Abstract
In order to replace chemical herbicides, which harm the environment and health, we seek sustainable methods to control weeds. We remove a seed-beetle species, Acanthoscelides atrocephalus, from synonymy with Acanthoscelides modestus and recognize it as a potential bioagent for Aeschynomene denticulata and A. indica. Belonging the megacornis group of the genus Acanthoscelides, its fine morphology was analyzed using high-resolution photography and scanning electron microscopy. The species differs from others of the A. megacornis group based on integument coloration, distinctive patterns of vestiture on the pronotum and pygidium, large and sexually dimorphic eyes, a strong frontal carina extending from the vertex of the head to the clypeus, and distinctive armature in the internal sac of the male genitalia. It stands out as a biological control agent due to the larvae’s habit of feeding on seeds, which hinders the development of the embryo. Through tetrazolium and germination tests, it was discovered that 100% of the infested seeds had no viable seed integument and did not germinate. A. atrocephalus is no longer a synonym of Acanthoscelides modestus. This species is a predator of A. denticulata and A. indica and prevents seed germination, becoming promising as a bioagent for the control of these weeds.
1. Introduction
Seed beetles (Chrysomelidae, Bruchinae) are phytophagous insects associated in a major part with the family Fabaceae Lindl. [1]. The females of these beetles lay their eggs on the pods or directly on the seeds, where the larvae penetrate, consume the seeds, and complete their development [2]. In general, each bruchine depends entirely upon a seed for its larval development. Bruchinae is the largest group of insects that engage in this feeding behavior, which happens in almost all species of the subfamily [3]. Today, the subfamily comprises almost 1750 species, with 1650 [4] and approximately 100 assigned to 65 genera worldwide [5,6].
The genus Acanthoscelides Schiskly, 1905 (Chrysomelidae, Bruchinae) is one of the largest genera in the subfamily, and its species are restricted to the New World [7]. The significant economic importance of the genus is currently concentrated on the bean weevil, Acanthoscelides obtectus (Say, 1831), a globally important seed-beetle pest [8]. However, bruchines have already shown that certain species can be beneficial when used in weed-control programs [4,9,10].
Weed control, a critical component of crop management, involves inhibiting growth and/or reducing the number of individuals per area to levels below the economic losses to the crop [11]. The most commonly used control method is chemical. However, the emergence of cases of weed resistance to herbicides in rice crops in Brazil is a pressing issue. Recent findings show that Aeschynomene denticulate is resistant to herbicides, highlighting the urgent need for integrated management practices. These practices, which combine various control methods, are necessary to effectively control weeds and prevent a reduction in crop productivity [12].
While the chemical method remains prominent among control methods, there is a growing interest in alternative weed-control methods [13]. These alternatives, which involve different means of control, promise to impact agribusiness positively. In this context, biological control has emerged as a significant player in the integrated management of weeds. The use of bioagents such as insects has proven to be insightful and holds great potential for the development of new, effective weed-control implementations [14].
Weeds of the genus Aeshynomene are known in Brazil as angiquinho, maricazinho, paquinha, pinheirinho, and corticeirinha. They grow in humid places and are very adapted to flooded soils; they are common in irrigated rice crops at the beginning and end of the crop cycle, even though they do not germinate in flooded environments [15,16]. It is considered the main broadleaf weed in rice crops and the third most likely to escape herbicides [17]. It can cause a 57.2% reduction in rice productivity when there are 25 to 31 angiquinho plants per square meter [18]. Chemical herbicides are still frequently used to control Aeschynomene. In rice cultivation in Rio Grande do Sul, Brazil, the herbicides most used post-emergence are ALS inhibitors, corresponding to more than 50% of the herbicides used to control angiquinho [19]. However, due to toxicity, these have environmental and human health impacts [20,21]. In addition, exclusive management with herbicides may fail due to the selection of resistant weeds, the increase in the “seed bank” in the soil, and the increasing need for high doses or multiple mixtures in the sprayer, which increases production costs [22].
Therefore, one way to mitigate these negative effects is to identify insects as biological control agents for weeds. This method tends to be a more permanent alternative than other control methods and meets various environmental and economic requirements [23]. It does not cause permanent damage to the environment, is more economically accessible, has a long-lasting effect, is safe for non-target plants, and is effective in uncultivated areas, instilling confidence in its potential [24].
In order to collaborate with this very important topic, in our research, we collected a species of Acanthoscelides in two species of weeds in rice cultivation (Aeschynomene denticulata and A. indica). We then conducted a series of experiments to determine the potential of these insects as biological control agents, which we initially believed was a new species within the megacornis group [25]. However, subsequent museum research revealed this to be Acanthoscelides atrocephalus (Pic, 1938), a species that had been placed in synonymy with Acanthoscelides modestus (Sharp, 1885) by Johnson 1990 [26] without explanation. A review of the holotype of A. modestus at The Museum of Natural History (London, UK) and numerous series present at the National Museum of Natural History (Washington, DC, USA), and subsequent comparison of types of A. atrocephalus at the Muséum National d’Histoire Naturelle (Paris, France) and El Museo de La Plata (La Plata Argentina), revealed these two species to be distinct. We, therefore, remove A. atrocephalus from synonymy with A. modestus. A. atrocephalus is herein comprehensively described. We provide high-resolution photographs of the syntypes and extensive high-resolution photographs and scanning electron micrographs of specimens recently collected in order to augment these descriptions. A differential diagnosis from the two most similar species, A. modestus and A. megacornis [27,28], is provided. We also discuss the potential of this species as a biological control agent for Aeschynomene denticulata and A. indica in rice crops. This is based on our investigation of its abundance in the field and its predation potential, assessed through germination and Tetrazolium tests on infested seeds.
2. Materials and Methods
2.1. Study Characterization
The study was conducted in experimental areas of the Herbology Center/CAP/UFPel, in Capão do Leão, RS (−31.8066940, −524824340) and at the Insect Ecology Laboratory (LABEI) of the Federal University of Pelotas Biology Institute. Ten collections were carried out during the 2022/23 and 2023/24 rice harvest periods, each consisting of 15 samples/plants of each weed species (A. denticulata and A. indica) every 15 days for 300 sampled plants. Samples were randomly collected in an area of 20 × 40 m, where in previous years, irrigated rice was grown. At that moment, the weeds continued to grow spontaneously. The area was prepared annually with a post-emergent narrow-leaf herbicide mixture (187.5 Clincher®—active ingredient cyhalofop-butyl) with a vegetable oil adjuvant (125 Veget®). No broadleaf herbicides or insecticides were applied. Fruits were removed from each sample, then counted, and stored in transparent plastic containers with perforated lids to allow airflow. When needed, and due to moisture in the samples, filter papers were placed at the bottom of the container before storing the fruits. Then, they were placed in a climate-controlled chamber with a temperature of 24° ± 2°, relative humidity of 70% ± 10%, and a 12-h photoperiod, where beetle emergence was analyzed every 24 h for two months. These beetles were counted, stored in 70% alcohol, and labeled. Data regarding the number of beetles collected from each Aeschynomene species per sample were recorded on a collection sheet and analyzed graphically.
2.2. Taxonomic Analysis
Colored images of external characters were captured of type specimens and reared specimens using stacking technology developed by Macroscopic Solutions (Tolland, CT, USA) using a Canon EOS 6D digital camera (Canon U.S.A., Melville, NY, USA) attached to a Canon Extension Tube EF25 II paired with a Canon Macro Photo Lens MP-E 65-mm 1×–5× (external morphology) or attached to a Canon USM II 70–200 mm f2.8 lens paired with a 10× Mitutoyo Plan Apo Infinity-Corrected Long Working Distance Objective (genitalia) (Mitutoyo Corporation, Kanagawa, Japan). The number of images taken per specimen was optimized according to the depth of the specimen and the magnification. The resulting photographs were compiled using Zerene Stacker v1.04 (Zerene Systems LLC, Richland, WA, USA) and saved and edited in JPEG format using the Adobe Photoshop 2024 application suite (Adobe Inc., San Jose, CA, USA). Measurements are based on calibrations at specific magnification levels and incorporated into Adobe Photoshop 2024. Length was measured from the apex of the pronotum to the apex of the elytra. Width was measured from the widest point of the insect (in seed beetles, this is always across some point in the elytra). The ocular index [28] was calculated by measuring the greatest width across the eyes divided by the narrowest width between the eyes when measured on a horizontal plane. For dissections and scanning electron micrography, dried specimens were left in 10% potassium hydroxide overnight (about 36 h), followed by a bath of acetic acid to neutralize the hydroxide and then water to remove residue. Dissections were performed under a stereoscopic microscope, and the habitus photograph and dissected parts were illustrated using a light or stereoscopic microscope. The micrographs were taken with a Vega3 Tescan scanning electron microscope at the Federal University of Paraná after gold coating the material. Dissected parts were mounted on transparent plastic boards covered with Canada balsam and pinned with the respective specimens. Label information is listed in sequence from top to bottom. The data from each type of label is enclosed within double quotes (“ ”), a forward slash (/) separates lines, and information enclosed by square brackets ([]) provides additional details. Data from additional material are provided in abbreviated format. The terminology used follows that of Johnson 1990 [26] and Manfio et al., 2013 [29].
The holotype of A. modestus from Guatemala was reviewed at The Museum of Natural History (BMNH, London, UK). Specimens were also reviewed from Mexico and the USA (Texas) deposited in the collections of the National Museum of Natural History (USNM, Washington, DC, USA) and Brigham Young University (BYU, Provo, UT, USA). The holotype of A. megacornis from Costa Rica was reviewed at the USNM. Specimens were also reviewed from Honduras, Mexico, and Nicaragua deposited in the USNM. A male paratype of A. atrocephalus at the Museo de La Plata (MLPA, La Plata, Argentina) was examined and imaged, and the genitalia were dissected. A type and one paratype at the Muséum National d’Histoire Naturelle (MNHN, Paris, France) were reviewed (they are conspecific with the paratype in La Plata). Further specimens were examined at the Museu de Entomologia Pe. Jesus Santiago Moure (DZUP, Universidade Federal do Paraná, Curitiba, Paraná, Brazil), El Museo Argentino de Ciencias Naturales Bernardino Rivadavia (MACN, Buenos Aires, Argentina), and a series reared from the seeds of Aeschynomene denticulata and A. indica deposited in the Coleção Entomológica do Setor Palotina (CESP, Palotina, Paraná, Brazil) and in the research collection of the University of San Diego, USA (USDC, with ultimate deposition in the San Diego Natural History Museum: SDMC).
2.3. Quantification of Seed Damage Caused by A. atrocephalus
Fruit collection was carried out to calculate the incidence of bruchines, and the percentage of seed predation (P) per harvest was calculated—which took into account an estimate of the number of seeds collected (number of fruits collected x average number of seeds per fruit) and the number of insects emerged in each harvest: P = (100.Ti)/Ts, where Ti means total insects per harvest and Ts represents the total seeds (estimated value) [30].
Seeds used for the germination test underwent dormancy overcoming through the method of Mechanical Scarification and were then disinfected by immersion in a solution of distilled water and chlorine hypochlorite (3%) for 5 min. Next, they were drained and immersed in a sequence of three containers with only distilled water for 1 min each.
