The Effects of the Addition of Secondary Phyllosilicate Minerals on the Decomposition Process and Products of Maize Straw in Black Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Microbial Inoculum
2.3. Preparation of Artificial Soils
2.4. Incubation Experiment
2.5. Sample Collection and Determination Index
2.6. Statistical Analysis
3. Results
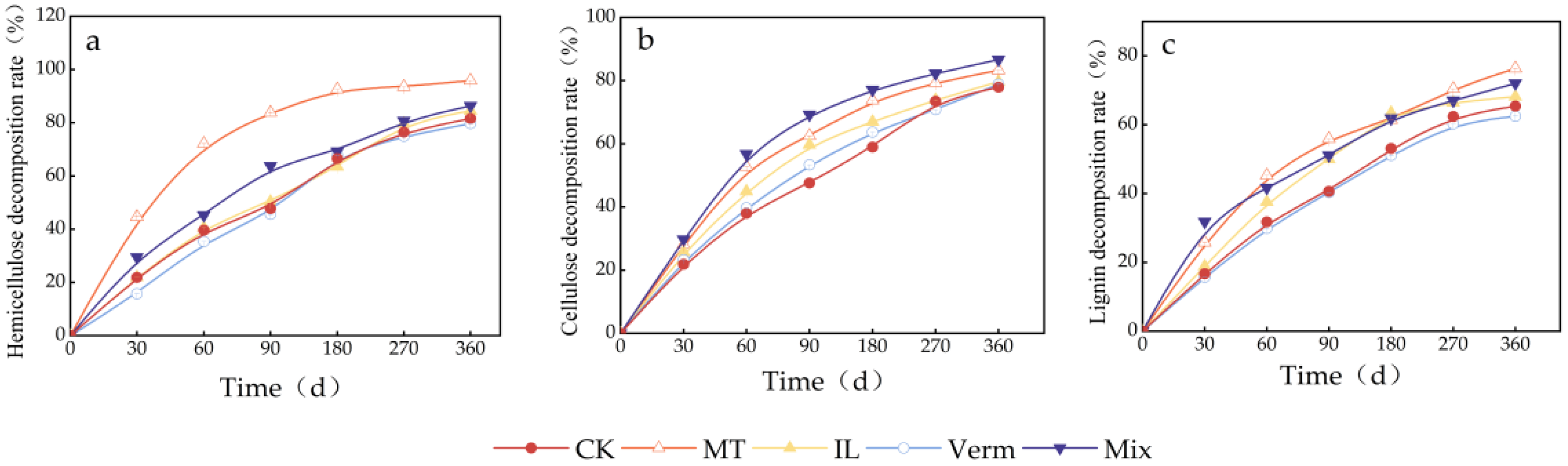
3.1. Changes in Hemicellulose, Cellulose, and Lignin in Maize Straw
3.2. Substance Changes During the Decomposition of Maize Straw
3.2.1. Low-Molecular-Weight Organic Acids
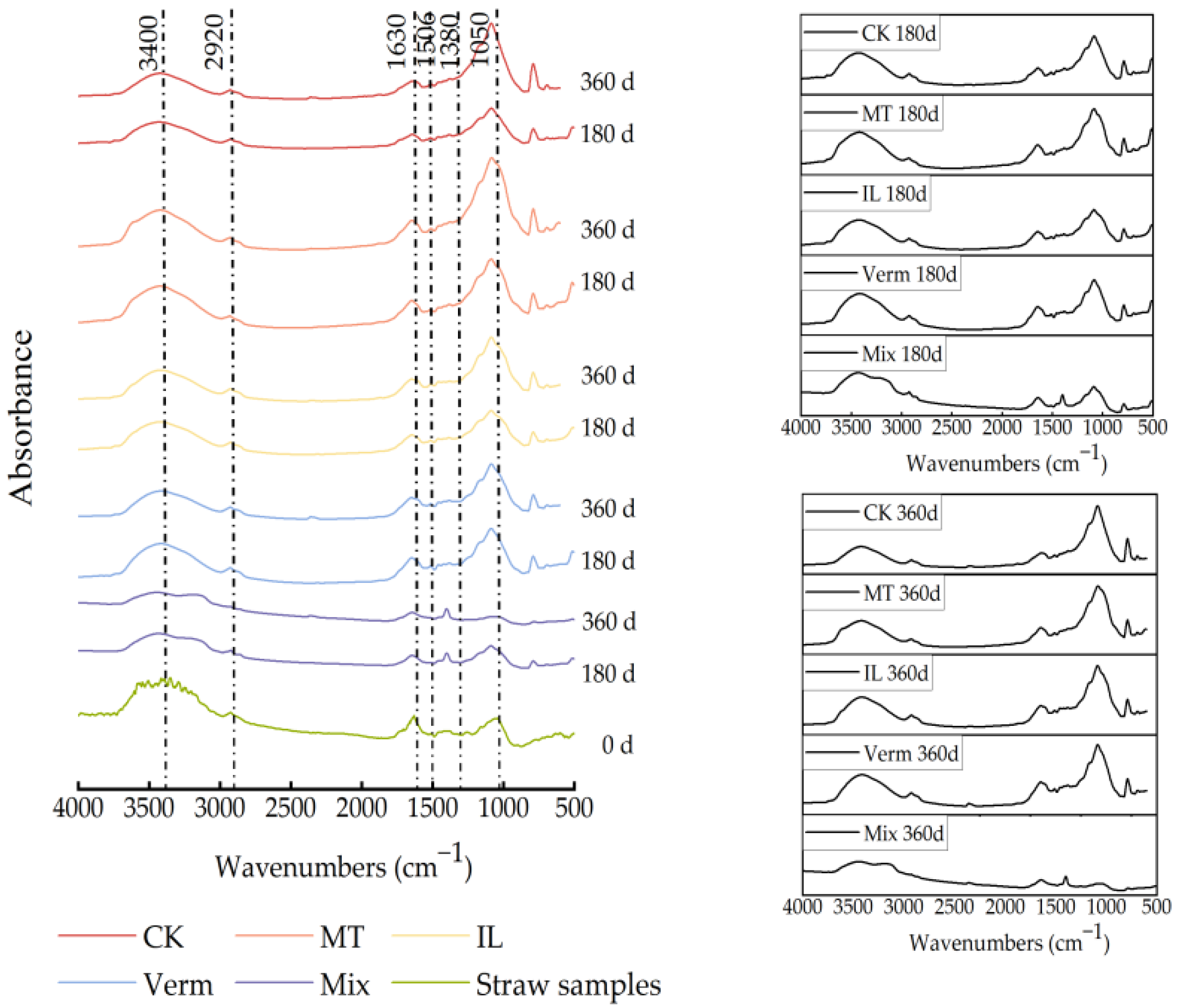
3.2.2. FTIR Analysis
3.2.3. Thermogravimetric Analysis
3.3. Straw Decomposition Products and Soil Organic–Mineral Complexes
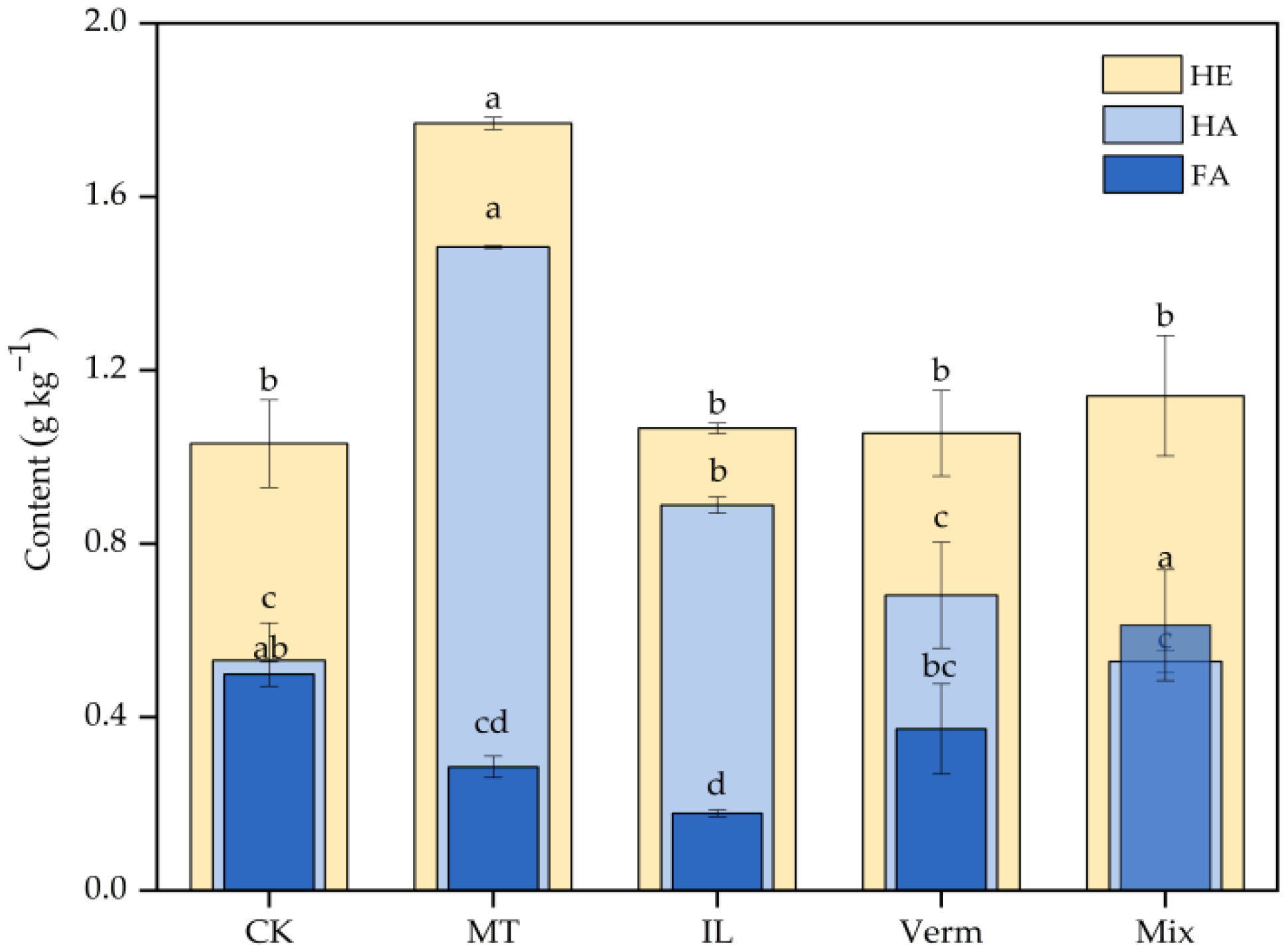
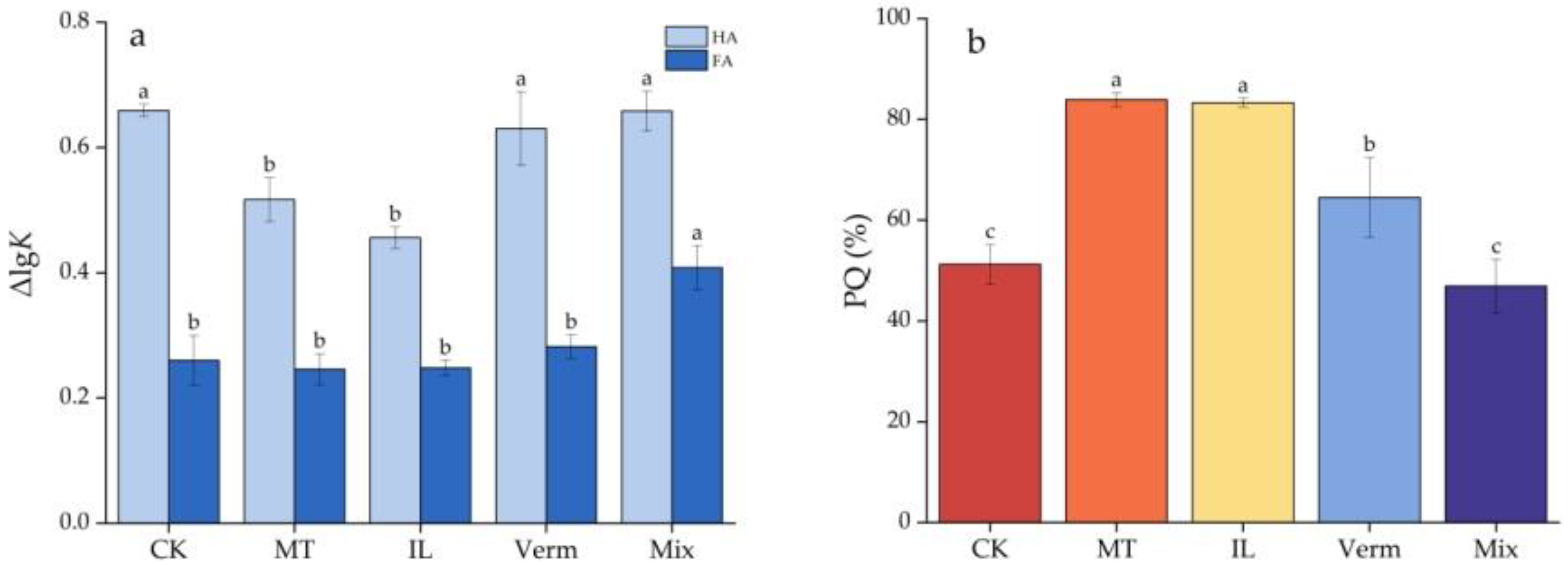
3.3.1. Effects of Secondary Phyllosilicate Minerals on HE, HA, and FA
3.3.2. Effects of Secondary Phyllosilicate Minerals on Organic–Mineral Complexes
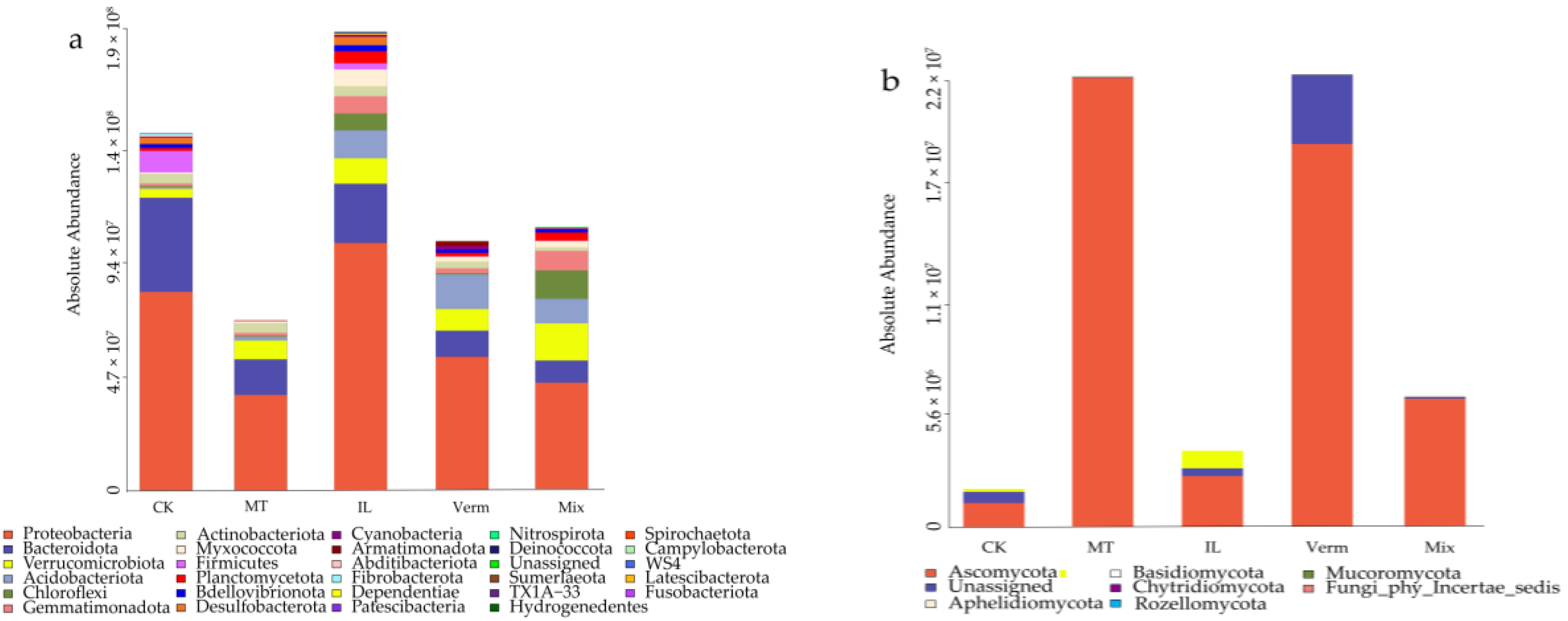
3.4. The Composition of Microbial Communities in Soil
4. Discussion
4.1. The Influence of Adding Secondary Phyllosilicate Minerals on the Organic Components of Maize Straw
4.2. The Influence of Secondary Phyllosilicate Minerals on the Formation of Low-Molecular-Weight Organic Acids During Maize Straw Decomposition
4.3. The Influence of Secondary Phyllosilicate Minerals on the Decomposition Products of Maize Straw
4.4. The Effect of Secondary Phyllosilicate Minerals on the Formation of Organic–Mineral Complexes During Maize Straw Decomposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Liu, C.; Wang, J.; Meng, Q.; Yuan, Y.; Ma, X.; Liu, X.; Zhu, Y.; Ding, G.; Zhang, J.; et al. Soil Aggregates Stability and Storage of Soil Organic Carbon Respond to Cropping Systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Possinger, A.R.; Zachman, M.J.; Enders, A.; Levin, B.D.A.; Muller, D.A.; Kourkoutis, L.F.; Lehmann, J. Organo–Organic and Organo–Mineral Interfaces in Soil at the Nanometer Scale. Nat. Commun. 2020, 11, 6103. [Google Scholar] [CrossRef]
- Singh, M.; Sarkar, B.; Bolan, N.S.; Ok, Y.S.; Churchman, G.J. Decomposition of Soil Organic Matter as Affected by Clay Types, Pedogenic Oxides and Plant Residue Addition Rates. J. Hazard. Mater. 2019, 374, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Höper, H. Soil Organic Matter Turnover as a Function of the Soil Clay Content: Consequences for Model Applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Dilustro, J.J.; Collins, B.; Duncan, L.; Crawford, C. Moisture and Soil Texture Effects on Soil CO2 Efflux Components in Southeastern Mixed Pine Forests. For. Ecol. Manag. 2005, 204, 87–97. [Google Scholar] [CrossRef]
- Fissore, C.; Giardina, C.P.; Kolka, R.K.; Trettin, C.C.; King, G.M.; Jurgensen, M.F.; Barton, C.D.; Mcdowell, S.D. Temperature and Vegetation Effects on Soil Organic Carbon Quality along a Forested Mean Annual Temperature Gradient in North America. Glob. Change Biol. 2008, 14, 193–205. [Google Scholar] [CrossRef]
