Exploring Organic Matter, Soil Enzymes, and Fungal Communities Under Land-Use Intensification in the Argentine Pampas
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Soil Sampling
2.2. Soil Chemical Analysis
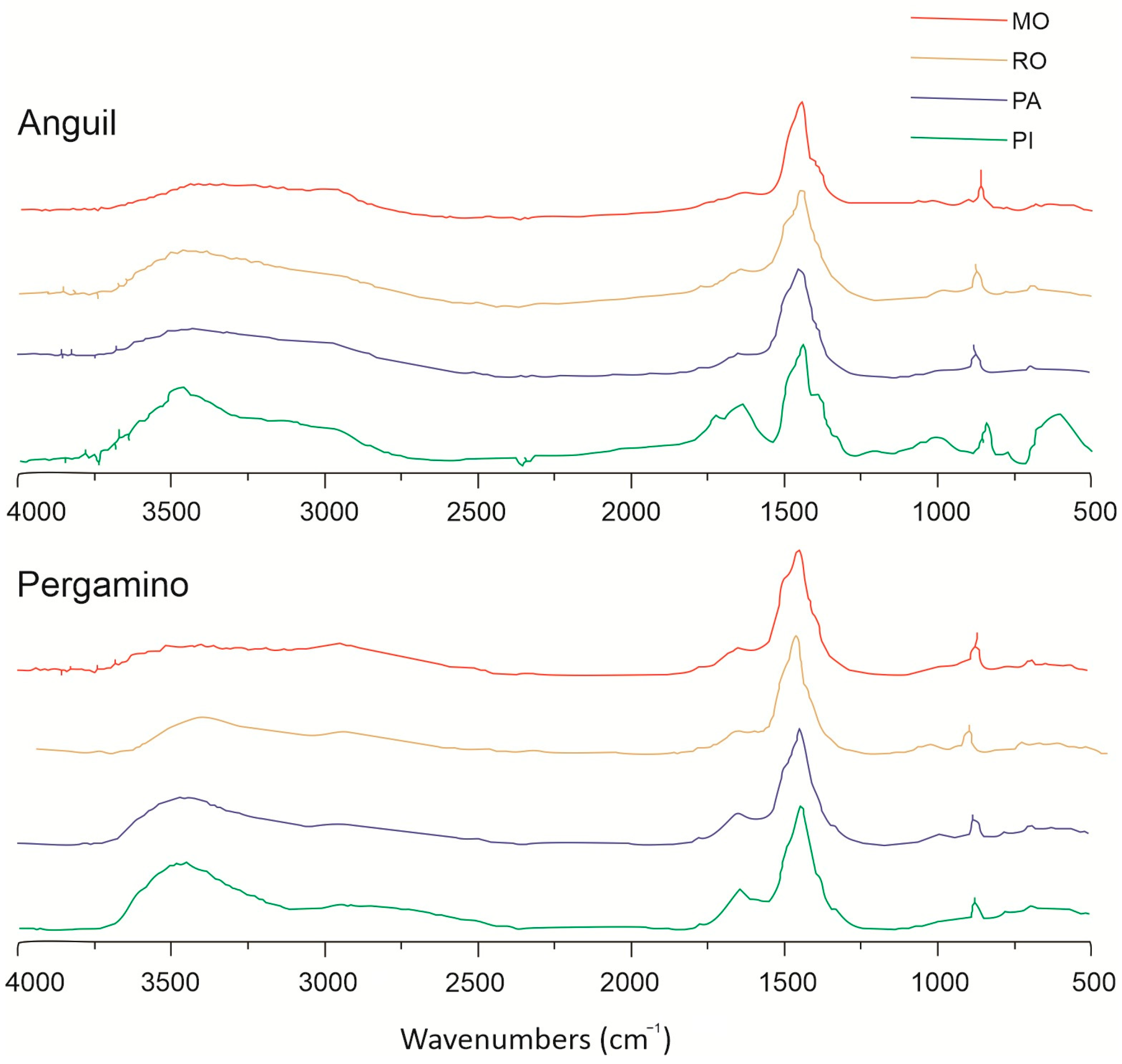
2.3. Soil Spectroscopic Analysis
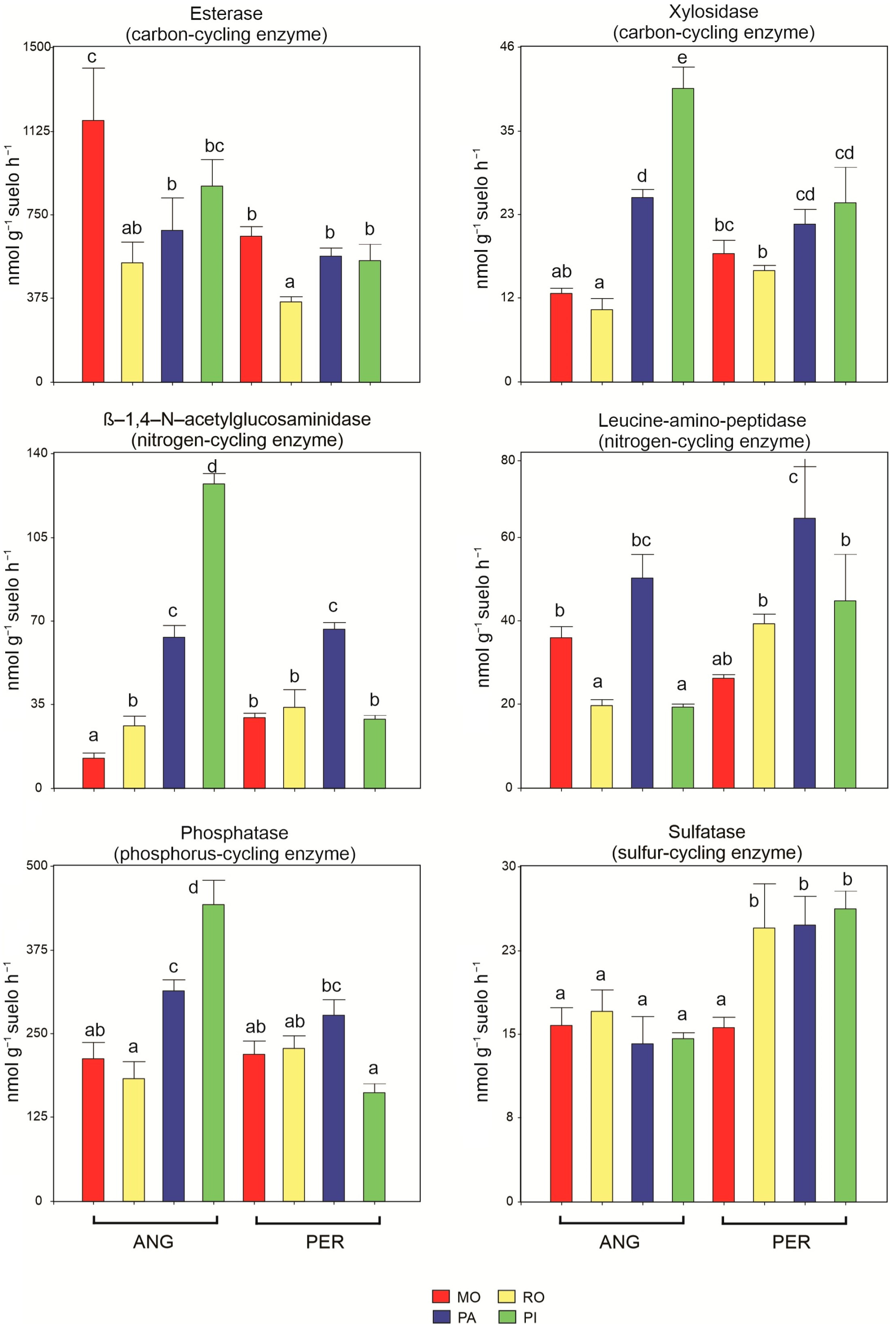
2.4. Soil Enzyme Analysis
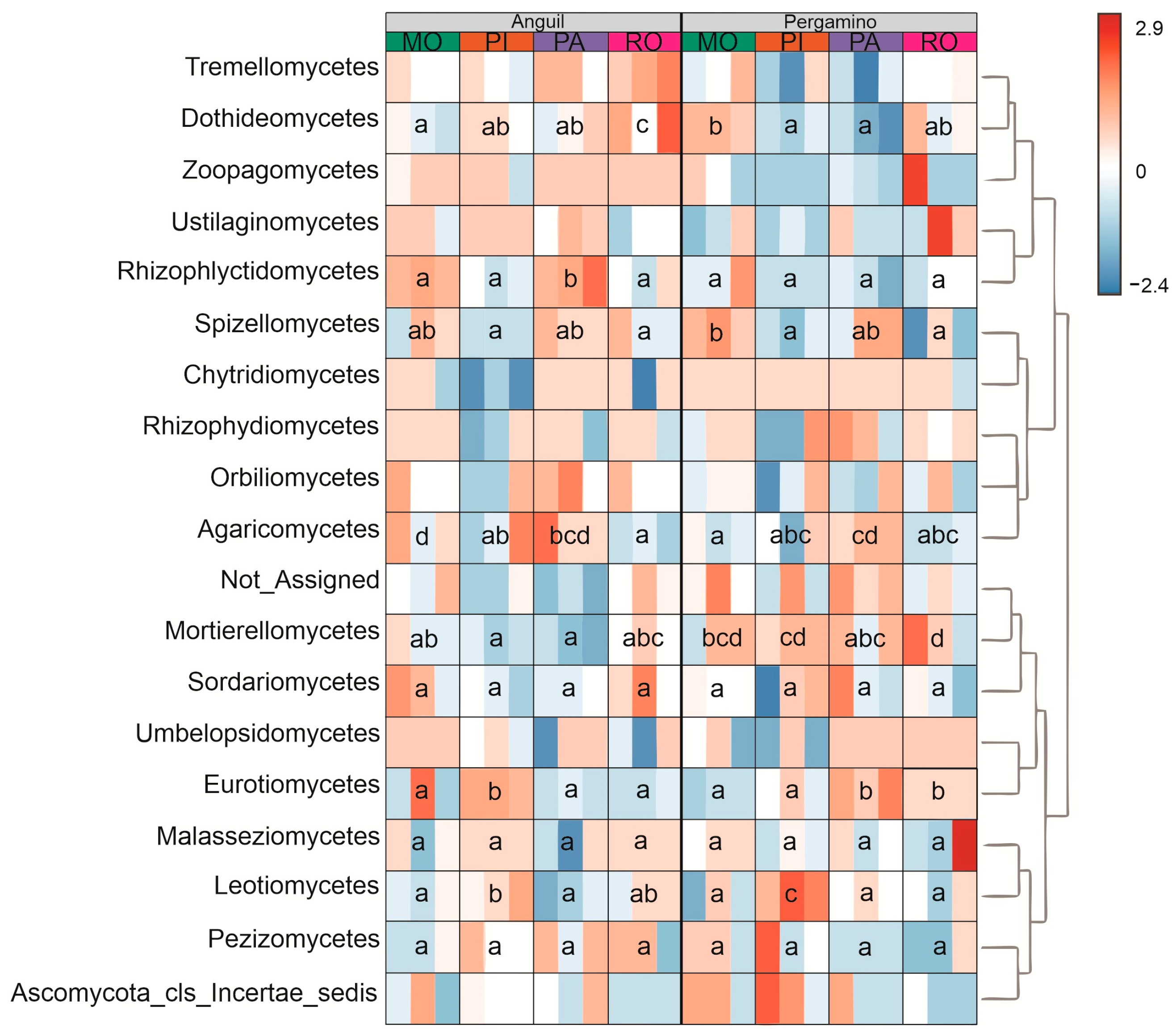
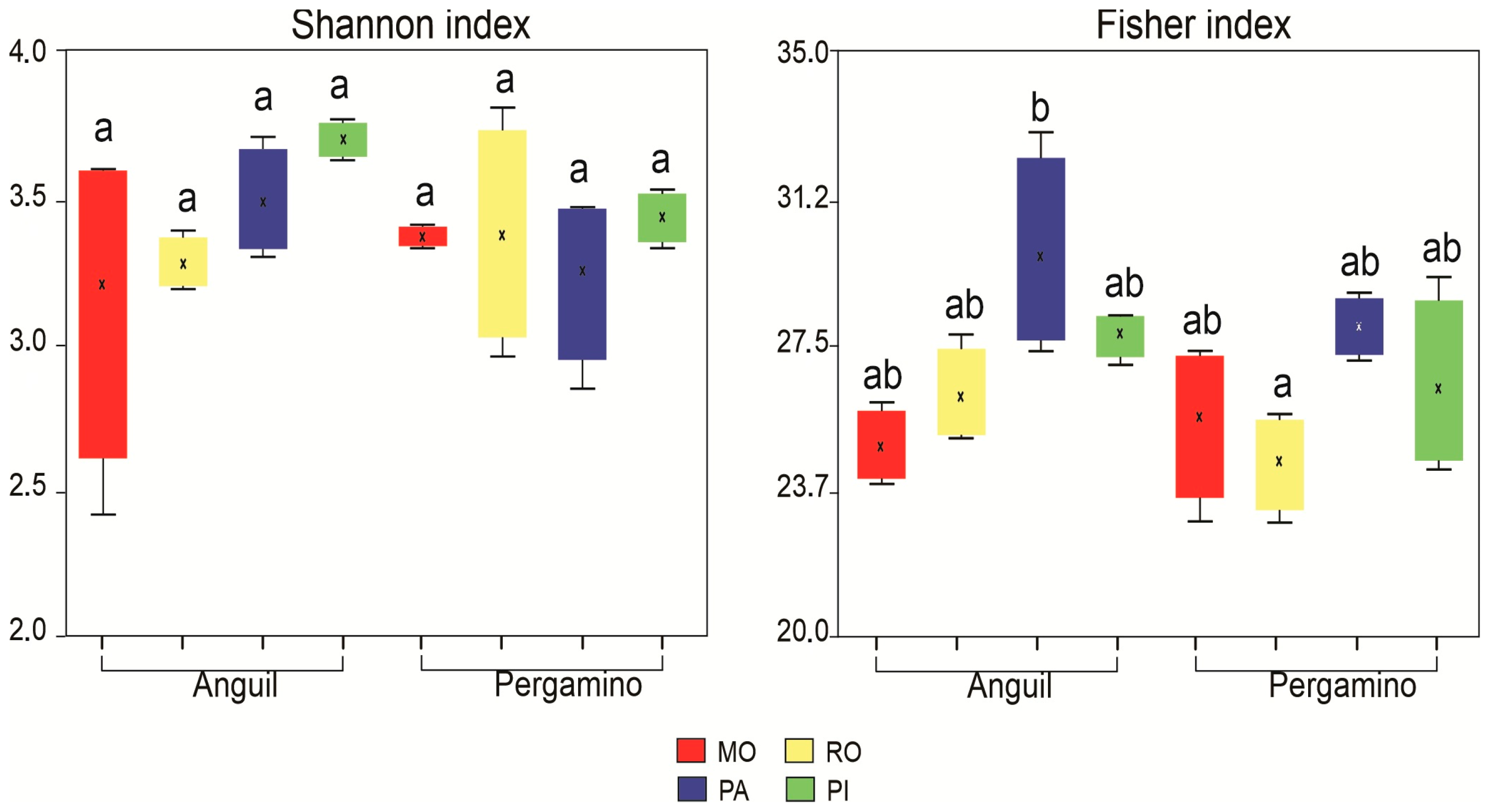
2.5. Fungal Community Characterization
2.6. Data Analysis
3. Results
3.1. Soil Chemical and Spectroscopic Analysis
3.2. Soil Enzyme Activities
3.3. Fungal Community
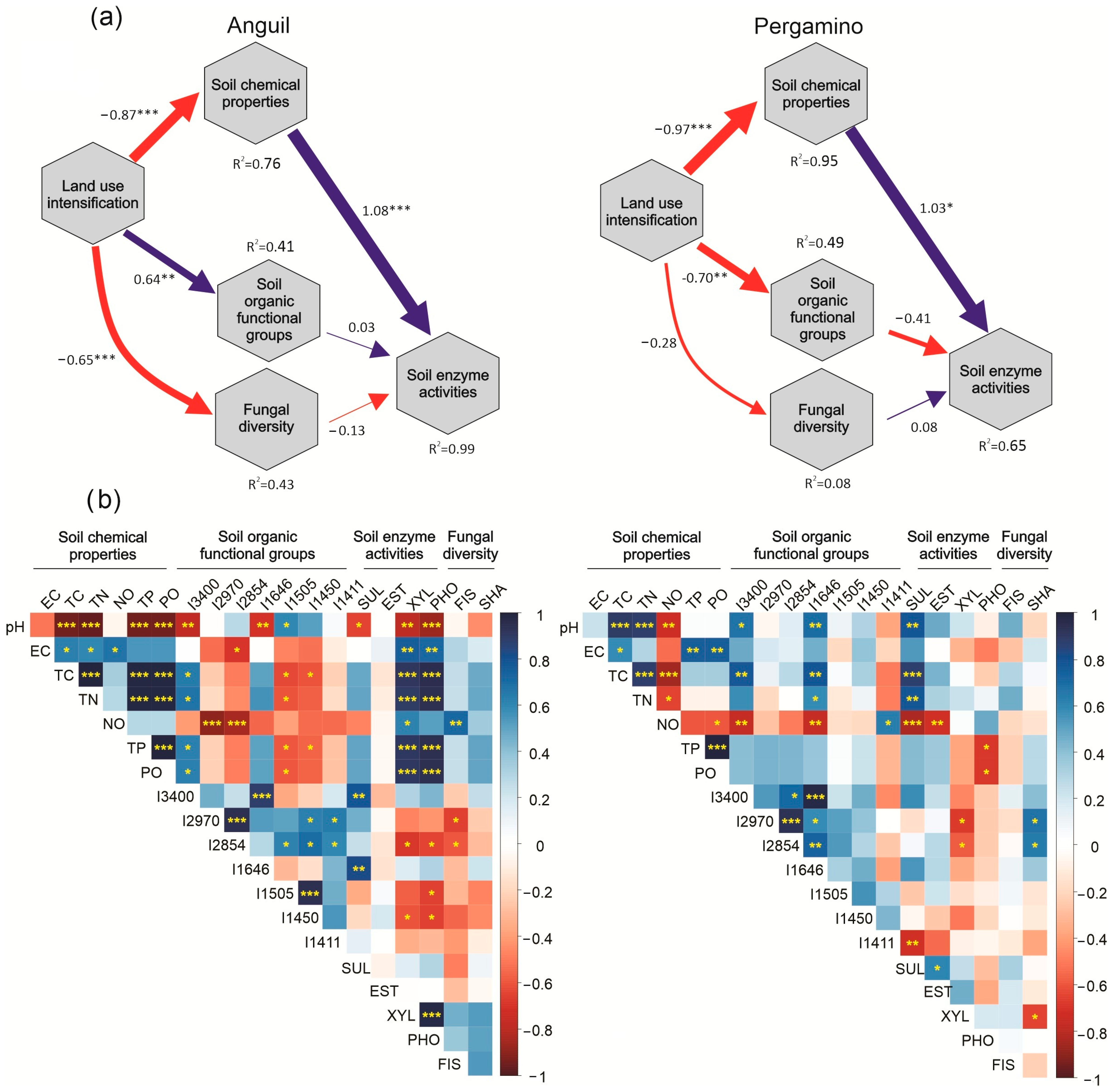
3.4. Ecological Linkages Between Soil Chemical Properties, Enzyme Activities, and Fungal Diversity
4. Discussion
4.1. Soil Properties and Organic Matter Composition Across Land Uses and Location
4.2. Soil Enzyme Activity and Its Relationship with Organic Matter Quality
4.3. Fungal Community Structure and Diversity in Response to Land Use and Location
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wyngaard, N.; Crespo, C.; García, G.V.; Reussi Calvo, N.I.; Rivero, C.; Carciochi, W.D.; Eyherabide, M.; Larrea, G.; Angelini, H.; Barbieri, P.; et al. Nitrogen Mineralization Potential Depletion in Pampas (Argentina) Croplands Following Conversion from Native Grasslands. Geoderma Reg. 2025, 40, e00925. [Google Scholar] [CrossRef]
- De Moraes, A.; Carvalho, P.C.d.F.; Crusciol, C.A.C.; Lang, C.R.; Pariz, C.M.; Deiss, L.; Mark Sulc, R. Integrated Crop-Livestock Systems as a Solution Facing the Destruction of Pampa and Cerrado Biomes in South America by Intensive Monoculture Systems. In Agroecosystem Diversity: Reconciling Contemporary Agriculture and Environmental Quality; Academic Press: Cambridge, MA, USA, 2018; pp. 257–273. [Google Scholar] [CrossRef]
- D’Andrea, M.F.; Letourneau, G.; Rousseau, A.N.; Brodeur, J.C. Sensitivity Analysis of the Pesticide in Water Calculator Model for Applications in the Pampa Region of Argentina. Sci. Total Environ. 2020, 698, 134232. [Google Scholar] [CrossRef]
- Barbero, F.M.; Dominchin, M.F.; Verdenelli, R.A.; Frasier, I.; Restovich, S.B.; Campilongo Mancilla, E.J.; Mlewski, E.C.; Labuckas, D.; Vargas Gil, S.; Meriles, J.M. Impact of Land Use Changes on Soil Chemical Properties, Enzyme Activities and Microbial Communities in Two Contrasting Localities of the Argentinian Pampas. Appl. Soil Ecol. 2025, 206, 105836. [Google Scholar] [CrossRef]
