Mechanistic Insights into Silicon-Enhanced Cadmium Detoxification in Rice: A Spatiotemporal Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Plant Cultivation
2.2. Determination of Cd and Si Contents in Roots and Leaves
2.3. Measurements of Photosynthetic Parameters and Rubisco Activity
2.4. Determinations of Oxidative Stress Markers and Antioxidant Enzyme Activities in Tissues
2.5. Statistical Analysis
3. Results
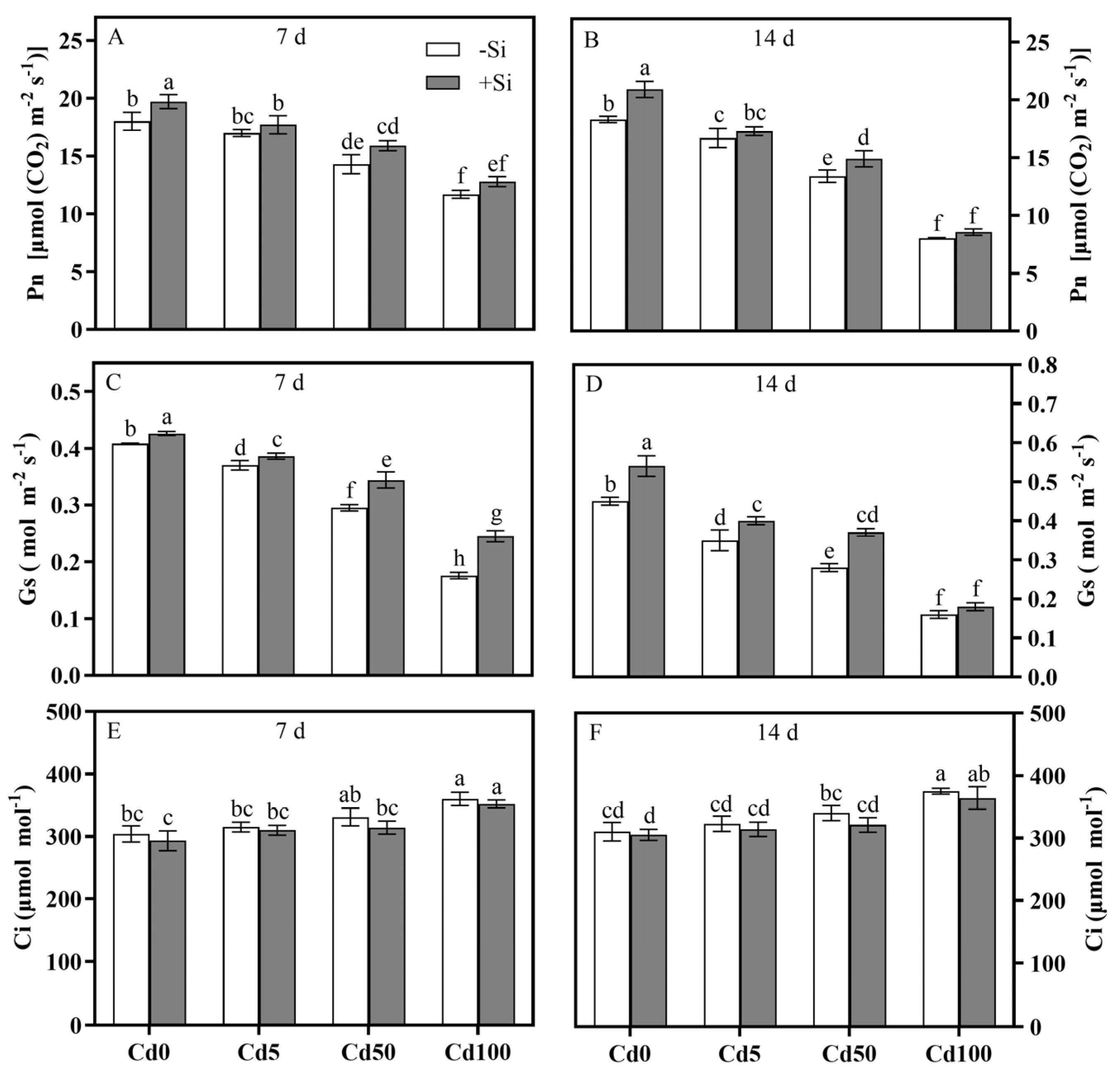
3.1. Photosynthetic Response and Si Mitigating Effect of Rice Plants Under Cd Stress
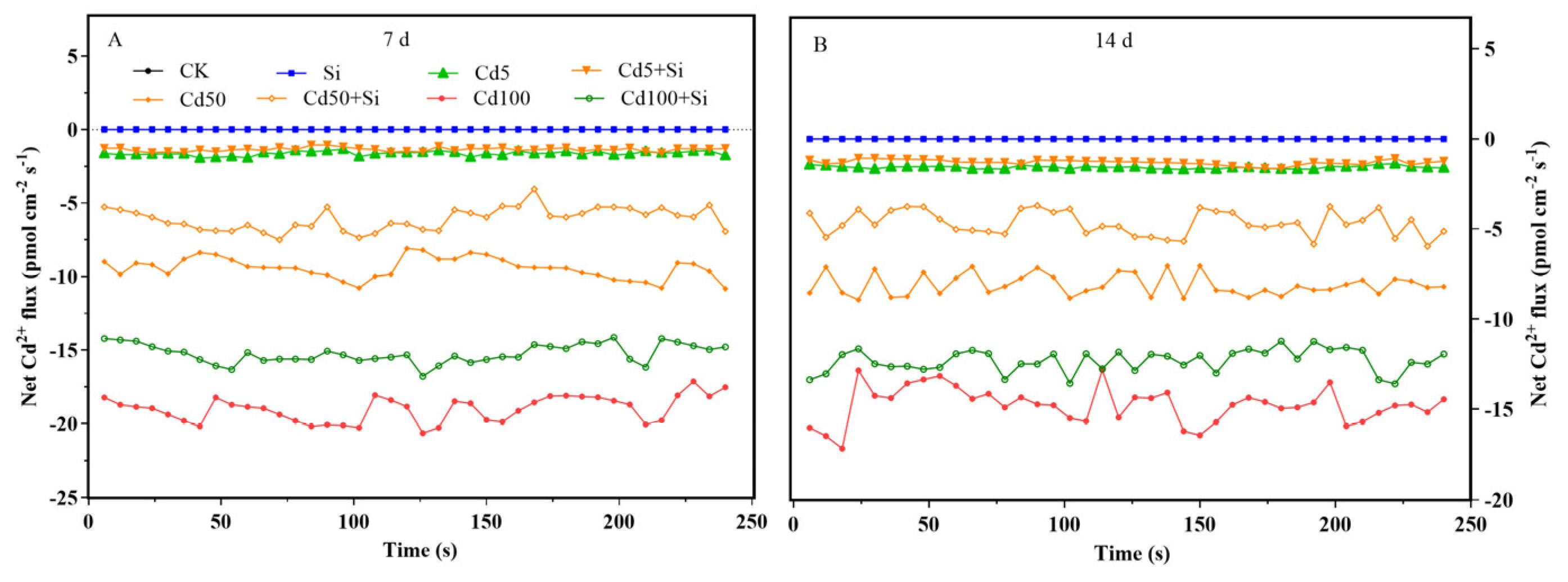
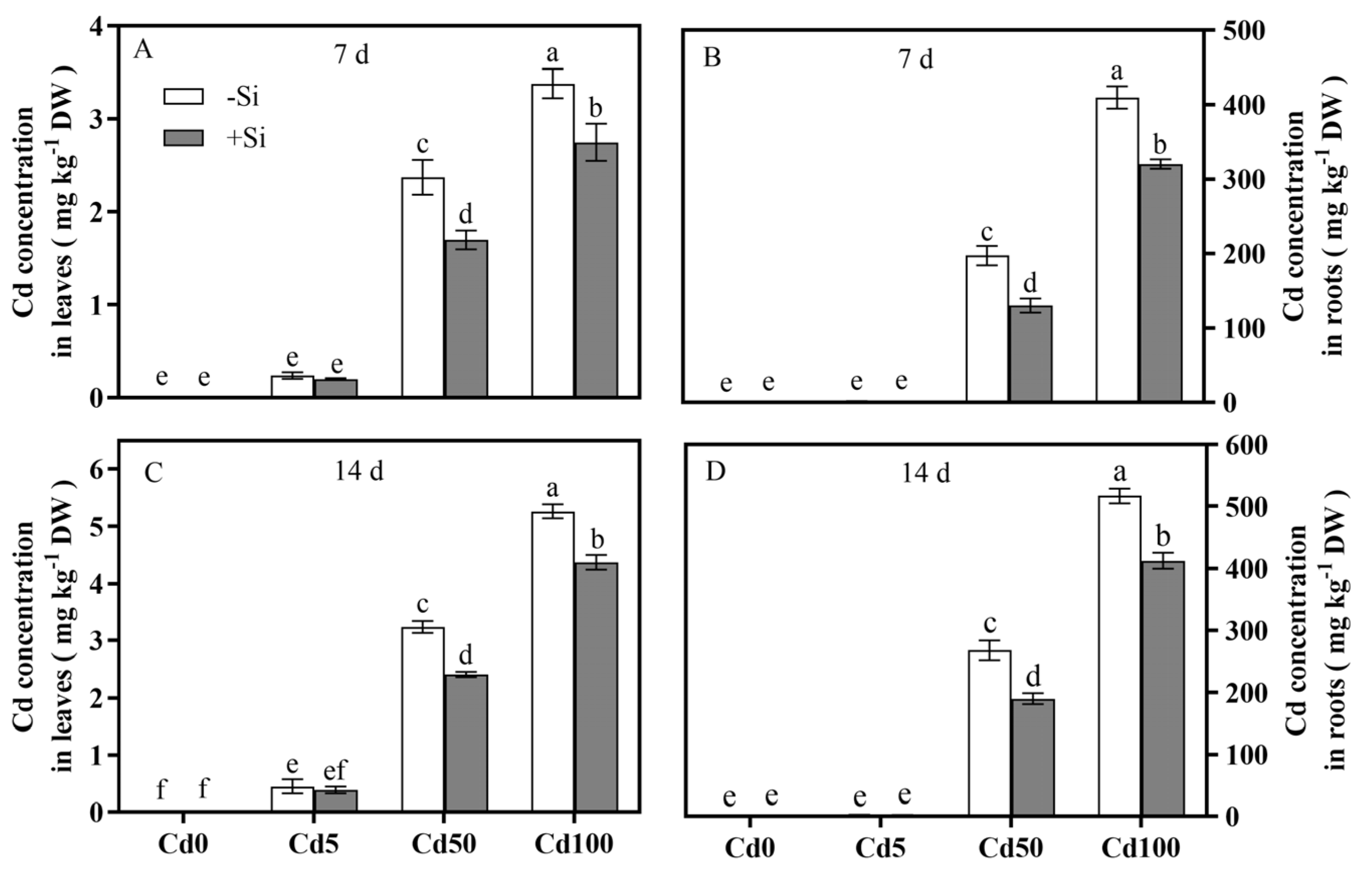
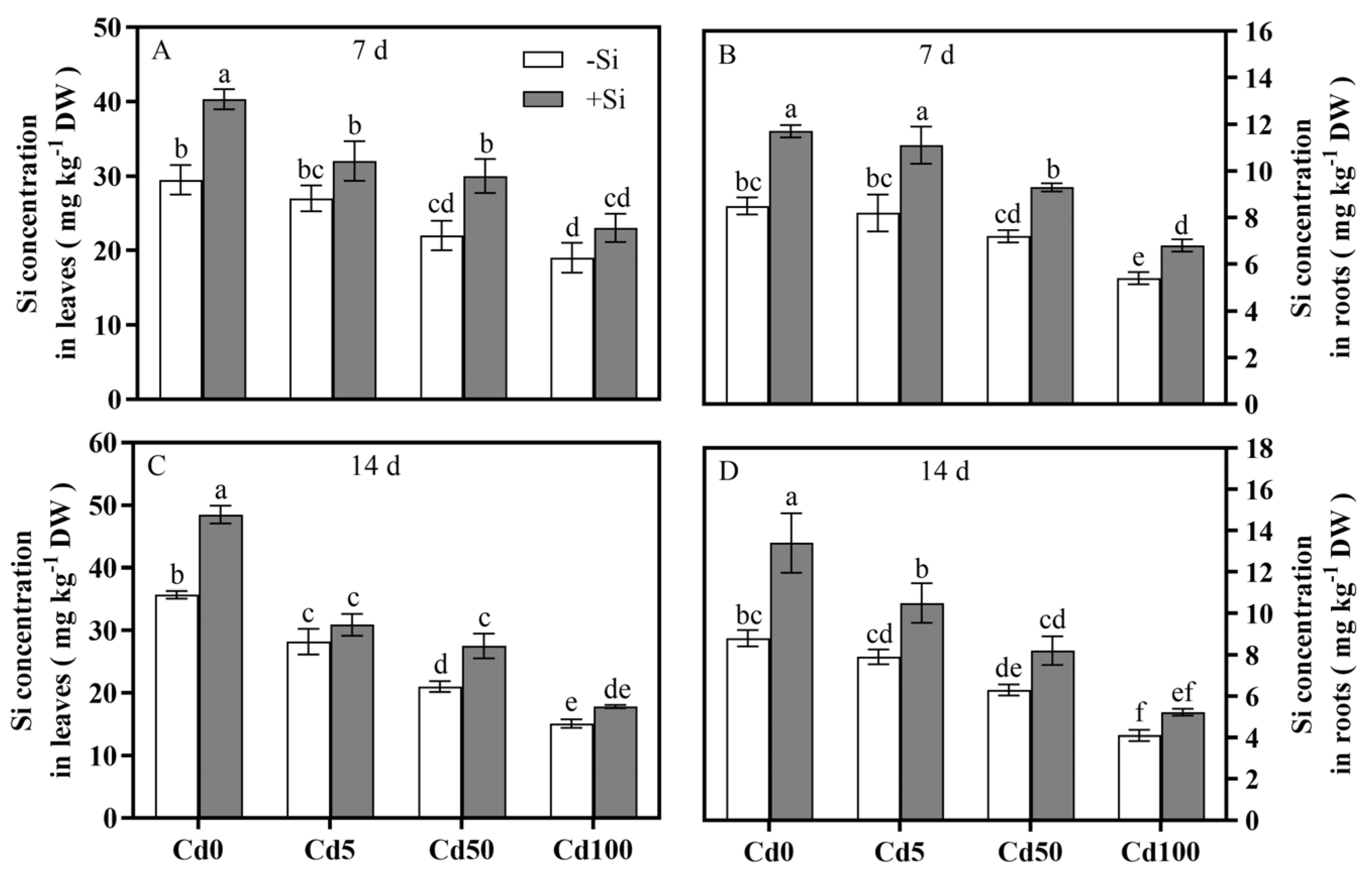
3.2. Si Spatiotemporally Regulated Cd Influx and Optimized Cd-Si Distribution in Rice Tissues
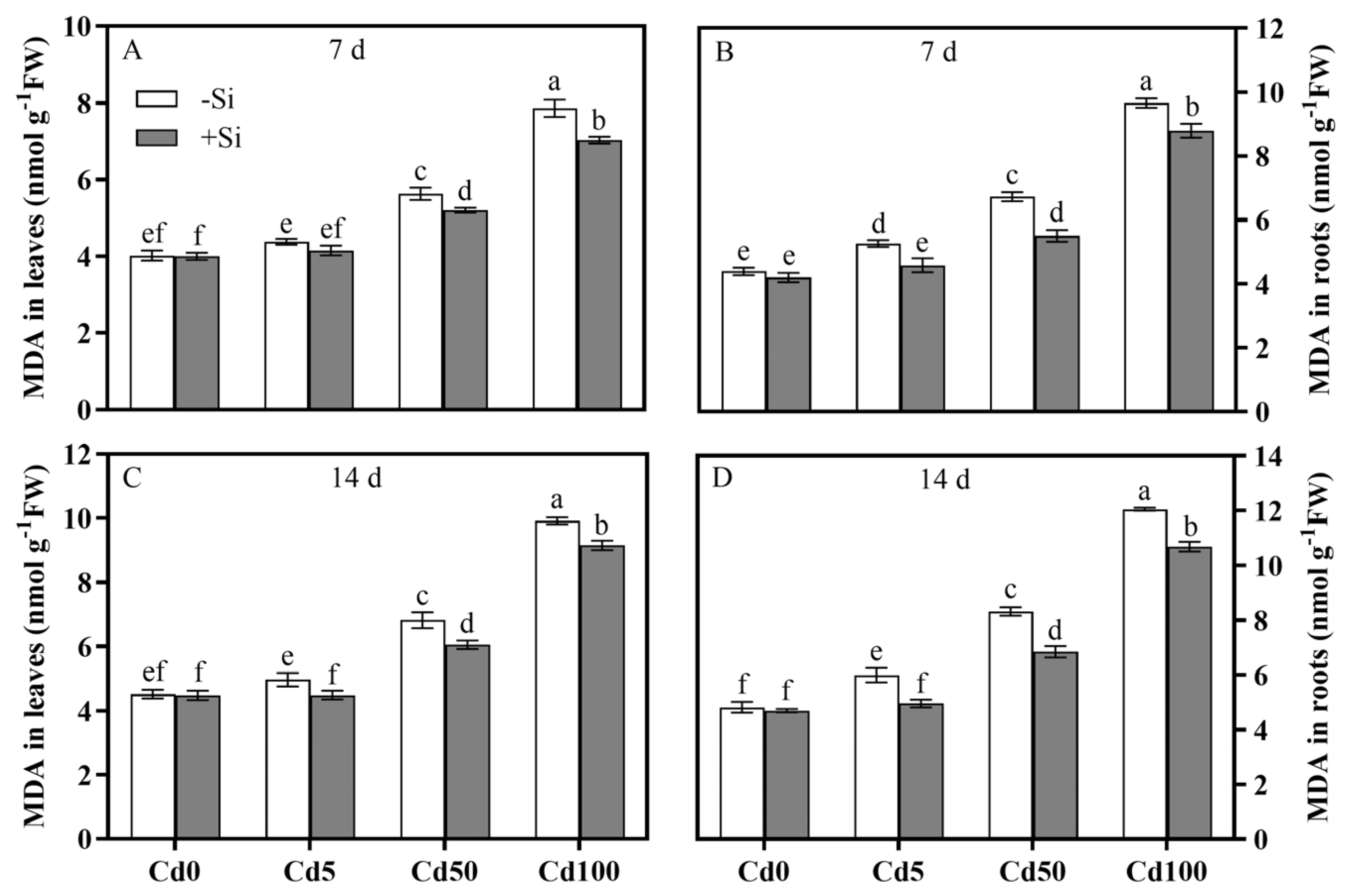
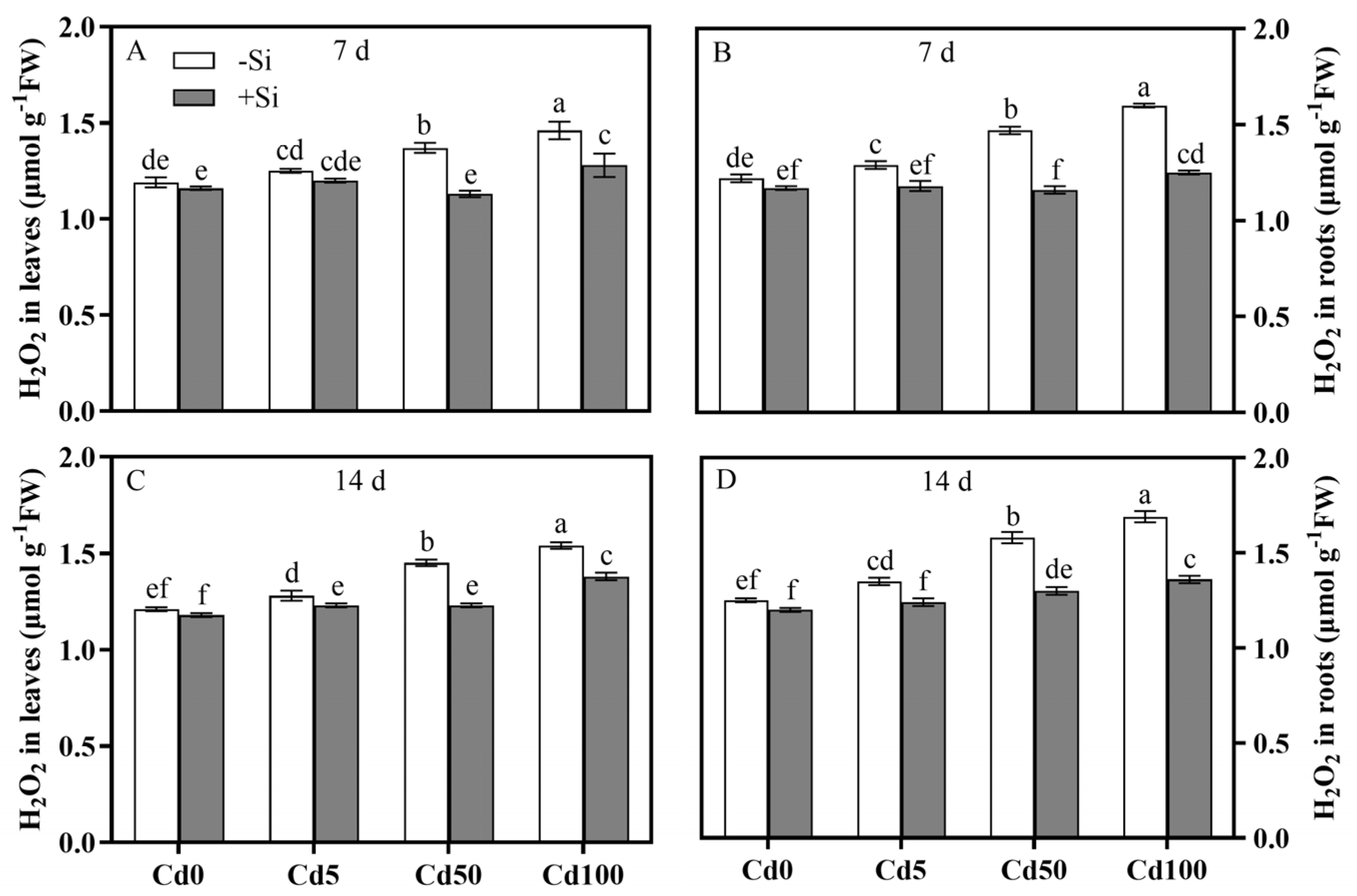
3.3. Spatiotemporal Characteristics of Si-Mediated Cd Toxicity Alleviation via MDA and H2O2 Reductions in Plant Tissues
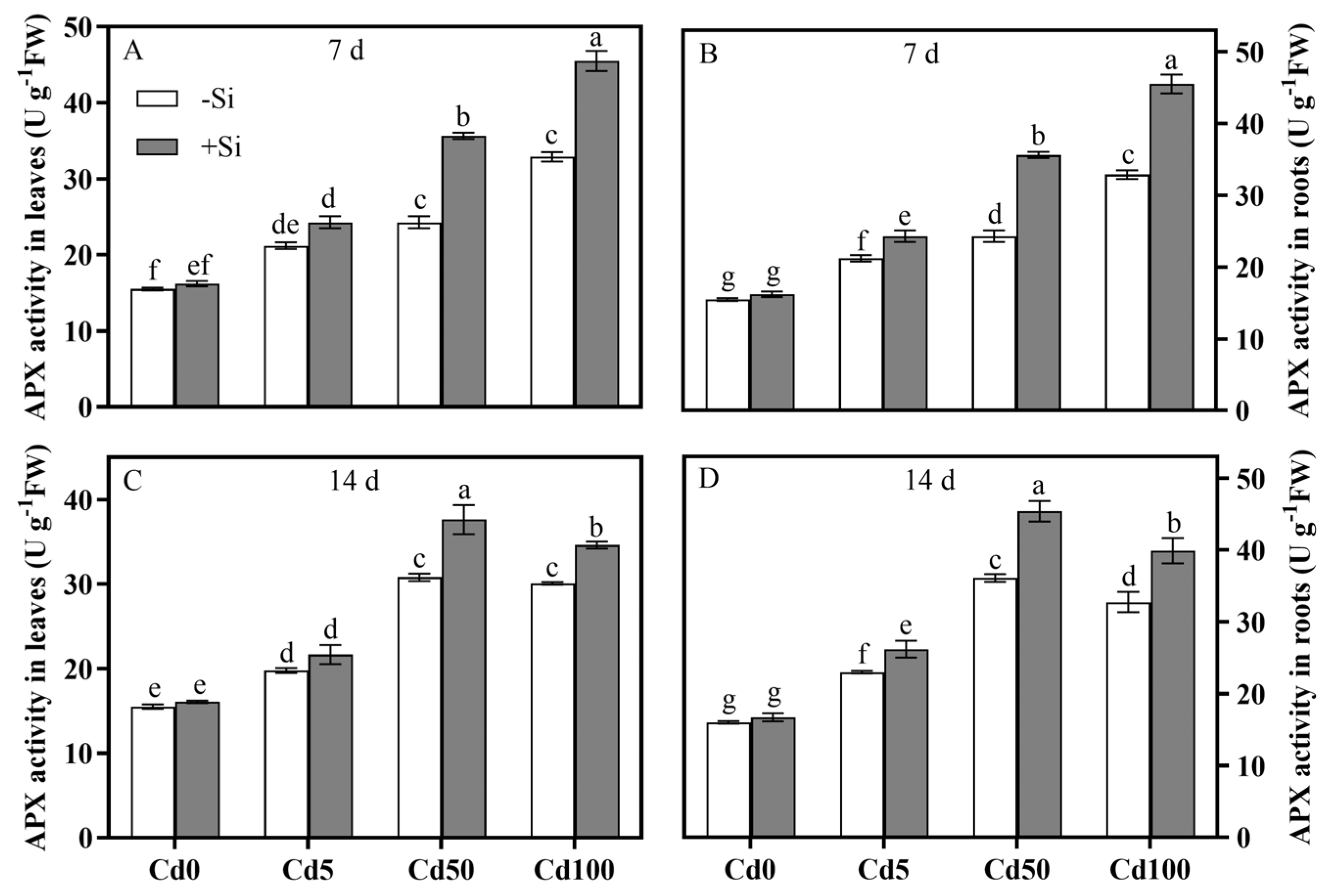
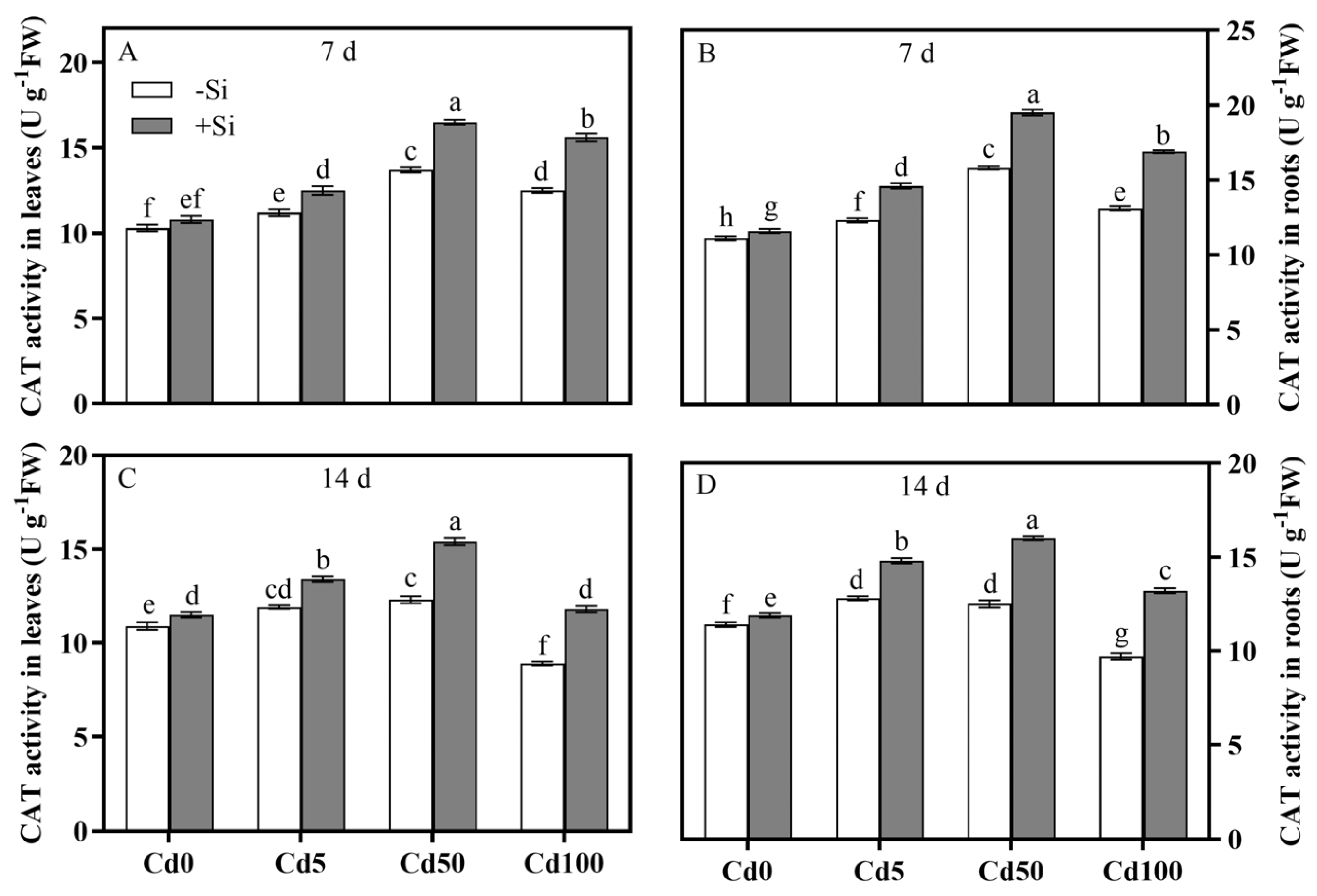
3.4. Spatiotemporal Characteristics of Si-Mediated Cd Toxicity Alleviation via Antioxidant Defense System Regulation of Rice Plants
4. Discussion
4.1. Cd-Induced Photosynthetic Inhibition and Si-Mediated Alleviation
4.2. Effects of Si on Cd Absorption and Translocation
4.3. Effects of Cd and Si on Antioxidant System and Glutathione Metabolism of Rice Plants
