Abstract
The advancement of hybrid japonica rice is pivotal for securing japonica rice supplies and bolstering food security. To address prevalent issues such as inconsistent yields, subpar rice quality, and inadequate seed production in existing cultivars, Shenyou R3 was developed using advanced high-density rice gene chip technology, which is characterized by the expression of specific genes. This late-season, premium aromatic variety, characterized by a popcorn-like aroma, was bred by the Crop Breeding and Cultivation Research Institute of the Shanghai Academy of Agricultural Sciences. Shenyou R3 incorporates superior genes such as badh2-E7, Pi2, Xa21, Sdt97, and Hd17, among which, badh2-E7 and Hd17 are inherited from the maternal line, while Pi2, Xa21, and Sdt97 are inherited from both the maternal and paternal lines. Shenyou R3 offers high-quality rice that adheres to national premium grade 2 standards, with level 1 resistance to blast disease, and yields surpassing the control variety Huayou 14 by over 5% in 2022 Shanghai trials. The new hybrid japonica rice Shenyou R3 has high yield potential and nitrogen utilization efficiency. This paper elaborates on the molecular marker-assisted selection process, key traits, quality metrics, and yield performance of Shenyou R3, while also outlining essential cultivation practices.
1. Introduction
Rice is China’s principal staple food, relied upon by over 60% of the population as their primary dietary source [1]. As the socio-economic landscape evolves and living standards rise, there is a growing consumer demand for premium rice varieties [2,3]. However, the main cultivated varieties in production generally suffer from poor resistance to rice blast disease in high-quality rice varieties or average taste in high-yield rice varieties. This necessitates the development of disease-resistant, high-quality varieties capable of consistently high yields [4]. Hybrid japonica rice significantly outperforms traditional japonica in terms of yield and disease resistance, and now comprises about 40% of the rice cultivated in Shanghai’s suburbs and is the leading variety nationally [5]. However, the quality indicators such as chalkiness rate and chalkiness degree of hybrid japonica rice are slightly lower than those of conventional japonica rice [6]. To meet market needs, employing modern molecular biotechnologies to rapidly develop superior parent lines and breed hybrid japonica varieties that are aromatic, flavorful, disease-resistant, and yield-stable is essential. These new, environmentally friendly high-quality varieties are poised for extensive cultivation in Shanghai and nearby areas [7,8].
Fragrance is a critical quality attribute of rice. Fragrant rice varieties, enriched with vitamins, aromatic amino acids, and other nutrients, are highly prized for their delightful aroma [9,10]. Research indicates that mutations in the OsBADH2 gene, which encodes betaine aldehyde dehydrogenase, lead to the accumulation of 2-acetyl-1-pyrroline, a compound directly linked to the rice’s aromatic qualities [11,12,13,14,15]. Located on chromosome 8, the OsBADH2 gene comprises 15 exons and 14 introns. Currently, eight allelic variations have been identified, with the most significant being an 8 bp deletion and a 3 bp substitution in exon 7, now utilized in breeding numerous aromatic rice varieties [16,17,18,19,20,21,22,23]. Evolutionary analysis suggests that the aromatic gene first emerged in japonica rice, challenging the traditional view that fragrant rice originated from indica varieties [24].
The gelatinization temperature is another crucial indicator of rice’s cooking quality, ranking just below amylose content. The China Rice Research Institute has made strides in isolating the ALK gene responsible for rice gelatinization temperature through positional cloning. This gene, encoding soluble starch synthase II, plays a vital role in starch structure and gelatinization properties [25]. Subsequent experiments confirm that mutations in the ALK gene’s coding region can alter the branched starch’s crystal structure, influencing gelatinization temperatures [25,26].
Disease resistance is a priority in rice breeding, highlighted by the characterization of several complex alleles at the Piz locus, such as Pi2, Pi9, Pi50, Pigm, Pizh, Piz, and Piz-t [27,28]. Among these, Pi2 is notable for its role in pathogen defense, encoded by a gene within the NBS-LRR protein family [29].
The Xa21 gene, derived from wild rice, encodes a receptor-like protein kinase and is crucial for resistance against rice bacterial wilt disease. This gene’s structure includes various functional regions essential for disease resistance and signaling [30,31,32,33,34].
Traits like semi-dwarfism are integral to rice agronomy. The Sdt97 gene, located on chromosome 6, influences plant height and is being studied for its potential to induce semi-dwarf phenotypes through genetic modifications [35].
Low-temperature germination is another focus, with genes like qLTG3-1 being identified for their role in enhancing germination under adverse conditions. This gene, expressed primarily in the aleurone layer and embryo, may regulate cellular processes that facilitate germination at low temperatures [36,37,38].
Hd17, a gene homologous to ELF3 in Arabidopsis, is involved in regulating rice flowering times and is crucial for adapting rice to different photoperiods [39].
Phenotype is determined by both genes and environment. Superior genotypes play a crucial role in rice breeding. By aggregating favorable gene or genotype sets, breeders can cultivate varieties with new or improved traits, which can significantly improve crop yield, quality, and resistance. These advancements in these superior genotypes mentioned above underscore the dynamic nature of rice genetics and the potential for integrating these superior traits into breeding programs to improve crop resistance, yield, and quality, ultimately benefiting global food security and agricultural sustainability.