The germination test was developed based on Seed Analysis Rules—SAR (2009). It consisted of 4 treatments (whole seeds of A. indica, broached seeds of A. indica, whole seeds of A. denticulata, and broached seeds of A. denticulata), with 10 seeds each/05 replicates. Treatments were stored in Gerbox boxes (11 cm × 11 cm × 3.5 cm) with blotting paper substrate moistened with distilled water and placed in a climate-controlled BOD at 25 °C ± 1°, RH 70% ± 10%, at a 12-h photoperiod. Germination process observation and seed counting were carried out on 3, 7, and 14 days. Finally, the germination percentage was calculated in order to determine the maximum germination potential of the seeds, which corresponds to the number of seeds that produced seedlings classified as normal. This makes it possible to determine whether pest-bored seeds (damaged by insects) can develop [31].
For the tetrazolium test, seeds were also disinfected in a solution of distilled water and chlorine hypochlorite (3%) and then underwent Mechanical Scarification. Ten whole seeds and 10 bored seeds were used for each weed species studied. The test consisted of pre-wetting the seeds, which were immersed directly in distilled water for 24 h. Then, a longitudinal cut was made using a scalpel in each seed to expose embryo tissues and remove the tegument. Seeds were then soaked in tetrazolium solution and were kept in a climate-controlled BOD at 25 °C ± 1°, RH 70% ± 10%, with a photoperiod of 12 h. At the end of the staining period, the solution was discarded, and the seeds were washed in running water and kept submerged until the end of the evaluation to stop them from drying [31]. Seeds were observed and photographed in a Discovery V20 Zeiss stereomicroscope equipped with the AxioVision system to record any damage found.
3. Results
3.1. Taxonomy
Acanthoscelides atrocephalus (Pic 1938) was removed from synonymy with Acanthoscelides modestus (Sharp 1885).
Bruchus atrocephalus Pic, 1938 (type: Pampas du Chili (MNHN); paratypes: Argentina: Buenos Aires (MNHN, MLPA)).
Bruchus atrocephalus: [26] (p. 433) (as jr. syn. of Acanthoscelides modestus); [32] (p. 2) (catalog).
Acanthoscelides atrocephalus: [33] (p. 746) (nov. comb., catalog); [34] (p. 37) (catalog).
3.2. Material Examined
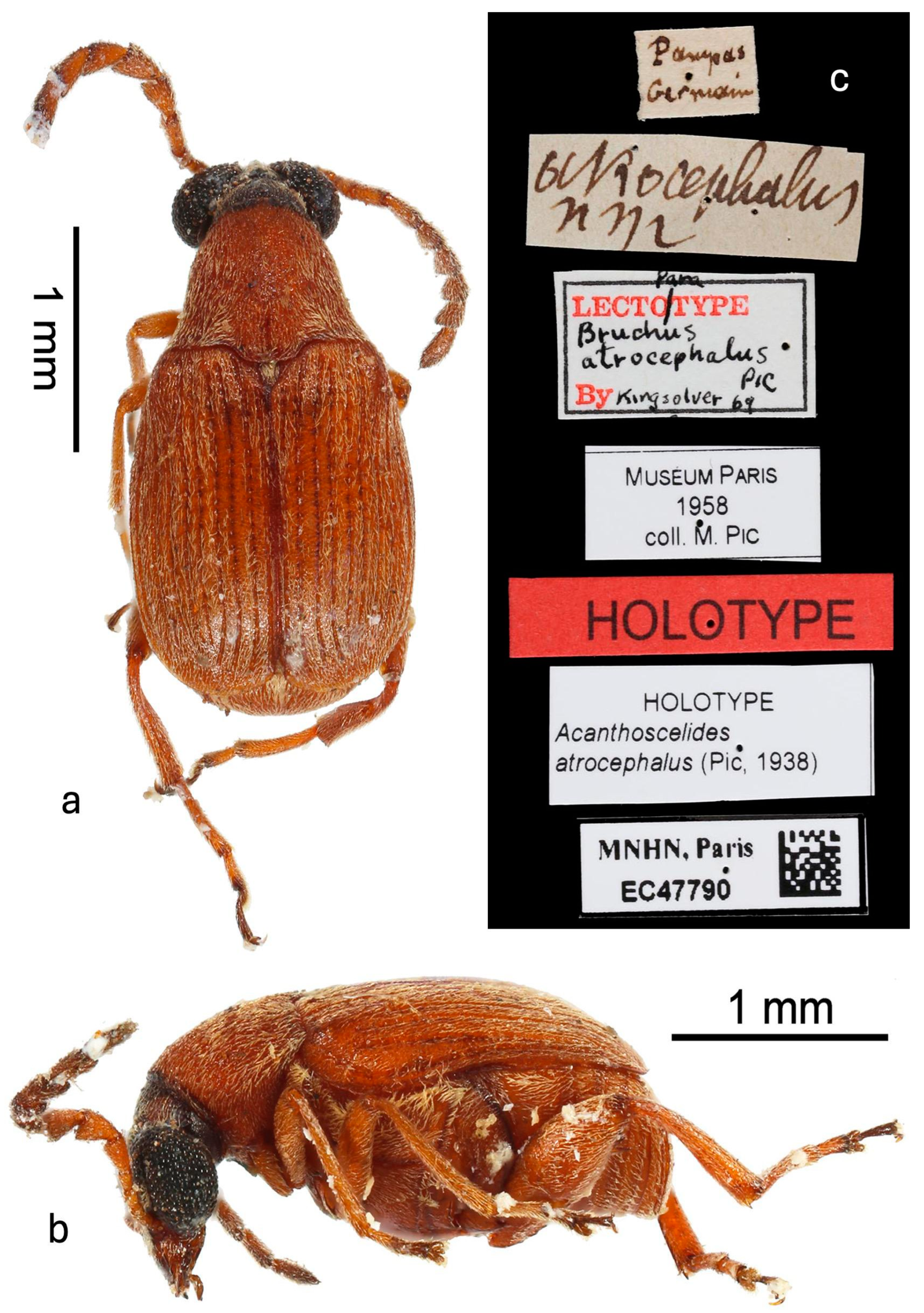
Type (Figure 1): Male deposited in MNHN, labels: (1) “Pampas/Germain” [handwritten in black ink]; (2) “atrocephalus/n sp” [Pic’s handwriting in black ink]; (3) “Para [handwritten in black ink/Lectotype [printed in red ink]/Bruchus [handwritten in black ink]/atrocephalus [handwritten in black ink]/Pic [handwritten in black ink]/By [printed in red ink] Kingsolver 69 [handwritten in black ink]” [label with black border]; (4) “Muséum Paris/1958/coll. M. Pic” [printed in black ink]; (5) “Holotype” [printed in black ink on red label]; (6) “Holotype/Acanthoscelides/atrocephalus (Pic, 1938)” [printed in black ink]; (7) “MNHN, Paris/EC47790” [printed in black ink on label with black border and QR code]. Kingsolver affixed a “paralectotype” label to this specimen. However, not only were lectotype and paralectotype designations never published, but this is also one of the few cases where Pic (1938) actually specified a single type and paratypes. This specimen in the MNHN collected by Germain from “Pampas du Chili” (p. 78) is indicated as the type and now has holotype labels added to it in 2024 by curator A. Mantilleri.
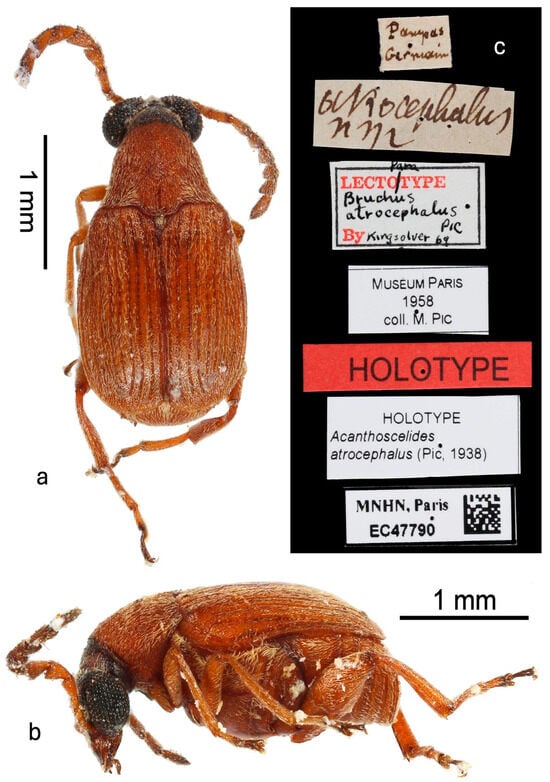
Figure 1.
Holotype male in MNHN: (a) dorsal habitus; (b) lateral habitus; (c) labels.
Paratype: Male deposited in MNHN, labels: (1) “Buenos Aires Argentina/Isla Martin Garcia/6-1936 M.J. Viana” [label printed in black with black border]; (2) “No 5” [handwritten in blank ink]; (3) atrocephalus/n sp” [Pic’s handwriting in black ink]; (4) “Lectotype [printed in red ink] ♂ [printed in black ink]/Bruchus [printed in black ink]/atrocephalus [printed in black ink]/Pic [printed in black ink]/By [printed in red ink] Kingsolver 69 [printed in black ink]” [label with black border] (5) “PARATYPE” [printed in black ink on red label]; “PARATYPE/Acanthoscelides/atrocephalus (Pic, 1938)” [label printed in black]; “MNHN, Paris/EC47791” [label printed in black ink with QR code]. This was designated as a paratype by Pic (1938) in the original description. The unpublished “lectotype” label affixed by Kingsolver is therefore not appropriate.
Paratype: Male deposited in MLPA, labels: (1) “Buenos Aires Argentina/Isla Martin Garcia/6-1936 M.J. Viana” [label printed in black with black border]; (2) “5” [printed in black on green label with black border]; (3) “B atrocephalus/Pic/paratype” [Pic’s handwriting in black ink]; (4) “956” [handwritten in black ink on green round label]; (5) Para [handwritten in black ink]/Lectotype [printed in red ink]/Bruchus [handwritten in black ink]/atrocephalus [handwritten in black ink]/Pic [handwritten in black ink]/By [printed in red ink]” [label with black border]. Genitalia dissected and in genitalia vial with specimen. Nearly identical to those imaged of reared specimens. This was designated as a paratype by Pic (1938) in the original description. The unpublished “paralectotype” label affixed by Kingsolver is, therefore, not appropriate.
New host associations: BRAZIL: Rio Grande do Sul: Capão do Leão (31.806694° W, 52.482434° S), 9.iii.2023, coll. M.G.C.E. Greco, reared from seeds of Aeschynomene denticulata Rudd (9 males: 4 in CESP, 3 in DZUP, 2 in USDC; 7 females: 3 in CESP, 2 in DZUP, 2 in USDC; 4 females and 4 males with dissected parts in CESP; numerous others stored in freezer); ibid., reared from seeds of Aeschynomene indica L. (7 males: 3 in CESP, 2 in DZUP, 2 in USDC; 7 females: 3 in CESP, 2 in DZUP, 2 in USDC); Santa Maria, 9.v.2005, coll. D. Link (1 male in DZUP).
Other material: ARGENTINA: Buenos Aires: Tigre (1 male in MACN); Santa Fe: Rosario de Santa Fe, coll. Stevenin (1 male in MACN).
It is possible that material cited from Brazil [26] is also A. atrocephalus, but these specimens should be examined using the criteria below.
