Complex Microbial Fertilizer Promotes the Growth of Summer-Sown Short-Season-Cultivated Cotton and Increases Cotton Yield in the Yangtze River Basin by Changing the Soil Microbial Community Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials, Sites, and Design
2.2. Measurement Indicators and Methods
2.2.1. Cotton Plant Height and Number of Fruit Branches
2.2.2. Enzyme Activity
2.2.3. Measurement of Soil Biodiversity
2.3. Yield and Yield Components
2.4. Data Processing and Analysis
3. Results and Analyses
3.1. Leaf Enzyme Activities
3.2. Plant Height and Number of Cotton Branches
3.3. Yield and Yield Composition Factors
3.4. Changes in Soil Microorganisms Under Different Treatments
3.4.1. Alpha-Diversity Analysis
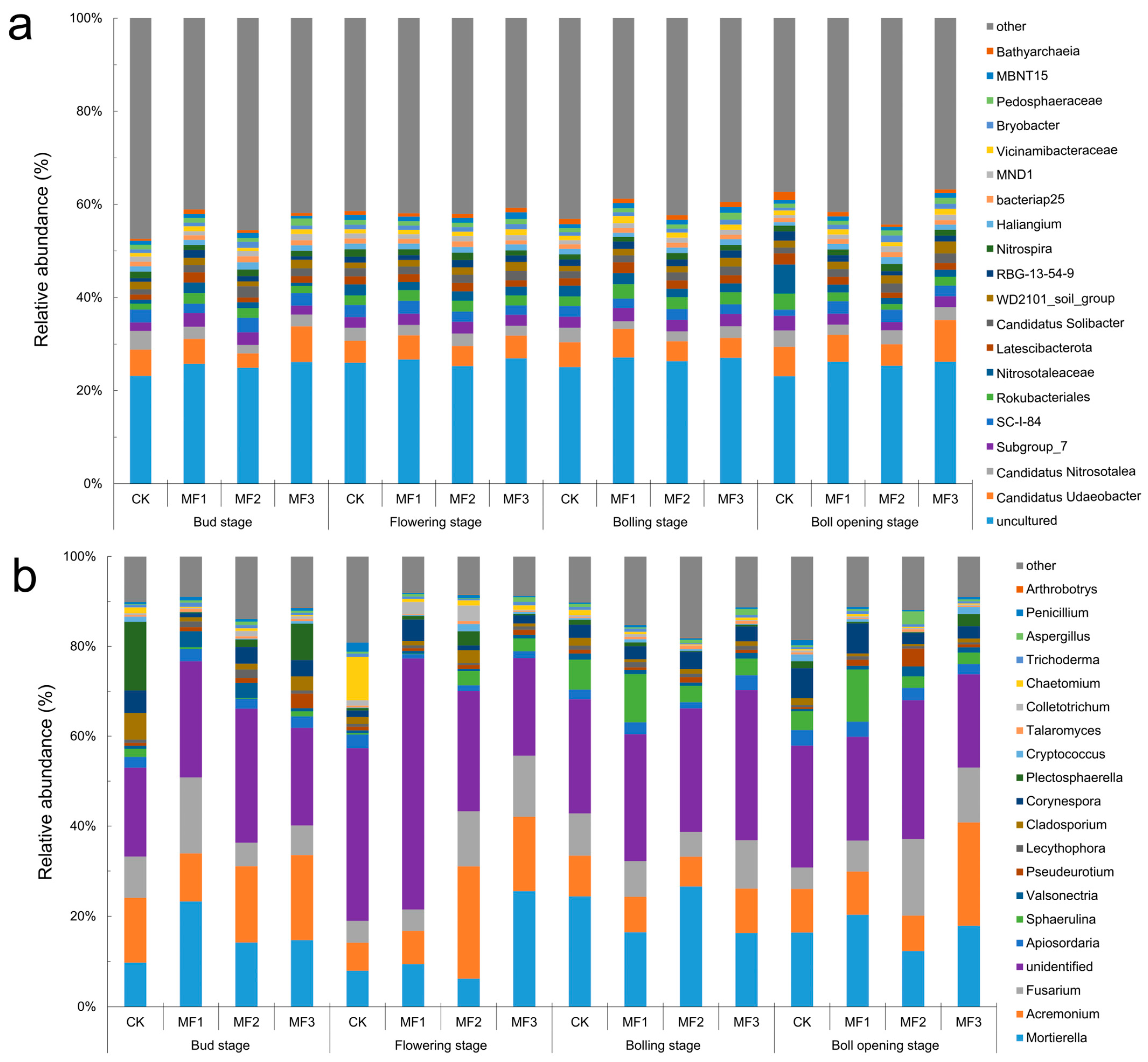
3.4.2. Community Distribution
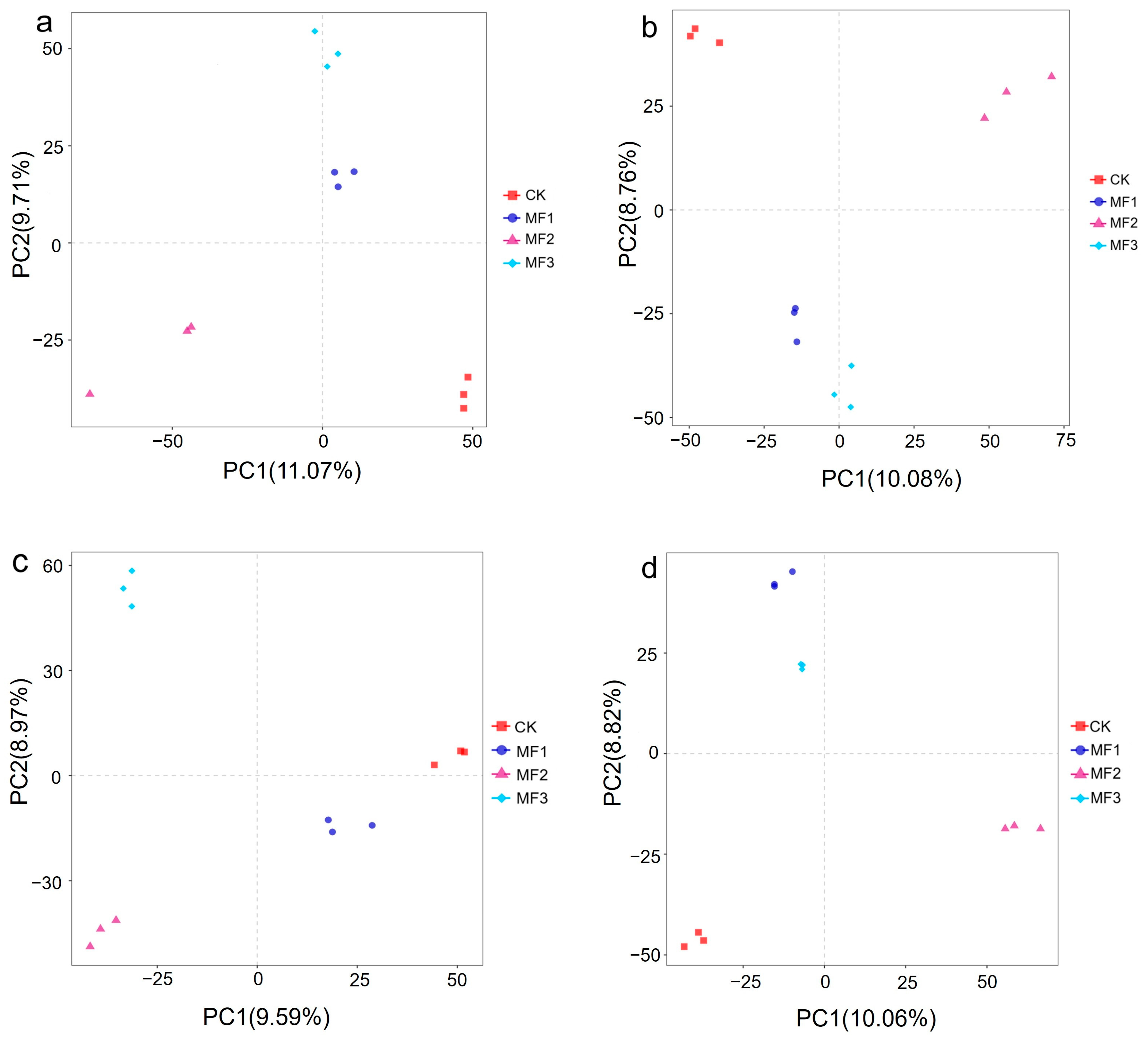
3.4.3. Beta Diversity
4. Discussion
4.1. Characteristics of Soil Microbial Diversity in Summer-Sown, Short-Season-Cultivated Cotton
4.2. Effect of Microbial Fertilizer on Cotton Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, L.; Chi, B.-J.; Dong, H.-Z. Cotton cultivation technology with Chinese characteristics has driven the 70-year development of cotton production in China. J. Integr. Agric. 2022, 21, 597–609. [Google Scholar] [CrossRef]
- Liu, A.; Chen, J.; Li, R. On short-season cultivation of cotton in the Yangtze River Basin. China Cotto 2014, 41, 7–10. (In Chinese) [Google Scholar]
- Liu, A.; Tu, X.; Zhou, Z.; Li, R. Light and simplified cultivation technology of cotton short-season summer sowing. Hunan Agric. Sci. 2022, 02, 28–31. (In Chinese) [Google Scholar]
- Feng, L.; Dai, J.; Tian, L.; Zhang, H.; Li, W.; Dong, H. Review of the technology for high-yielding and efficient cotton cultivation in the northwest inland cotton-growing region of China. Field Crops Res. 2017, 208, 18–26. [Google Scholar] [CrossRef]