2. Materials and Methods
2.1. Plant Materials and Pedigree Family Tree of Shenyou R3
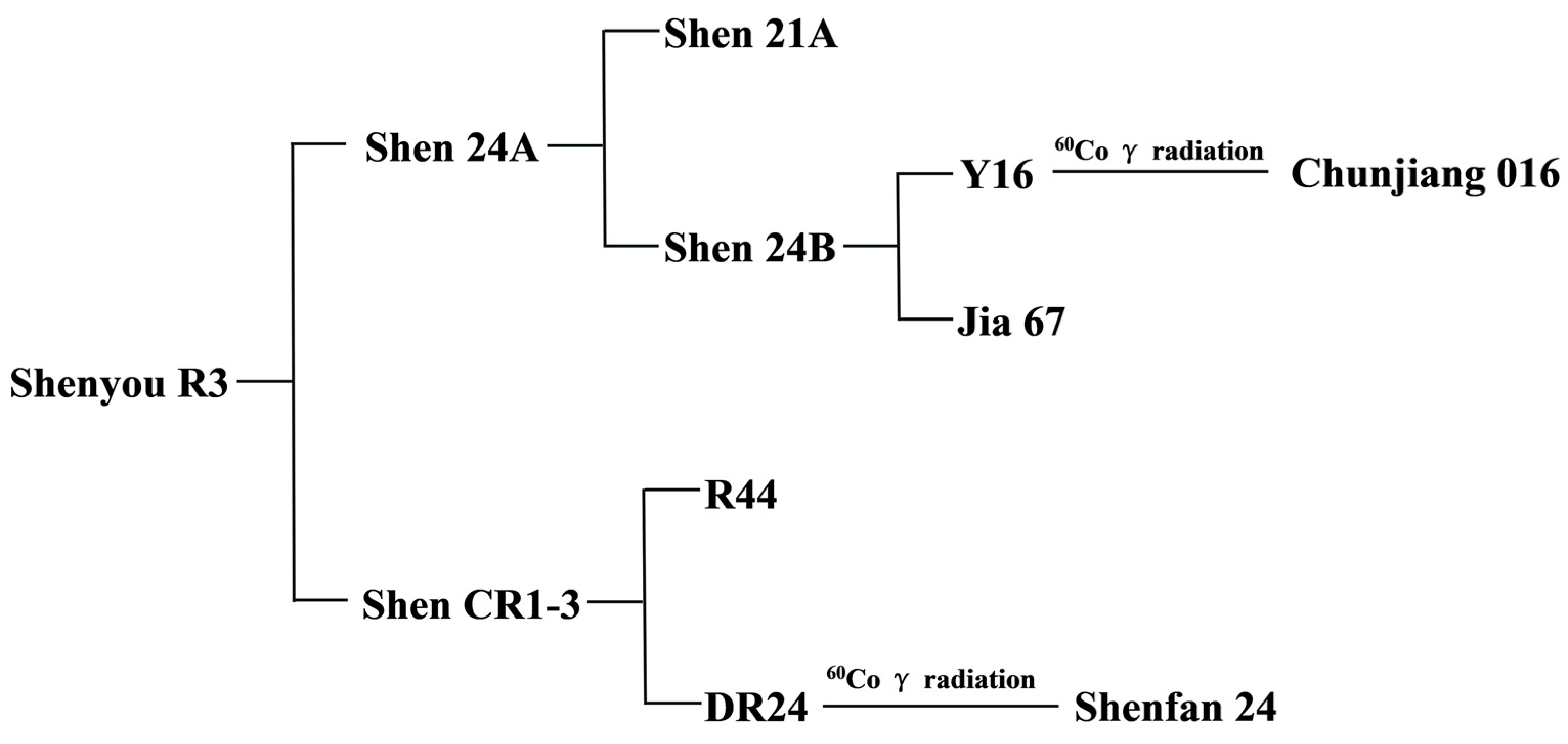
The parental lines used are the Chinsurah Boro II/Taichung 65 (BT) type japonica rice sterile line Shen 24A and the restorer line Shen CR1-3. The pedigree family tree of Shenyou R3 is as follows: Shenyou R3 was obtained by hybridizing Shen 24A with ShenCR1-3. The BT-type japonica rice sterile line Shen 21A is the cytoplasmic donor of Shen 24A. Shen 24B is a stable strain obtained quickly by crossbreeding Chunjiang 016 induced by cobalt-60 gamma rays and Jia 67 using anther culture technology. Shen CR1-3 is a stable strain obtained quickly by crossbreeding DR24 induced by cobalt-60 gamma rays and R44 using anther culture technology.
2.2. Molecular Marker Detection
Haplotype analysis is a method used to identify the specific combination of alleles (variants) present on a single chromosome. It helps researchers understand how different genetic variants interact with each other and contribute to a particular trait or disease. Haplotype analysis can be performed using various methods, such as DNA sequencing, microsatellite analysis, or SNP (single nucleotide polymorphism) genotyping.
Gene chip technology, also known as DNA chip or biochar technology, is a high-throughput detection technology that uses microarray technology to fix a large number of gene probes on carriers such as silicon wafers, glass slides, and plastic sheets, and then hybridize these with labeled samples to achieve rapid and parallel detection of gene sequences. The gene chip used in this study is the GSR40K, which can make breeding improvement faster and more accurate [40].
Haplotype analysis was conducted on the resistance genes for rice blast and bacterial blight in Shen 24A and Shen CR1-3, utilizing high-density rice gene chips. This analysis identifies specific polymorphic markers within the gene region and the surrounding 100 kb areas to determine the presence of specific resistance genes. The methodology is based on the work of Chen et al. [41].
2.3. Field Agronomic Conditions for Yield Data
Production trials and consortium trials were conducted in the Yangtze River Delta region, consisting primarily of the provinces of Shanghai, Jiangsu, Zhejiang, and Anhui, China. The field management conditions follow the local cultivation conditions.
2.4. Data Sources
The results of the gene chip are provided by the Wuhan Shuanglvyuan Chuangxin Technology Research Institute Co., Ltd, Wuhan, Hubei province, China. The results of rice quality testing were provided by the Rice Product Quality Supervision and Inspection Center Ministry of Agriculture and Rural Affairs, and the testing standards were based on the NY/T 593-2021 “Quality of Edible Rice Varieties”.
2.5. Identification of Rice Blast Disease
The identification of rice blast disease adopts a combination of manual inoculation identification and field-induced identification of neck blast disease. Suyunuo was used as the susceptible control variety for rice blast disease identification. The identified strains are 2021-3, 2021-43, 2021-71, 2021-150, and 2021-497, all of which were provided by the Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences. The detailed implementation steps and grading standards of the experiment refer to the local standard DB32/T 1123-2007 “Examining rules for identification method and evaluating standard of resistance of rice cultivars (lines) to rice blast”. The disease resistance test report of rice varieties was issued by the Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences.