3.3. Diagnosis
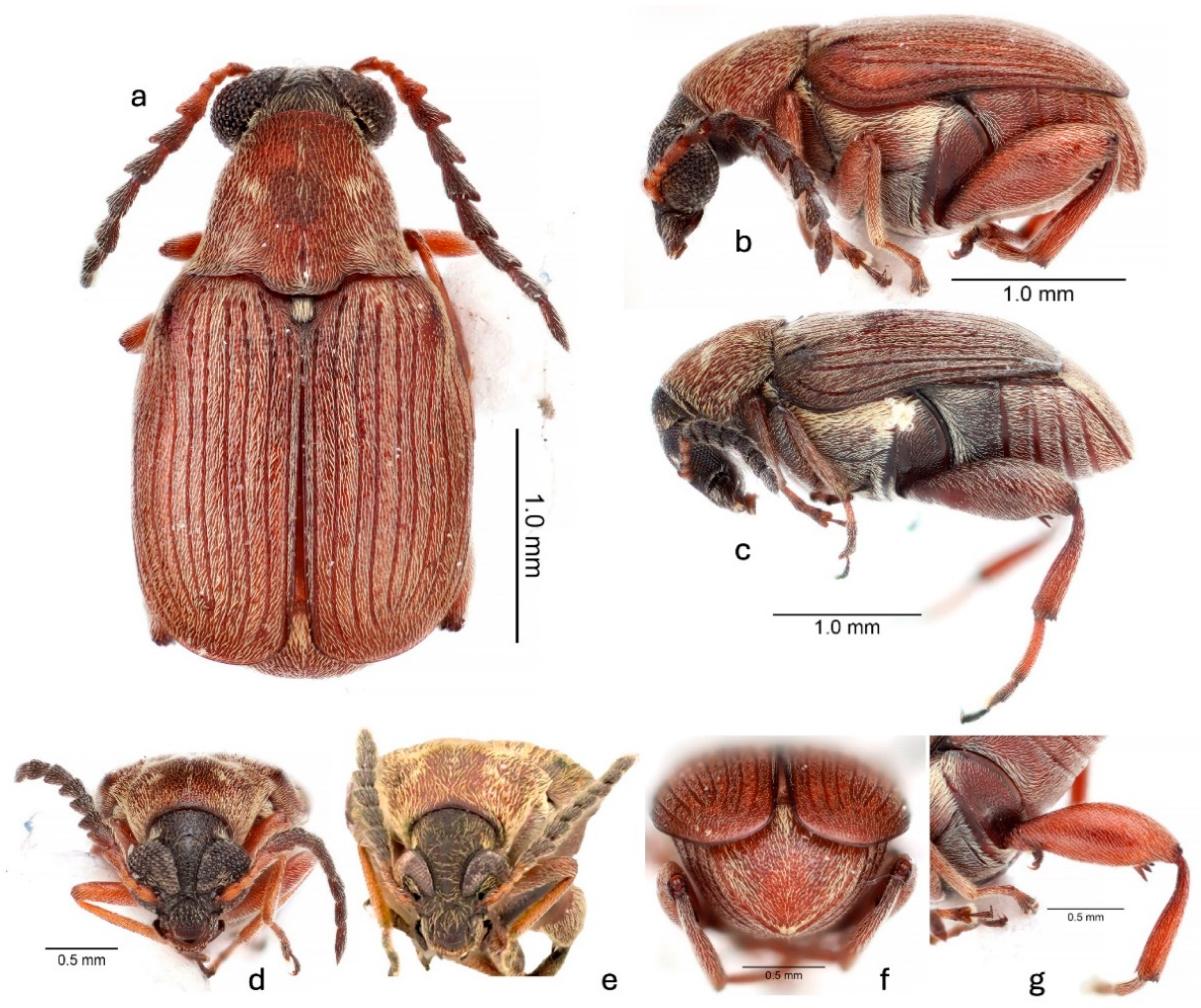
A. atrocephalus belongs to the megacornis species group [26,35] based on its long and serrate male antennae, large eyes, small scutellum, the median lobe of the male genitalia with an elongate and acuminate ventral valve, and the lack of large sclerites in the internal sac of the median lobe. The mostly uniform pubescence and short mucro that is subequal in length to the lateral coronal denticle near it only to A. modestus and A. megacornis. The nearly completely reddish-brown integument, except a black head, dark purple antennomeres 5–11, dark purple humeral angles, and dark purple thoracic ventrites (Figure 1 and Figure 2) distinguish A. atrocephalus from most specimens of these two species, although some color variants of A. modestus are similar. The pubescence on the pronotum is composed of a narrow median white stripe flanked by wide stripes of golden-yellow pubescence, with these then flanked by white pubescence laterally (Figure 1a and Figure 2a–e). The pronota of both A. modestus and A. megacornis have uniform white pubescence, sometimes with more dense pubescence forming a median stripe. A. atrocephalus and A. megacornis both have a pronounced frontal carina (Figure 2d,e) extending from the frontoclypeal suture to the vertex, while A. modestus has a frontal glabrous area. A. megacornis has eyes that are not noticeably sexually dimorphic, with an interocular ratio of 4.0–4.3. Both A. modestus and A. atrocephalus have noticeably sexually dimorphic eyes, with those of males larger and set more closely together. The eyes of A. atrocephalus (Figure 2d,e and Figure 3a) are larger and closer together than those of A. modestus in both sexes, however. The interocular ratio of A. atrocephalus is 9.4–11.0 in males and 5.0–5.5 in females, while in A. modestus, it ranges from 5.6 to 7.8 in males and 4.2 to 4.8 in females. Finally, the male genitalia of the three species are different. A. megacornis has a ventral valve that is subtly convex and has a base about 0.8× as wide as the apex of the median lobe. Both A. modestus and A. atrocephalus have a ventral valve that is slightly concave to nearly straight and have a base that is about 0.6× as wide as the apex of the median lobe (Figure 4a,b). The armature of the internal sac of the median lobe of all three species lacks large sclerites. That of A. megacornis is the least similar, with a cluster of fine spicules medially at the base, scattered denticles through the middle of the internal sac, and clusters of discrete spines at the apex. Both A. modestus and A. atrocephalus have a cluster of fine spicules medially at the base, and a series of densely packed spines angled toward the base and the interior extending medially from the apex and through the middle of the internal sac. A. modestus has a break separating the fine spicules at the base from the series of spines, and the series of spines is attached to a pair of slightly arcuate spines basally; A. atrocephalus, on the other hand, has no break and lacks the pair of small arcuate spines, and instead has the extensive series of small spines gradually decreasing in size towards base until becoming spicules (Figure 4a,b).
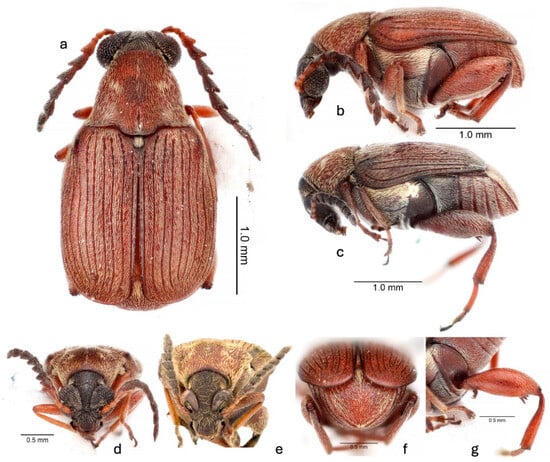
Figure 2.
Acanthoscelides atrocephalus (Pic, 1938): (a) dorsal habitus, male; (b) lateral habitus, male; (c) lateral habitus, female; (d) frontal habitus, male; (e) frontal habitus, female; (f) caudal habitus, male; (g) details of hindleg.
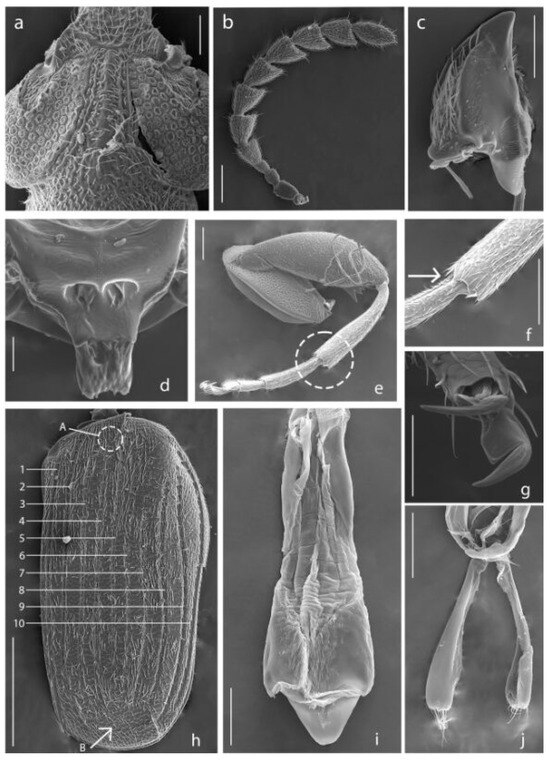
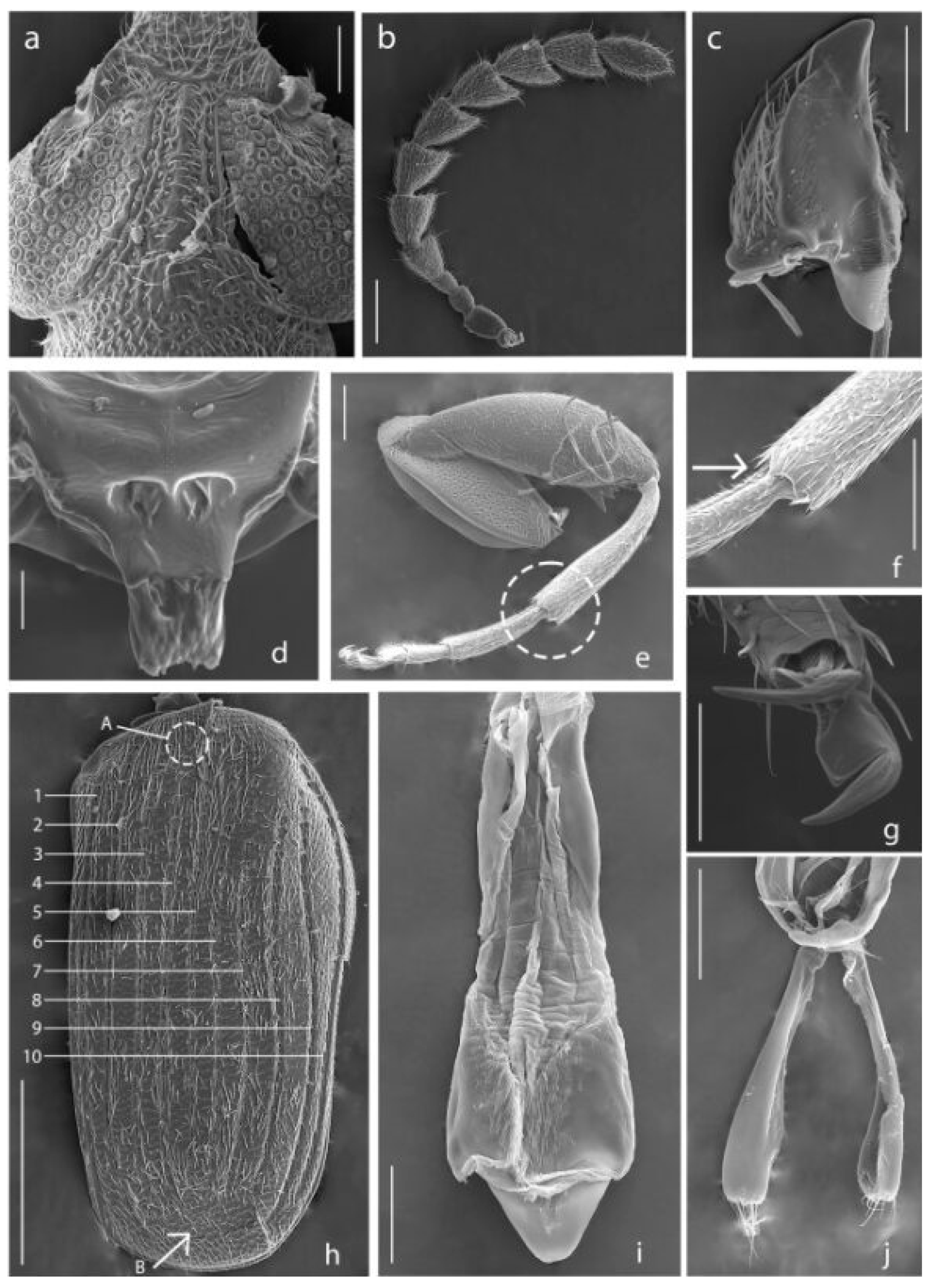
Figure 3.