- Yang, G.; Tang, H.; Tong, J.; Nie, Y.; Zhang, X. Effect of fertilization frequency on cotton yield and biomass accumulation. Field Crops Res. 2012, 125, 161–166. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Chu, K.-Y.; Tang, H.-Y.; Nie, Y.-C.; Zhang, X.-L. Fertilizer 15N accumulation, recovery and distribution in cotton plant as affected by N rate and split. J. Integr. Agric. 2013, 12, 999–1007. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Ahsan, T.; Tian, P.C.; Gao, J.; Wang, C.; Liu, C.; Huang, Y.Q. Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. J. Adv. Res. 2024, 64, 1–13. [Google Scholar] [CrossRef]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of compound microbial fertilizer on soil characteristics and yield of wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Yuan, Z.; Feng, J.; Chen, S.; Sun, G.; Wei, Z.; Hu, T. Effects of microbial fertilizer and irrigation amount on growth, physiology and water use efficiency of tomato in greenhouse. Sci. Hortic. 2024, 323, 112553. [Google Scholar] [CrossRef]
- DB43/T 2379-2022; Hunan Provincial Bureau of Technology and Quality Supervision. Technical Code of Cotton-Rape Rotation Double Direct Seeding. China Standard Press: Beijing, China, 2022. Available online: https://dbba.sacinfo.org.cn/stdDetail/201e8f2ad829bb935b9fd273721eeffc0eb769b10fdbf07ffdadf5bfc696f10b (accessed on 2 February 2025). (In Chinese)
- Hou, Z.; Zhao, L.; Wang, Y.; Liao, X. Purification and characterization of superoxide dismutases from sea buckthorn and chestnut rose. J. Food Sci. 2019, 84, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Ma, Y.; Zhang, L.; Jiang, Y.; Liang, H.; Wang, D. Inhibitory mechanism of low-oxygen-storage treatment in postharvest internal bluing of radish (Raphanus sativus) roots. Food Chem. 2021, 364, 130423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, Y.; Han, Y.; Huang, L.; Zhou, W.; Zhou, C.; Hu, Y.; Lu, R.; Wang, F.; Shi, W.; et al. Immunotoxicity of pentachlorophenol to a marine bivalve species and potential toxification mechanisms underpinning. J. Hazard. Mater. 2022, 439, 129681. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, C.; Xie, X.; Li, K.; Yang, D.; Tu, X.; Li, F.; Qin, Y.; Xu, D.; Li, J.; et al. A novel seed balling technology and its effect on cotton emergence, yield and fiber quality. Ital. J. Agron. 2023, 18, 3. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Zhao, Z.B.; He, J.Z.; Quan, Z.; Wu, C.-F.; Sheng, R.; Zhang, L.-M.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Anderson, I.C.; Cairney, J.W.G. Diversity and ecology of soil fungal communities: Increased understanding through the application of molecular techniques. Environ. Microbiol. 2004, 6, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Nawaz, F.; Rafeeq, R.; Majeed, S.; Ismail, M.S.; Ahsan, M.; Ahmad, K.S.; Akram, A.; Haider, G. Biochar amendment in combination with endophytic bacteria stimulates photosynthetic activity and antioxidant enzymes to improve soybean yield under drought stress. J. Soil Sci. Plant Nutr. 2023, 23, 746–760. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.; Wong, M. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]


| Period | Treatment | POD/(U·g−1) | SOD/(U·g−1) | MDA /(nmol·g−1) |
|---|---|---|---|---|
| Bud stage | CK | 2384.56 ± 57.15 d | 209.67 ± 16.84 b | 340.21 ± 10.21 a |
| MF1 | 2756.74 ± 58.88 c | 245.45 ± 19.02 ab | 321.97 ± 11.45 b | |
| MF2 | 2948.05 ± 90.51 b | 252.15 ± 28.23 ab | 300.60 ± 8.85 c | |
| MF3 | 3156.81 ± 145.72 a | 282.81 ± 15.36 a | 263.58 ± 6.29 d | |
| Flowering stage | CK | 2569.80 ± 77.55 d | 284.78 ± 6.51 c | 405.31 ± 8.02 a |
| MF1 | 2910.86 ± 59.52 c | 345.13 ± 16.04 b | 358.72 ± 3.88 b | |
| MF2 | 3406.85 ± 84.08 b | 366.14 ± 15.23 b | 356.93 ± 15.96 b | |
| MF3 | 3781.00 ± 30.88 a | 395.43 ± 11.37 a | 340.95 ± 9.12 b | |
| Bolling stage | CK | 2963.58 ± 93.48 d | 335.48 ± 7.94 c | 462.61 ± 13.86 a |
| MF1 | 4085.58 ± 197.55 c | 388.62 ± 14.03 b | 406.87 ± 11.10 b | |
| MF2 | 4448.56 ± 65.45 b | 419.71 ± 15.81 ab | 388.62 ± 31.11 bc | |
| MF3 | 4700.38 ± 75.50 a | 447.82 ± 12.68 a | 350.70 ± 2.64 c | |
| Boll-opening stage | CK | 1545.48 ± 61.78 c | 240.85 ± 7.46 c | 547.62 ± 7.59 a |