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xi, Y.; Gan, L.; Johnson, D.; Wu, Y.; Ren, D.; Liu, H. Effects of lead and cadmium on photosynthesis in Amaranthus spinosus and assessment of phytoremediation potential. Int. J. Phytoremediat. 2019, 21, 1041–1049. [Google Scholar] [CrossRef]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–3. [Google Scholar] [CrossRef]
- Shaari, N.E.M.; Tajudin, M.T.F.M.; Khandaker, M.M.; Majrashi, A.; Alenazi, M.M.; Abdullahi, U.A.; Mohd, K.S. Cadmium toxicity symptoms and uptake mechanism in plants: A review. Braz. J. Biol. 2022, 84, e252143. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Jia, H.; Peng, M.; Zhu, T.; Liu, Y.; Wei, J. Effects of Cd treatment on morphology, chlorophyll content and antioxidant enzyme activity of Elymus nutans Griseb., a native plant in Qinghai-Tibet Plateau. Plant Signal. Behav. 2023, 18, 2187561. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; Ye, X.; Yang, L.; Hu, R.; Ma, J.; Ma, S.; Li, D.; Zhou, J.; Nie, G.; et al. Unraveling cadmium toxicity in Trifolium repens L. seedling: Insight into regulatory mechanisms using comparative transcriptomics combined with physiological analyses. Int. J. Mol. Sci. 2022, 23, 4612. [Google Scholar] [CrossRef]
- Ji, X.; Liu, S.; Juan, H.; Bocharnikova, E.A.; Matichenkov, V.V. Effect of silicon fertilizers on cadmium in rice (Oryza sativa) tissue at tillering stage. Environ. Sci. Pollut. Res. 2017, 24, 10740–10748. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.P.; Giehl, R.F.H.; Pitann, B.; Mühling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef]
- Kollárová, K.; Kusá, Z.; Vatehová-Vivodová, Z.; Lišková, D. The response of maize protoplasts to cadmium stress mitigated by silicon. Ecotoxicol. Environ. Saf. 2019, 170, 488–494. [Google Scholar] [CrossRef]