- Tong, Y.; Xiang, H.; Jiang, J.; Chen, W. Interfacial Interactions between Minerals and Organic Matter: Mechanisms and Characterizations. Chemosphere 2024, 359, 142383. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, H.; Ding, S.; Hu, Z.; Tie, B.; Luo, S. A New Insight into the Straw Decomposition Associated with Minerals: Promoting Straw Humification and Cd Immobilization. J. Environ. Sci. 2025, 148, 553–566. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Mason, K.E.; Goodall, T.; Taylor, A.; Zhao, P.; Otero-Fariña, A.; Chen, H.; Peacock, C.L.; Ostle, N.J.; Griffiths, R.; et al. Microbial and Mineral Interactions Decouple Litter Quality from Soil Organic Matter Formation. Nat. Commun. 2024, 15, 10063. [Google Scholar] [CrossRef] [PubMed]
- Finley, B.K.; Mau, R.L.; Hayer, M.; Stone, B.W.; Morrissey, E.M.; Koch, B.J.; Rasmussen, C.; Dijkstra, P.; Schwartz, E.; Hungate, B.A. Soil Minerals Affect Taxon-Specific Bacterial Growth. ISME J. 2022, 16, 1318–1326. [Google Scholar] [CrossRef]
- Rakhsh, F.; Golchin, A.; Beheshti Al Agha, A.; Nelson, P.N. Mineralization of Organic Carbon and Formation of Microbial Biomass in Soil: Effects of Clay Content and Composition and the Mechanisms Involved. Soil Biol. Biochem. 2020, 151, 108036. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, G.; Chen, S.; Rasmussen, C.; Liu, B. Assessing Soil Thickness in a Black Soil Watershed in Northeast China Using Random Forest and Field Observations. Int. Soil Water Conserv. Res. 2021, 9, 49–57. [Google Scholar] [CrossRef]
- Pronk, G.J.; Heister, K.; Ding, G.-C.; Smalla, K.; Kögel-Knabner, I. Development of Biogeochemical Interfaces in an Artificial Soil Incubation Experiment; Aggregation and Formation of Organo-Mineral Associations. Geoderma 2012, 189–190, 585–594. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Dou, S.; Guan, S.; Yang, C.; Wang, Z. Deep Straw Burial Accelerates Straw Decomposition and Improves Soil Water Repellency. Agronomy 2023, 13, 1927. [Google Scholar] [CrossRef]
- Zhu, H.; Bing, H.; Wu, Y.; Sun, H.; Zhou, J. Low Molecular Weight Organic Acids Regulate Soil Phosphorus Availability in the Soils of Subalpine Forests, Eastern Tibetan Plateau. Catena 2021, 203, 105328. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Hui, L.; Wang, H. Diols as Solvent Media for Liquefaction of Corn Stalk at Ambient Pressure. BioResources 2018, 13, 6818–6836. [Google Scholar] [CrossRef]
- Barros, N.; Salgado, J.; Villanueva, M.; Rodriquez-Añón, J.; Proupin, J.; Feijóo, S.; Martín-Pastor, M. Application of DSC–TG and NMR to Study the Soil Organic Matter. J. Therm. Anal. Calorim. 2011, 104, 53–60. [Google Scholar] [CrossRef]
- Wu, J.; Yao, W.; Zhao, L.; Zhao, Y.; Qi, H.; Zhang, R.; Song, C.; Wei, Z. Estimating the Synergistic Formation of Humus by Abiotic and Biotic Pathways during Composting. J. Clean. Prod. 2022, 363, 132470. [Google Scholar] [CrossRef]
- Huang, X.; Kang, W.; Guo, J.; Wang, L.; Tang, H.; Li, T.; Yu, G.; Ran, W.; Hong, J.; Shen, Q. Highly Reactive Nanomineral Assembly in Soil Colloids: Implications for Paddy Soil Carbon Storage. Sci. Total Environ. 2020, 703, 134728. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chen, X.; Han, B.-Z.; Xue, Y. Insights into the Bacterial, Fungal, and Phage Communities and Volatile Profiles in Different Types of Daqu. Food Res. Int. 2022, 158, 111488. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Bao, Z.; Li, H.; Lyu, Y.; Zan, Y.; Wu, Y.; Cheng, L.; Fang, Y.; Wu, K.; et al. Graph Pangenome Captures Missing Heritability and Empowers Tomato Breeding. Nature 2022, 606, 527–534. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Yu, Y.-N.; Gao, R.-W.; Wang, H.; Zhang, J.; Li, R.; Long, X.-H.; Shen, Q.-R.; Chen, W.; Cai, F. High-Throughput Absolute Quantification Sequencing Reveals the Effect of Different Fertilizer Applications on Bacterial Community in a Tomato Cultivated Coastal Saline Soil. Sci. Total Environ. 2019, 687, 601–609. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Li, S.; Delgado-Baquerizo, M.; Ding, J.; Hu, H.; Huang, W.; Sun, Y.; Ni, H.; Kuang, Y.; Yuan, M.M.; Zhou, J.; et al. Intrinsic Microbial Temperature Sensitivity and Soil Organic Carbon Decomposition in Response to Climate Change. Glob. Change Biol. 2024, 30, e17395. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Xu, G.; Deng, J.; Dong, M.; Murugadoss, V.; Liu, C.; Shao, Q.; Wu, S.; Guo, Z. Structural Characterization of Lignin from D. Sinicus by FTIR and NMR Techniques. Green Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Ma, F.; Zhang, S.; Zhou, Q.; Huang, A. Three-Step Identification of Infrared Spectra of Similar Tree Species to Pterocarpus santalinus Covered with Beeswax. J. Mol. Struct. 2020, 1218, 128484. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Tan, Z.; Zhou, T. Cellulose Extraction from Rice Straw Waste for Biodegradable Ethyl Cellulose Films Preparation Using Green Chemical Technology. J. Clean. Prod. 2024, 439, 140839. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Zhang, X.; Xu, J.; Lei, W.; Zhou, C.; Chen, C.; Li, F.; Wang, N. Humus Composition of Mineral–Microbial Residue from Microbial Utilization of Lignin Involving Different Mineral Types. Can. J. Soil Sci. 2019, 99, 208–216. [Google Scholar] [CrossRef]
- Yotsuji, K.; Tachi, Y.; Sakuma, H.; Kawamura, K. Effect of Interlayer Cations on Montmorillonite Swelling: Comparison between Molecular Dynamic Simulations and Experiments. Appl. Clay Sci. 2021, 204, 106034. [Google Scholar] [CrossRef]
- Schulze, D.G. Clay Minerals. In Encyclopedia of Soils in the Environment, 1st ed.; Hillel, D., Ed.; Academic Press: New York, NY, USA, 2005; pp. 246–254. [Google Scholar]
- Hearon, S.E.; Orr, A.A.; Moyer, H.; Wang, M.; Tamamis, P.; Phillips, T.D. Montmorillonite Clay-Based Sorbents Decrease the Bioavailability of per- and Polyfluoroalkyl Substances (PFAS) from Soil and Their Translocation to Plants. Environ. Res. 2022, 205, 112433. [Google Scholar] [CrossRef]
- Wei, H.; Guenet, B.; Vicca, S.; Nunan, N.; Asard, H.; AbdElgawad, H.; Shen, W.; Janssens, I.A. High Clay Content Accelerates the Decomposition of Fresh Organic Matter in Artificial Soils. Soil Biol. Biochem. 2014, 77, 100–108. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhang, Z.; Shen, F.; Zhang, G.; Qin, R.; Li, X.; Xiao, R. Nutrient Transformations during Composting of Pig Manure with Bentonite. Bioresour. Technol. 2012, 121, 362–368. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Food Waste and Montmorillonite Contribute to the Enhancement of Green Waste Composting. Process Saf. Environ. Prot. 2023, 170, 983–998. [Google Scholar] [CrossRef]
- Riley, W.J.; Maggi, F.; Kleber, M.; Torn, M.S.; Tang, J.Y.; Dwivedi, D.; Guerry, N. Long Residence Times of Rapidly Decomposable Soil Organic Matter: Application of a Multi-Phase, Multi-Component, and Vertically Resolved Model (BAMS1) to Soil Carbon Dynamics. Geosci. Model Dev. 2014, 7, 1335–1355. [Google Scholar] [CrossRef]