3. Results
3.1. Gene Chip Identification of Parents
Shenyou R3, a newly developed aromatic late japonica hybrid rice variety, originates from a strategic cross between the BT-type japonica male sterile line Shen 24A (approved by the Shanghai Municipal Variety Approval Committee on 7 October 2022) and the restorer line Shen CR1-3 (variety right application number: 20191001690) (Figure 1). The female parent, Shen 24A, is a product of hybridizing the newly bred line Shen 24B with the japonica sterile line Shen 21A (a BT-type cytoplasmic donor), followed by seven generations of backcrossing to enhance desirable traits (Figure 1). This line is characterized by its strong photoperiod sensitivity, height of 90–95 cm, compact stature, green and upright leaves, large panicles, robust tillering, and rapid grain filling. Its grains are transparent and of high quality, with an amylose content of 11.7%, and it exhibits strong resistance to rice blast, bacterial blight, and stripe leaf blight. Shen 24A is noted for its favorable flowering traits, including early and concentrated flowering (approximately 20 min earlier than the commonly cultivated Shen 9A), wide glume opening, about 60% stigma exposure, and high seed production yields. Genetic analysis using high-density gene chips and InDel markers identified the presence of genes such as Pi2 and Pita (Table 1). The male parent, Shen CR1-3, is an aromatic long-grain late japonica restorer line, bred from crossing R44 with DR24 and refined through anther culture in the F1 generation with molecular marker-assisted selection (Figure 1). It possesses the fragrance gene badh2-E7 and is resistant to blast disease (Pi2 gene) and bacterial blight (Xa21 gene), reaching about 105 cm in height (semi-dwarf gene Sdt97). It features strong tillering, large panicles, rapid grain filling, about 180 grains per panicle, and a 1000-grain weight of 26 g. Due to carrying the Hd17 gene, Shen CR1-3 blooms later and can better meet the flowering time of the mother plant (Table 1). Moreover, Shen CR1-3 contains a large amount of pollen, which is very suitable for mechanized seed production. In 2019, Shen CR1-3 underwent hybridization trials with Shen 24A in Hainan, exhibiting strong yield advantages, excellent grain quality with a distinct aroma, and robust overall resistance. From 2021 to 2022, it was included in regional and production trials of hybrid japonica rice in Shanghai, successfully applying for a national plant variety right in 2022 (20221008341). It also surpassed the national joint regional trials from 2021 to 2022, achieving agricultural industry standard grade 2, outperforming the control, and achieving a yield increase of over 1%.
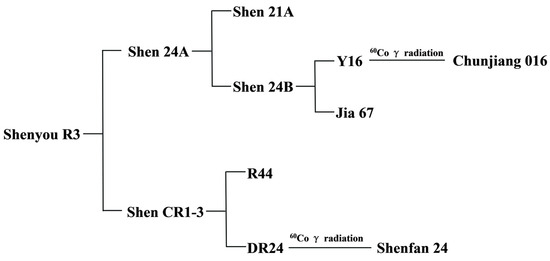
Figure 1.
Pedigree family tree of Shenyou R3.

Table 1.
Gene Chip Detection for Shen CR1-3 and Shen 24A.
3.2. Characteristics of Hybrid Japonica Rice Shenyou R3
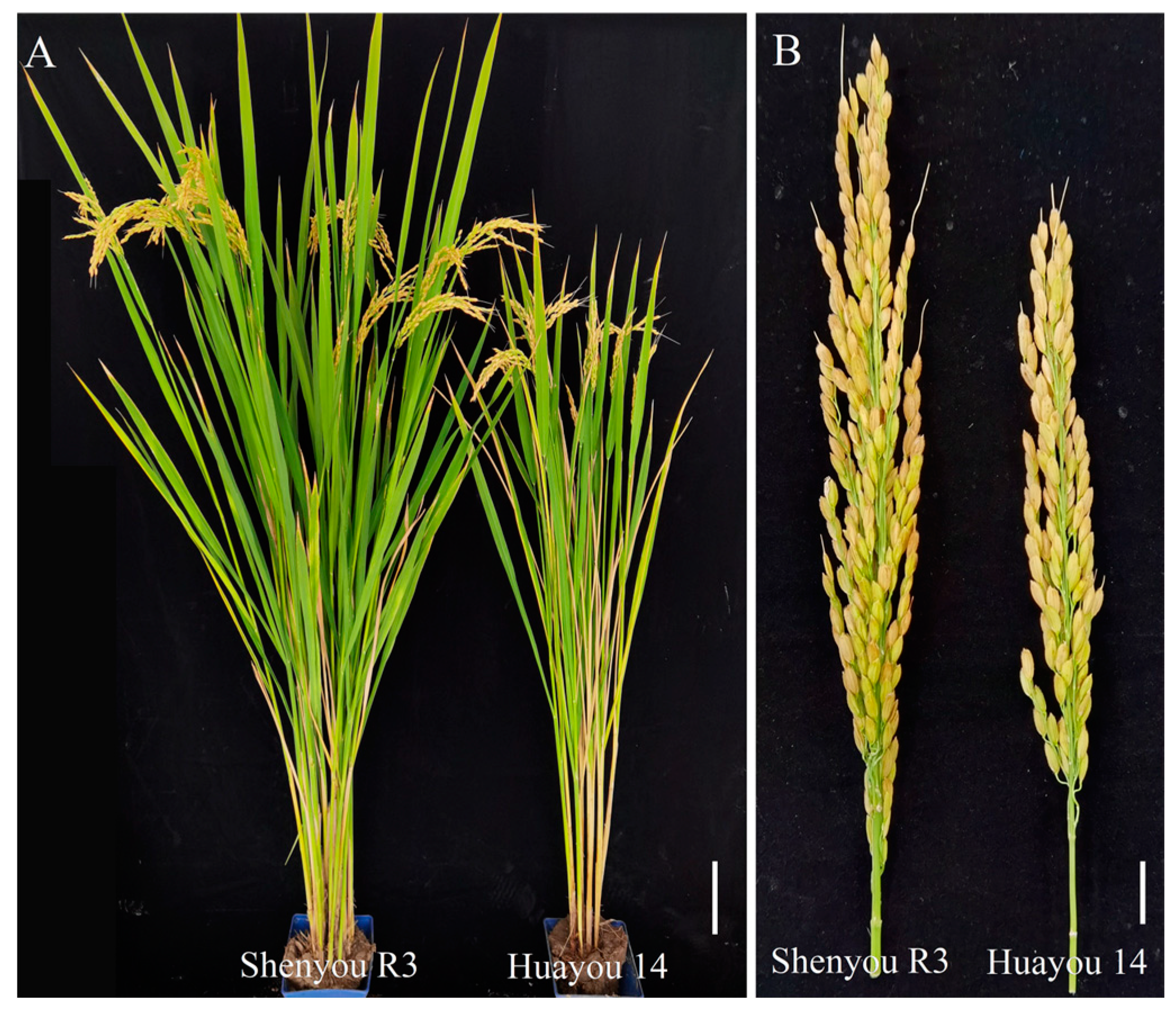