- Hou, L.; Ji, S.; Zhang, Y.; Wu, X.; Zhang, L.; Liu, P. The mechanism of silicon on alleviating cadmium toxicity in plants: A review. Front. Plant Sci. 2023, 14, 1141138. [Google Scholar] [CrossRef]
- Song, A.; Li, Z.; Zhang, J.; Xue, G.; Fan, F.; Liang, Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J. Hazard. Mater. 2009, 172, 74–83. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Chen, H.; Zhang, S.; Fan, C.; Fu, T.; He, T.; Gao, Z. Effect of silicon spraying on rice photosynthesis and antioxidant defense system on cadmium accumulation. Sci. Rep. 2024, 14, 15265. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; He, C.; Li, X.; Zhang, W.; Xu, F.; Lin, Y.; Wang, L. Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol. 2013, 200, 691–699. [Google Scholar] [CrossRef]
- Lin, H.M.; Fang, C.X.; Li, Y.Z.; Lin, W.W.; He, J.Y.; Lin, R.Y.; Lin, W.X. Effect of silicon on grain yield of rice under cadmium-stress. Acta Physiol. Plant. 2016, 38, 186. [Google Scholar] [CrossRef]
- Lin, H.M.; He, J.Y.; Lin, W.W.; Li, Y.Z.; Fang, C.X.; Lin, W.X. Lsi1-regulated Cd uptake and phytohormones accumulation in rice seedlings in presence of Si. Plant Growth Regul. 2018, 86, 149–157. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Yu, G.; Jiang, P.; You, S.; Zhang, L.; Lin, H.; Liu, J.; Shu, Y. Application of Non-invasive Micro-test Technology (NMT) in environmental fields: A comprehensive review. Ecotoxicol. Environ. Saf. 2022, 240, 113706. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, R.; Liu, Z.; Ding, Y.; Li, T. Non-invasive microelectrode cadmium flux measurements reveal the spatial characteristics and real-time kinetics of cadmium transport in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii. J. Plant Physiol. 2013, 170, 355–359. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, C.; Wang, H.; Zhang, F. Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 2005, 272, 53–60. [Google Scholar] [CrossRef]
- Lin, H.M.; Fang, C.X.; Li, Y.Z.; Lin, W.W.; He, J.Y.; Lin, R.Y.; Lin, W.X. Cadmium-stress mitigation through gene expression of rice and silicon addition. Plant Growth Regul. 2017, 81, 91–101. [Google Scholar] [CrossRef]
- Sulpice, R.; Tschoep, H.; Von Korff, M.; Büssis, D.; Usadel, B.; HÖHne, M.; Witucka-Wall, H.; Altmann, T.; Stitt, M.; Gibon, Y. Description and applications of a rapid and sensitive non-radioactive microplate-based assay for maximum and initial activity of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2007, 30, 1163–1175. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000, 30, 151–155. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Method in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Jakoby, W.B. The glutathione S-transferases a group of multifunctional detoxification proteins. Adv. Enzymol. Relat. Areas Mol. Biol. 1978, 46, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Song, J.; Sun, Z.; Saud, S.; Fahad, S.; Nawaz, T. Exploring the deleterious effects of heavy metal cadmium on antioxidant defense and photosynthetic pathways in higher plants. Plant Stress 2025, 15, 100716. [Google Scholar] [CrossRef]