Acanthoscelides atrocephalus. (a) Head, dorsal view; (b) Antenna; (c) Mandible; (d) Scutellum; (e) Hind leg, apex of metatibia is indicated by dashed line; (f) Apex of metatibia, mucro is indicated by the arrow; (g) Claws; (h) Elytron, striae are indicated by numbers, small tooth is indicated by the letter A, striae not reaching the apical margin are indicated by the letter B; (i) Median lobe of aedeagus, dorsal view; (j) Lateral lobes of aedeagus, dorsal view. Scale bars 5, 7 = 100 μm; 6, 9, 10 = 200 μm; 8, 11, 13 = 50 μm; 12 = 500 μm; 14 = 10 μm.
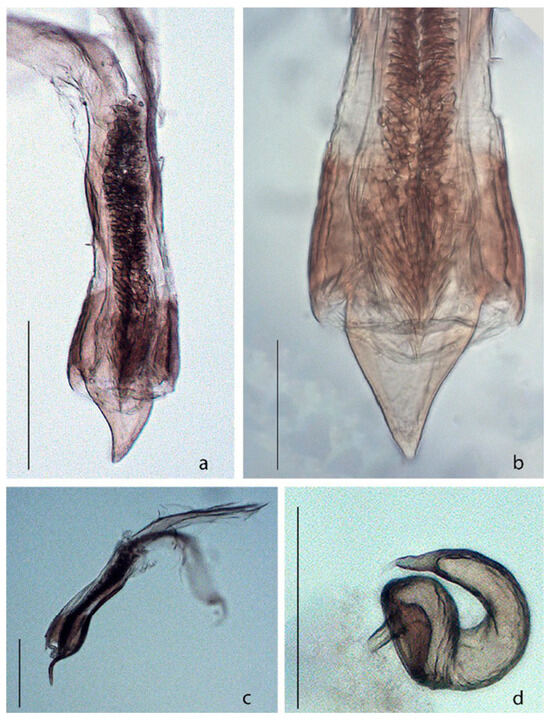
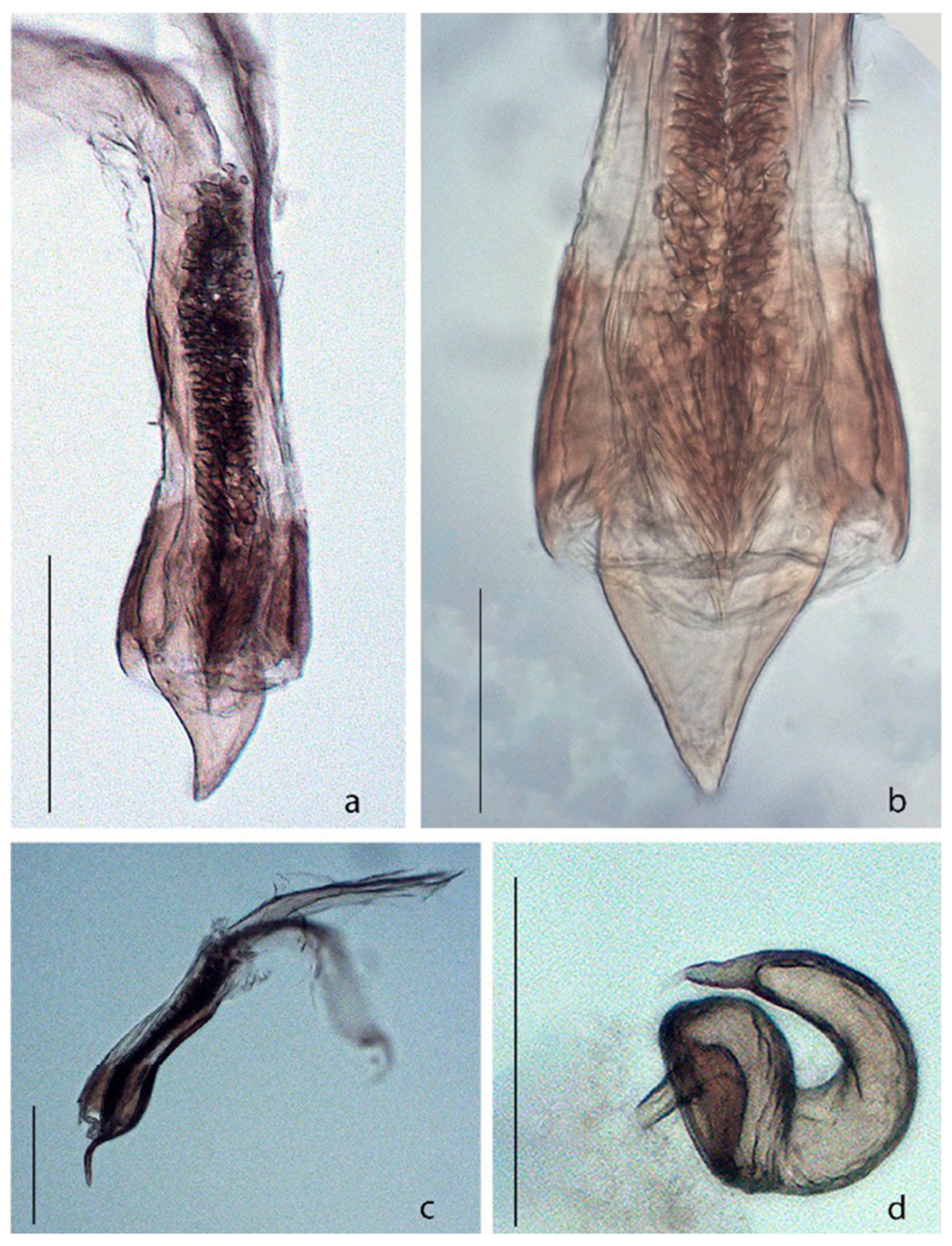
Figure 4.
Acanthoscelides atrocephalus. (a) Median lobe of aedeagus, dorsal view; (b) Apex of median lobe of aedeagus, dorsal view; (c) Median lobe of aedeagus, lateral view; (d) Spermatheca. Scale bars a, b, d = 0.15 mm; c = 0.07 mm.
3.4. Description
Male. Body length 2.6–2.8 mm, elytral width 1.6–1.9 mm. Body ovate (Figure 1a and Figure 2a). Integument color (Figure 1a,b and Figure 2) is mostly reddish-brown, except with a nearly black head, dark purple antennomeres 5–11, dark purple humeral angles, and dark purple thoracic ventrites. Vestiture (Figure 1a,b and Figure 2) is mostly light yellow, with humeral angles glabrous; denser pubescence on ocular sinus, vertex behind eyes (post-ocular lobe), each one-third lateral of pronotum, narrow median stripe of pronotum, scutellum, pleural thoracic regions (lateral view), and median basal region of pygidium; narrow median stripe of pronum and one-third lateral areas of pronotum separated by distinctly deep golden-yellow pubescence. Head (Figure 2d and Figure 3a): As wide as long; frontal carina conspicuous; gena very short; eyes large and prominent, each eye more than 4 times as wide as frons resulting in an interocular ratio of 9.4–11.0; supercilium along all internal margin of eyes. Antenna (Figure 2a,d and Figure 3b) long, reaching to mid-length of elytra; fine pubescence increasing gradually on antennomeres 4–11; scape, pedicel, and antennomere 3 each longer than wide; antennomere 4–10 serrate, gradually increasing in width towards apex; antennomere 11 ovate, longer than wide, slightly longer than antennomere 10, apical margin with a small projection. Clypeus as long as wide, basal margin emarginated. Labrum transverse, apical margin arcuate. Mandibles (Figure 3c) symmetrical, short, without internal teeth, and lateral margin with setae. Maxilla with galea and lacinia elongate, almost the same length; cardo elongate; distal lobe of galea short and densely ciliate; apical two-thirds of internal margin of lacinia densely ciliate; palpomere 1 shortest; palpomere 2 longer than 3; palpomere 4 longest, almost 3 times length of palpomere 3. Mentum transverse; apical angle rounded, two times longer than median third; median third truncated. Labium with palpomere 1 shortest, 2 twice as long as 1, 3 longest, three times longer than palpomere 1; subapical sclerite trapezoidal and with pair of macrosetae; glossa with apical margin emarginate. Gular plate broad, with subparallel sides. Thorax (Figure 1a,b and Figure 2a,b,d): Pronotum convex, as wide as long, gradually decreasing in width towards apex; weak longitudinal median sulcus on the basal third; apical margin truncate, with submarginal sulcus on anterolateral margin behind eyes; without lateral carina but with ridgelike lateral margin extending from base to procoxal cavity; posterior margin sinuate, with a slight emargination on median three-fifths. Prosternum transverse, short; intercoxal process triangular; procoxal cavities almost closed. Scutellum (Figure 3d) small, with two setose concavities at the base and bifurcate at the posterior margin. Elytra (Figure 1a, Figure 2a and Figure 3h) as long as wide, longer and wider than pronotum; each elytron with ten longitudinal striae, not reaching the apical margin (see Figure 3h, “B”); stria 4 slightly abbreviated at the base by subtle tooth, posterior to a line drawn between the starting of striae 3 and 5; posterior margin of each elytron with internal half slightly serrate; base of striae 3 and 4 as well as base of striae 5 and 6 closer to each than to adjacent striae. Hind wings developed. Mesocoxae separated by half of the width of mesocoxa, mesoventrite process longer than metaventrite process, former truncate; metaventrite with longitudinal sulcus on apical half; metacoxae feebly separated. Forelegs with procoxae almost contiguous, each coxa twice as long as wide; profemur two times longer than procoxa; protibia equal in length with profemur; protarsomere 1 slightly longer than protarsomere 2; protarsomere 2 equal in length with protarsomere 5. Midlegs with mesocoxa globose; mesofemur and mesotibia similar to foreleg; mesotarsomere 1 twice as long as mesotarsomere 2; mesotarsomere 2 equal in length with mesotarsomere 5. Hindlegs (Figure 2g and Figure 3e–g) with metacoxa three times wider than long, external face with setigerous pores, except on basal third; metafemur slightly swollen, 2.6–2.7× longer than wide and somewhat wider than metacoxa, slightly longer than metatibia; three robust subapical spines on inner margin with length of basal about 1.8× width of base of metatibia, second about 0.6× the length of the first and third about 0.3× the length of the first; metatibia with apical two-thirds straight, with complete ventral, ventrolateral, lateral, and dorsomesal carinae, mucro about 0.05 as long as metatarsomere 1 and slightly shorter than triangulate lateral coronal denticle from which it is separated by a shallow sinus. Metatarsomere 1 almost three times longer than metatarsomere 2; metatarsomere 2 equal in length to metatarsomere 5, with lateral, ventral, and mesal carinae. Tarsal formula 5-5-5 (pseudopentamere). Claws with a sharp apex and somewhat quadrate base (Figure 3g). Pygidium (Figure 2f) subtriangulate, slightly longer than wide, basal margin broadly arcuate, apex pointed. Abdomen with five visible sternites; first as long as four others combined; apical sternite slightly emarginated at apex. Male genitalia (Figure 3i,j and Figure 4a–c): Aedeagus with median lobe gradually increasing in width towards to apex (dorsal view); dorsal face of apical fold with a set of spines; apical half sinuate in lateral view. Ventral valve subtriangulate, apex pointed, as long as wide, lateral margin straight to slightly concave, internal margin deeply emarginated. Internal sac with small denticles, all of them somewhat directed to apex and to internal region, gradually decreasing in size towards to apex. Lateral lobes deeply cleft to near base (Figure 3j), internal margin concave, external margin straight, apical margin truncate and with long setae; tegminal rings and struts together with the same length of lateral lobe.