- da Silva, H.F.; de Sousa, P.V.F.; Escobar, M.E.O.; de Oliveira, T.S. Changes in the Composition of Soil Organic Matter Caused by Organic and Conventional Management in the Long Term. J. Environ. Manag. 2025, 374, 124018. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Z.; He, C.; Zhang, Y.; Dong, F.; Huang, W. Effect of Soil Microbial Community Structure on the Chemical Compositions of Different Soil Organic Matter Fractions in Land Uses of the Pearl River Estuary. Appl. Soil Ecol. 2024, 193, 105126. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, Z.; Walden, L.; Sepanta, F.; Luo, Z.; Gao, L.; Serrano, O.; Viscarra Rossel, R.A. Deep Learning of the Particulate and Mineral-Associated Organic Carbon Fractions Using a Compositional Transform and Mid-Infrared Spectroscopy. Geoderma 2025, 455, 117207. [Google Scholar] [CrossRef]
- Zhao, C.; Toufigh, V.; Zhang, J.; Liu, Y.; Fan, W.; He, X.; Cao, B.; Xiao, Y. Enhancing Biomineralization Process Efficiency with Trained Bacterial Strains: A Technical Perspective. Biogeotechnics 2023, 1, 100017. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Lanzén, A.; Mijangos, I.; Garbisu, C. The Application of Fresh and Composted Horse and Chicken Manure Affects Soil Quality, Microbial Composition and Antibiotic Resistance. Appl. Soil Ecol. 2019, 135, 73–84. [Google Scholar] [CrossRef]
- Islam, W.; Zeng, F.; Alotaibi, M.O.; Khan, K.A. Unlocking the Potential of Soil Microbes for Sustainable Desertification Management. Earth-Sci. Rev. 2024, 252, 830011. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Yang, Y.; Bissett, A.; Behrens, T.; Dixon, K.; Nevil, P.; Li, S. Environmental Controls of Soil Fungal Abundance and Diversity in Australia’s Diverse Ecosystems. Soil Biol. Biochem. 2022, 170, 108694. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal Fungi Influence Soil Structure BT—Arbuscular Mycorrhizas: Physiology and Function; Kapulnik, Y., Douds, D.D., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 3–18. ISBN 978-94-017-0776-3. [Google Scholar]
- Cordero, I.; Leizeaga, A.; Hicks, L.C.; Rousk, J.; Bardgett, R.D. High Intensity Perturbations Induce an Abrupt Shift in Soil Microbial State. ISME J. 2023, 17, 2190–2199. [Google Scholar] [CrossRef]
- Bonanomi, G.; D’Ascoli, R.; Antignani, V.; Capodilupo, M.; Cozzolino, L.; Marzaioli, R.; Puopolo, G.; Rutigliano, F.A.; Scelza, R.; Scotti, R.; et al. Assessing Soil Quality under Intensive Cultivation and Tree Orchards in Southern Italy. Appl. Soil Ecol. 2011, 47, 184–194. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gu, X.; Liu, L.; Gou, J. Biochar Application Ameliorated the Nutrient Content and Fungal Community Structure in Different Yellow Soil Depths in the Karst Area of Southwest China. Front. Plant Sci. 2022, 13, 1020832. [Google Scholar] [CrossRef]