- He, S.; Lian, X.; Zhang, B.; Liu, X.; Yu, J.; Gao, Y.; Zhang, Q.; Sun, H. Nano silicon dioxide reduces cadmium uptake, regulates nutritional homeostasis and antioxidative enzyme system in barley seedlings (Hordeum vulgare L.) under cadmium stress. Environ. Sci. Pollut. Res. 2023, 30, 67552–67564. [Google Scholar] [CrossRef]
- An, M.; Wang, H.; Fan, H.; Ippolito, J.A.; Meng, Y.E.; Li, Y.; Wang, K.; Wei, C. Effects of modifiers on the growth, photosynthesis, and antioxidant enzymes of cotton under cadmium toxicity. J. Plant Growth Regul. 2019, 38, 1196–1205. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Kumar, D.; Chauhan, D.K. Rice seedlings under cadmium stress: Effect of silicon on growth, cadmium uptake, oxidative stress, antioxidant capacity and root and leaf structures. Chem. Ecol. 2012, 28, 281–291. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 35. [Google Scholar] [CrossRef]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Kumar, K. Plant Metal and Metalloid Transporters; Srivastava, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef]
- Wu, J.W.; Shi, Y.; Zhu, Y.X.; Wang, Y.C.; Gong, H.J. Mechanisms of enhanced heavy metal tolerance in plants by silicon: A review. Pedosphere 2013, 23, 815–825. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Tamai, K.; Mitani, N. Genotypic difference in silicon uptake and expression of silicon transporter genes in rice. Plant Physiol. 2007, 145, 919–924. [Google Scholar] [CrossRef]