| MF1 | 2652.64 ± 122.19 b | 286.73 ± 4.47 b | 441.00 ± 10.06 b | |
| MF2 | 3072.31 ± 200.99 a | 298.15 ± 8.30 ab | 399.71 ± 15.98 c | |
| MF3 | 3232.49 ± 50.25 a | 312.88 ± 4.06 a | 388.68 ± 7.64 c |
| Period | Treatment | Plant Height/cm | Number of Cotton Branches |
|---|---|---|---|
| Bud stage | CK | 48.10 ± 1.25 c | 3.53 ± 0.29 b |
| MF1 | 53.93 ± 1.10 b | 5.16 ± 0.57 a | |
| MF2 | 56.03 ± 1.25 ab | 6.00 ± 0.36 a | |
| MF3 | 57.23 ± 0.55 a | 5.93 ± 0.25 a | |
| Flowering stage | CK | 86.43 ± 1.10 c | 6.67 ± 0.61 b |
| MF1 | 95.83 ± 1.25 b | 10.87 ± 0.31 a | |
| MF2 | 96.30 ± 0.66 ab | 11.06 ± 0.81 a | |
| MF3 | 98.20 ± 0.46 a | 11.67 ± 0.42 a | |
| Bolling stage | CK | 107.37 ± 2.22 b | 10.83 ± 0.51 b |
| MF1 | 119.07 ± 8.21 a | 11.80 ± 0.20 ab | |
| MF2 | 120.13 ± 4.87 a | 12.47 ± 0.58 a | |
| MF3 | 122.60 ± 5.50 a | 12.77 ± 0.51 a | |
| Boll-opening stage | CK | 107.37 ± 2.22 b | 11.20 ± 0.53 b |
| MF1 | 119.07 ± 8.21 a | 12.90 ± 0.85 a | |
| MF2 | 120.13 ± 4.87 a | 13.27 ± 0.40 a | |
| MF3 | 122.60 ± 5.50 a | 13.53 ± 0.65 a |
| Treatment | Boll Number per Plant/Pieces | Boll Weight/g | Lint Percentage/% | Seed Cotton Yield/(kg·ha−1) | Lint Yield/(kg·ha−1) |
|---|---|---|---|---|---|
| CK | 21.00 ± 1.59 b | 2.53 ± 0.06 b | 35.96 ± 0.83 a | 4066.74 ± 255.07 c | 1461.23 ± 65.93 c |
| MF1 | 23.77 ± 0.42 ab | 2.73 ± 0.06 b | 39.60 ± 1.47 a | 4970.46 ± 177.25 b | 1969.62 ± 135.91 b |
| MF2 | 24.70 ± 0.92 a | 3.07 ± 0.15 a | 39.97 ± 2.78 a | 5793.60 ± 333.41 ab | 2313.73 ± 170.52 ab |
| MF3 | 25.37 ± 0.31 a | 3.23 ± 0.15 a | 40.81 ± 0.70 a | 6276.83 ± 373.11 a | 2562.49 ± 184.65 a |
| Classification | Period | Treatment | Chao1 | Observed Species | PD Whole Tree | Shannon |
|---|---|---|---|---|---|---|
| Bacteria | Bud stage | CK | 8185.19 ± 466.34 a | 6769.20 ± 443.47 a | 335.26 ± 22.17 a | 10.53 ± 0.25 a |
| MF1 | 8081.91 ± 124.49 a | 6626.00 ± 120.02 a | 325.15 ± 7.02 a | 10.51 ± 0.15 a | ||
| MF2 | 8221.69 ± 162.26 a | 6848.83 ± 70.56 a | 333.73 ± 2.92 a | 10.71 ± 0.12 a | ||