- Wu, T.; Sabula, M.; Milner, H.; Strickland, G.; Liu, G. Agricultural Practice Negatively Affects Soil Bacterial Diversity and Nitrogen Functional Genes Comparing to Adjacent Native Forest Soils. Appl. Soil Ecol. 2023, 186, 104856. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, S.; Hu, Q.; Huang, F.; Bao, D.; Li, X.; Dong, C.; Zhu, S.; Fu, J.; Yan, P. Long-Term Agricultural Management Alters Soil Fungal Communities and Soil Carbon and Nitrogen Contents in Tea Plantations. Agronomy 2024, 14, 2779. [Google Scholar] [CrossRef]
- Zhang, H.; He, M.; Pandey, S.P.; Liu, L.; Zhou, S. Soil Fungal Community Is More Sensitive than Bacterial to Mining and Reforestation in a Tropical Rainforest. Land Degrad. Dev. 2023, 34, 4035–4045. [Google Scholar] [CrossRef]
- Bremner, J.M.; Jenkinson, D.S. Determination of Organic Carbon in Soil: Oxidation by Dichromate of Organic Matter in Soil and Plant Materials. J. Soil Sci. 1960, 11, 394–402. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen Total. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- United States Environmental Protection Agency. Methods for the Determination of Inorganic Substances in Environmental Samples; United States Environmental Protection Agency: Cincinnati, OH, USA, 1993. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Giletto, C.M.; Echeverria, H.E.; Sadras, V.; Fertilization, N.; Potato, O.F.; The, I.N.; Pampas, S. Fertilizacion nitrogenada de cultivares de papa (Solanum tuberosum) en el sudeste bonaerense. Cien. Suelo 2003, 21, 44–51. [Google Scholar]
- Marinari, S.; Dell’Abate, M.T.; Brunetti, G.; Dazzi, C. Differences of Stabilized Organic Carbon Fractions and Microbiological Activity along Mediterranean Vertisols and Alfisols Profiles. Geoderma 2010, 156, 379–388. [Google Scholar] [CrossRef]
- Vázquez, C.; Iriarte, A.G.; Merlo, C.; Abril, A.; Kowaljow, E.; Meriles, J.M. Land Use Impact on Chemical and Spectroscopical Characteristics of Soil Organic Matter in an Arid Ecosystem. Environ. Earth Sci. 2016, 75, 883. [Google Scholar] [CrossRef]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A Microplate Fluorimetric Assay for the Study of Enzyme Diversity in Soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of Hydrolytic and Oxidative Enzyme Methods for Ecosystem Studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Usyk, M.; Zolnik, C.P.; Patel, H.; Levi, M.H.; Burk, R.D. Novel ITS1 Fungal Primers for Characterization of the Mycobiome. mSphere 2017, 2, e00488-17. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive Statistical, Functional and Integrative Analysis of Microbiome Data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.D.; Macklaim, J.M.; Linn, T.G.; Reid, G.; Gloor, G.B. ANOVA-Like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. PLoS ONE 2013, 8, e67019. [Google Scholar] [CrossRef]
