreil, D.P.; Matzke, M.; Matzke, A.J.M. A structural-maintenance-of-chromosomes hinge domain-containing protein is required for RNA-directed DNA methylation. Nat. Genet. 2008, 40, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Du, X.; Hu, H.; Li, S.; Cao, X.; Jacobsen, S.E.; Du, J. Structure and mechanism of the plant RNA polymerase V. Science 2023, 379, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Hale, C.J.; Law, J.A.; Johnson, L.M.; Feng, S.; Tu, A.; Jacobsen, S.E. DDR complex facilitates global association of RNA polymerase V to promoters and evolutionarily young transposons. Nat. Struct. Mol. Biol. 2012, 19, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.W.; Shao, C.R.; Zhang, C.J.; Zhou, J.X.; Zhang, S.W.; Li, L.; Chen, S.; Huang, H.-W.; Cai, T.; He, X.-J.; et al. The SET domain proteins SUVH2 and SUVH9 are required for Pol V occupancy at RNA-directed DNA methylation loci. PLoS Genet. 2014, 10, e1003948. [Google Scholar] [CrossRef]
- Johnson, L.M.; Du, J.; Hale, C.J.; Bischof, S.; Feng, S.; Chodavarapu, R.K.; Zhong, X.; Marson, G.; Pellegrini, M.; Segal, D.J.; et al. SRA- and SET-domain-containing proteins link RNA polymerase V occupancy to DNA methylation. Nature 2014, 507, 124–128. [Google Scholar] [CrossRef]
- Wierzbicki, A.T.; Haag, J.R.; Pikaard, C.S. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 2008, 135, 635–648. [Google Scholar] [CrossRef]
- Xie, G.; Du, X.; Hu, H.; Du, J. Molecular mechanisms of the RNA polymerases in plant RNA-directed DNA methylation. Trends Biochem. Sci. 2024, 49, 247–256. [Google Scholar] [CrossRef]
- Li, S.; Vandivier, L.E.; Tu, B.; Gao, L.; Won, S.Y.; Li, S.; Zheng, B.; Gregory, B.D.; Chen, X. Detection of Pol IV/RDR2-dependent transcripts at the genomic scale in Arabidopsis reveals features and regulation of siRNA biogenesis. Genome Res. 2015, 25, 235–245. [Google Scholar] [CrossRef]
- Zhai, J.; Bischof, S.; Wang, H.; Feng, S.; Lee, T.-F.; Teng, C.; Chen, X.; Park, S.Y.; Liu, L.; Gallego-Bartolome, J.; et al. A one precursor one siRNA model for Pol IV-dependent siRNA biogenesis. Cell 2015, 163, 445–455. [Google Scholar] [CrossRef]
- Yang, D.L.; Zhang, G.; Tang, K.; Li, J.; Yang, L.; Huang, H.; Zhang, H.; Zhu, J.K. Dicer-independent RNA-directed DNA methylation in Arabidopsis. Cell Res. 2016, 26, 1264. [Google Scholar] [CrossRef]
- Ye, R.; Chen, Z.; Lian, B.; Rowley, M.J.; Xia, N.; Chai, J.; Li, Y.; He, X.-J.; Wierzbicki, A.T.; Qi, Y. A Dicer-independent route for biogenesis of siRNAs that direct DNA methylation in Arabidopsis. Mol. Cell 2016, 61, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, Z.; Li, S.; Yu, B.; Liu, J.Y.; Chen, X. Intergenic transcription by RNA polymerase II coordinates Pol IV and Pol V in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes Dev. 2009, 23, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shoji, K.; Naganuma, M.; Tomari, Y.; Iwakawa, H.O. The mechanisms of siRNA selection by plant Argonaute proteins triggering DNA methylation. Nucleic Acids Res. 2022, 50, 12997–13010. [Google Scholar] [CrossRef] [PubMed]
- McCue, A.D.; Panda, K.; Nuthikattu, S.; Choudury, S.G.; Thomas, E.N.; Slotkin, R.K. ARGONAUTE 6 bridges transposable element mRNA-derived siRNAs to the establishment of DNA methylation. EMBO J. 2015, 34, 20–35. [Google Scholar] [CrossRef]
- Marí-Ordóñez, A.; Marchais, A.; Etcheverry, M.; Martin, A.; Colot, V.; Voinnet, O. Reconstructing de novo silencing of an active plant retrotransposon. Nat. Genet. 2013, 45, 1029–1039. [Google Scholar] [CrossRef]
- Huang, C.F.; Miki, D.; Tang, K.; Zhou, H.R.; Zheng, Z.; Chen, W.; Ma, Z.-Y.; Yang, L.; Zhang, H.; Liu, R.; et al. A pre-mRNA-splicing factor is required for RNA-directed DNA methylation in Arabidopsis. PLoS Genet. 2013, 9, e1003779. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519–532. [Google Scholar] [CrossRef]
- Riggs, A.D. X inactivation, differentiation, and DNA methylation. Cytogenet. Cell Genet. 1975, 14, 9–25. [Google Scholar] [CrossRef]
- Holliday, R.; Pugh, J.E. DNA modification mechanisms and gene activity during development. Science 1975, 187, 226–232. [Google Scholar] [CrossRef]