| MF3 | 7978.57 ± 205.43 a | 6630.17 ± 187.07 a | 325.31 ± 10.77 a | 10.54 ± 0.18 a | ||
| Flowering stage | CK | 8356.91 ± 124.63 a | 6990.60 ± 81.31 a | 317.23 ± 4.16 a | 10.66 ± 0.10 a | |
| MF1 | 8222.83 ± 34.74 a | 6922.63 ± 138.80 a | 316.39 ± 4.44 a | 10.67 ± 0.09 a | ||
| MF2 | 8269.78 ± 59.30 a | 7024.97 ± 76.03 a | 319.70 ± 6.53 a | 10.71 ± 0.12 a | ||
| MF3 | 8339.52 ± 112.32 a | 6954.97 ± 58.82 a | 315.65 ± 1.38 a | 10.66 ± 0.06 a | ||
| Bolling stage | CK | 8066.15 ± 239.82 a | 6789.63 ± 182.82 a | 291.79 ± 11.26 a | 10.65 ± 0.12 a | |
| MF1 | 8119.73 ± 155.86 a | 6754.63 ± 114.06 a | 288.75 ± 3.20 a | 10.61 ± 0.16 a | ||
| MF2 | 8081.62 ± 267.10a | 6757.60 ± 150.03a | 292.55 ± 5.04a | 10.65 ± 0.06a | ||
| MF3 | 8245.98 ± 213.49 a | 6842.00 ± 135.53 a | 293.36 ± 5.39 a | 10.71 ± 0.05 a | ||
| Boll-opening stage | CK | 8332.57 ± 232.85 a | 6996.83 ± 113.47 a | 312.62 ± 3.72 a | 10.68 ± 0.11 a | |
| MF1 | 8449.62 ± 114.24 a | 7003.47 ± 124.94 a | 315.68 ± 7.94 a | 10.68 ± 0.06 a | ||
| MF2 | 8308.74 ± 274.94 a | 6926.87 ± 241.90 a | 310.52 ± 6.91 a | 10.62 ± 0.12 a | ||
| MF3 | 8171.77 ± 486.10 a | 6836.27 ± 360.10 a | 307.89 ± 11.39 a | 10.53 ± 0.33 a | ||
| Fungi | Bud stage | CK | 1281.65 ± 64.20 a | 1136.30 ± 42.02 a | 205.56 ± 6.47 b | 6.33 ± 0.30 a |
| MF1 | 1239.97 ± 46.04 a | 1064.33 ± 36.23 a | 207.28 ± 6.25 b | 6.01 ± 0.32 a | ||
| MF2 | 1238.76 ± 51.38 a | 1055.97 ± 5.33 a | 210.31 ± 5.61 ab | 6.10 ± 0.25 a | ||
| MF3 | 1274.35 ± 49.62 a | 1075.00 ± 27.87 a | 229.04 ± 5.79 a | 6.25 ± 0.22 a | ||
| Flowering stage | CK | 1309.30 ± 90.17 a | 1104.00 ± 107.46 a | 247.93 ± 23.14 a | 5.70 ± 0.43 a | |
| MF1 | 1296.56 ± 103.83 a | 1123.33 ± 75.27 a | 242.93 ± 18.94 a | 6.44 ± 0.15 a | ||
| MF2 | 1215.02 ± 133.57 a | 1038.67 ± 121.13 a | 228.08 ± 22.19 a | 5.08 ± 0.75 a | ||
| MF3 | 1311.08 ± 92.62 a | 1144.00 ± 105.30 a | 238.24 ± 10.23 a | 5.80 ± 0.66 a | ||
| Bolling stage | CK | 1231.32 ± 96.88 a | 1019.00 ± 128.75 a | 241.27 ± 36.89 a | 5.12 ± 0.32 a | |
| MF1 | 1204.80 ± 35.11 a | 1013.63 ± 59.79 a | 234.94 ± 17.32 a | 5.38 ± 0.62 a | ||