Female. Similar to male, except: eyes less prominent, each eye 2.5× as wide as frons, resulting in an interocular ratio of 5.0–5.5 (Figure 3e); antenna shorter, reaching only to base of elytra (Figure 3c); antennomere 4 longer than wide, equal in length the two precedents; antennomere 11 ovate, but as long as wide; pygidium subtriangulate, but as long as wide, basal margin truncate; abdomen with apical sternite pointed at apex; sternites 8 with apical margin truncate and with long setae, basal projection two times longer than the width of apical margin; coxites together with apical margin arcuate, with long setae, stylus quadrate; spermatheca gradually decreasing in width towards apex, C-shaped, base concave, apex pointed (Figure 4d).
3.5. Distribution
The species is known in Brazil from the southern state of Rio Grande do Sul, in Argentina in the northeastern provinces of Buenos Aires and Santa Fe, and in the Pampas region of northern Chile. Given the extent of rice cultivation in neighboring regions, it is likely to have a wider distribution.
3.6. Biological Notes
The specimens were collected from seeds of Aeschynomene indica L. and A. denticulata Rudd, pest weed plants in a rice monoculture. We are adding A. atrocephalus Pic to the list of Acanthoscelides associated with Aeschynomene and citing for the first time two new host plant species for Acanthoscelides: Aeschynomene indica L. and A. denticulata Rudd.
3.7. Natural Occurrence of Angiquinho Seed-Beetle Acanthoscelides atrocephalus
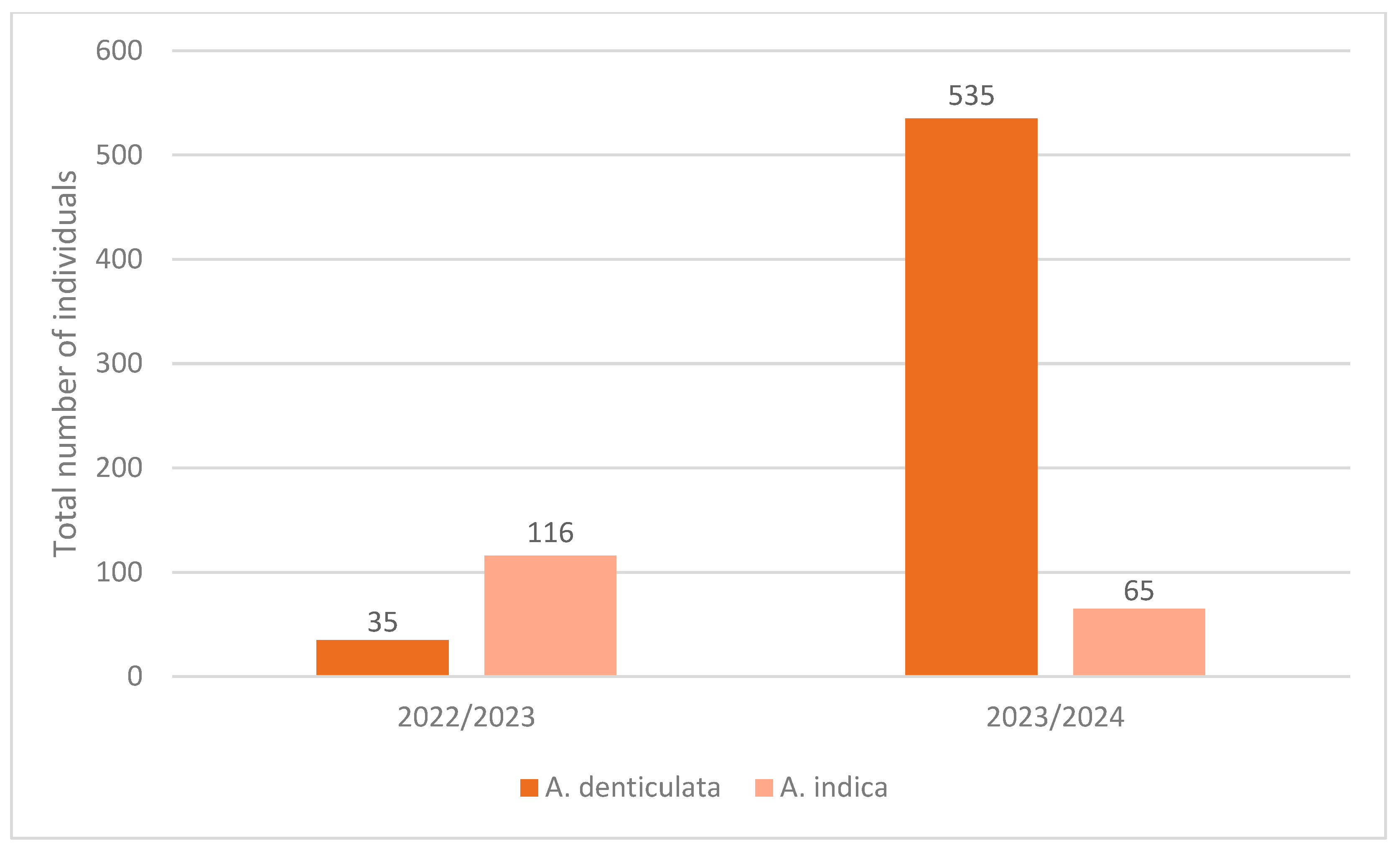
Acanthoscelides atrocephalus is very abundant in Aeschynomene seeds. The ten seed collections carried out resulted in a total of 751 individuals. During the 2022–2023 collection period, 35 individuals were collected in A. denticulata and 116 in A. indica, whereas in the 2023–2024 collections, a total of 535 and 65 individuals were obtained in A. denticulata and A. indica, respectively (Figure 5).
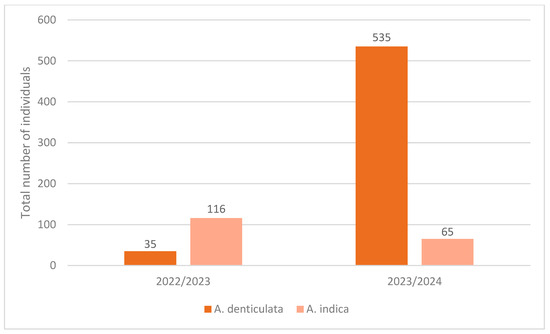
Figure 5.
Total number of individuals of Acanthoscelides atrocephalus to Aeschynomene denticulata and Aeschynomene indica, collected between 2022/2023 and 2023/2024.
3.8. Damage to Aeschynomene Seeds Caused by A. atrocephalus
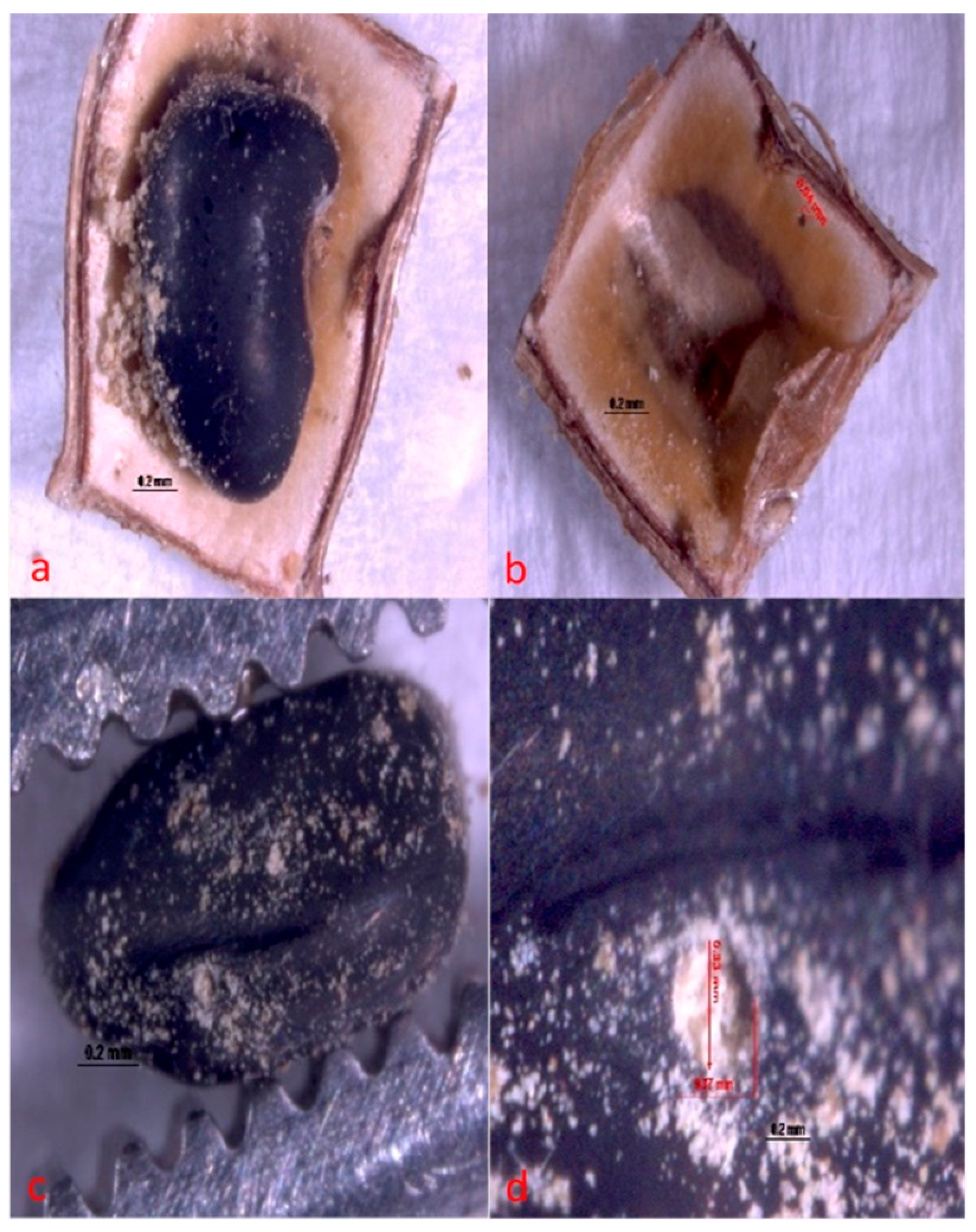
The larvae of A. atrocephalus penetrate the fruit and then the seed through holes measuring approximately 0.33 mm × 0.37 mm (Figure 6). Larvae then feed on seed cotyledon until the adult emerges. Within the seed, it makes a circular opening in both seed and fruit of approximately 0.36 mm × 0.29 mm (Figure 7) to exit (Figure 8).