- Luo, P.; Wu, J.; Li, T.T.; Shi, P.; Ma, Q.; Di, D.W. An overview of the mechanisms through which plants regulate ROS homeostasis under cadmium stress. Antioxidants 2024, 13, 1174. [Google Scholar] [CrossRef]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plantarum 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef]
- Sun, H.; He, S.; Liu, T.; Zhang, Q.; Yu, J.; Gao, Y.; Wang, X. Alleviation of cadmium toxicity by nano-silicon dioxide in Momordica charantia L. Seedlings. J. Soil Sci. Plant Nutr. 2023, 23, 1060–1069. [Google Scholar] [CrossRef]
- Mišúthová, A.; Slováková, Ľ.; Kollárová, K.; Vaculík, M. Effect of silicon on root growth, ionomics and antioxidant performance of maize roots exposed to As toxicity. Plant Physiol. Biochem. 2021, 168, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.N.; Amaral Carvalho, M.E.; Piotto, F.A.; Batagin-Piotto, K.D.; Nogueira, M.L.; Gaziola, S.A.; Azevedo, R.A. Antioxidant defense response in plants to cadmium stress. In Cadmium Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 423–461. [Google Scholar] [CrossRef]
- Heile, A.O.; Zaman, Q.u.; Aslam, Z.; Hussain, A.; Aslam, M.; Saleem, M.H.; Abualreesh, M.H.; Alatawi, A.; Ali, S. Alleviation of cadmium phytotoxicity using silicon fertilization in wheat by altering antioxidant metabolism and osmotic adjustment. Sustainability 2021, 13, 11317. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Zhang, Y.; Chai, T. Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol. Biochem. 2013, 68, 1–7. [Google Scholar] [CrossRef]
- Fan, X.M.; Qin, L.; Wang, J.X.; Zhan, F.D.; He, Y.M.; Zu, Y.Q. A review of glutathione metabolism and cadmium tolerance in plants. J. West China For. Sci. 2019, 48, 50–56. (In Chinese) [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Rios, J.A.; Nascimento, K.J.T.; Silva, L.C. The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathol. 2014, 63, 581–589. [Google Scholar] [CrossRef]