Shenyou R3 is a premium aromatic hybrid japonica rice developed by the Crop Breeding and Cultivation Research Institute of the Shanghai Academy of Agricultural Sciences. This new late japonica variety is celebrated for its popcorn-like aroma and superior grain quality (classified as national grade 2 premium rice). It shows strong resistance to rice blast (rated level 1), produces high yields (exceeding the control variety Huayou 14 by over 5% in the 2022 Shanghai trials), and is ideal for mechanized seed production due to its early flowering and highly exposed stigmas, yielding over 3000 kg per hectare (ha). The total growth period spans 161.4 days, which is 1.6 days shorter than Huayou 14, making it ideal for single late-season or early double-season cropping in Shanghai and surrounding areas. The plant stands at 108.5 cm, with panicle lengths of 18.9 cm, and features vibrant green leaves, robust tillering, a high rate of spikelet formation, medium-sized panicles, and small grains that fill rapidly and mature well. It averages 262,500 effective panicles per ha, with 191.7 grains per panicle, a seed-setting rate of 88.6%, and a 1000-grain weight of 25.7 g. The stems are thick and resistant to lodging, and the variety exhibits comprehensive disease resistance. In 2021, the Shanghai Agricultural Technology Extension Service Center submitted it for evaluation to the Jiangsu Academy of Agricultural Sciences Plant Protection Research Institute, where it received a blast disease comprehensive index of 1.75, a disease rating of level 1, and was classified as resistant. It is also resistant to sheath blight. The grains are transparent and fragrant, with an aroma score of 70. According to analyses by the Ministry of Agriculture’s Rice and Products Quality Inspection and Testing Center, commissioned by the Shanghai Agricultural Technology Extension Service Center, it achieved a whole milled rice rate of 73.0%, a chalkiness level of 1.6%, transparency grade 1, alkali spreading value of 7.0, gel consistency of 73 mm, and an amylose content of 17.0%. All primary quality indicators meet the standards for national grade 1 premium rice (Table 2).

Table 2.
Inspection Report of the Quality Supervision, Inspection and Testing Center for Rice and Products of the Ministry of Agriculture and Rural Affairs in 2022.
3.3. The Yield Performance of Shenyou R3
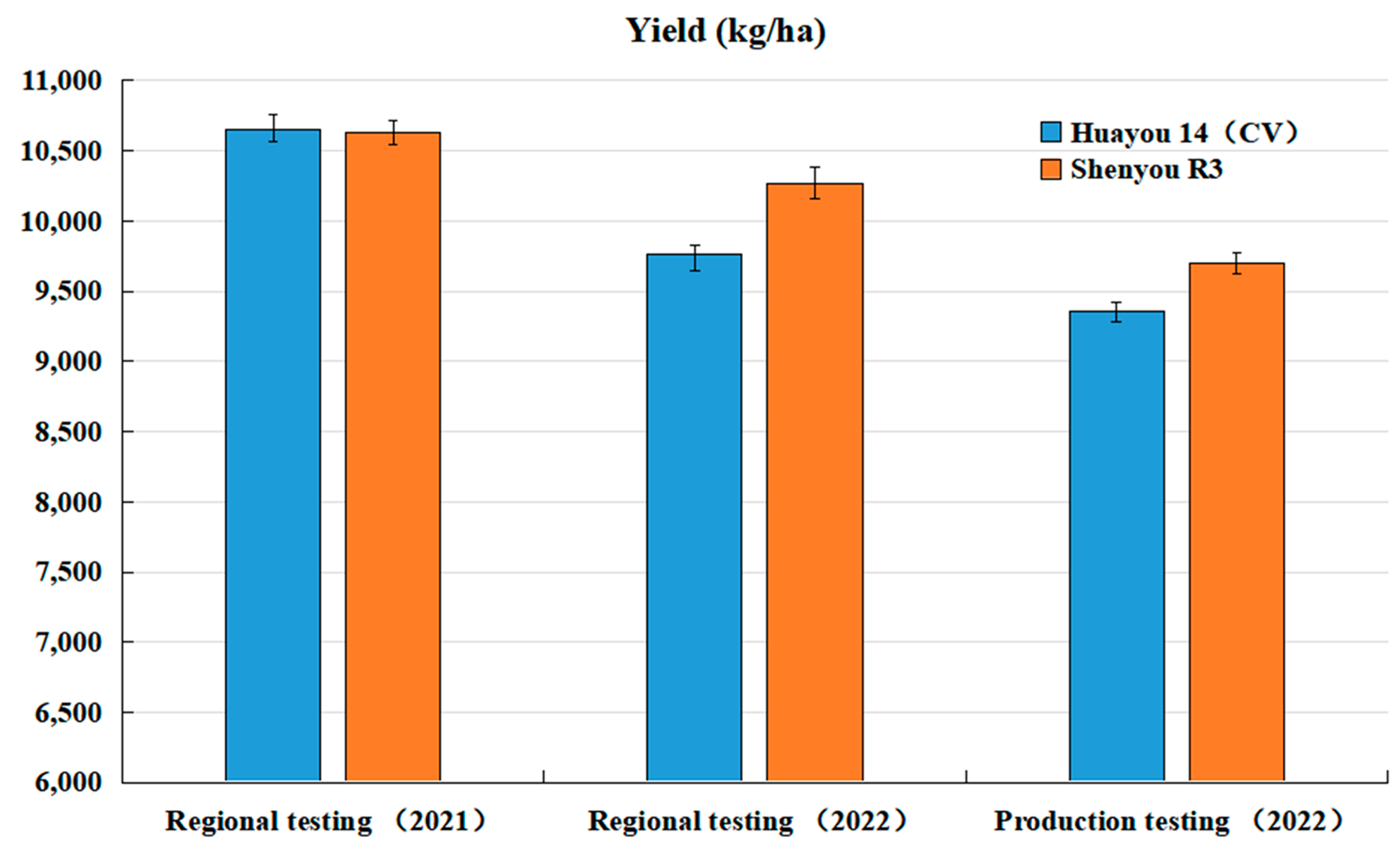
The new premium aromatic hybrid japonica rice, Shenyou R3, was included in Shanghai’s regional and production trials for hybrid japonica rice from 2021 to 2022. In the 2021 regional trials, Shenyou R3 achieved an average yield of 708.7 kg per mu and 10.63 tons per hectare, showing no significant decrease compared to the control variety, Huayou 14. In the continuation trials of 2022, it yielded an average of 10,630.5 kg per ha, marking a significant yield increase of 5.2% over the control. Furthermore, in the 2022 production trials conducted by the Shanghai hybrid japonica rice group, it recorded an average yield of 9697.5 kg per ha, which was 3.6% higher than the control (Figure 2 and Figure 3).