- Bashtrykov, P.; Jankevicius, G.; Smarandache, A.; Jurkowska, R.Z.; Ragozin, S.; Jeltsch, A. Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem. Biol. 2012, 19, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Teplova, M.; Ishibe-Murakami, S.; Patel, D.J. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science 2012, 335, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Klingel, V.; Radde, N.E.; Bashtrykov, P.; Jeltsch, A. On the accuracy of the epigenetic copy machine: Comprehensive specificity analysis of the DNMT1 DNA methyltransferase. Nucleic Acids Res. 2023, 51, 6622–6633. [Google Scholar] [CrossRef] [PubMed]
- Bostick, M.; Kim, J.K.; Estève, P.O.; Clark, A.; Pradhan, S.; Jacobsen, S.E. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science 2007, 317, 1760–1764. [Google Scholar] [CrossRef]
- Sharif, J.; Muto, M.; Takebayashi, S.-I.; Suetake, I.; Iwamatsu, A.; Endo, T.A.; Shinga, J.; Mizutani-Koseki, Y.; Toyoda, T.; Okamura, K.; et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 2007, 450, 908–912. [Google Scholar] [CrossRef]
- Woo, H.R.; Dittmer, T.A.; Richards, E.J. Three SRA-domain methylcytosine-binding proteins cooperate to maintain global CpG methylation and epigenetic silencing in Arabidopsis. PLoS Genet. 2008, 4, e1000156. [Google Scholar] [CrossRef]
- Petryk, N.; Bultmann, S.; Bartke, T.; Defossez, P.A. Staying true to yourself: Mechanisms of DNA methylation maintenance in mammals. Nucleic Acids Res. 2021, 49, 3020–3032. [Google Scholar] [CrossRef]
- Rocha, P.S.; Sheikh, M.; Melchiorre, R.; Fagard, M.; Boutet, S.; Loach, R.; Moffatt, B.; Wagner, C.; Vaucheret, H.; Furner, I. The Arabidopsis HOMOLOGY-DEPENDENT GENE SILENCING1 gene codes for an S-adenosyl-L-homocysteine hydrolase required for DNA methylation-dependent gene silencing. Plant Cell 2005, 17, 404–417. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Miki, D.; Cutler, S.; La, H.; Hou, Y.-J.; Oh, J.; Zhu, J.-K. Sulfamethazine suppresses epigenetic silencing in Arabidopsis by impairing folate synthesis. Plant Cell 2012, 24, 1230–1241. [Google Scholar] [CrossRef]
- Zhou, H.R.; Zhang, F.F.; Ma, Z.Y.; Huang, H.W.; Jiang, L.; Cai, T.; Zhu, J.K.; Zhang, C.; He, X.J. Folate polyglutamylation is involved in chromatin silencing by maintaining global DNA methylation and histone H3K9 dimethylation in Arabidopsis. Plant Cell 2013, 25, 2545–2559. [Google Scholar] [CrossRef]
- Gong, Z.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T.; David, L.; Zhu, J.K. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 2002, 111, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Gehring, M.; Huh, J.H.; Hsieh, T.F.; Penterman, J.; Choi, Y.; Harada, J.J.; Goldberg, R.B.; Fischer, R.L. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 2006, 124, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Agius, F.; Kapoor, A.; Zhu, J.K. Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc. Natl. Acad. Sci. USA 2006, 103, 11796–11801. [Google Scholar] [CrossRef]
- Morales-Ruiz, T.; Ortega-Galisteo, A.P.; Ponferrada-Marín, M.I.; Martínez-Macías, M.I.; Ariza, R.R.; Roldán-Arjona, T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl. Acad. Sci. USA 2006, 103, 6853–6858. [Google Scholar] [CrossRef]
- Penterman, J.; Zilberman, D.; Huh, J.H.; Ballinger, T.; Henikoff, S.; Fischer, R.L. DNA demethylation in the Arabidopsis genome. Proc. Natl. Acad. Sci. USA 2007, 104, 6752–6757. [Google Scholar] [CrossRef]
- Huh, J.H.; Bauer, M.J.; Hsieh, T.F.; Fischer, R.L. Cellular programming of plant gene imprinting. Cell 2008, 132, 735–744. [Google Scholar] [CrossRef]
- Martínez-Macías, M.I.; Qian, W.; Miki, D.; Pontes, O.; Liu, Y.; Tang, K.; Liu, R.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T.; et al. A DNA 3’ phosphatase functions in active DNA demethylation in Arabidopsis. Mol. Cell 2012, 45, 357–370. [Google Scholar] [CrossRef]
- Lee, J.; Jang, H.; Shin, H.; Choi, W.L.; Mok, Y.G.; Huh, J.H. AP endonucleases process 5-methylcytosine excision intermediates during active DNA demethylation in Arabidopsis. Nucleic Acids Res. 2014, 42, 11408–11418. [Google Scholar] [CrossRef]