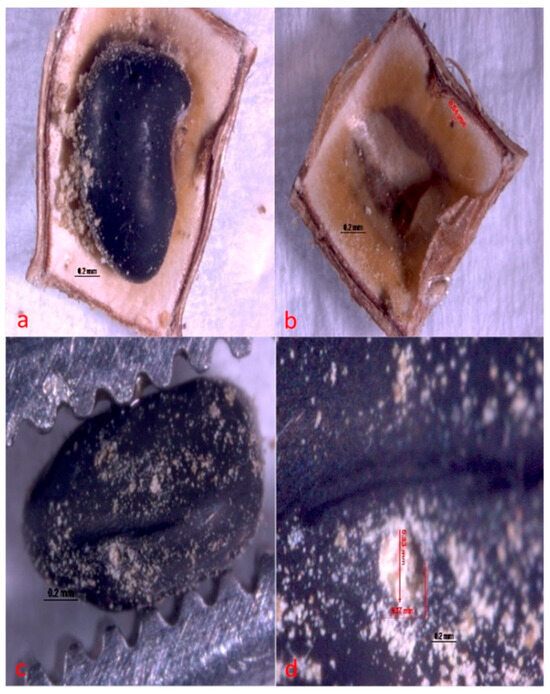
Figure 6.
Hole made by Acanthoscelides atrocephalus larva (Coleoptera: Chrysomelidae, Bruchinae), newly hatched on fruit and seed of angiquinho (Aeschynomene denticulata and Aeschynomene indica). (a) general view of a seed with a hole and residue indicating where damage has begun; (b) hole in the fruit shell, inside view; (c) hole in the seed, general view; and (d) close-up view of a hole in the seed.
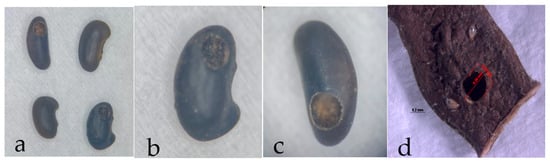
Figure 7.
Hole pattern of angiquinho seed (Aeschynomene denticulata and Aeschynomene indica) made for the adult Acanthoscelides atrocephalus (Coleoptera: Chrysomelidae, Bruchinae) to emerge. (a) comparison between whole and drilled seeds; (b,c) close-up view of drilled seeds after weevil emergence; (d) hole on seed and through fruit, with seed inside.

Figure 8.
Images of emerging adult Acanthoscelides atrocephalus (Coleoptera: Chrysomelidae, Bruchinae).
After conducting the counts, the amounts of A. atrocephalus in each weed species per crop were recorded. The seed predation percentages (P) were 0.55% for A. denticulata and 1.34% for A. indica during the 2022–2023 period. In the following collection period of 2023–2024, the seed predation percentages increased to 6.28% for A. denticulata and 2.28% for A. indica (Table 1). This rise in predation percentages suggests an increased abundance of these species, which may reduce the likelihood of A. atrocephalus establishing itself in the region.

Table 1.
Relationship of Predation Percentages (%) in Aeschynomene denticulata and Aeschynomene indica for the 2022–2023 and 2023–2024 Field Collection Periods.
The germination test of 50 seeds for each treatment (whole and drilled seeds of A. indica, whole and drilled seeds of A. denticulate) found that the seeds infested with both weeds by Acanthoscelides atrocephalus were unable to germinate since there was 0% germination, whereas the whole seeds of A. denticulata had 40% germination, and whole seeds of A. indica had 52% germination (Table 2).

Table 2.
Relationship of germination percentage (%) for whole and bored seeds of A. denticulate and A. indica.
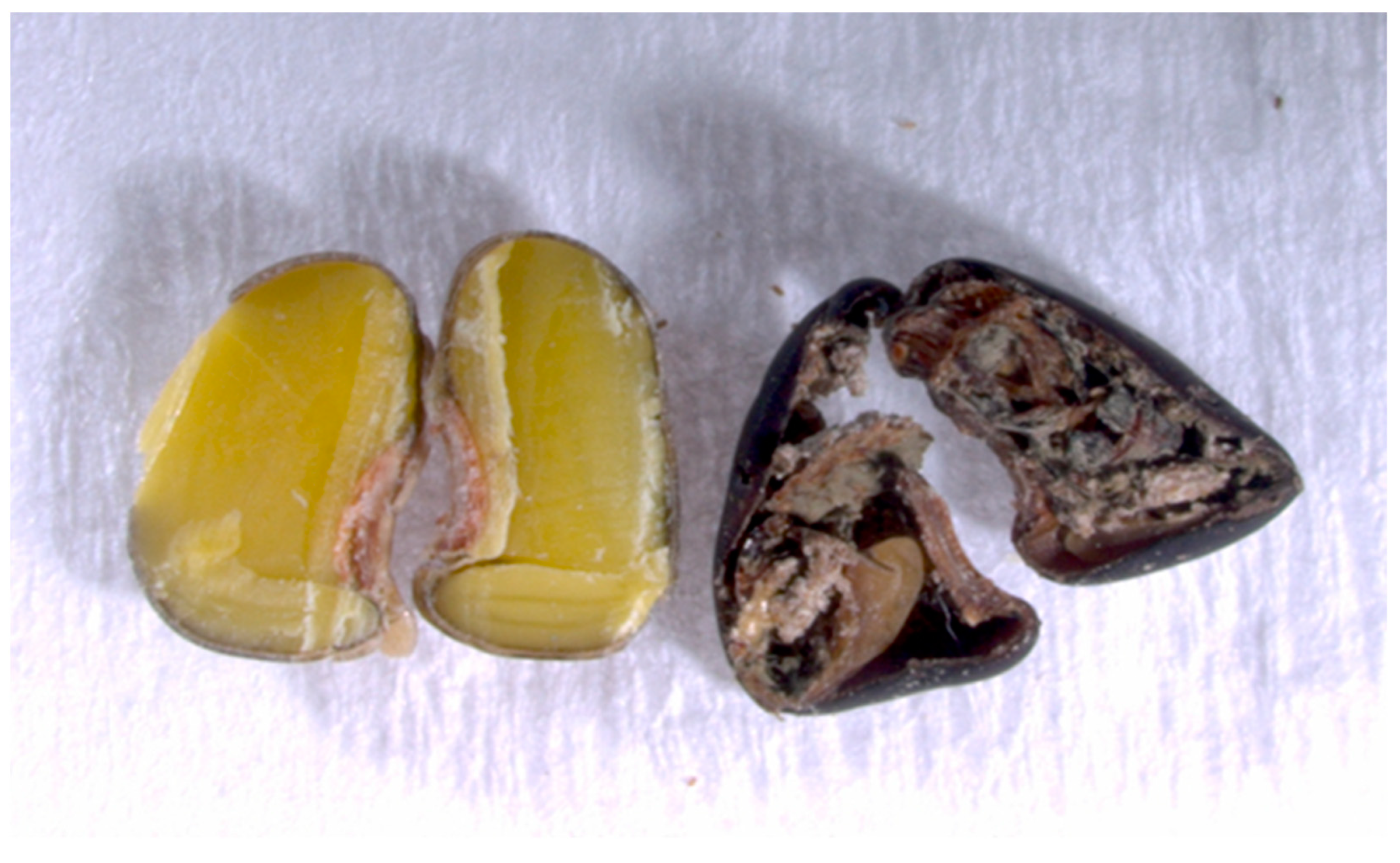
And the tetrazolium test has found that 100% of the seeds infested by Acanthoscelides atrocephalus did not have plant tissue to stain, thus showing that larvae feed entirely on plant tissue, leaving only residues inside the seed, which eventually rot (Figure 9).
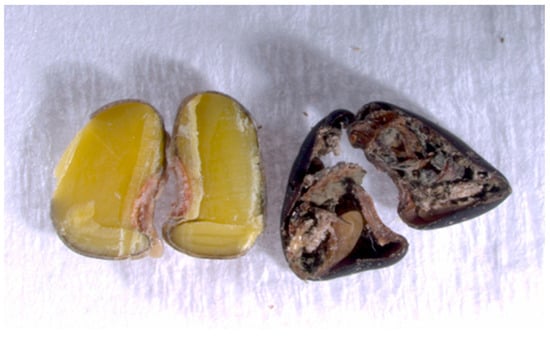
Figure 9.
Inside comparison of an angiquinho whole seed and a drilled seed (Aeschynomene denticulata and Aeschynomene indica) by Acanthoscelides atrocephalus (Coleoptera: Chrysomelidae, Bruchinae) with remains of rotting waste.
These data lead to inferences that the drilled seeds lack vigor and germination potential due to the damage caused by the development of A. atrocephalus inside them. This probably results in a reduction of the seed bank, which indicates that this insect has promising potential as a biological control agent.
4. Discussion
Using insects for biological control of plants tends to be a more permanent alternative than other control methods, as well as meeting several environmental and economic requirements [23]. Moreover, it does not leave permanent harmful effects on the environment; its implementation is relatively less expensive; it has a long-lasting effect, being safe for non-target plants and effective for non-cultivated areas [24]. Due to their wide distribution and easy management, the insects undergo suitability tests for biological control programs of exotic and native weeds, mainly because of their recognized host specificity [36], which agrees with this work’s bias.
Beetles are important possible biological control agents for weeds, for when their larvae feed on seeds, they cause damage to the embryo and neutralize it [37]. In other words, seeds are important structures for the development of plants once they ensure dispersion and permanence in the environment. Therefore, consumption by predation directly affects their germination and dispersion [38,39,40,41]. Furthermore, if weed control consists of inhibiting growth and/or reducing the number of individuals per area below the economic losses for the crop [11], then reducing seed banks is convenient for integrating weed management (IMPD).
In agricultural systems, a soil seed bank is the main source of new, yearly weed infestations. Its effective management is essential for successful weed control since the size of a seed bank varies according to seed production and loss. Many species produce seeds in large quantities, allowing populations to recover quickly even with inadequate control [42]. Therefore, predation is one of the factors that can result in substantial seed losses, both in epigean and hypogean banks [43,44]. Even minor seed losses that are due to predation affect abundance and distribution in the soil, impacting subsequent stages of the weed life cycle. As a result, there are changes in population dynamics and structure of the communities where these plants are inserted [43]. Bruchinae subfamily already has a history of seed predation in the literature [45], with Fabaceae being their preferred hosts. Bruchines also have already been suggested as biological control agents by others [45,46].
There are four species of Acanthoscelides associated with five species of Aeschynomene [26]. Two of these bruchines are in the megacornis group: A. megacornis occurring in Aeschynomene americana L. and A. sp. (an unidentified species from Venezuela); and A. modestus occurring in Aeschynomene ciliata Vogel, A. rudis Benth., A. scabra G. Don, and A. sensitiva Sw. [26,47]. Therefore, we are adding A. atrocephalus Pic to this list and citing for the first time two new species of host plants to Acanthoscelides: Aeschynomene indica L. and A. denticulata Rudd.