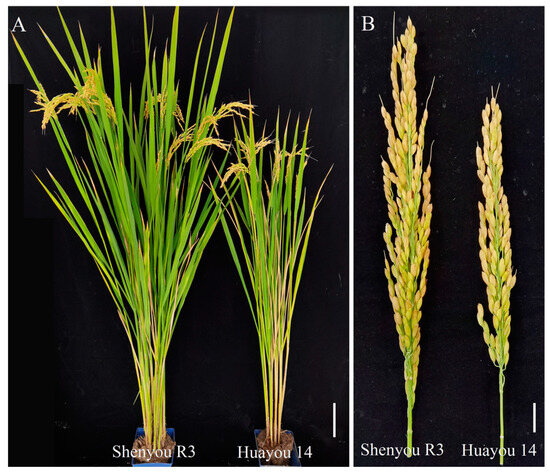
Figure 2.
Phenotypes of Shenyou R3 and Huayou 14. (A) Scale bars = 100 mm. (B) Scale bars = 10 mm.
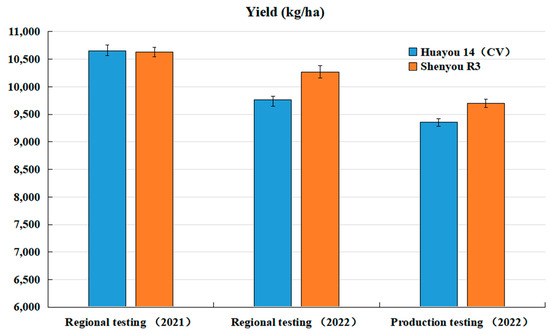
Figure 3.
Performance of regional and production trials for Shenyou R3 and Huayou 14 in 2021 and 2022. Data are means (SD) and duplicate measurements were performed for each sample (n = 3).
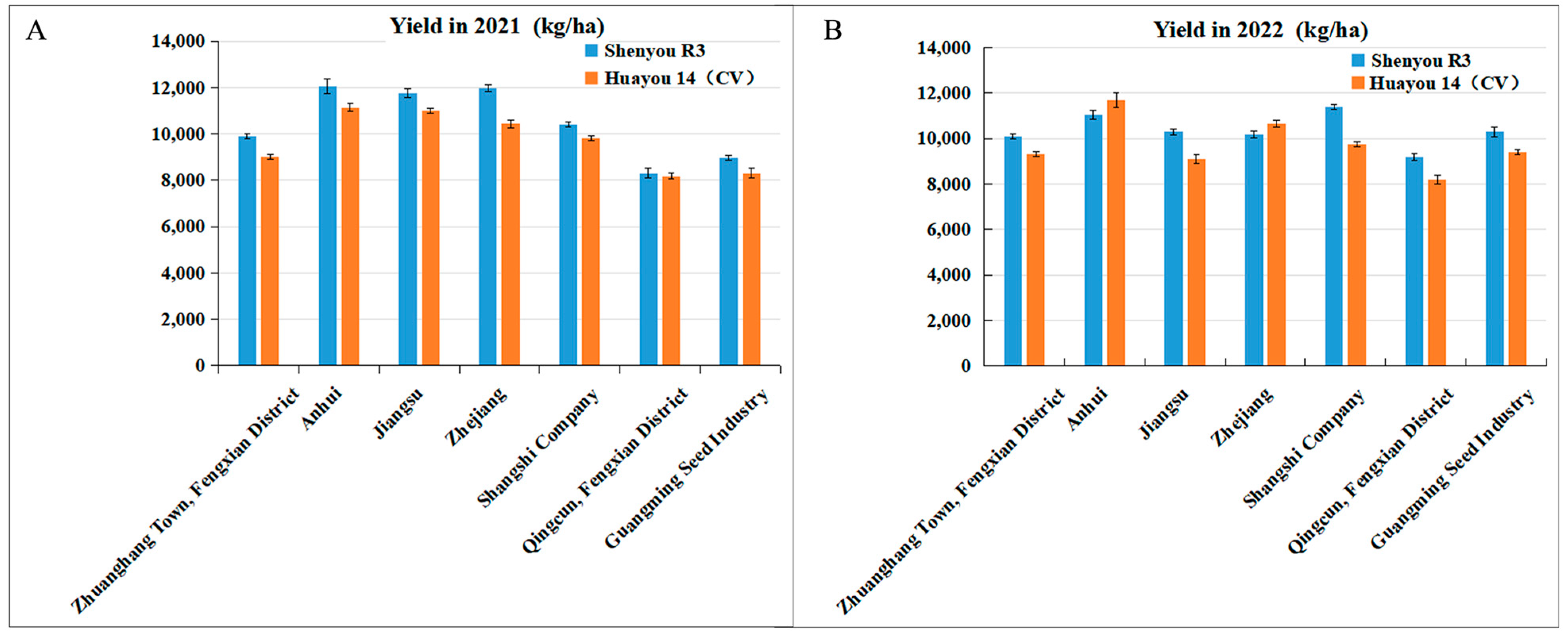
During 2021–2022, Shenyou R3 underwent evaluation across multiple sites in Zhejiang, Anhui, Jiangsu, Guangming Seed Industry, Shanghai Real Estate Company, and Fengxian Zhuanghang. In 2021, it posted an average yield of 10,479.0 kg per ha, which was 8.1% higher than the control, Huayou 14. In 2022, it maintained strong performance with an average yield of 10,345.5 kg per ha, 6.3% above the control (Figure 4 and Table 3). Additionally, in 2022, Shenyou R3 was showcased at five demonstration sites in Shanghai and its adjacent areas. The Quzhou site in Zhejiang reported an exemplary average yield of 11,344.5 kg per ha, highlighting its high quality (popcorn-like aroma), excellent productivity, robust lodging resistance, appealing maturity, and overall strong resistance. The demonstration site at Fangping recorded an average yield of 10,644.0 kg per ha, effectively demonstrating its high yield potential and nitrogen utilization efficiency (Table 4).