- Raja, P.; Sanville, B.C.; Buchmann, R.C.; Bisaro, D.M. Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 2008, 82, 8997–9007. [Google Scholar] [CrossRef]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Miki, D.; Zhang, H.; Liu, Y.; Zhang, X.; Tang, K.; Kan, Y.; La, H.; Li, X.; Li, S.; et al. A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science 2012, 336, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.P.; Pignatta, D.; Henikoff, S.; Gehring, M. Methylation-sensitive expression of a DNA demethylase gene serves as an epigenetic rheostat. PLOS Genet. 2015, 11, e1005142. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Bardet, A.F.; Adrian Ginno, P.; Hartl, D.; Burger, L.; Schübeler, D. Competition between DNA methylation and transcription factors determines binding of NRF1. Nature 2015, 528, 575–579. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, G.H.; Qian, J. Transcription factors as readers and effectors of DNA methylation. Nat. Rev. Genet. 2016, 17, 551–565. [Google Scholar] [CrossRef]
- Tang, K.; Lang, Z.; Zhang, H.; Zhu, J.K. The DNA demethylase ROS1 targets genomic regions with distinct chromatin modifications. Nat. Plants 2016, 2, 16169. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, H.; Julian, R.; Tang, K.; Xie, S.; Zhu, J.K. Regulatory link between DNA methylation and active demethylation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3553–3557. [Google Scholar] [CrossRef]
- Feng, Z.; Zhan, X.; Pang, J.; Liu, X.; Zhang, H.; Lang, Z.; Zhu, J.K. Genetic analysis implicates a molecular chaperone complex in regulating epigenetic silencing of methylated genomic regions. J. Integr. Plant Biol. 2021, 63, 1451–1461. [Google Scholar] [CrossRef]
- Qian, W.; Miki, D.; Lei, M.; Zhu, X.; Zhang, H.; Liu, Y.; Li, Y.; Lang, Z.; Wang, J.; Tang, K.; et al. Regulation of active DNA demethylation by an alpha-crystallin domain protein in Arabidopsis. Mol. Cell 2014, 55, 361–371. [Google Scholar] [CrossRef]
- Boone, B.A.; Ichino, L.; Wang, S.; Gardiner, J.; Yun, J.; Jami-Alahmadi, Y.; Sha, J.; Mendoza, C.P.; Steelman, B.J.; van Aardenne, A.; et al. ACD15, ACD21, and SLN regulate the accumulation and mobility of MBD6 to silence genes and transposable elements. Sci. Adv. 2023, 9, eadi9036. [Google Scholar] [CrossRef]
- Duan, C.G.; Wang, X.; Xie, S.; Pan, L.; Miki, D.; Tang, K.; Hsu, C.C.; Lei, M.; Zhong, Y.; Hou, Y.J.; et al. A pair of transposon-derived proteins function in a histone acetyltransferase complex for active DNA demethylation. Cell Res. 2017, 27, 226–240. [Google Scholar] [CrossRef]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Cokus, S.J.; Feng, S.; Zhang, X.; Chen, Z.; Merriman, B.; Haudenschild, C.D.; Pradhan, S.; Nelson, S.F.; Pellegrini, M.; Jacobsen, S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 2008, 452, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhong, X.; Bernatavichute, Y.V.; Stroud, H.; Feng, S.; Caro, E.; Vashisht, A.A.; Terragni, J.; Chin, H.G.; Tu, A.; et al. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell 2012, 151, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gent, J.I.; Zynda, G.; Song, J.; Makarevitch, I.; Hirsch, C.D.; Hirsch, C.N.; Dawe, R.K.; Madzima, T.F.; McGinnis, K.M.; et al. RNA-directed DNA methylation enforces boundaries between heterochromatin and euchromatin in the maize genome. Proc. Natl. Acad. Sci. USA 2015, 112, 14728–14733. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, F.; Schmidt, M.; Van Lijsebettens, M.; Schmidt, T. DNA methylation of retrotransposons, DNA transposons and genes in sugar beet (Beta vulgaris L.). Plant J. 2017, 90, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Gouil, Q.; Baulcombe, D.C. DNA methylation signatures of the plant chromomethyltransferases. PLoS Genet. 2016, 12, e1006526. [Google Scholar] [CrossRef]
- Grob, S.; Schmid, M.W.; Grossniklaus, U. Hi-C analysis in Arabidopsis identifies the KNOT, a structure with similarities to the flamenco Locus of Drosophila. Mol. Cell 2014, 55, 678–693. [Google Scholar] [CrossRef]