- Mikutta, R.; Mikutta, C.; Kalbitz, K.; Scheel, T.; Kaiser, K.; Jahn, R. Biodegradation of Forest Floor Organic Matter Bound to Minerals via Different Binding Mechanisms. Geochim. Cosmochim. Acta 2007, 71, 2569–2590. [Google Scholar] [CrossRef]
- Baldock, J.A.; Skjemstad, J.O. Role of the Soil Matrix and Minerals in Protecting Natural Organic Materials against Biological Attack. Org. Geochem. 2000, 31, 697–710. [Google Scholar] [CrossRef]
- Ruiz, F.; Barreto, M.S.C.; Rumpel, C.; Nóbrega, G.N.; Oliveira, H.A.; Menandro, A.S.; Péres, L.O.; Montes, C.R.; Ferreira, T.O. Adsorption and Thermal Stability of Dissolved Organic Matter on Ca- and Mg-Exchanged Montmorillonite: Implications for Persistence in Soils and Sediments. Chem. Geol. 2024, 643, 121813. [Google Scholar] [CrossRef]
- Fan, Q.H.; Tanaka, M.; Tanaka, K.; Sakaguchi, A.; Takahashi, Y. An EXAFS Study on the Effects of Natural Organic Matter and the Expandability of Clay Minerals on Cesium Adsorption and Mobility. Geochim. Cosmochim. Acta 2014, 135, 49–65. [Google Scholar] [CrossRef]
- Shi, L.; Qiu, J.; Wang, W.; Ding, Z.; Zhang, W.; Liang, J.; Li, P.; Fan, Q. Influence of Cations and Low Molecular Weight Organic Acids on Cs(I) Adsorption on Montmorillonite and Vermiculite. J. Mol. Liq. 2024, 402, 124778. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; He, X.; Sun, H.; Yan, H.; Zhao, S.; Zhao, K.; Liu, W. The Joint Action of Nano-Montmorillonite and Plant Roots on the Remediation of Cadmium-Contaminated Soil and the Improvement of Rhizosphere Bacterial Characterization. J. Environ. Chem. Eng. 2025, 13, 115462. [Google Scholar] [CrossRef]
- Su, M.; Han, F.; Wu, Y.; Yan, Z.; Lv, Z.; Tian, D.; Wang, S.; Hu, S.; Shen, Z.; Li, Z. Effects of Phosphate-Solubilizing Bacteria on Phosphorous Release and Sorption on Montmorillonite. Appl. Clay Sci. 2019, 181, 105227. [Google Scholar] [CrossRef]
- Tietjen, T.; Wetzel, R.G. Extracellular Enzyme-Clay Mineral Complexes: Enzyme Adsorption, Alteration of Enzyme Activity, and Protection from Photodegradation. Aquat. Ecol. 2003, 37, 331–339. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, S.; Liu, B.; Pu, Y.; Gao, Z.; Wang, J.; Jin, R.; Zhou, J. Microbial Reduction of Fe (III)-Bearing Clay Minerals in the Presence of Humic Acids. Sci. Rep. 2017, 7, 45354. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Fornasier, F.; Paletto, A.; Ceotto, E.; De Meo, I. Ascomycota and Basidiomycota Fungal Phyla as Indicators of Land Use Efficiency for Soil Organic Carbon Accrual with Woody Plantations. Ecol. Indic. 2024, 160, 111796. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Matsukura, K. Decay Stages of Wood and Associated Fungal Communities Characterise Diversity–Decomposition Relationships. Sci. Rep. 2021, 11, 8972. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Guo, Y.-X.; Chen, Q.-J.; Wang, Y.-Y.; Wang, Q.-Y.; Yang, Y.-R.; Zhang, G.-Q. Metagenomic Insights into the Lignocellulose Degradation Mechanism during Short-Term Composting of Peach Sawdust: Core Microbial Community and Carbohydrate-Active Enzyme Profile Analysis. Environ. Technol. Innov. 2025, 37, 103959. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, M.; Liu, H.; Xiao, J.; Men, J.; Cernava, T.; Deng, Y.; Jin, D. Comparison of Plastisphere Microbiomes during the Degradation of Conventional and Biodegradable Mulching Films. J. Hazard. Mater. 2025, 487, 137243. [Google Scholar] [CrossRef]