- Gao, L.; Chang, J.; Chen, R.; Li, H.; Lu, H.; Tao, L.; Xiong, J. Comparison on cellular mechanisms of iron and cadmium accumulation in rice: Prospects for cultivating Fe-rich but Cd-free rice. Rice 2016, 9, 39. [Google Scholar] [CrossRef]



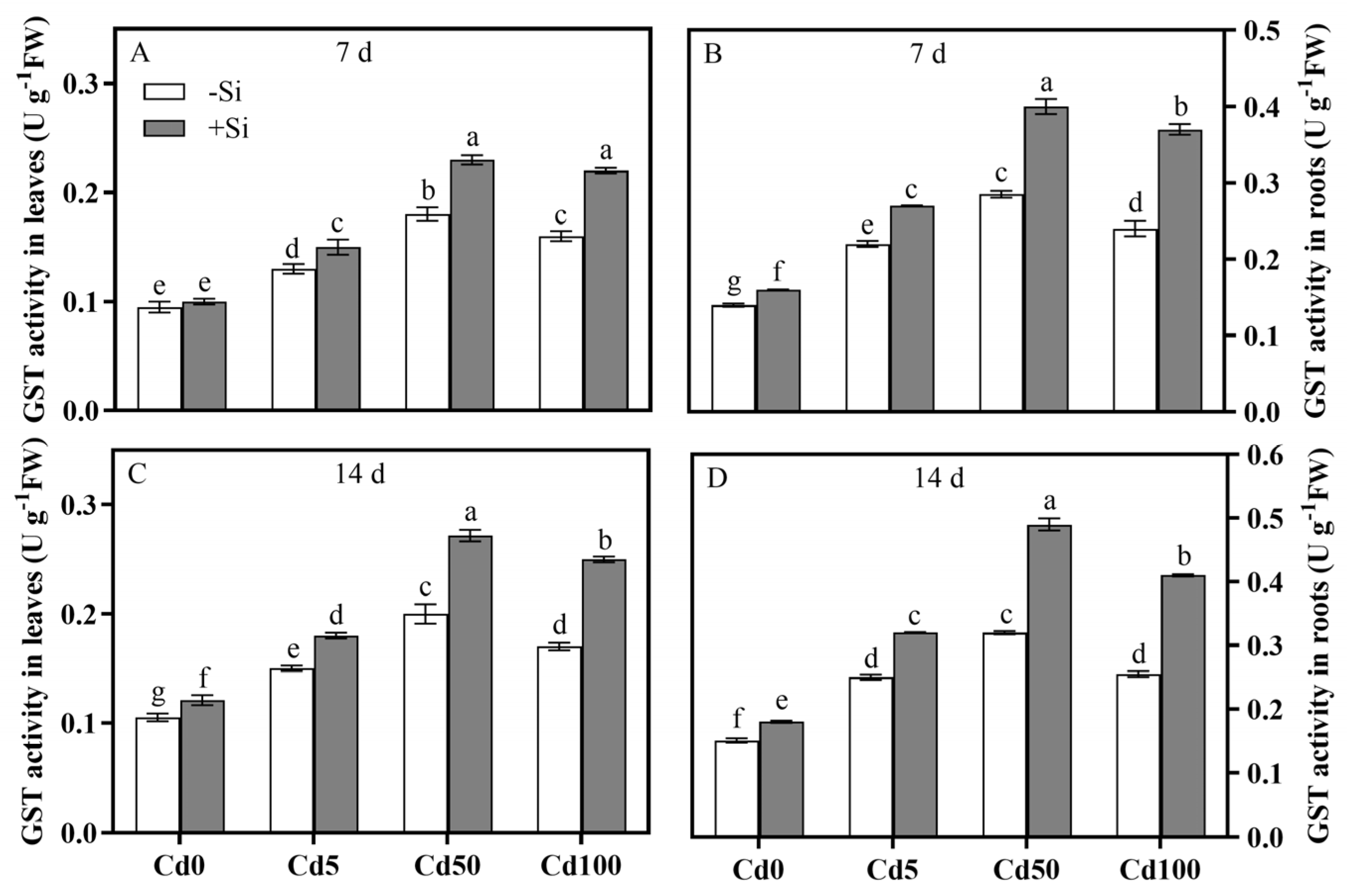
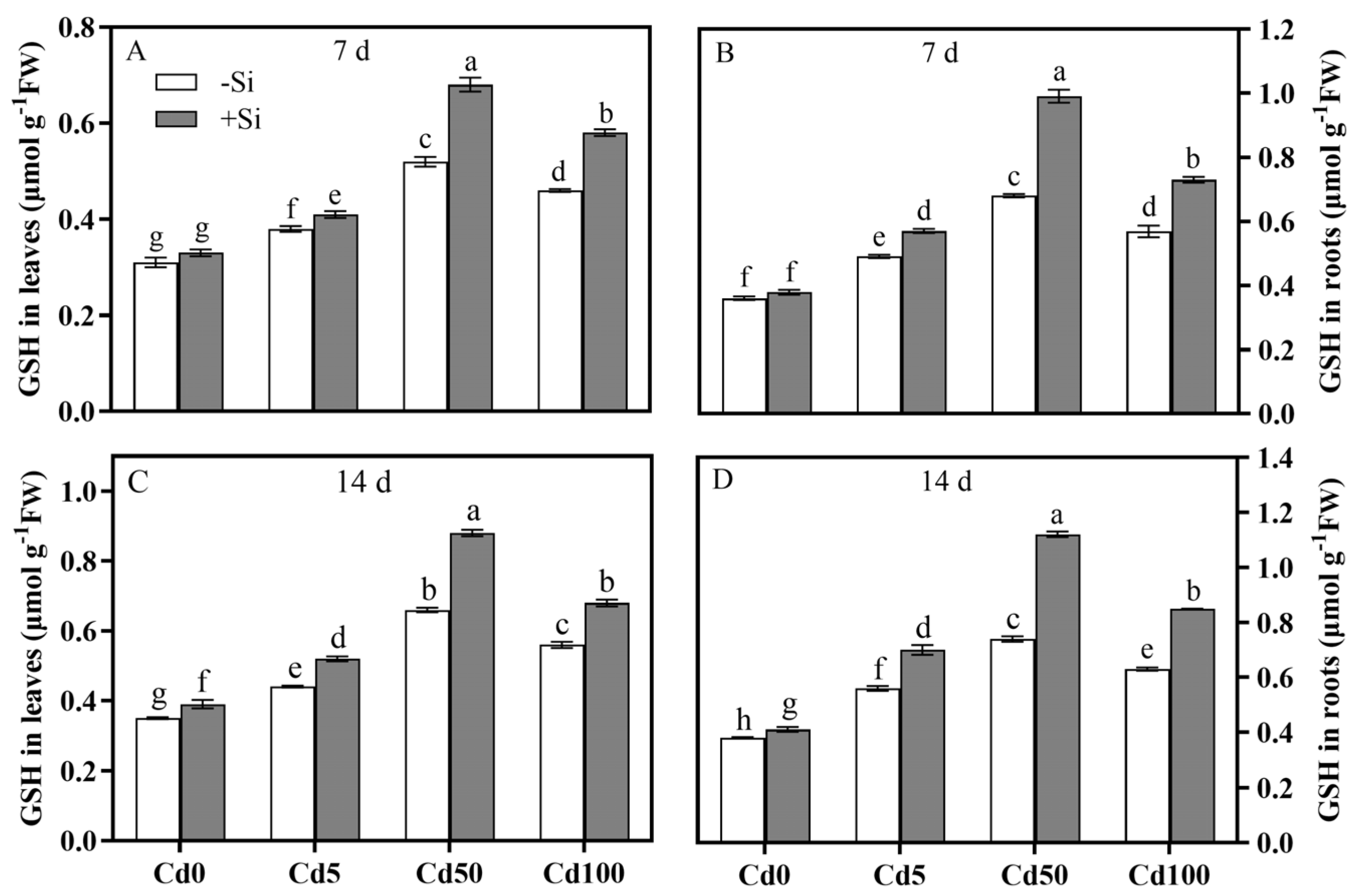
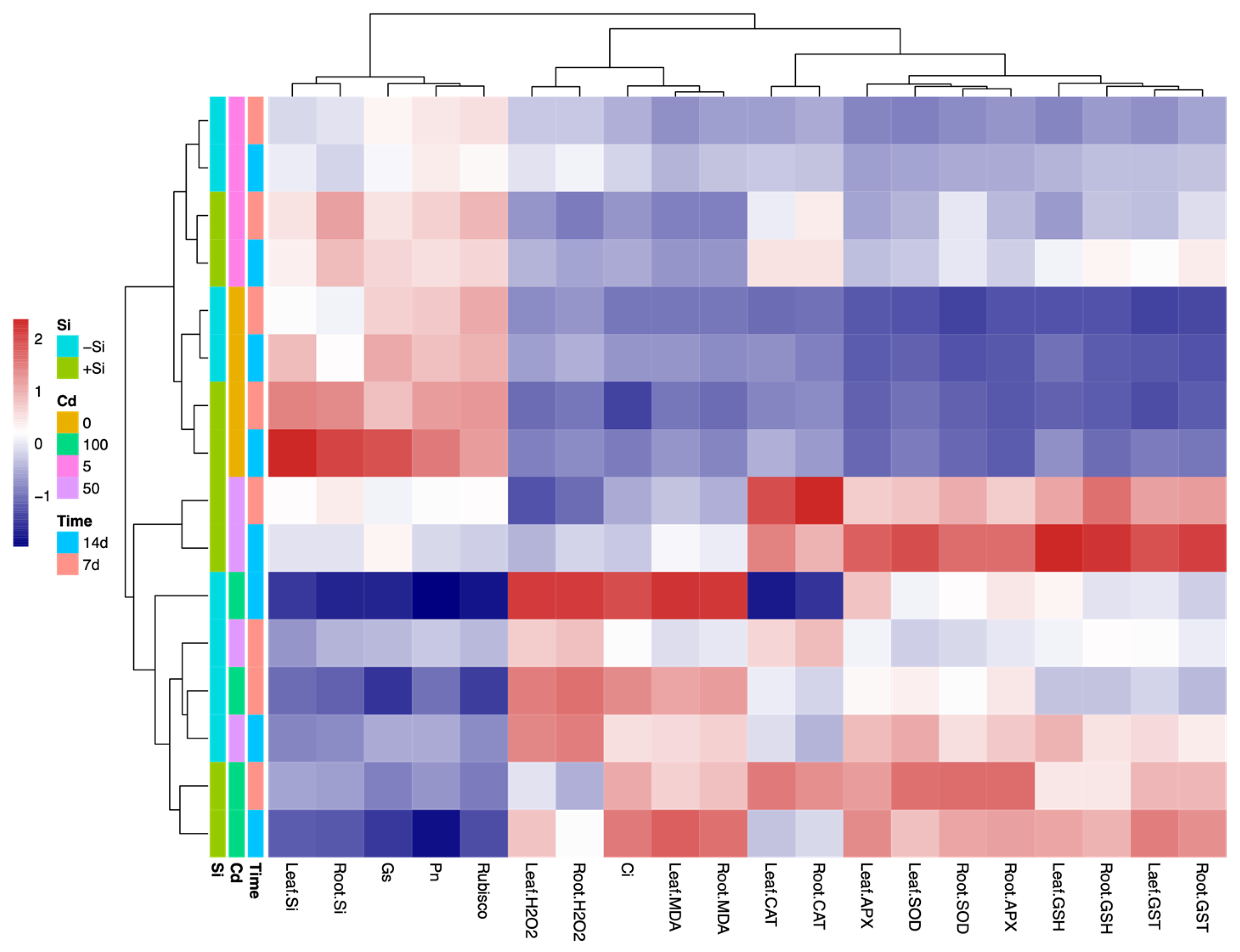
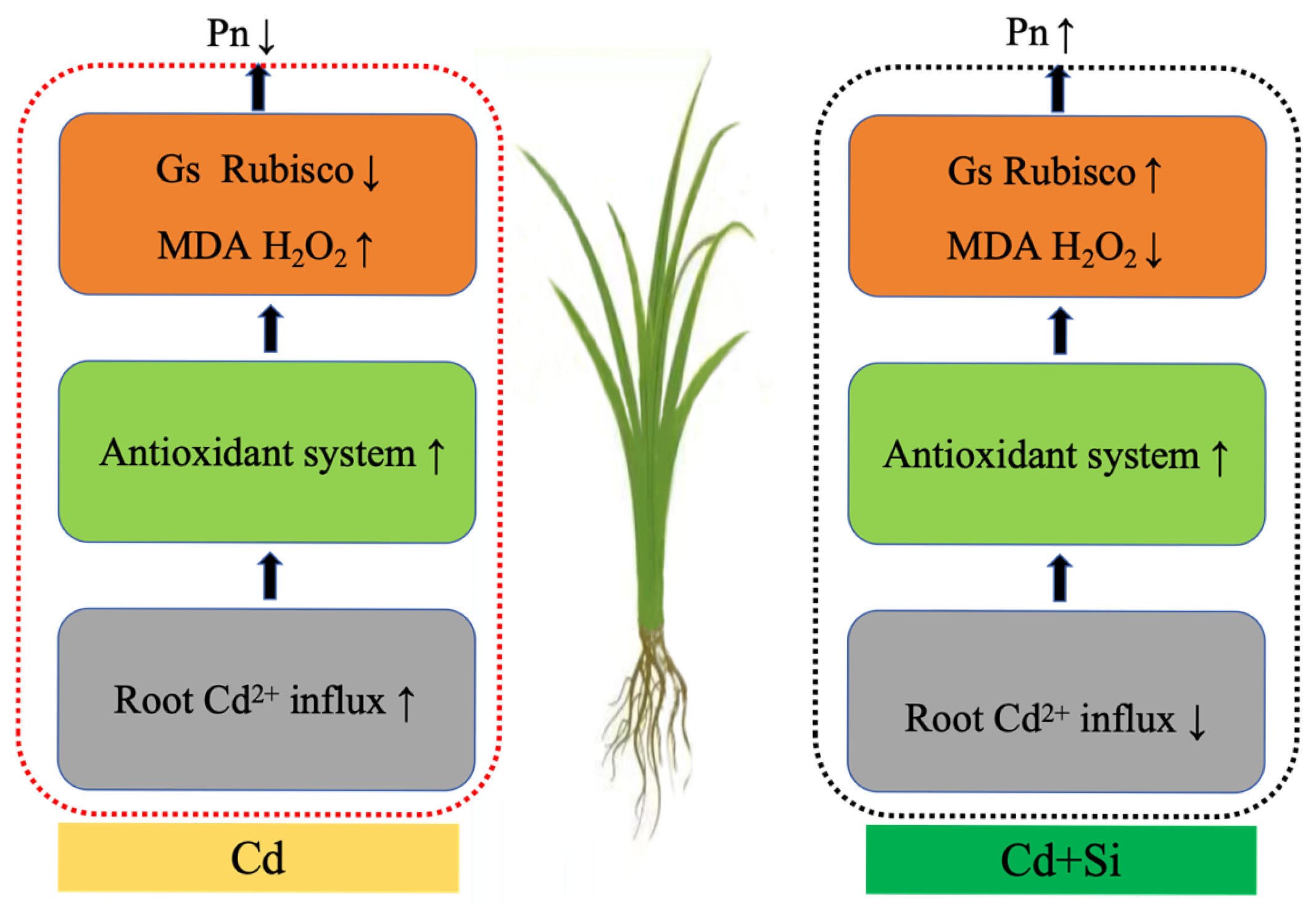
| Parameter | Cd | Si | Cd × Si | |||
|---|---|---|---|---|---|---|
| 7 d | 14 d | 7 d | 14 d | 7 d | 14 d | |
| Net Cd2+ flux | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Cd concentration in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Cd concentration in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Si concentration in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0405 | 0.0001 |
| Si concentration in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0159 | 0.0037 |
| Pn | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.4588 | 0.0115 |
| Gs | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0033 |
| Ci | 0.0001 | 0.0001 | 0.0394 | 0.0433 | 0.8148 | 0.7932 |
| Rubisco | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.004 | 0.0409 |
| MDA in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0005 | 0.0043 |
| MDA in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0005 | 0.0001 |