- Feng, S.; Cokus, S.J.; Schubert, V.; Zhai, J.; Pellegrini, M.; Jacobsen, S.E. Genome-wide Hi-C analyses in wild-type and mutants reveal high-resolution chromatin interactions in Arabidopsis. Mol. Cell 2014, 55, 694–707. [Google Scholar] [CrossRef]
- Rowley, M.J.; Rothi, M.H.; Bohmdorfer, G.; Kucinski, J.; Wierzbicki, A.T. Long-range control of gene expression via RNA-directed DNA methylation. PLoS Genet. 2017, 13, e1006749. [Google Scholar] [CrossRef]
- Gehring, M.; Bubb, K.L.; Henikoff, S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 2009, 324, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.F.; Ibarra, C.A.; Silva, P.; Zemach, A.; Eshed-Williams, L.; Fischer, R.L.; Zilberman, D. Genome-wide demethylation of Arabidopsis endosperm. Science 2009, 324, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, C.A.; Feng, X.; Schoft, V.K.; Hsieh, T.-F.; Uzawa, R.; Rodrigues, J.A.; Zemach, A.; Chumak, N.; Machlicova, A.; Nishimura, T.; et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 2012, 337, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, M.Y.; Vickers, M.; Park, J.-S.; Hyun, Y.; Okamoto, T.; Zilberman, D.; Fischer, R.L.; Feng, X.; Choi, Y.; et al. DNA demethylation is initiated in the central cells of Arabidopsis and rice. Proc. Natl. Acad. Sci. USA 2016, 113, 15138–15143. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W.; Kulma, A.; Dziadas, M.; Kostyn, K.; Dymińska, L.; Hanuza, J.; Żuk, M.; Szopa, J. 3-Hydroxybutyrate Is Active Compound in Flax That Upregulates Genes Involved in DNA Methylation. Int. J. Mol. Sci. 2020, 21, 2887. [Google Scholar] [CrossRef]
- Martínez, G.; Panda, K.; Köhler, C.; Slotkin, R.K. Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nat. Plants. 2016, 2, 16030. [Google Scholar] [CrossRef]
- Ingouff, M. Live-cell analysis of DNA methylation during sexual reproduction in Arabidopsis reveals context and sex-specific dynamics controlled by noncanonical RdDM. Genes Dev. 2017, 31, 72–83. [Google Scholar] [CrossRef]
- Walker, J. Sexual-lineage-specific DNA methylation regulates meiosis in Arabidopsis. Nat. Genet. 2018, 50, 130–137. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, S.; Dong, X.; Zhao, X.; Zeng, B.; Chen, J.; Li, H.; Yang, W.; Zhao, H.; Wang, G.; et al. Genome-wide high resolution parental-specific DNA and histone methylation maps uncover patterns of imprinting regulation in maize. Genome Res. 2014, 24, 167–176. [Google Scholar] [CrossRef]
- Klosinska, M.; Picard, C.L.; Gehring, M. Conserved imprinting associated with unique epigenetic signatures in the Arabidopsis genus. Nat. Plants 2016, 2, 16145. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Ruan, R.; Nishimura, T.; Sharma, M.K.; Sharma, R.; Ronald, P.C.; Fischer, R.L.; Zilberman, D. Imprinted expression of genes and small RNA is associated with localized hypomethylation of the maternal genome in rice endosperm. Proc. Natl. Acad. Sci. USA 2013, 110, 7934–7939. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Saze, H.; Kinoshita, T.; Miura, A.; Soppe, W.J.; Koornneef, M.; Kakutani, T. Control of FWA gene silencing in Arabidopsis thaliana by SINE-related direct repeats. Plant J. 2007, 49, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-F.; Shin, J.; Uzawa, R.; Silva, P.; Cohen, S.; Bauer, M.J.; Hashimoto, M.; Kirkbride, R.C.; Harada, J.J.; Zilberman, D.; et al. Regulation of imprinted gene expression in Arabidopsis endosperm. Proc. Natl. Acad. Sci. USA 2011, 108, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Miura, A.; Choi, Y.; Kinoshita, Y.; Cao, X.; Jacobsen, S.E.; Fischer, R.L.; Kakutani, T. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 2004, 303, 521–523. [Google Scholar] [CrossRef]
- Bratzel, F.; Yang, C.; Angelova, A.; López-Torrejón, G.; Koch, M.; del Pozo, J.C.; Calonje, M. Regulation of the new Arabidopsis imprinted gene AtBMI1C requires the interplay of different epigenetic mechanisms. Mol. Plant 2012, 5, 260–269. [Google Scholar] [CrossRef]