Regarding the distribution, Acanthoscelides megacornis and A. modestus have similar geographical records, the first from the USA to Peru and the second from Mexico to Northeast Brazil [26]. While A. atrocephalus. is only known only in the extreme southern region of Brazil and northern Argentina and Chile at present. However, the South American specimens of A. modestus should be examined based on the differential diagnosis presented above.
Studies involving bruchines have been conducted in countries of the European Union, as well as in Argentina, South Africa, and others, explaining their distribution and the impacts caused by their spermatophagous feeding habits, highlighting their potential action as a biological control agent. One example is the Asian seed weevil Megabruchidius tonkineus (PIC, 1904) (Coleoptera: Chrysomelidae: Bruchinae), which is reported to be a seed predator of Gleditsia triacanthos (Fabaceae) in South Africa, a fast-growing plant native to North America. M. tonkineus weevil can damage around 9% of G. triacanthos seeds and is also established throughout the population range of this weed in South Africa since it has been found in the field after research, and it is known for being easily dispersed [48,49]. A different study has recently surveyed bruchine species in the European Union region, listing their host specificities. A total of 1584 species/subspecies/varieties of legumes were included in the study, and approximately 16% of them are known hosts for the 175 bruchine species found in Europe, where the genus Acanthoscelides was recorded for different species of three Leguminous Tribes (five species of Amorphae Boriss., and 10 of Phaseoleae (Bronn) AD. and five of Cicereae Alef.) [50]. In Chiapas, Mexico, two specimens of Acanthoscelides (A. flavescens and A. dani) were registered, both in species of Rhynchosia (R. longoacemosa, R. minima, R. phaseoloides) [51]. However, A. flavescens, a polyphagous bruchine, has also been recorded, feeding on three other different genera of Fabaceae (Eriosema, Galactia, and Rhynchosia) [25].
In South Africa, nine species of bruchines have been evaluated as biological control agents. Four were never released (three were rejected, one was archived), one failed to establish, three had negligible impact, and only one achieved significant success. Algarobius prosopis (Le Conte) (Coleoptera: Chrysomelidae: Bruchinae), introduced in 1989, has been effective in reducing viable seeds of Neltuma (published as Prosopis; Fabaceae), being established throughout the distribution range of the target plant. Despite cattle interference, which consumes the pods, control is usually effective, with seed damage often exceeding 90% when the pods remain on the ground, and cattle are absent [52,53]. Still, little is known about how biological control acts and impacts organisms that consume seeds on weed populations in agricultural ecosystems [54]. However, we have other examples, such as the Acanthoscelides macrophthalmus (Chrysomelidae: Bruchinae) beetle, which was introduced in 1999 in South Africa to control excessive seed production of Leucaena leucocephala (Fabaceae) invasive tree without compromising its agroforestry benefits. Despite becoming widely established, its impact on weed management is still moderate, which indicates the need for further studies to improve management [55].
On the other hand, the study carried out in Santiago del Estero, in Argentina, with predation of Ipomoea nil (Convolvulaceae) by Megacerus maculiventris (Coleoptera: Chrysomelidae: Bruchinae), indicated a predation percentage ranging from 0.84 to 15.66% in the 2004–2008 cotton crops in irrigated areas [56]. With the Acanthoscelides macropthalmus (Coleoptera: Chrysomelidae: Bruchinae) seed weevil controlling Leucaena leucocephala (Fabaceae) in southeastern Queensland, Australia, observed predation levels ranged from 11 to 54%, depending on how long fruit remained on the plant [57]. Studies show that for legumes with high production of seeds, seed predators must destroy at least 95% and, ideally, more than 99% of seeds produced in order to assure population regulation [57,58]. Despite lower percentages of predation registered in collections for the present study for A. denticulata and A. indica caused by A. atrocephalus seed weevil in the 2022–2023 harvest—with an increase in percentage in 2023–2024, it is worth noting that such percentage was based on a natural incidence collection since they have not yet been released for effective control testing—thus indicating a promising potential to establish them in the distribution range of such weeds in South America.
Finally, it is worth mentioning that the literature finds that a successful bioagent is successful when it has specific hosts (they should not attack other plants), ease of multiplication (how easy natural reproduction is), feeding habit (bioagents are more effective in controlling weeds when they attack flowers, seeds or when they pierce the stems, compared to those that feed on roots or leaves), and bioagent resistance (the bioagent must be free of parasites and predators of its own). In addition, the bioagent must be able to survive short or longer periods of food shortage if the population of the target weed is reduced to very low levels) [24]. Therefore, Bhaliya and Shekhada 2023 [24] corroborate that bioagents that feed on seeds are very efficient and thus encourage the study of this species in other contexts as well, in order to verify its host specificity as well as its bioecological features.
5. Conclusions
With this research, Acanthoscelides macrocephalus (Pic, 1938) is no longer synonymous with Acanthoscelides modestus (Sharp, 1885). This is the first record of Coleoptera causing injuries to Aeschynomene denticulate and Aeschynomene indica seeds. When feeding on the seed, A. macrocephalus prevents its germination, making the embryo unviable. This helps to reduce the seed bank of these weeds, thus indicating a high potential as a biological control agent for weeds of the genus Aeschynomene.
Due to the importance of the topic addressed here, additional studies need to be continued in order to verify the bioecology of A. atrocephalus, as well as its host specificity and breeding techniques that may enable the use of this insect in integrated weed management programs (MIPD). In order to encourage the reduction of the use of chemical products in rice crops will open a path of ideas for studies with other weed species, as this is a topic little discussed in Brazil.
Author Contributions
Conceptualization, M.G.d.C.E.G., D.A. and F.R.M.G.; Methodology, M.G.d.C.E.G., E.C. and G.M.; Formal Analysis, M.G.d.C.E.G., D.A., E.S.C., E.C., G.M. and F.R.M.G.; Research, M.G.d.C.E.G., E.S.C., G.M. and E.C.; Resources, E.C., D.A. and F.R.M.G.; Writing—Preparation of the Original Draft, M.G.d.C.E.G., E.S.C., G.M. and E.C.; Writing—Review and Editing, M.G.d.C.E.G., E.C., G.M. and F.R.M.G.; Supervision, F.R.M.G., D.A. and E.C.; Project Administration, M.G.d.C.E.G., E.C. and F.R.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
CESP is supported by The Network of Biological Collections of Paraná—Taxonline, which receives funds from the Araucaria Foundation for Supporting Scientifc and Technological Development of the State of Paraná—FA (Brazil, Paraná). Travel funds to examine type specimens were provided to G.M. by a generous gift from D. Rockefeller. The National Council for Scientific and Technological Development—Brazil (CNPq) for the productivity scholarships (311390/2021-8) for E.C, F.R.M.G and D.A. And Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Code 001 for granting doctoral scholarships for M.G.C.E.G.
Data Availability Statement
The datasets analyzed in the present study are available from the corresponding authors on reasonable request.
Acknowledgments
We are grateful to Eduardo L. C. Balester and Luana Costa for the scanning electron micrographs. We acknowledge Adriana E. Marvaldi (CONICET, Universidad Nacional de La Plata, Buenos Aires, Argentina) for dissecting, clearing, and providing photographs of the genitalia of the paratype of A. atrocephalus (Pic). We thank A. Mantilleri (Muséum National d’Histoire Naturelle, Paris, France) for providing images of the holotype and one paratype of A. atrocephalus. And we thank Daniel Soares Duarte and Lúcia Maciel for the English translation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Johnson, C.D. Seed beetles host specifity and the systematics of the Leguminosae. In Advances in Leguminosae Systematics; Polhill, R.M., Raven, P.H., Eds.; Royal Botanic Gardens, Kew: London, UK, 1981; Volume 2, pp. 995–1027. [Google Scholar]
- Ribeiro-Costa, C.S.; Almeida, L.M. Seed-Chewing Beetles (Coleoptera: Chrysomelidae, Bruchinae). In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.R.P., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA; EUA: Brussels, Belgium, 2012; pp. 325–352. [Google Scholar]
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Evol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Morse, G.E. Bruchinae Latreille, 1802. In Morphology and Systematics: Phytophaga; Leschen, R.A.B., Beutel, R.G., Eds.; Arthropoda: Insecta: Coleoptera; De Gruyter: Berlin, Germany; Boston, MA, USA, 2014; Volume 3, pp. 189–198. [Google Scholar]
- Anton, K.W. New nomenclatural and taxonomic acts. Chrysomelidae: Bruchinae. In Chrysomeloidea II (Orsodacnidae, Megalopodidae, Chrysomelidae); Updated and Revised Second Edition; Catalogue of Palaearctic Coleoptera, Volume 6/2/1; Bezděk, J., Sekerka, L., Eds.; Koninklijke Brill: Leiden, The Netherlands, 2024; pp. 3–24. [Google Scholar]
- Borowiec, L. The genera of seed-beetles (Coleoptera: Bruchidae). Pol. J. Entomol. 1987, 57, 3–207. [Google Scholar]
- Kergoat, G.J.; Alvarez, N.; Hossaert-McKey, M.; Faure, N.; Silvain, J.-F. Parallels in the evolution of the two largest New and Old World seed-beetle genera (Coleoptera, Bruchidae). Mol. Ecol. 2005, 14, 4003–4021. [Google Scholar] [CrossRef] [PubMed]
- Kljajic, P.; Andric, G.; Golic, M.; Jovicic, I. Bean weevil Acanthoscelides obtectus (Say) survival and progeny production affected by residual insecticide deposits, and related damage of two types of bean. J. Stored Prod. Res. 2022, 98, 102004. [Google Scholar] [CrossRef]
- Redmon, S.G.; Forrest, T.G.; Markin, G.P. Biology of Bruchidius villosus (Coleoptera: Bruchidae) on Scotch broom in North Carolina. Fla. Entomol. 2000, 83, 242–253. [Google Scholar] [CrossRef]
- Radford, I.J.; Nicholas, M.; Brown, J.R. Assessment of the biological control impact of seed predators on the invasive shrub Acacia nilotica (prickly acacia) in Australia. Biol. Control 2001, 20, 261–268. [Google Scholar] [CrossRef]
- Agostinetto, D.; Ulguim, A.D.R.; Vargas, L. Controle de plantas daninhas: Manejo de plantas daninhas em sistema plantio direto. In Capítulo 04 em Sistema de Plantio Direto no Brasil, 1st ed.; Aldeia Norte Editora: Passo Fundo, Brazil, 2022; pp. 106–118. [Google Scholar]
- Nunes, F.S.; Schaedler, C.E.; Chiapinotto, D.M. Levantamento Fitossociológico de Plantas Daninhas na Cultura do Arroz Irrigado. Planta Daninha 2018, 36. [Google Scholar] [CrossRef]
- Constantin, J. Métodos de Manejo. In Biologia e Manejo de Plantas Daninhas; Ominipax: Curitiba, Brazil, 2011; pp. 67–78. [Google Scholar]
- Adegas, F.S.; Silva, A.F.; Concenço, G. Controle biológico de plantas daninhas. In Bioinsumos na Cultura da Soja; Meyer, M.C., Bueno, A.F., Mazaro, S.M., Silva, J.C.d., Eds.; Embrapa: Brasília, Brazil, 2022; Chapter 16; pp. 285–295, 550. [Google Scholar]
- Takanose, Y.; Ishida, S.; Kudo, N.; Kamitani, T. Effects of tillage and irrigation on the occurrence and establishment of native wetland plant species in fallow paddy fields. Paddy Water Environ. 2013, 11, 45–58. [Google Scholar] [CrossRef]
- Pei, Q.; Yuanlain, C.; Huanhão, H.; Bo, L. Efeito da irrigação por inundação e padrões de irrigação intermitente na diversidade da comunidade de ervas daninhas em campos de arroz tardios. Trans. Queixo. Soc. Eng. Agríc. 2015, 31, 115–121. [Google Scholar]
- Fruet, B.L.; Merotto, A., Jr.; Ulguim, A.R. Levantamento sobre manejo de plantas daninhas de arroz e características de consultores públicos e privados no sul do Brasil. Weed Technol. J. 2019, 1, 1–22. [Google Scholar]
- Andres, A.; Theisen, G. Épocas de Controle de Angiquinho e Prejuízos em Arroz Irrigado cv. BRS Querência; Boletim de Pesquisa e Desenvolvimento 93; Embrapa Clima Temperado: Pelotas, Brazil, 2009. [Google Scholar]
- Martins, M.B.; Agostinetto, D.; Fogliatto, S.; Vidotto, F.; Andrés, A. Aeschynomene spp. Identification and Weed Management in Rice Fields in Southern Brazil. Agronomy 2021, 11, 453. [Google Scholar] [CrossRef]
- Montagner, C.C.; Vidal, C.; Acayaba, R.D. Contaminantes emergentes em matrizes aquáticas do Brasil: Cenário atual e aspectos analíticos, ecotoxicológicos e regulatórios. Quim. Nova 2017, 40, 1094–1110. [Google Scholar] [CrossRef]
- Ngwu, C.M.; Odoemelam, S.A.; Nnaji, J.C. A Review on the Synthesis and Application of Nanomaterials for the Removal of Emerging Contaminants from Industrial Wastewater. Comms. Phys. Sci. 2020, 5, 343–357. [Google Scholar]
- Correia, N.M. Comportamento dos Herbicidas no Ambiente; Embrapa Hortaliças: Brasília, Brazil, 2018; 30p. [Google Scholar]
- Vitorino, M.D.; Pedrosa, M.J.H. Controle biológico—Uma alternativa para o controle de invasões biológicas. In O Araçazeiro: Ecologia e Controle Biológico; Pedrosa, M.J.H., Dalmolin, A., Smith, C.W., Eds.; FUPEF: Curitiba, Brazil, 2007; pp. 55–69. [Google Scholar]
- Bhaliya, C.M.; Shekhada, H.A. Role of biological control in plant management. Plant 2023, 7, 1328–1340. [Google Scholar]
- Johnson, C.D. Adaptive radiation of Acanthoscelides in seeds: Examples of legume-bruchid interactions. In Stirton C H, Zarucchi J L (eds) Advances in legume biology. Monogr. Syst. Bot. 1989, 29, 747–779. [Google Scholar]
- Johnson, C.D. Systematics of the seed beetle genus Acanthoscelides (Bruchidae) of Northern South America. Trans. Am. Entomol. Soc. 1990, 116, 297–618. [Google Scholar]
- Kingsolver, J.M. Eighteen new species of Bruchidae, principally from Costa Rica, with host records and distributional notes (Insecta:Coleoptera). In Proceedings of the Biological Society of Washington; Biological Society of Washington: Washington, DC, USA, 1980; Volume 93, pp. 229–283. [Google Scholar]
- Kingsolver, J.M. Handbook of the Bruchidae of the United States and Canada (Insecta, Coleoptera); U.S.D.A. Technical Bulletin Number 1912; United States Department of Agriculture: Washington, DC, USA, 2004; pp. 1–324.