- Wilhelm, R.C.; Singh, R.; Eltis, L.D.; Mohn, W.W. Bacterial Contributions to Delignification and Lignocellulose Degradation in Forest Soils with Metagenomic and Quantitative Stable Isotope Probing. ISME J. 2019, 13, 413–429. [Google Scholar] [CrossRef]
- Gao, X.; Liu, W.; Li, X.; Zhang, W.; Bu, S.; Wang, A. A Novel Fungal Agent for Straw Returning to Enhance Straw Decomposition and Nutrients Release. Environ. Technol. Innov. 2023, 30, 103064. [Google Scholar] [CrossRef]
- Wegner, C.; Liesack, W. Microbial Community Dynamics during the Early Stages of Plant Polymer Breakdown in Paddy Soil. Environ. Microbiol. 2016, 18, 2825–2842. [Google Scholar] [CrossRef]
- Nevins, C.J.; Nakatsu, C.; Armstrong, S. Characterization of Microbial Community Response to Cover Crop Residue Decomposition. Soil Biol. Biochem. 2018, 127, 39–49. [Google Scholar] [CrossRef]
- Liu, X.; Laipan, M.; Zhang, C.; Zhang, M.; Wang, Z.; Yuan, M.; Guo, J. Microbial Weathering of Montmorillonite and Its Implication for Cd (II) Immobilization. Chemosphere 2024, 349, 140850. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yuan, X.; Xiong, T.; Tan, Y.Z.; Wang, H. Physicochemical Properties, Metal Availability and Bacterial Community Structure in Heavy Metal-Polluted Soil Remediated by Montmorillonite-Based Amendments. Chemosphere 2020, 261, 128010. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in Straw Decomposition Rate and Soil Microbial Community Composition after Straw Addition in Different Long-Term Fertilization Soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, J.; Ni, L.; Li, J. Effect of Hot-Pressing Temperature on Characteristics of Straw-Based Binderless Fiberboards with Pulping Effluent. Materials 2019, 12, 922. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Ma, H.-X.; Qi, Y.; Zhu, R.-X.; Li, X.-W.; Yu, W.-J.; Rao, F. Study of the Long-Term Degradation Behavior of Bamboo Scrimber under Natural Weathering. npj Mater. Degrad. 2022, 6, 63. [Google Scholar] [CrossRef]
- Sun, C.; Yao, Z.; Wang, Q.; Guo, L.; Shen, X. Theoretical Study on the Organic Acid Promoted Dissolution Mechanism of Forsterite Mineral. Appl. Surf. Sci. 2023, 614, 156063. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, Roles and Fate of Organic Acids in Soils: A Review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, C.; Wang, X.; Cheng, K.; Wang, C.; Xu, X.; Lin, L. Modeling Bacterial Interactions Uncovers the Importance of Outliers in the Coastal Lignin-Degrading Consortium. Nat. Commun. 2025, 16, 639. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Fitz, D.; Jakschitz, T.; Rode, M. Selective Adsorption and Chiral Amplification of Amino Acids in Vermiculite Clay -Implications for the Origin of Biochirality. Phys. Chem. Chem. Phys. 2010, 13, 831–838. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujii, K.; Hayakawa, C.; Inagaki, Y.; Ono, K. Sorption Reduces the Biodegradation Rates of Multivalent Organic Acids in Volcanic Soils Rich in Short-Range Order Minerals. Geoderma 2019, 333, 188–199. [Google Scholar] [CrossRef]