- Moreno-Romero, J.; Jiang, H.; Santos-Gonzalez, J.; Kohler, C. Parental epigenetic asymmetry of PRC2-mediated histone modifications in the Arabidopsis endosperm. EMBO J. 2016, 35, 1298–1311. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, M.; Chen, J.; Peng, L.; Zhang, N.; Wang, X.; Lai, J. Dynamic and antagonistic allele-Specific epigenetic modifications controlling the expression of imprinted genes in maize endosperm. Mol. Plant 2017, 10, 442–455. [Google Scholar] [CrossRef]
- Baubec, T.; Finke, A.; Scheid, O.M.; Pecinka, A. Meristem-specific expression of epigenetic regulators safeguards transposon silencing in Arabidopsis. EMBO Rep. 2014, 15, 446–452. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Stuart, T.; Valdes, M.; Breakfield, N.; Schmitz, R.J.; Nery, J.R.; Urich, M.A.; Han, X.; Lister, R.; Benfey, P.N.; et al. Unique cell-type-specific patterns of DNA methylation in the root meristem. Nat. Plants 2016, 2, 16058. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Zhou, C.; Zhou, Q.; Zhao, Y.; Li, G.; Zhou, D.X. Cooperation between the H3K27me3 chromatin mark and non-CG methylation in epigenetic regulation. Plant Physiol. 2016, 172, 1131–1141. [Google Scholar]
- Candaele, J.; Demuynck, K.; Mosoti, D.; Beemster, G.T.; Inzé, D; Nelissen, H. Differential methylation during maize leaf growth targets developmentally regulated genes. Plant Physiol. 2014, 164, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, C.; Miki, D.; Zheng, Z.; Ma, J.; Wang, J.; Yang, Z.; Dong, J.; Zhu, J.-K. Overproduction of stomatal lineage cells in Arabidopsis mutants defective in active DNA demethylation. Nat. Commun. 2014, 5, 4062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Xue, X.Y.; Zhu, J.K.; Dong, J. Demethylation of ERECTA receptor genes by IBM1 histone demethylase affects stomatal development. Development 2016, 143, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Hieber, A.D.; Mudalige-Jayawickrama, R.G.; Kuehnle, A.R. Color genes in the orchid Oncidium Gower Ramsey: Identification, expression, and potential genetic instability in an interspecific cross. Planta 2006, 223, 521–531. [Google Scholar] [CrossRef]
- Chiou, C.Y.; Pan, H.A.; Chuang, Y.N.; Yeh, K.W. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in floral tissues of Oncidium cultivars. Planta 2010, 232, 937–948. [Google Scholar] [CrossRef]
- Liu, X.J.; Chuang, Y.N.; Chiou, C.Y.; Chin, D.C.; Shen, F.Q.; Yeh, K.W. Methylation effect on chalcone synthase gene expression determines anthocyanin pigmentation in floral tissues of two Oncidium orchid cultivars. Planta 2012, 236, 401–409. [Google Scholar] [CrossRef]
- Han, M.L.; Yin, J.; Zhao, Y.H.; Sun, X.W.; Meng, J.X.; Zhou, J.; Shen, T.; Li, H.H.; Zhang, F. How the Color Fades from Malus halliana Flowers: Transcriptome Sequencing and DNA Methylation Analysis. Front. Plant Sci. 2020, 11, 576054. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, K.; Zhang, M.; Wang, J.; Zhang, Q. Integration of Transcriptome and Methylome Analyses Provides Insight Into the Pathway of Floral Scent Biosynthesis in Prunus mume. Front. Genet. 2021, 12, 779557. [Google Scholar] [CrossRef]
- Zhong, S.; Fei, Z.; Chen, Y.R.; Zheng, Y.; Huang, M.; Vrebalov, J.; McQuinn, R.; Gapper, N.; Liu, B.; Xiang, J.; et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 2013, 31, 154–159. [Google Scholar] [CrossRef]
- Liu, R.; How-Kit, A.; Stammitti, L.; Teyssier, E.; Rolin, D.; Mortain-Bertrand, A.; Liu, M.; Kong, J.; Wu, C.; Degraeve-Guibaul, C.; et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl. Acad. Sci. USA 2015, 112, 10804–10809. [Google Scholar] [CrossRef]
- Lang, Z.; Wang, Y.; Tang, K.; Tang, D.; Datsenka, T.; Cheng, J.; Zhang, Y.; Handa, A.K.; Zhu, J.K. Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc. Natl. Acad. Sci. USA 2017, 114, E4511–E4519. [Google Scholar] [CrossRef] [PubMed]
- Telias, A.; Lin-Wang, K.; Stevenson, D.E.; Cooney, J.M.; Hellens, R.P.; Allan, A.C.; Hoover, E.E.; Bradeen, J.M. Apple skin patterning is associated with differential expression of MYB10. BMC Plant Biol. 2011, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, I.; Liang, D.; Xu, K.N. Transcriptome analysis of an apple (Malus x domestica) yellow fruit somatic mutation identifies a gene network module highly associated with anthocyanin and epigenetic regulation. J. Exp. Bot. 2015, 66, 7359–7376. [Google Scholar] [CrossRef] [PubMed]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Tollefson, J. How hot will earth get by 2100? Nature 2020, 580, 443–445. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Li, H.; Li, F.; Xu, K.; Yan, G.; Chen, B.; Qiao, J.; Wu, X. Comparison of the heat stress induced variations in DNA methylation between heat-tolerant and heat-sensitive rapeseed seedlings. Breed Sci. 2014, 64, 125–133. [Google Scholar] [CrossRef]