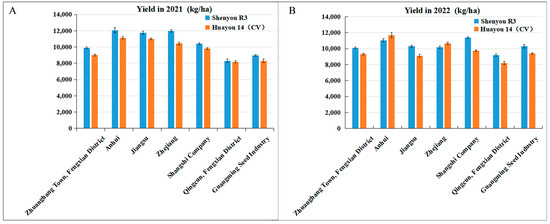
Figure 4.
Yield performance of new hybrid japonica rice Shenyou R3 in multiple-site identification trails from 2021 to 2022 (kg/ha).

Table 3.
Yield Performance of New Hybrid Japonica Rice Shenyou R3 in Multiple-Site Identification Trials from 2021 to 2022 (kg/ha).

Table 4.
Demonstration Planting Situation of New Hybrid Japonica Rice Shenyou R3 in 2022 (kg/ha).
3.4. Key Technologies for Seed Production
For seed production, the male parent Shen CR1-3 is sown in two phases on May 25 and June 6, and the female parent Shen 24A is sown on June 10. Seedlings, raised for 18–25 days, are suitable for machine transplanting. The row and plant spacing are set between the male and female parents at 2:6 to 2:8 and 25 × 14 cm, respectively, targeting about 750,000 plants per ha for the female line, and light and staged flooding is employed during field preparation. The flowering period is periodically predicted and timely adjusted, typically using fertilizer and water adjustments. For rapidly maturing parents, 150–225 kg of urea per ha is applied coupled with deep watering. The leaves of both parents are trimmed 2–3 days before heading, removing two-thirds of the flag leaves to minimize pollination barriers and enhance the female’s pollination efficiency. A total of 120 g of gibberellic acid per ha is applied when 5% of the male parents are heading, and 90 g per ha when 10% of both parents are heading. Mechanical aids are used for pollination post-heading. Contaminants must be rigorously removed during all phases of seed production (before transplanting, during tillering, before leaf cutting, at flowering, during grain filling, and post-harvest of the male parent) to maintain purity. Timely harvest of the seed production field is crucial to ensure viable germination rates.
4. Discussion
Single Nucleotide Polymorphism (SNP) markers, derived from sequencing technologies, have become integral to rice molecular breeding. Recently, the development of high-density rice gene chips has enabled rapid and precise identification of key functional genes associated with rice traits. This is achieved through the joint analysis of polymorphic markers within and around gene regions (haplotype analysis) [42]. Our study utilized these high-density gene chips to pinpoint genes influencing yield, quality, disease resistance, and other vital traits in hybrid japonica rice’s backbone parents (Table 1). These findings provide a robust foundation for breeding new, high-quality, high-yielding, and disease-resistant hybrid japonica rice combinations.
Rice blast is a globally significant disease, potentially reducing annual yields by 35% to 50%, and in extreme cases, leading to total crop failure [43]. Utilizing high-quality genetic resources to develop disease-resistant rice varieties has proven to be the most economical, sustainable, and effective strategy for managing rice blast [44]. Our gene chip analysis of Shenyou R3, a new hybrid japonica rice combination, revealed that the female sterile line Shen 24A carries the Pi2 and Pita genes, while the male restorer line Shen CR1-3 harbors the Pi2 gene. Leveraging a complementary breeding strategy for dominant resistance genes against rice blast, Shenyou R3 effectively combines these genes, showcasing exceptional resistance to the disease (Table 1).
The advancement and deployment of hybrid rice have been critical in maintaining the consistent and stable growth of China’s grain production for over a decade [45]. However, the area planted with hybrid indica rice has recently decreased, primarily due to low seed yields and high costs, which significantly slow the introduction of new hybrid combinations. Fully mechanizing the seed production of hybrid rice is essential for enhancing efficiency and furthering the development of this crop. The female parent Shen 24A of the new hybrid japonica rice combination Shenyou R3 exhibits favorable flowering traits, a wide glume opening angle, and a high outcrossing rate. The male parent Shen CR1-3 is characterized by high pollen production, concentrated flowering periods, and extended bloom duration. Therefore, Shenyou R3 is well-suited for high-yield, mechanized seed production. We have developed a comprehensive high-yield mechanized seed production technology for Shenyou R3, incorporating varied sowing times, mechanized seed production ratios, and management practices such as water and fertilizer application, mechanical leaf trimming, gibberellin application, and mechanized pollination. This approach not only ensures full mechanization of Shenyou R3’s seed production but also increases yield and reduces costs, thereby supporting its widespread promotion and application.
The genetic basis of rice quality traits is complex. Although the hybrid generation typically displays stable phenotypes, the F2 generation exhibits segregation in rice quality traits, making improvements challenging, especially in terms of appearance. Generally, hybrid japonica rice falls short of conventional japonica in quality [46]. Nevertheless, using sterile and restorer lines with superior rice quality facilitates the development of high-quality hybrid combinations. Following this strategy, we selected the high-quality sterile line Shen 24A and the high-quality restorer line Shen CR1-3 for pairing and successfully bred the new high-quality hybrid japonica rice Shenyou R3. This variety matures early, delivers excellent rice quality, yields high and stable production, high nitrogen utilization efficiency, provides strong disease resistance, and is conducive to mechanized seed production. It holds promising potential for widespread adoption in the middle and lower Yangtze River regions.
5. Conclusions
This study addresses several challenges in the production of hybrid japonica rice, including delayed growth periods, unstable yields, and the necessity to enhance both rice quality and seed production yields. We utilized high-density rice gene chip technology to facilitate the development of a new hybrid japonica rice combination, Shenyou R3. This variety is characterized by early maturity, superior quality, high and stable yields, high nitrogen utilization efficiency, resistance to blast disease, and ease of seed production. Shenyou R3 incorporates outstanding genes such as badh2-E7, Pi2, Xa21, Sdt97, and Hd17, which confer traits such as exceptional rice quality, robust resistance to rice blast, high yield, and suitability for mechanized seed production. Shenyou R3 offers high-quality rice that adheres to national premium grade 2 standards and level 1 resistance to blast disease. The general yield of Shenyou R3 can reach 10,500.0 kg to 11,250.0 kg per ha. Given these attributes, Shenyou R3 has significant potential for widespread adoption in the middle and lower reaches of the Yangtze River region.