- Giudici, P.; Marcos, M.; Olivera, N.L. Effects of the Cessation of Fish-Processing Effluent Discharges on the Soil Microbiome Associated with Saltbush Patches. Appl. Soil Ecol. 2023, 187, 104832. [Google Scholar] [CrossRef]
- Alvarez, R.; Gimenez, A.; Caffaro, M.; Pagnanini, F.; Gangi, D.; Berhongaray, G. Land Use Effects on Soil Organic Carbon Fractions in the Pampas of Argentina Determined via Inverse Analysis of Carbon Mineralization Data. Commun. Soil Sci. Plant Anal. 2022, 53, 2094–2104. [Google Scholar] [CrossRef]
- Peltre, C.; Bruun, S.; Du, C.; Thomsen, I.K.; Jensen, L.S. Assessing Soil Constituents and Labile Soil Organic Carbon by Mid-Infrared Photoacoustic Spectroscopy. Soil Biol. Biochem. 2014, 77, 41–50. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Qi, J.; Zhang, H.; Tao, F.; Zhang, R. Priming Effects of Soil Organic Matter Decomposition with Addition of Different Carbon Substrates. J. Soils Sediments 2019, 19, 1171–1178. [Google Scholar] [CrossRef]
- Merino, A.; Omil, B.; Piñeiro, V.; Barros, N.; Souza-Alonso, P.; Campo, J. Soil C Dynamics after Deforestation and Subsequent Conversion of Arable Cropland to Grassland in Humid Temperate Areas. Sci. Total Environ. 2023, 901, 165793. [Google Scholar] [CrossRef] [PubMed]
- Pisani, O.; Hills, K.M.; Courtier-Murias, D.; Haddix, M.L.; Paul, E.A.; Conant, R.T.; Simpson, A.J.; Arhonditsis, G.B.; Simpson, M.J. Accumulation of Aliphatic Compounds in Soil with Increasing Mean Annual Temperature. Org. Geochem. 2014, 76, 118–127. [Google Scholar] [CrossRef]
- Jokic, A.; Cutler, J.N.; Ponomarenko, E.; van der Kamp, G.; Anderson, D.W. Organic Carbon and Sulphur Compounds in Wetland Soils: Insights on Structure and Transformation Processes Using K-Edge XANES and NMR Spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 2585–2597. [Google Scholar] [CrossRef]
- Kaufman, D.D. Structure of Pesticides and Decomposition by Soil Microorganisms. In Pesticides and Their Effects on Soils and Water; Soil Science Society of America: Madison, WI, USA, 1966; Volume 8. [Google Scholar] [CrossRef]
- Margenot, A.J.; Calderón, F.J.; Bowles, T.M.; Parikh, S.J.; Jackson, L.E. Soil Organic Matter Functional Group Composition in Relation to Organic Carbon, Nitrogen, and Phosphorus Fractions in Organically Managed Tomato Fields. Soil Sci. Soc. Am. J. 2015, 79, 772–782. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. FTIR Spectral Band Shifts Explained by OM–Cation Interactions. J. Plant Nutr. Soil Sci. 2021, 184, 388–397. [Google Scholar] [CrossRef]
- Ferreras, L.A.; Toresani, S.M.I.; Faggioli, V.S.; Galarza, C.M. Sensibilidad de Indicadores Biológicos Edáficos En Un Argiudol de La Región Pampeana Argentina. Span. J. Soil Sci. 2015, 5, 227–242. [Google Scholar] [CrossRef]