- Singh, R.K.; Jaishankar, J.; Muthamilarasan, M.; Shweta, S.; Dangi, A.; Prasad, M. Genome-wide analysis of heat shock proteins in C4 model, foxtail millet identifies potential candidates for crop improvement under abiotic stress. Sci. Rep. 2016, 6, 32641. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, W.; Liao, J.; Zhang, J.; Ren, Q. The dynamics of DNA methylation in the maize (Zea mays L.) inbred line B73 response to heat stress at the seedling stage. Biochem. Biophys. Res. Commun. 2019, 512, 742–749. [Google Scholar] [CrossRef]
- Lamelas, L.; Valledor, L.; Escandón, M.; Pinto, G.; Cañal, M.J.; Meijón, M. Integrative analysis of the nuclear proteome in Pinus radiata reveals thermopriming coupled to epigenetic regulation. J. Exp. Bot. 2020, 71, 2040–2057. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2021, 11, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, S.; Wang, X.; Zhu, J.; Wei, Y.; Wang, Y.; Wen, Y.; Wang, L.; Huang, Y.; Zhang, B.; et al. DNA methylation dynamics: Identification and functional annotation. Brief Funct. Genom. 2016, 15, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Moussa, M. DNA methylation changes stimulated by drought stress in ABA-deficient maize mutant vp10. Plant Physiol. Biochem. 2021, 160, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, M.; He, Y. DNA methylation analysis of the Citrullus lanatus response to cucumber green mottle mosaic virus infection by whole-genome bisulfite sequencing. Genes 2019, 10, 344. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E. DNA methylation mediates stress-induced transgenerational plasticity in plants. Proc. Natl. Acad. Sci. USA 2016, 113, 8804–8809. [Google Scholar]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Godwin, J.; Farrona, S. Plant Epigenetic Stress Memory Induced by Drought: A Physiological and Molecular Perspective. Methods Mol. Biol. 2020, 2093, 243–259. [Google Scholar]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, D.; Zhang, Y.; Li, G.; Sun, D.; Zhou, B.; Li, J. Insights into the Epigenetic Basis of Plant Salt Tolerance. Int. J. Mol. Sci. 2024, 25, 11698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Sahu, P.P.; Pandey, G.; Sharma, N.; Puranik, S.; Muthamilarasan, M.; Prasad, M. Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Rep. 2013, 32, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Dowen, R.H.; Pelizzola, M.; Schmitz, R.J.; Lister, R.; Dowen, J.M.; Nery, J.R.; Dixon, J.E.; Ecker, J.R. Widespread dynamic DNA methylation in response to biotic stress. Proc. Natl. Acad. Sci. USA 2012, 109, E2183–E2191. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Lepère, G.; Jay, F.; Wang, J.; Bapaume, L.; Wang, Y.; Abraham, A.-L.; Penterman, J.; Fischer, R.L.; Voinnet, O.; et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 2013, 110, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Ramírez, V.; García-Andrade, J.; Flors, V.; Vera, P. The RNA silencing enzyme RNA polymerase V is required for plant immunity. PLoS Genet. 2011, 7, e1002434. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. DNA methylation is critical for defense against the fungal pathogen Verticillium dahliae in Arabidopsis. Plant Cell 2016, 28, 1237–1251. [Google Scholar]
- Gohlke, J.; Scholz, C.J.; Kneitz, S.; Weber, D.; Fuchs, J.; Hedrich, R.; Deeken, R. DNA methylation mediated control of gene expression is critical for development of crown gall tumors. PLoS Genet. 2013, 9, e1003267. [Google Scholar] [CrossRef]