- Mendonça, F.G.; Filho, E.J.S.; Bertoli, A.C.; Fernández, M.A.; Torres Sánchez, R.M.; Lago, R.M. Use of Montmorillonite to Recover Carboxylic Acids from Aqueous Medium. Sep. Purif. Technol. 2019, 229, 115751. [Google Scholar] [CrossRef]
- Su, Y.; Yu, M.; Xi, H.; Lv, J.; Ma, Z.; Kou, C.; Shen, A. Soil Microbial Community Shifts with Long-Term of Different Straw Return in Wheat-Corn Rotation System. Sci. Rep. 2020, 10, 6360. [Google Scholar] [CrossRef]
- Muhanmaitijiang, N.; Feng, Y.; Xie, Y.; Du, X.; Li, J.; Chen, H. Outside-in Enhancement of Phosphate Solubilizing Bacteria by Sludge Biochar for Phenol Remediation in Soil: Pollution Stress Reduction, Electron Transfer Gain and Secretion Regulation. Chem. Eng. J. 2024, 500, 157541. [Google Scholar] [CrossRef]
- Yadav, M.; Joshi, C.; Paritosh, K.; Thakur, J.; Pareek, N.; Masakapalli, S.K.; Vivekanand, V. Organic Waste Conversion through Anaerobic Digestion: A Critical Insight into the Metabolic Pathways and Microbial Interactions. Metab. Eng. 2022, 69, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Saber, W.I.A.; El-Naggar, N.E.-A.; AbdAl-Aziz, S.A. Bioconversion of Lignocellulosic Wastes into Organic Acids by Cellulolytic Rock Phosphate-Solubilizing Fungal Isolates Grown under Solid-State Fermentation Conditions. Res. J. Microbiol. 2010, 5, 1–20. [Google Scholar] [CrossRef]
- Casella, P.; Loffredo, R.; Rao, M.A.; Balducchi, R.; Liuzzi, F.; De Bari, I.; Molino, A. Inhibitors Derived from Wheat Straw Hydrolysate Can Affect the Production of Succinic Acid by Actinobacillus Succinogenes. Process Biochem. 2024, 147, 228–239. [Google Scholar] [CrossRef]
- Chatgasem, C.; Suwan, W.; Attapong, M.; Siripornadulsil, W.; Siripornadulsil, S. Single-Step Conversion of Rice Straw to Lactic Acid by Thermotolerant Cellulolytic Lactic Acid Bacteria. Biocatal. Agric. Biotechnol. 2023, 47, 102546. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C.; et al. Application and Prospect of Organic Acid Pretreatment in Lignocellulosic Biomass Separation: A Review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, P.; Li, H.; Yu, H.; Ouyang, J.; Zheng, Z. Sustainable Wheat Straw Pretreatment Process by Self-Produced and Cyclical Crude Lactic Acid. Bioresour. Technol. 2024, 402, 130788. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Rodrigues, R.C.L.B.; Kim, H.J.; Choi, I.-G.; Jeffries, T.W. The Roles of Xylan and Lignin in Oxalic Acid Pretreated Corncob during Separate Enzymatic Hydrolysis and Ethanol Fermentation. Bioresour. Technol. 2010, 101, 4379–4385. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J.; Guan, H.; Tian, D.; Gao, H. Decomposition of Maize Straw between Two Phosphate Solubilizing Fungi: Aspergillus Niger and Penicillium Chrysogenum. E3S Web Conf. 2022, 350, 1028. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, E.; Ma, F.; Jiang, K. An Integrated Biorefinery Process for Co-Production of Xylose and Glucose Using Maleic Acid as Efficient Catalyst. Bioresour. Technol. 2021, 325, 124698. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, J.; Fu, Q.; Hu, H.; Huang, Q. Sorption of Cu by Humic Acid from the Decomposition of Rice Straw in the Absence and Presence of Clay Minerals. J. Environ. Manag. 2017, 200, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lv, J.; Peng, Y.; Yao, Y.; Wei, X.; Wang, X. Mechanisms Controlling the Stability and Sequestration of Mineral Associated Organic Carbon upon Erosion and Deposition. Catena 2024, 242, 108119. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Zhao, Y.; Tao, W.; Wei, Z.; Song, C. Investigating the Effect of Fenton-like Pretreatment-Clay Mineral Addition on Humic Substance during Straw Composting. Chem. Eng. J. 2024, 496, 154199. [Google Scholar] [CrossRef]