- Ding, J.; Yu, S. Impacts of Land Use on Soil Nitrogen-Cycling Microbial Communities: Insights from Community Structure, Functional Gene Abundance, and Network Complexity. Life 2025, 15, 466. [Google Scholar] [CrossRef]
- Pratibha, G.; Manjunath, M.; Raju, B.M.K.; Srinivas, I.; Rao, K.V.; Shanker, A.K.; Prasad, J.V.N.S.; Rao, M.S.; Kundu, S.; Indoria, A.K.; et al. Soil Bacterial Community Structure and Functioning in a Long-Term Conservation Agriculture Experiment under Semi-Arid Rainfed Production System. Front. Microbiol. 2023, 14, 1102682. [Google Scholar] [CrossRef]
- Di Gerónimo, P.F.; Cecilia Del carmen ViDela, P. laclau. Efecto de La Agriculturización Sobre La Calidad Biológica Del Suelo. Cienc. Suelo 2018, 36, 124–137. [Google Scholar]
- Suran, P.; Balík, J.; Kulhánek, M.; Sedlář, O.; Černý, J. The Relationship of Soil Sulfur with Glomalin-Related Soiprotein and Humic Substances under Different Mineral and Organic Fertilisation. Plant Soil Environ. 2024, 70, 93–100. [Google Scholar] [CrossRef]
- Khan, M.F.; Liao, J.; Liu, Z.; Chugh, G. Bacterial Cytochrome P450 Involvement in the Biodegradation of Fluorinated Pyrethroids. J. Xenobiotics 2025, 15, 58. [Google Scholar] [CrossRef]
- Ye, F.; Gong, D.; Pang, C.; Luo, J.; Zeng, X.; Shang, C. Analysis of Fungal Composition in Mine-Contaminated Soils in Hechi City. Curr. Microbiol. 2020, 77, 2685–2693. [Google Scholar] [CrossRef]
- Chen, L.R.; Lin, J.N.; Shen, R.; Zhu, G.Y.; Lai, J.S.; Lin, D.M. Characteristic of Soil Fungal Community and the Influencing Factors of Pinus Massoniana Forests in the Three Gorges Reservoir Region. Chin. J. Appl. Ecol. 2022, 33, 2397–2404. [Google Scholar] [CrossRef]
- Hou, N.; Jin, Q.; Liu, X.; Li, X.; Lin, S.; Zhang, Y.; Wang, W. Community Characteristics of Soil Fungi in Tea Plantations under Different Management Modes and Prediction on Their Functions. J. Plant Resour. Environ. 2024, 33, 77–86. [Google Scholar] [CrossRef]
- Johnston, P.R.; Quijada, L.; Smith, C.A.; Baral, H.O.; Hosoya, T.; Baschien, C.; Pärtel, K.; Zhuang, W.Y.; Haelewaters, D.; Park, D.; et al. A Multigene Phylogeny toward a New Phylogenetic Classification of Leotiomycetes. IMA Fungus 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; Etayo, J.; Olariaga, I. A New Lineage of Mazaediate Fungi in the Eurotiomycetes: Cryptocaliciomycetidae Subclass. Nov., Based on the New Species Cryptocalicium Blascoi and the Revision of the Ascoma Evolution. Mycol. Prog. 2021, 20, 889–904. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Barrasa, J.M.; Sánchez-García, M.; Camarero, S.; Miyauchi, S.; Serrano, A.; Linde, D.; Babiker, R.; Drula, E.; Ayuso-Fernández, I.; et al. Genomic Analysis Enlightens Agaricales Lifestyle Evolution and Increasing Peroxidase Diversity. Mol. Biol. Evol. 2021, 38, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Noya, Y.E.; Montoya-Ciriaco, N.; Muñoz-Arenas, L.C.; Hereira-Pacheco, S.; Estrada-Torres, A.; Dendooven, L. Conversion of a High-Altitude Temperate Forest for Agriculture Reduced Alpha and Beta Diversity of the Soil Fungal Communities as Revealed by a Metabarcoding Analysis. Front. Microbiol. 