- Singh, N.; Titov, V.; Ayemere, I.; Byeon, B.; Kovalchuk, I. Multigenerational exposure to heat stress induces phenotypic resilience, and genetic and epigenetic variations in Arabidopsis thaliana offspring. Front. Plant Sci. 2020, 13, 728167. [Google Scholar]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 1999, 401, 157–161. [Google Scholar] [CrossRef]
- Gallego-Bartolome, J. DNA methylation in plants: Mechanisms and tools for targeted manipulation. New Phytol. 2020, 227, 38–44. [Google Scholar] [CrossRef]
- Le, T.N.; Schumann, U.; Smith, N.A.; Tiwari, S.; Au, P.C.; Zhu, Q.H.; Taylor, J.M.; Kazan, K.; Llewellyn, D.J.; Zhang, R.; et al. DNA demethylases target promoter transposable elements to positively regulate stress responsive genes in Arabidopsis. Genome Biol. 2014, 15, 458. [Google Scholar] [CrossRef]
- Manning, K.; Tör, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006, 38, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, T.; Stelly, D.M.; Chen, Z.J. Epigenomic and functional analyses reveal roles of epialleles in the loss of photoperiod sensitivity during domestication of allotetraploid cottons. Genome Biol. 2017, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Pilu, R.; Panzeri, D.; Cassani, E.; Badone, F.C.; Landoni, M.; Nielsen, E. A paramutation phenomenon is involved in the genetics of maize low phytic acid1-241 (lpa1-241) trait. Heredity 2009, 102, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, T.; Ishikura, S.; Miyaji, N.; Sasaki, T.; Wu, L.M.; Itabashi, E.; Takada, S.; Shimizu, M.; Takasaki-Yasuda, T.; Osabe, K.; et al. Role of DNA methylation in hybrid vigor in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, E6704–E6711. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xu, T.; Srivastava, A.K.; Wang, D.; Zeng, L.; Yang, L.; He, L.; Zhang, H.; Zheng, Z. The chromatin remodeler DDM1 promotes hybrid vigor by regulating salicylic acid metabolism. Cell Discov. 2016, 2, 16027. [Google Scholar] [CrossRef]
- Brachi, B.; Morris, G.P.; Borevitz, J.O. Genome-wide association studies in plants: The missing heritability is in the field. Genome Biol. 2011, 12, 232. [Google Scholar] [CrossRef]
- Dubin, M.J.; Zhang, P.; Meng, D.; Remigereau, M.S.; Osborne, E.J.; Paolo Casale, F.; Drewe, P.; Kahles, A.; Jean, G.; Vilhjalmsson, B.; et al. DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. Elife 2015, 4, e05255. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Huang SS, C.; Jupe, F.; Sasaki, E.; Schmitz, R.J.; Urich, M.A.; Schork, N.J. Epigenomic diversity in a global collection of Arabidopsis thaliana accessions. Cell 2016, 166, 492–505. [Google Scholar]
- Eichten, S.R.; Briskine, R.; Song, J.; Li, Q.; Swanson-Wagner, R.; Hermanson, P.J.; Waters, A.J.; Starr, E.; West, P.T.; Tiffin, P.; et al. Epigenetic and genetic influences on DNA methylation variation in maize populations. Plant Cell 2013, 25, 2783–2797. [Google Scholar] [CrossRef]
- Xu, G.; Lyu, J.; Li, Q.; Liu, H.; Wang, D.; Zhang, M.; Springer, N.M.; Ross-Ibarra, J.; Yang, J. Evolutionary and functional genomics of DNA methylation in maize domestication and improvement. Nat. Commun. 2020, 11, 5539. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Liu, Y.; Liu, S.; Liu, Z.; Duan, Z.; Wang, Z.; Zhu, B.; Guo, Y.-L.; Tian, Z. DNA methylation footprints during soybean domestication and improvement. Genome Biol. 2018, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xie, L.; Zhang, Q.; Ouyang, W.; Deng, L.; Guan, P.; Ma, M.; Li, Y.; Zhang, Y.; Xiao, Q.; et al. Integrative analysis of reference epigenomes in 20 rice varieties. Nat. Commun. 2020, 11, 2658. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, L.-J.; Joynson, R.; Omony, J.; Rusholme-Pilcher, R.; Olohan, L.; Lang, D.; Bai, C.; Hawkesford, M.; Salt, D.; Spannagl, M.; et al. Hidden variation in polyploid wheat drives local adaptation. Genome Res. 2018, 28, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, G.; Hermanson, P.J.; Xu, Q.; Sun, C.; Chen, W.; Kan, Q.; Li, M.; Crisp, P.A.; Yan, J.; et al. Population-level analysis reveals the widespread occurrence and phenotypic consequence of DNA methylation variation not tagged by genetic variation in maize. Genome Biol. 2019, 20, 243. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Schultz, M.D.; Urich, M.A.; Nery, J.R.; Pelizzola, M.; Libiger, O.; Alix, A.; McCosh, R.B.; Chen, H.; Schork, N.J.; et al. Patterns of population epigenomic diversity. Nature 2013, 495, 193–198. [Google Scholar] [CrossRef]