| H2O2 in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| H2O2 in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| SOD in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0392 |
| SOD in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 |
| APX in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| APX in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| CAT in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| CAT in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| GST in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| GST in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| GSH in leaf | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| GSH in root | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Jiang, M.; Jin, S.; Chen, S. Mechanistic Insights into Silicon-Enhanced Cadmium Detoxification in Rice: A Spatiotemporal Perspective. Agronomy 2025, 15, 2331. https://doi.org/10.3390/agronomy15102331
Lin H, Jiang M, Jin S, Chen S. Mechanistic Insights into Silicon-Enhanced Cadmium Detoxification in Rice: A Spatiotemporal Perspective. Agronomy. 2025; 15(10):2331. https://doi.org/10.3390/agronomy15102331
Chicago/Turabian StyleLin, Hongmei, Miaohua Jiang, Shaofei Jin, and Songbiao Chen. 2025. "Mechanistic Insights into Silicon-Enhanced Cadmium Detoxification in Rice: A Spatiotemporal Perspective" Agronomy 15, no. 10: 2331. https://doi.org/10.3390/agronomy15102331
APA StyleLin, H., Jiang, M., Jin, S., & Chen, S. (2025). Mechanistic Insights into Silicon-Enhanced Cadmium Detoxification in Rice: A Spatiotemporal Perspective. Agronomy, 15(10), 2331. https://doi.org/10.3390/agronomy15102331