2021, 12, 667566. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Röhrig, L.; Dussart, F. Does Abiotic Host Stress Favour Dothideomycete-Induced Disease Development? Plants 2022, 11, 1615. [Google Scholar] [CrossRef]
- Nai, C.; Wong, H.Y.; Pannenbecker, A.; Broughton, W.J.; Benoit, I.; de Vries, R.P.; Gueidan, C.; Gorbushina, A.A. Nutritional Physiology of a Rock-Inhabiting, Model Microcolonial Fungus from an Ancestral Lineage of the Chaetothyriales (Ascomycetes). Fungal Genet. Biol. 2013, 56, 54–66. [Google Scholar] [CrossRef]
- Gonçalez, E.; Aparecido, C.C.; Felicio, J.D. Fusarium: Epidemiology, Environmental Sources and Prevention; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 75–99. ISBN 978-161942539-2. [Google Scholar]
- Lozupone, C.A.; Klein, D.A. Molecular and Cultural Assessment of Chytrid and Spizellomyces Populations in Grassland Soils. Mycologia 2002, 94, 411–420. [Google Scholar] [CrossRef]
- Sharma Ghimire, P.; Ouyang, H.; Wang, Q.; Luo, Y.; Shi, B.; Yang, J.; Lü, Y.; Jin, C. Insight into Enzymatic Degradation of Corn, Wheat, and Soybean Cell Wall Cellulose Using Quantitative Secretome Analysis of Aspergillus Fumigatus. J. Proteome Res. 2016, 15, 4387–4402. [Google Scholar] [CrossRef]
- Bainard, L.D.; Navarro-Borrell, A.; Hamel, C.; Braun, K.; Hanson, K.; Gan, Y. Increasing the Frequency of Pulses in Crop Rotations Reduces Soil Fungal Diversity and Increases the Proportion of Fungal Pathotrophs in a Semiarid Agroecosystem. Agric. Ecosyst. Environ. 2017, 240, 206–214. [Google Scholar] [CrossRef]
- Szink, I.; Davis, E.L.; Ricks, K.D.; Koide, R.T. New Evidence for Broad Trophic Status of Leaf Endophytic Fungi of Quercus Gambelii. Fungal Ecol. 2016, 22, 2–9. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, W.; Wu, C.; Feng, H.; Peng, Y.; Shahid, H.; Cui, Z.; Ding, P.; Shan, T. Diversity and Antibacterial Activity of Fungal Endophytes from Eucalyptus Exserta. BMC Microbiol. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic Fungi: Resource for Gibberellins and Crop Abiotic Stress Resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, C.L. The Phylogenetic Relationship Revealed Three New Wood-Inhabiting Fungal Species From Genus Trechispora. Front. Microbiol. 2021, 12, 650195. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Bardgett, R.D. Soil Biodiversity and Carbon Cycling: A Review and Synthesis of Studies Examining Diversity-Function Relationships. Eur. J. Soil Sci. 2011, 62, 105–116. [Google Scholar] [CrossRef]