- Kou, H.P.; Li, Y.; Song, X.X.; Ou, X.F.; Xing, S.C.; Ma, J.; Von Wettstein, D.; Liu, B. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance in rice (Oryza sativa L.). J. Plant Pyhsiol. 2011, 65, 655–664. [Google Scholar] [CrossRef]
- Gouil, Q.; Baulcombe, D.C. Paramutation-like features of multiple natural epialleles in tomato. BMC Genom. 2018, 19, 203. [Google Scholar] [CrossRef]
- Cao, S.; Wang, L.; Han, T.; Ye, W.; Liu, Y.; Sun, Y.; Moose, S.P.; Song, Q.; Chen, Z.J. Small RNAs mediate transgenerational inheritance of genome-wide trans-acting epialleles in maize. Genome Biol. 2022, 23, 53. [Google Scholar] [CrossRef]
- Cortijo, S.; Wardenaar, R.; Colomé-Tatché, M.; Gilly, A.; Etcheverry, M.; Labadie, K.; Caillieux, E.; Hospital, F.; Aury, J.-M.; Wincker, P.; et al. Mapping the epigenetic basis of complex traits. Science 2014, 343, 1145–1148. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Latzel, V.; Fischer, M.; Bossdorf, O. Understanding the evolutionary potential of epigenetic variation: A comparison of heritable phenotypic variation in epiRILs, RILs, and natural ecotypes of Arabidopsis thaliana. Heredity 2018, 121, 257–265. [Google Scholar] [CrossRef]
- Schmidt, M.; Byzova, M.; Martens, C.; Peeters, M.; Raj, Y.; Shukla, S.; Verwulgen, T.; De Block, M.; Van Lijsebettens, M. Methylome and epialleles in rice epilines selected for energy use efficiency. Agronomy 2018, 8, 163. [Google Scholar] [CrossRef]
- Hauben, M.; Haesendonckx, B.; Standaert, E.; Van Der Kelen, K.; Azmi, A.; Akpo, H.; Van Breusegem, F.; Guisez, Y.; Bots, M.; Lambert, B.; et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc. Natl. Acad. Sci. USA 2009, 106, 20109–20114. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Li, G.; Malzahn, A.A.; Cheng, Y.; Leyson, B.; Sretenovic, S.; Gurel, F.; Coleman, G.D.; Qi, Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 2022, 8, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Bartolome, J.; Gardiner, J.; Liu, W.; Papikian, A.; Ghoshal, B.; Kuo, H.Y.; Zhao, J.M.-C.; Segal, D.J.; Jacobsen, S.E. Targeted DNA demethylation of the Arabidopsis genome using the human TET1 catalytic domain. Proc. Natl. Acad. Sci. USA 2018, 115, E2125–E2134. [Google Scholar] [CrossRef]
- Ji, L.; Jordan, W.T.; Shi, X.; Hu, L.; He, C.; Schmitz, R.J. TET-mediated epimutagenesis of the Arabidopsis thaliana methylome. Nat. Commun. 2018, 9, 895. [Google Scholar] [CrossRef]
- Papikian, A.; Liu, W.; Gallego-Bartolome, J.; Jacobsen, S.E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 2019, 10, 729. [Google Scholar] [CrossRef]
- Ghoshal, B.; Picard, C.L.; Vong, B.; Feng, S.; Jacobsen, S.E. CRISPR-based targeting of DNA methylation in Arabidopsis thaliana by a bacterial CG-specific DNA methyltransferase. Proc. Natl. Acad. Sci. USA 2021, 118, e2125016118. [Google Scholar] [CrossRef]
- Galonska, C.; Charlton, J.; Mattei, A.L.; Donaghey, J.; Clement, K.; Gu, H.; Mohammad, A.W.; Stamenova, E.K.; Cacchiarelli, D.; Klages, S.; et al. Genome-wide tracking of dCas9-methyltransferase footprints. Nat. Commun. 2018, 9, 597. [Google Scholar] [CrossRef]
- Pflueger, C.; Tan, D.; Swain, T.; Nguyen, T.; Pflueger, J.; Nefzger, C.; Polo, J.M.; Ford, E.; Lister, R. A modular dCas9-SunTag DNMT3A epigenome editing system overcomes pervasive off-target activity of direct fusion dCas9-DNMT3A constructs. Genome Res. 2018, 28, 1193–1206. [Google Scholar] [CrossRef]
- Chan, W.F.; Coughlan, H.D.; Chen, Y.; Keenan, C.R.; Smyth, G.K.; Perkins, A.C.; Johanson, T.M.; Allan, R.S. Activation of stably silenced genes by recruitment of a synthetic de-methylating module. Nat. Commun. 2022, 13, 5582. [Google Scholar] [CrossRef]
- Swain, T.; Pflueger, C.; Freytag, S.; Poppe, D.; Pflueger, J.; Nguyen, T.V.; Li, J.K.; Lister, R. A modular dCas9-based recruitment platform for combinatorial epigenome editing. Nucleic Acids Res. 2024, 52, 474–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).