Author Contributions
The writing of the original draft, A.Z. and J.Z. (Jianming Zhang); data curation, C.C., F.N. and J.Z. (Jihua Zhou); visualization, B.S., Y.D. and K.X.; writing review and editing, H.C.; supervision, H.C. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chongming Agriculture Innovation Project, grant number 2023CNKC-01-03, Shanghai Agriculture Applied Technology Development Program, China, grant number 2022-02-08-00-12-F01127, and Shanghai Science and Technology Innovation Action Plan Project, grant number 21N11900100.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Yuan, L.P. Development of Hybrid Rice to Ensure Food Security. Rice Sci. 2014, 21, 1–2. [Google Scholar] [CrossRef]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; He, Y. Rice grain quality-traditional traits for high quality rice and health-plus substances. Mol. Breed. 2020, 40, 1. [Google Scholar] [CrossRef]
- Niu, F.A.; Chu, H.W.; Sun, B.; Zhou, J.H.; Zhang, A.P.; Huang, Y.W.; Li, Y.; Yao, Y.; Cheng, C.; Cao, L.M. Molecular Marker Assisted Breeding of a New Japonica Hybrid Rice ‘Shenyou28’ with High Quality and Disease Resistance. Mol. Plant Breed. 2023, 1, 198–204. [Google Scholar]
- Du, Z.M.; Yang, Y.C.; Xia, Y.Y.; Gong, Y.L.; Yan, Z.Q.; Xu, H. The Effect of Harvest Period on the Quality of Northern Hybrid Japonica Rice and Conventional Japonica Rice. Crops 2018, 1, 147–151. [Google Scholar]
- Xi, M.; Ji, Y.L.; Wu, W.G.; Xu, Y.Z.; Sun, X.Y.; Zhou, Y.J. Research progress and prospects of factors affecting rice eating quality. Chin. Agric. Sci. Bull. 2020, 36, 159–164. [Google Scholar]
- Yu, G.P.; Xu, C.C.; Wu, Y.W.; Xiu, X.J.; Tong, H.H. Thoughts on the supply side reform of China’s rice industry. Chin. J. Agric. Resour. Reg. Plan. 2020, 41, 53–62. [Google Scholar]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Peng, B.; Kong, D.; Song, X.; Li, H.L.; He, L.L.; Gong, A.; Sun, Y.; Pang, R.; Liu, L.; Li, J.T.; et al. A Method for Detection of Main Metabolites in Aromatic Rice Seeds. Agric. Biotechnol. 2018, 1, 112–116. [Google Scholar]
- Bardburyl, M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.S.; Waters, D.L.E. The gene for fragrance in rice. Plant Biotech. J. 2005, 3, 363–371. [Google Scholar]
- Fukuda, T.; Takeda, T.; Yoshida, S. Comparison of volatiles in cooked rice with various amylose contents. Food Sci. Technol. Res. 2014, 20, 1251–1259. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.S.; Jeong, O.Y.; Kim, K.J.; Kays, S.J. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2008, 56, 235–240. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Huang, D.Q.; Wang, Z.R.; Li, H.; Zhou, D.G.; Wang, Z.D.; Chen, Y.B.; Zhao, L.; Gong, R.; Zhou, S.C. Research progress in haplotype of Badh2 gene and metabolic pathway of aroma component 2-acetyl-1-pyrroline in fragrant rice. Guangdong Agric. Sci. 2021, 48, 9–16. [Google Scholar]
- Kovach, M.J.; Calingacion, M.N.; Fitzgerald, M.A.; Mccouch, S.R. The origin and evolution of fragrance in rice (Oryza sativa L.). Proc. Natl. Acad. Sci. USA 2009, 106, 14444–14449. [Google Scholar] [CrossRef]
- Amarawathi, Y.; Singh, R.; Singh, A.K.; Singh, V.P.; Mohapatra, T.; Sharma, T.R.; Singh, N.K. Mapping of quantitative trait loci for basmati quality traits in rice (Oryza sativa L.). Mol. Breed. 2008, 21, 49–65. [Google Scholar] [CrossRef]
- He, Q.; Park, Y.J. Discovery of a novel fragrant allele and development of functional markers for fragrance in rice. Mol. Breed. 2015, 35, 217–226. [Google Scholar] [CrossRef]
- Ootsuka, K.; Takahashi, I.; Tanaka, K.; Itani, T.; Tabuchi, H.; Yoshihashi, T.; Tonouchi, A.; Ishikawa, R. Genetic polymorphisms in Japanese flagrant landraces and novel fragrant allele domesticated in Northern Japan. Breed. Sci. 2014, 64, 115–124. [Google Scholar] [CrossRef]
- Shao, G.N.; Tang, A.; Tang, S.Q.; Luo, J.; Jiao, G.A.; Wu, J.L.; Hu, P.S. A new deletion mutation of fragrant gene and the development of three molecular markers for fragrance in rice. Plant Breed. 2011, 130, 172–176. [Google Scholar] [CrossRef]
- Shao, G.N.; Tang, S.Q.; Chen, M.L.; Wei, X.J.; He, J.W.; Luo, J.; Jiao, G.A.; Hu, Y.C.; Xie, L.H.; Hu, P.S. Haplotype variation at Badh2, the gene determining fragrance in rice. Genomics 2013, 101, 157–162. [Google Scholar] [CrossRef]
- Shi, W.W.; Yang, Y.; Chen, S.H.; Xu, M.L. Discovery of a new fragrance allele and the development of functional markers for the breeding of fragrant rice varieties. Mol. Breed. 2008, 22, 185–192. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Zhao, G.C.; Xu, X.L.; Li, J.Y. Discovery of a new fragrance allele and development of functional markers for identifying diverse fragrant genotypes in rice. Mol. Breed. 2014, 33, 701–708. [Google Scholar] [CrossRef]
- Sun, P.Y.; Zhang, W.H.; Shu, F.; He, Q.; Zhang, L.; Peng, Z.R.; Deng, H.F. Analysis of Mutation Sites of OsBADH2 Gene in Fragrant Rice and Development of Related Functional Marker. Biotechnol. Bull. 2021, 37, 1–7. [Google Scholar]