- Manfio, D.; Ribeiro-Costa, C.S.; Caron, E. Phylogeny and revision of the new world seed-feeding bruchine genus Gibbobruchus Pic (Coleoptera: Chrysomelidae). Invertebr. Syst. 2013, 27, 1–37. [Google Scholar] [CrossRef]
- Rodrigues, L.M.d.S. Insetos predadores de sementes e suas relações com a qualidade e a morfologia de frutos e sementes. Ph.D. Thesis, Universidade Estadual Paulista, Instituto de Biociências de Botucatu, Botucatu, Brazil, 2013; 117p. [Google Scholar]
- Brasil Ministério da Agricultura, Pecuária e Abastecimento; Secretaria de Defesa Agropecuária. Regras para Análise de Sementes; MAPA/ACS: Brasília, Brazil, 2009; 399p. [Google Scholar]
- Cabrera, L.; Fernández, L.A. Los ejemplares tipo de Bruchidae (Insecta, Coleoptera) depositados en la coleccion del Museo de La Plata. Rev. Mus. Plata 1998, 30, 1–5. [Google Scholar]
- Blackwelder, R.E. Checklist of the coleopterous insects of Mexico, Central America, the West Indies, and South America. Bull. U. S. Natl. Mus. 1946, 185, 551–763. [Google Scholar] [CrossRef]
- Udayagiri, S.; Wadhi, S.R. Catalog of Bruchidae. Mem. Amer. Ent. Inst. 1989, 45, 1–300. [Google Scholar]
- Johnson, C.D. Ecosystematics of Acanthoscelides (Coleoptera: Bruchidae) of Southern Mexico and Central America. Misc. Publ. Entomol. Soc. Am. 1983, 56, 1–370. [Google Scholar]
- Pereira, A.I.d.A.; Cantuário, F.S.; De Sousa, P.V.; Tavares, W.S.; De Medeiros, M.G.H.; De Melo, W.F. Especificidade de Disonycha (Chrysomelidade) em plantas de caruru e genótipos de batata-doce. In Congresso Brasileiro de Olericultura—Anais; ABH (Associação Brasileira de Horticultura): Minas Gerais, Brazil, 2011; Volume 51, pp. 1100–1107. [Google Scholar]
- Pereira, P.R.V.S.; Salvadori, J.R. Identificação dos principais Coleoptera (Insecta) associados a produtos armazenados. Embrapa Trigo 2006, 75, 33. [Google Scholar]
- Orozco-Almanza, M.S.; León-Gárcia, L.P.; Grether, R.; Gácia-Moya, E. Germination of four species of the genus Mimosa (Leguminosae) in a semi-arid zone of Central Mexico. J. Arid Environ. 2003, 55, 75–92. [Google Scholar] [CrossRef]
- Schelin, M.; Tigabu, M.; Eriksson, I.; Sawadogo, L.; Óden, P.C. Predispersal seed predation in Acacia macrostachya, its impact on seed viability, and germination responses to scarification and dry heat treatments. New For. 2004, 27, 251–267. [Google Scholar] [CrossRef]
- Szentesi, A. Pre-dispersal seed predation by Bruchidius villosus (Coleoptera: Bruchidae) in Laburnum anagyroides (Fabaceae: Genisteae). Community Ecol. 2006, 7, 13–22. [Google Scholar] [CrossRef]
- Kolb, A.; Ehrlén, J.; Eriksson, O. Ecological and evolutionary consequences of spatial and temporal variation in pre-dispersal seed predation. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 79–100. [Google Scholar] [CrossRef]
- Acosta, L.; Agüero, R. El banco de propágulos de malezas en el agroecosistema: Conocimiento actual y propuesta metodológica para su estudio. Agron. Mesoam. 2001, 12, 141–151. [Google Scholar] [CrossRef]
- Nisensohn, L.; Faccini, D.; Montero, G.; Lietti, M. Predación de semillas de Amaranthus quitensis H.B.K. en un cultivo de soja: Influencia del sistema de siembra. Pesq. Agropec. Bras. 1999, 34, 377–384. [Google Scholar] [CrossRef]
- Puricelli, E.J.M.; Faccini, D.; Orioli, G.; Sabbatini, M.R. Seed survival and predation of Anoda cristata in soybean crops. Weed Res. 2005, 45, 477–482. [Google Scholar] [CrossRef]
- Southgate, B.J. Biology of the Bruchidae. Annu. Rev. Entomol. 1979, 24, 449–473. [Google Scholar] [CrossRef]
- Rogers, C.E.; Garrison, J. Seed destruction in indigobush Amorpha by a seed beetle. J. Range Manag. 1975, 28, 241–242. [Google Scholar] [CrossRef]
- Kingsolver, J.M.; Whitehead, D.R. The North and Central American species of Meibomeus (Coleoptera: Bruchidae: Bruchinae); Technical Bulletin 1523; Agricultural Reserach Service, United States Department of Agriculture: Washington, DC, USA, 1976; pp. 1–60.
- Di Iorio, O.R. A new previously predicted larval host for the Asian seed beetle Megabruchidius tonkineus (Pic, 1904), and the incorporation of M. dorsalis (Fahraeus, 1839) to the Argentinian fauna of Bruchinae (Coleoptera: Chrysomelidae). Bol. SEA 2015, 56, 327–334. [Google Scholar]
- Salgado, S.E.; Martin, G.D. Distribution and impact of the Asian seed beetle, Megabruchidius tonkineus (Pic, 1904) (Coleoptera: Chrysomelidae: Bruchinae) on Gleditsia triacanthos L. seeds in South Africa. Afr. Entomol 2023, 31, e13386. [Google Scholar] [CrossRef]
- Szentesi, A. Legume (Fabaceae) and seed beetle (Coleoptera, Chrysomelidae, Bruchinae) species of Europe: Distribution and host specialization. Arthropod-Plant Interact. 2024, 18, 579–598. [Google Scholar] [CrossRef]
- Nápoles, J.R.; Kingsolver, J.M. A New Species of Acanthoscelides Schilsky (Coleoptera: Bruchidae) from México with Some Biological Notes. Neotrop. Entomol. 2009, 38, 497–500. [Google Scholar] [CrossRef]
- Kleinjan, C.A.; Hoffmann, J.H.; Heystek, F.; Ivey, P.; Kistensamy, Y. Developments and prospects for biological control of Prosopis (Leguminosae) in South Africa. Afr. Entomol. 2021, 29, 859–874. [Google Scholar] [CrossRef]
- Zachariades, C. A catalogue of natural enemies of invasive alien plants in South Africa: Classical biological control agents considered, released and established, exotic natural enemies present in the field, and bioherbicides. Afr. Entomol. 2021, 29, 1077–1142. [Google Scholar] [CrossRef]
- Cardina, J.; Norquay, H.M.; Stinner, B.R.; McCartney, D.A. Postdispersal predation of velvetleaf (Abutilon theophrasti) seed. Weed Sci. 1996, 44, 534–539. [Google Scholar] [CrossRef]
- Sharratt, M.E.J.; Olckers, T. The Biological Control Agent Acanthoscelides macrophthalmus (Chrysomelidae: Bruchinae) Inflicts Moderate Levels of Seed Damage on Its Target, the Invasive Tree Leucaena leucocephala (Fabaceae), in the KwaZulu-Natal Coastal Region of South Africa. Afr. Entomol. 2012, 20, 44–51. [Google Scholar] [CrossRef]
- Helman, S.; Sobrero, M.T.; Raña, E. Bruchine-predated seeds of Ipomoea nil (L.) Roth., a cotton crop weed in Santiago del Estero, Argentina. Planta Daninha 2020, 38, e020194688. [Google Scholar] [CrossRef]
- Raghu, S.; Wiltshire, C.; Dhileepan, K. Intensity of pre-dispersal seed predation in the invasive legume Leucaena leucocephala is limited by the duration of pod retention. Austral Ecol. 2005, 30, 310. [Google Scholar] [CrossRef]
- Hoffmann, J.H.; Moran, V.C. The population dynamics of an introduced tree, Sesbania punicea, in South Africa, in response to long-term damage caused by different combinations of three species of biological control agents. Oecologia 1998, 114, 343–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).