- Miura, A.; Okabe, R.; Izumo, K.; Fukushima, M. Influence of the Physicochemical Properties of Clay Minerals on the Degree of Darkening via Polycondensation Reactions between Catechol and Glycine. Appl. Clay Sci. 2009, 46, 277–282. [Google Scholar] [CrossRef]
- Fukuchi, S.; Miura, A.; Okabe, R.; Fukushima, M.; Sasaki, M.; Sato, T. Spectroscopic Investigations of Humic-like Acids Formed via Polycondensation Reactions between Glycine, Catechol and Glucose in the Presence of Natural Zeolites. J. Mol. Struct. 2010, 982, 181–186. [Google Scholar] [CrossRef]
- Wattel-Koekkoek, E.J.W.; van Genuchten, P.P.L.; Buurman, P.; van Lagen, B. Amount and Composition of Clay-Associated Soil Organic Matter in a Range of Kaolinitic and Smectitic Soils. Geoderma 2001, 99, 27–49. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Chang, L.; Yang, W.; Si, L.; Nan, H.; Peng, W.; Cao, Y. Functionality Developments in Montmorillonite Nanosheet: Properties, Preparation, and Applications. Chem. Eng. J. 2024, 499, 156186. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Tsang, D.C.W.; Li, G. Effects of External Additives: Biochar, Bentonite, Phosphate, on Co-Composting for Swine Manure and Corn Straw. Chemosphere 2020, 248, 125927. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, Q.; Liu, W.; Wang, J.; Guo, W.; Jia, E.; Zeng, Q.; Qin, R.; Zheng, J.; Hofmockel, K.S.; et al. Molecular Determination of Organic Adsorption Sites on Smectite during Fe Redox Processes Using ToF-SIMS Analysis. Environ. Sci. Technol. 2021, 55, 7123–7134. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Tan, J.; Zang, C.; Yu, Z. Chemical Composition and Structure of Humus Relative to Sources. Acta Pedol. Sin. 2018, 56, 386–397. [Google Scholar]
- Zhao, T.; Xu, S.; Hao, F. Differential Adsorption of Clay Minerals: Implications for Organic Matter Enrichment. Earth-Sci. Rev. 2023, 246, 104598. [Google Scholar] [CrossRef]
- Guimarães, V.; Bobos, I. Role of Clay Barrier Systems in the Disposal of Radioactive Waste. In Sorbents Materials for Controlling Environmental Pollution; Núñez-Delgado, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 513–541. [Google Scholar]
- Fernández-Ugalde, O.; Barré, P.; Hubert, F.; Virto, I.; Girardin, C.; Ferrage, E.; Caner, L.; Chenu, C. Clay Mineralogy Differs Qualitatively in Aggregate-size Classes: Clay-mineral-based Evidence for Aggregate Hierarchy in Temperate Soils. Eur. J. Soil Sci. 2013, 64, 410–422. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lin, X.; Nie, X.; Xie, R.; Qin, Z.; Ran, M.; Wan, Q.; Wang, J. Heteroaggregation and Deposition Behaviors of Carboxylated Nanoplastics with Different Types of Clay Minerals in Aquatic Environments: Important Role of Calcium (II) Ion-Assisted Bridging. Ecotoxicol. Environ. Saf. 2024, 280, 116533. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, G.; Tian, R.; Ding, W.; Hu, F.; Liu, X.; Li, H. Formation of Sandwich Structure through Ion Adsorption at the Mineral and Humic Interfaces: A Combined Experimental Computational Study. J. Mol. Struct. 2015, 1093, 96–100. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Wu, Z.; Feng, H.; Xue, X.; Xie, L.; Zhang, T.; Zhang, J. Retention of Organic Matter on the Surface of Illite Particle under the Influence of Different Cations: A Molecular Dynamics Simulation Study. Appl. Clay Sci. 2023, 232, 106810. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Wang, J.; Zhang, Y.; Chen, Y. Vermiculite Changed Greenhouse Gases Emission and Microbial Community Succession in Vermicomposting: Particle Size Investigation. Bioresour. Technol. 2025, 416, 131769. [Google Scholar] [CrossRef]
- Bleam, W.F. The Nature of Cation-Substitution Sites in Phyllosilicates. Clays Clay Miner. 1990, 38, 527–536. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.; Dou, Y.; Cheng, H.; Liu, L.; An, S. Linkage between Soil Ectoenzyme Stoichiometry Ratios and Microbial Diversity Following the Conversion of Cropland into Grassland. Agric. Ecosyst. Environ. 2021, 314, 107418. [Google Scholar] [CrossRef]


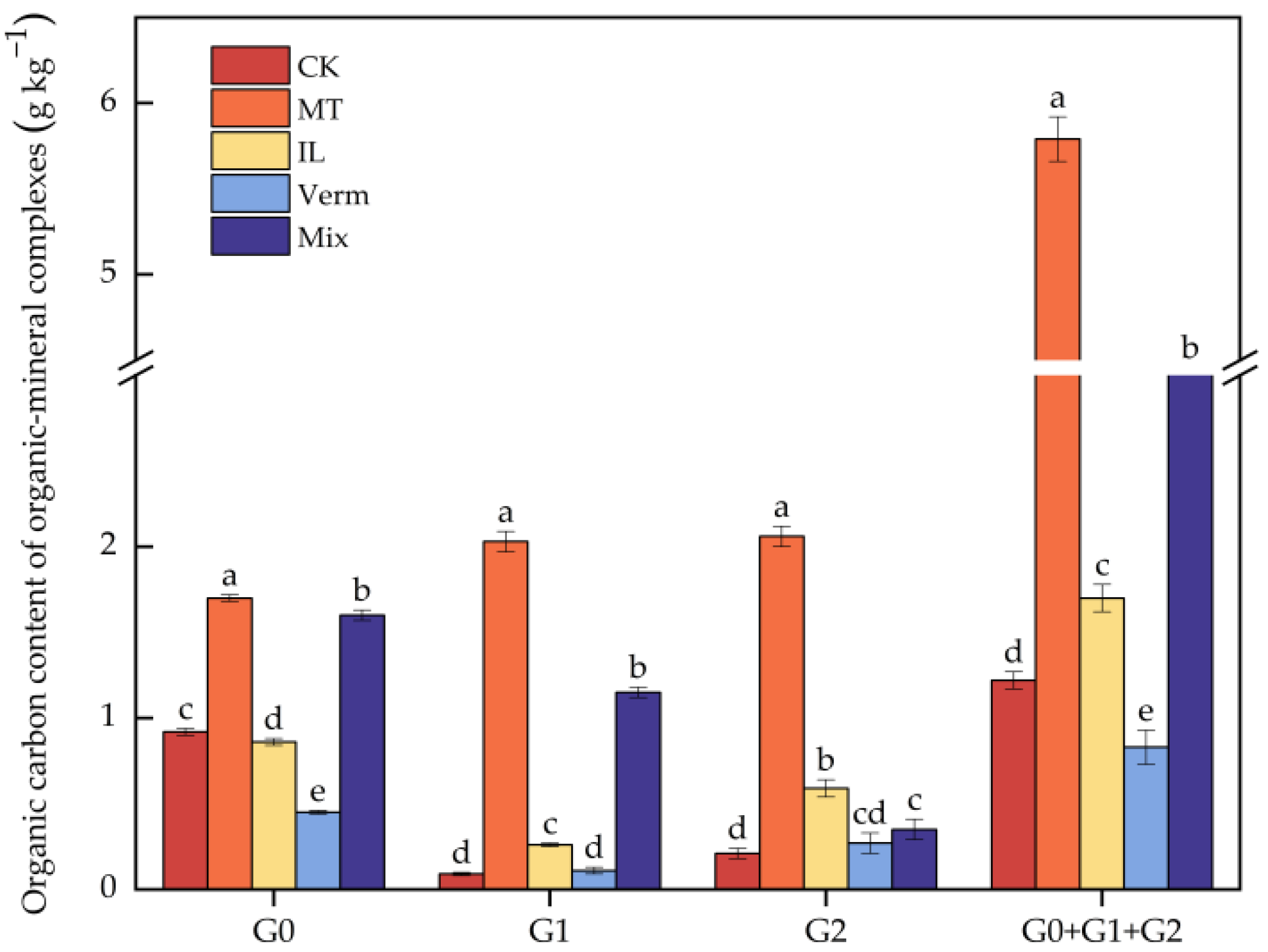
| Treatment | Complex Composition (%) | Complex Content (g kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G0 | G1 | G2 | G1 + G2 | G0 + G1 + G2 | |
| CK | 89.51 ± 0.91 a | 6.05 ± 0.70 e | 4.44 ±0.53 c | 110.67 ± 1.59 b | 7.50 ± 1.02 d | 5.50 ± 0.74 d | 13.00 ± 1.41 d | 123.67 ± 2.97 c |
| MT | 61.42 ± 0.24 d | 23.84 ± 0.13 a | 14.74 ± 0.11 a | 119.75 ± 2.04 a | 46.50 ± 1.22 a | 28.75 ± 0.82 a | 75.25 ± 2.04 a | 195.00 ± 4.08 a |
| IL | 71.03 ± 0.94 c | 17.34 ± 0.29 c | 11.63 ± 0.80 b | 77.75 ± 1.74 c | 19.00 ± 0.89 c | 12.75 ± 1.14 b | 31.75 ± 1.87 c | 109.50 ± 3.36 d |
| Verm | 76.29 ± 3.41 b | 12.28 ± 1.64 d | 11.42 ± 1.83 b | 44.50 ± 1.02 d | 7.25 ± 1.43 d | 6.75 ± 1.47 d | 14.00 ± 2.88 d | 58.50 ± 3.89 e |
| Mix | 73.93 ± 0.79 bc | 21.27 ± 0.07 b | 4.80 ± 0.77c | 119.00 ± 1.62 a | 34.25 ± 0.82 b | 7.75 ± 1.43 d | 42.00 ± 2.25 b | 161.00 ± 3.81 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wang, H.; Zhao, C.; Liu, J.; Huang, N.; Sui, B.; Yang, L.; Wang, N.; Zhao, X. The Effects of the Addition of Secondary Phyllosilicate Minerals on the Decomposition Process and Products of Maize Straw in Black Soil. Agronomy 2025, 15, 316. https://doi.org/10.3390/agronomy15020316
Zhao Q, Wang H, Zhao C, Liu J, Huang N, Sui B, Yang L, Wang N, Zhao X. The Effects of the Addition of Secondary Phyllosilicate Minerals on the Decomposition Process and Products of Maize Straw in Black Soil. Agronomy. 2025; 15(2):316. https://doi.org/10.3390/agronomy15020316
Chicago/Turabian StyleZhao, Qi, Hongbin Wang, Chenyu Zhao, Jinhua Liu, Ning Huang, Biao Sui, Luze Yang, Nan Wang, and Xingmin Zhao. 2025. "The Effects of the Addition of Secondary Phyllosilicate Minerals on the Decomposition Process and Products of Maize Straw in Black Soil" Agronomy 15, no. 2: 316. https://doi.org/10.3390/agronomy15020316
APA StyleZhao, Q., Wang, H., Zhao, C., Liu, J., Huang, N., Sui, B., Yang, L., Wang, N., & Zhao, X. (2025). The Effects of the Addition of Secondary Phyllosilicate Minerals on the Decomposition Process and Products of Maize Straw in Black Soil. Agronomy, 15(2), 316. https://doi.org/10.3390/agronomy15020316