- Gao, Z.Y.; Zeng, D.L.; Cui, X.; Zhou, Y.H.; Yan, M.X.; Huang, D.N.; Li, J.Y.; Qian, Q. Map-based cloning and sequence analysis of a gene, ALK, responsible for gelatinization temperature in rice (Oryza sativa L.). Sci. China 2003, 46, 661–668. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Zeng, D.L.; Cheng, F.M.; Tian, Z.X.; Guo, L.B.; Su, Y.; Yan, M.X.; Jiang, H.; Dong, G.J.; Huang, Y.C.; et al. ALK, the Key Gene for Gelatinization Temperature, is a Modifier Gene for Gel Consistency in Rice. J. Integr. Plant Biol. 2011, 53, 756–765. [Google Scholar]
- Xie, Z.; Yan, B.X.; Shou, J.Y.; Tang, J.; Wang, X.; Zhai, K.R.; Liu, J.Y.; Li, Q.; Luo, M.Z.; Deng, Y.W.; et al. A nucleotide-binding site-leucine-rich repeat receptor pair confers broad-spectrum disease resistance through physical association in rice. Philos. Trans. R. Soc. B—Biol. Sci. 2019, 374, 20180308. [Google Scholar] [CrossRef]
- Deng, Y.W.; Zhai, K.R.; Xie, Z.; Yang, D.Y.; Zhu, X.D.; Liu, J.Z.; Wang, X.; Qin, P.; Yang, Y.Z.; Zhang, G.M.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Zhou, B.; Qu, S.H.; Liu, G.F.; Dolan, M.; Sakai, H.; Lu, G.D.; Bellizzi, M.; Wang, G.L. The Eight Amino-Acid Differences Within Three Leucine-Rich Repeats Between Pi2 and Piz-t Resistance Proteins Determine the Resistance Specificity to Magnaporthe grisea. Mol. Plant-Microbe Interact. 2006, 19, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Chern, M.; Canlas, P.E.; Ruan, D.L.; Jiang, C.Y.; Ronald, P.C. An ATPase promotes autophosphorylation of the pattern recognition receptor XA21 and inhibits XA21-mediated immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 8029–8034. [Google Scholar] [CrossRef]
- Chen, F.; Gao, M.J.; Miao, Y.S.; Yuan, Y.X.; Wang, M.Y.; Li, Q.; Mao, B.Z.; Jiang, L.W.; He, Z.H. Plasma Membrane Localization and Potential Endocytosis of Constitutively Expressed XA21 Proteins in Transgenic Rice. Mol. Plant 2010, 3, 917–926. [Google Scholar] [CrossRef]
- Ercoli, M.F.; Luu, D.D.; Rim, E.Y.; Shigenaga, A.; Araujo, J.A.T.; Chern, M.; Jain, R.; Ruan, R.; Joe, A.; Stewart, V.; et al. Plant immunity: Rice XA21-mediated resistance to bacterial infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2121568119. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.X.; Zhu, L.H.; et al. A Receptor Kinase-Like Protein Encoded by the Rice Disease Resistance Gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef]
- Luo, Y.C.; Sangha, J.S.; Wang, S.H.; Li, Z.F.; Yang, J.B.; Yin, Z.C. Marker-assisted breeding of Xa4, Xa21 and Xa27 in the restorer lines of hybrid rice for broad-spectrum and enhanced disease resistance to bacterial blight. Mol. Breed. 2012, 30, 1601–1610. [Google Scholar] [CrossRef]
- Tong, J.P.; Han, Z.S.; Han, A.N.; Liu, X.J.; Zhang, S.Y.; Fu, B.Y.; Hu, J.; Su, J.P.; Li, S.Q.; Wang, S.J.; et al. Sdt97: A Point Mutation in the 5′ Untranslated Region Confers Semidwarfism in Rice. G3 Genes Genomes Genet. 2016, 6, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Sekiguchi, H.; Matsuda, Y.; Sugimoto, K.; Ono, K.; Yano, M. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 12623–12628. [Google Scholar] [CrossRef]
- Fujino, K.; Matsuda, Y. 64. Global expression profiling of genes targeted by qLTG3-1 controlling low temperature tolerance at the germination stage in rice. Cryobiology 2009, 59, 387–388. [Google Scholar] [CrossRef]
- Fujino, K.; Sekiguchi, H. Origins of functional nucleotide polymorphisms in a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Plant Mol. Biol. 2011, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Ogiso-Tanaka, E.; Hori, K.; Ebana, K.; Ando, T.; Yano, M. Natural Variation in Hd17, a Homolog of Arabidopsis ELF3 That is Involved in Rice Photoperiodic Flowering. Plant Cell Physiol. 2012, 53, 709–716. [Google Scholar] [CrossRef]
- Guo, N.H.; An, R.H.; Ren, Z.L.; Jiang, J.; Cai, B.N.; Hu, S.K.; Shao, G.N.; Jiao, G.A.; Xie, L.H.; Wang, L.; et al. Developing super rice varieties resistant to rice blast with enhanced yield and improved quality. Plant Biotechnol. J. 2025, 23, 232–234. [Google Scholar] [CrossRef]
- Chen, H.D.; Xie, W.B.; He, H.; Yu, H.H.; Chen, W.; Li, J.; Yu, R.B.; Yao, Y.; Zhang, W.H.; He, Y.Q.; et al. A high-density SNP genotyping array for rice biology and molecular breeding. Mol. Plant 2014, 7, 541–553. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Lu, Q.; Yu, H.H.; Ni, X.M.; Zhang, G.Y.; He, H.; Xie, W.B.; Zhou, F.S. The development and application of rice whole genome selection breeding platform. Chin. Bull. Life Sci. 2018, 30, 1120–1128. [Google Scholar]
- Singh, W.H.; Kapila, R.K.; Sharma, T.R.; Rathour, R. Genetic and physical mapping of a new allele of Pik locus from japonica rice ‘Liziangxintuanheigu’. Euphytica 2015, 205, 889–901. [Google Scholar] [CrossRef]
- Liang, T.M.; Chen, Z.J.; Chen, S.B. Research and progress of the application of genomewide analysis strategy in gene identification of rice blast resistance. Mol. Plant Breed. 2019, 17, 1525–1530. [Google Scholar]
- Tang, W.B.; Zhang, G.L.; Deng, H.B. Technology exploration and practice of hybrid rice mechanized seed production. Rice Sci. 2020, 34, 95–103. [Google Scholar]
- Xu, Q.G.; Huang, F. Studies and progress on seed production mechanization technology in hybrid rice. Trans. Chin. Soc. Agric. Eng. 2010, 26, 37–41. [Google Scholar]
- Min, J.; Zhu, Z.W.; Xu, L.; Mou, R.X. Studies on grain quality and high quality rate of japonica hybrid rice in China. Hybrid Rice 2007, 22, 67–70. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).