- Li, Y.Z.; Bao, X.L.; Zhu, X.F.; Deng, F.B.; Yang, Y.L.; Zhao, Y.; Xie, H.T.; Tang, S.X.; Ge, C.J.; Liang, C. Parent Material Influences Soil Properties to Shape Bacterial Community Assembly Processes, Diversity, and Enzyme-Related Functions. Sci. Total Environ. 2024, 927, 172064. [Google Scholar] [CrossRef] [PubMed]

| Location | Land Use | Land Management | Management History |
|---|---|---|---|
| Anguil | PI | Natural grassland dominated by Stipa tenuis, Poa ligularis, Bromus brevis, Piptochaetium napostaense, and Prosopis caldenia Burkart (Caldén woodland) | Agriculture experimental plots were established in 2009 in a production field belonging to the CREA group in La Pampa Province, Argentina. Soybean was sown under no-tillage in December at 24 plants m−2 with 0.52 m row spacing and harvested in May. Cover crops were sown at 80 seeds m−2 in late May and fertilized with 40 kg N ha−1, and terminated with glyphosate in October. |
| PA | Alfalfa (Medicago sativa L.) and Festuca (Festuca arundinacea) | ||
| RO | Soybean/corn rotation with Centeno (Secalecereale L.) | ||
| MO | Soybean monoculture | ||
| Pergamino | PI | Natural grassland dominated by Stipa spp., Cynodon dactylon, Paspalum spp., along with various forbs like thistles (Carduus pycnocephalus and Dipsacus fullonum). | Agricultural experimental plots were established in 2011 at the Experimental Station of INTA Pergamino in Buenos Aires Province, Argentina. Soybean (Glycine max L., var. DM 5.1) was sown under no-tillage in November at 50 plants m−2 with 0.52 m row spacing, and weeds were controlled with post-emergent glyphosate. Cover crops were sown in April–May at 80 and 20 kg ha−1, respectively, fertilized with 14.7 kg P2O5 ha−1, and terminated with glyphosate in October. |
| PA | Festuca roja (Festuca rubra) | ||
| RO | Soybean/corn rotation with oats and tillage radish (Avena sativa L., and Raphanus sativus L.) | ||
| MO | Soybean monoculture |
| Infrared Band | Anguil | Pergamino | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Funcional Group | MO | RO | PA | PI | MO | RO | PA | PI | |
| I3400 | Amide A OH/NH | 0.47 ± 0.30 ab | 0.74 ± 0.06 bc | 0.30 ± 0.04 a | 0.90 ± 0.08 c | 0.33 ± 0.05 a | 0.37 ± 0.07 a | 0.44 ± 0.09 ab | 0.62 ± 0.05 abc |
| I2970 | Aliphatic CH | 0.43 ± 0.10 bc | 0.55 ± 0.04 c | 0.23 ± 0.02 a | 0.37 ± 0.06 abc | 0.30 ± 0.03 ab | 0.27 ± 0.06 ab | 0.24 ± 0.03 ab | 0.30 ± 0.01 ab |
| I2854 | Aliphatic CH | 0.34 ± 0.03 ab | 0.49 ± 0.04 b | 0.19 ± 0.01 a | 0.23 ± 0.05 a | 0.24 ± 0.02 a | 0.18 ± 0.05 a | 0.20 ± 0.04 a | 0.26 ± 0.01 a |