| MF2 | 1283.12 ± 105.02 a | 1106.00 ± 74.30 a | 268.82 ± 29.42 a | 5.76 ± 0.82 a | ||
| MF3 | 1357.49 ± 134.39 a | 1165.67 ± 51.79 a | 270.06 ± 15.25 a | 5.94 ± 0.40 a | ||
| Boll-opening stage | CK | 1241.06 ± 77.17 a | 990.63 ± 118.59 a | 224.14 ± 44.08 a | 6.05 ± 0.60 a | |
| MF1 | 1307.51 ± 57.92 a | 1052.30 ± 25.55 a | 244.01 ± 10.60 a | 5.99 ± 0.14 a | ||
| MF2 | 1246.91 ± 213.65 a | 1010.63 ± 147.94 a | 223.36 ± 24.83 a | 5.93 ± 0.80 a | ||
| MF3 | 1257.38 ± 75.36 a | 1001.27 ± 66.14 a | 223.10 ± 8.82 a | 5.70 ± 0.37 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Wang, X.; Xie, X.; Yang, D.; Zhou, Z.; Wang, Q.; Liu, A.; Tu, X. Complex Microbial Fertilizer Promotes the Growth of Summer-Sown Short-Season-Cultivated Cotton and Increases Cotton Yield in the Yangtze River Basin by Changing the Soil Microbial Community Structure. Agronomy 2025, 15, 404. https://doi.org/10.3390/agronomy15020404
Xie Z, Wang X, Xie X, Yang D, Zhou Z, Wang Q, Liu A, Tu X. Complex Microbial Fertilizer Promotes the Growth of Summer-Sown Short-Season-Cultivated Cotton and Increases Cotton Yield in the Yangtze River Basin by Changing the Soil Microbial Community Structure. Agronomy. 2025; 15(2):404. https://doi.org/10.3390/agronomy15020404
Chicago/Turabian StyleXie, Zhangshu, Xiaorong Wang, Xuefang Xie, Dan Yang, Zhonghua Zhou, Qiming Wang, Aiyu Liu, and Xiaoju Tu. 2025. "Complex Microbial Fertilizer Promotes the Growth of Summer-Sown Short-Season-Cultivated Cotton and Increases Cotton Yield in the Yangtze River Basin by Changing the Soil Microbial Community Structure" Agronomy 15, no. 2: 404. https://doi.org/10.3390/agronomy15020404
APA StyleXie, Z., Wang, X., Xie, X., Yang, D., Zhou, Z., Wang, Q., Liu, A., & Tu, X. (2025). Complex Microbial Fertilizer Promotes the Growth of Summer-Sown Short-Season-Cultivated Cotton and Increases Cotton Yield in the Yangtze River Basin by Changing the Soil Microbial Community Structure. Agronomy, 15(2), 404. https://doi.org/10.3390/agronomy15020404