| I1750 | Carboxyl. C=O | 0.35 ± 0.06 ab | 0.35 ± 0.04 ab | 0.11 ± 0.02 a | 0.52 ± 0.12 b | 0.12 ± 0.01 a | 0.16 ± 0.04 a | 0.12 ± 0.02 a | 0.14 ± 0.00 a |
| I1646 | Aromatic/Amide I | 0.41 ± 0.05 abc | 0.48 ± 0.02 bc | 0.22 ± 0.02 a | 0.56 ± 0.06 c | 0.24 ± 0.02 a | 0.25 ± 0.04 a | 0.29 ± 0.04 ab | 0.38 ± 0.03 abc |
| I1505 | Aromatic C=C | 0.66 ± 0.04 a | 0.71 ± 0.03 a | 0.58 ± 0.07 a | 0.45 ± 0.14 a | 0.63 ± 0.04 a | 0.59 ± 0.03 a | 0.61 ± 0.06 a | 0.62 ± 0.02 a |
| I1450 | Alkane CH2/CH3 | 0.99 ± 0.01 a | 1.00 ± 0.00 a | 0.86 ± 0.05 a | 0.81 ± 0.10 a | 0.89 ± 0.10 a | 1.00 ± 0.00 a | 0.91 ± 0.09 a | 0.99 ± 0.01 a |
| I1411 | Carboxyl. COO− | 0.64 ± 0.04 a | 0.67 ± 0.03 a | 0.55 ± 0.06 a | 0.60 ± 0.06 a | 0.55 ± 0.07 a | 0.62 ± 0.02 a | 0.51 ± 0.08 a | 0.44 ± 0.02 a |
| I1337 | Polysac. C–O | 0.38 ± 0.06 a | 0.34 ± 0.06 a | 0.17 ± 0.02 a | 0.39 ± 0.08 a | 0.16 ± 0.02 a | 0.21 ± 0.03 a | 0.19 ± 0.03 a | 0.20 ± 0.02 a |
| I1226 | Polysac. C–O–C | 0.33 ± 0.09 b | 0.26 ± 0.05 ab | 0.08 ± 0.02 a | 0.27 ± 0.07 ab | 0.05 ± 0.01 a | 0.09 ± 0.04 a | 0.05 ± 0.01 a | 0.05 ± 0.00 a |
| I1023 | Polysac. C–O | 0.35 ± 0.09 b | 0.31 ± 0.04 b | 0.10 ± 0.02 a | 0.35 ± 0.02 b | 0.08 ± 0.01 a | 0.14 ± 0.03 a | 0.10 ± 0.01 a | 0.10 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, F.M.; Verdenelli, R.A.; Dominchin, M.F.; Frasier, I.; Restovich, S.B.; Serri, D.L.; Campilongo-Mancilla, E.J.; Faggioli, V.S.; Iriarte, A.G.; Vargas-Gil, S.; et al. Exploring Organic Matter, Soil Enzymes, and Fungal Communities Under Land-Use Intensification in the Argentine Pampas. Agronomy 2025, 15, 2469. https://doi.org/10.3390/agronomy15112469
Barbero FM, Verdenelli RA, Dominchin MF, Frasier I, Restovich SB, Serri DL, Campilongo-Mancilla EJ, Faggioli VS, Iriarte AG, Vargas-Gil S, et al. Exploring Organic Matter, Soil Enzymes, and Fungal Communities Under Land-Use Intensification in the Argentine Pampas. Agronomy. 2025; 15(11):2469. https://doi.org/10.3390/agronomy15112469
Chicago/Turabian StyleBarbero, Florencia M., Romina A. Verdenelli, María F. Dominchin, Ileana Frasier, Silvina B. Restovich, Dannae L. Serri, Ernesto J. Campilongo-Mancilla, Valeria S. Faggioli, Ana G. Iriarte, Silvina Vargas-Gil, and et al. 2025. "Exploring Organic Matter, Soil Enzymes, and Fungal Communities Under Land-Use Intensification in the Argentine Pampas" Agronomy 15, no. 11: 2469. https://doi.org/10.3390/agronomy15112469
APA StyleBarbero, F. M., Verdenelli, R. A., Dominchin, M. F., Frasier, I., Restovich, S. B., Serri, D. L., Campilongo-Mancilla, E. J., Faggioli, V. S., Iriarte, A. G., Vargas-Gil, S., & Meriles, J. M. (2025). Exploring Organic Matter, Soil Enzymes, and Fungal Communities Under Land-Use Intensification in the Argentine Pampas. Agronomy, 15(11), 2469. https://doi.org/10.3390/agronomy15112469







