Potential of New Plant Sources as Raw Materials for Obtaining Natural Pigments/Dyes
Abstract
1. Introduction
2. Data Source
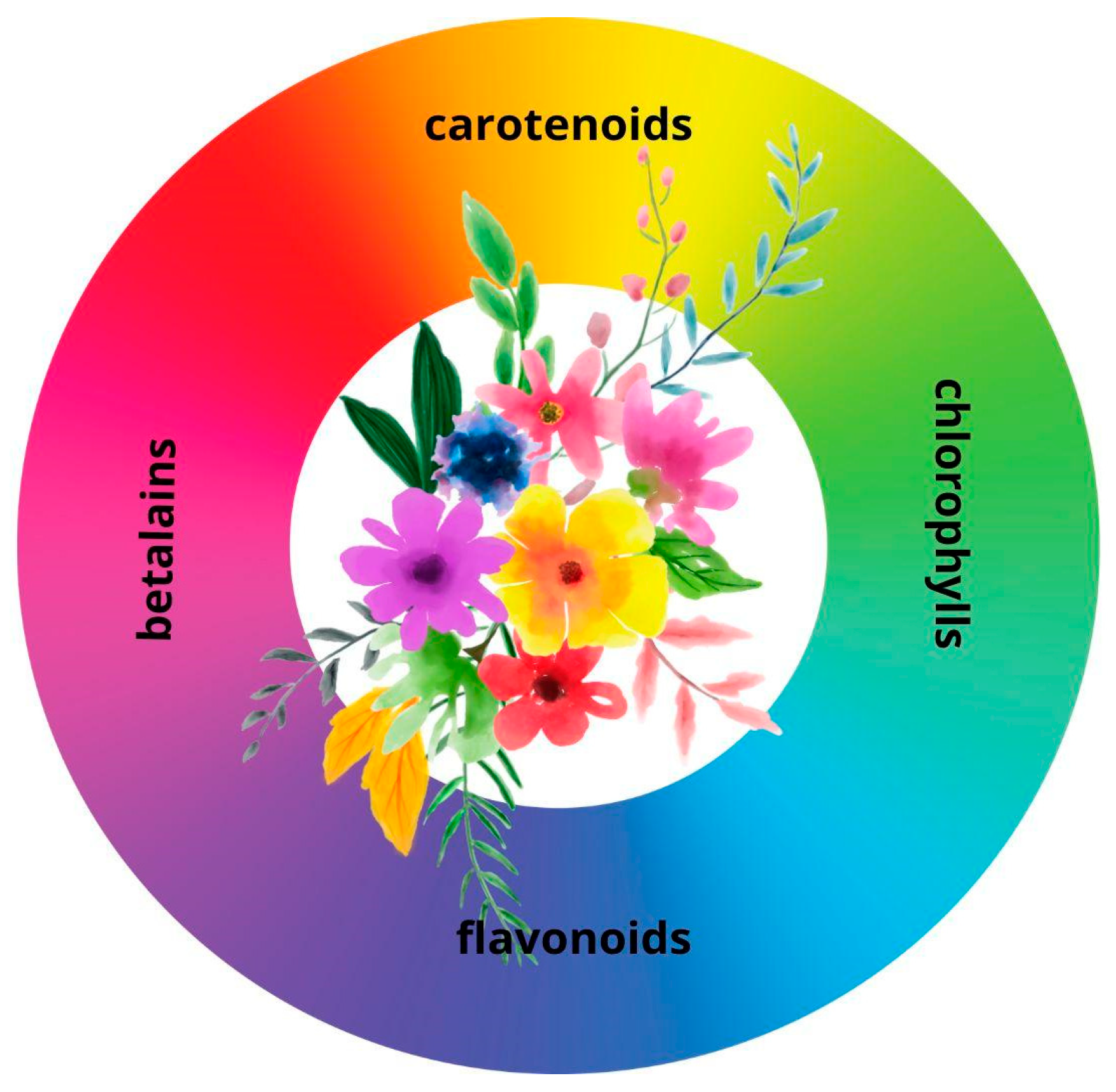
3. Vegetables with Dye Potential
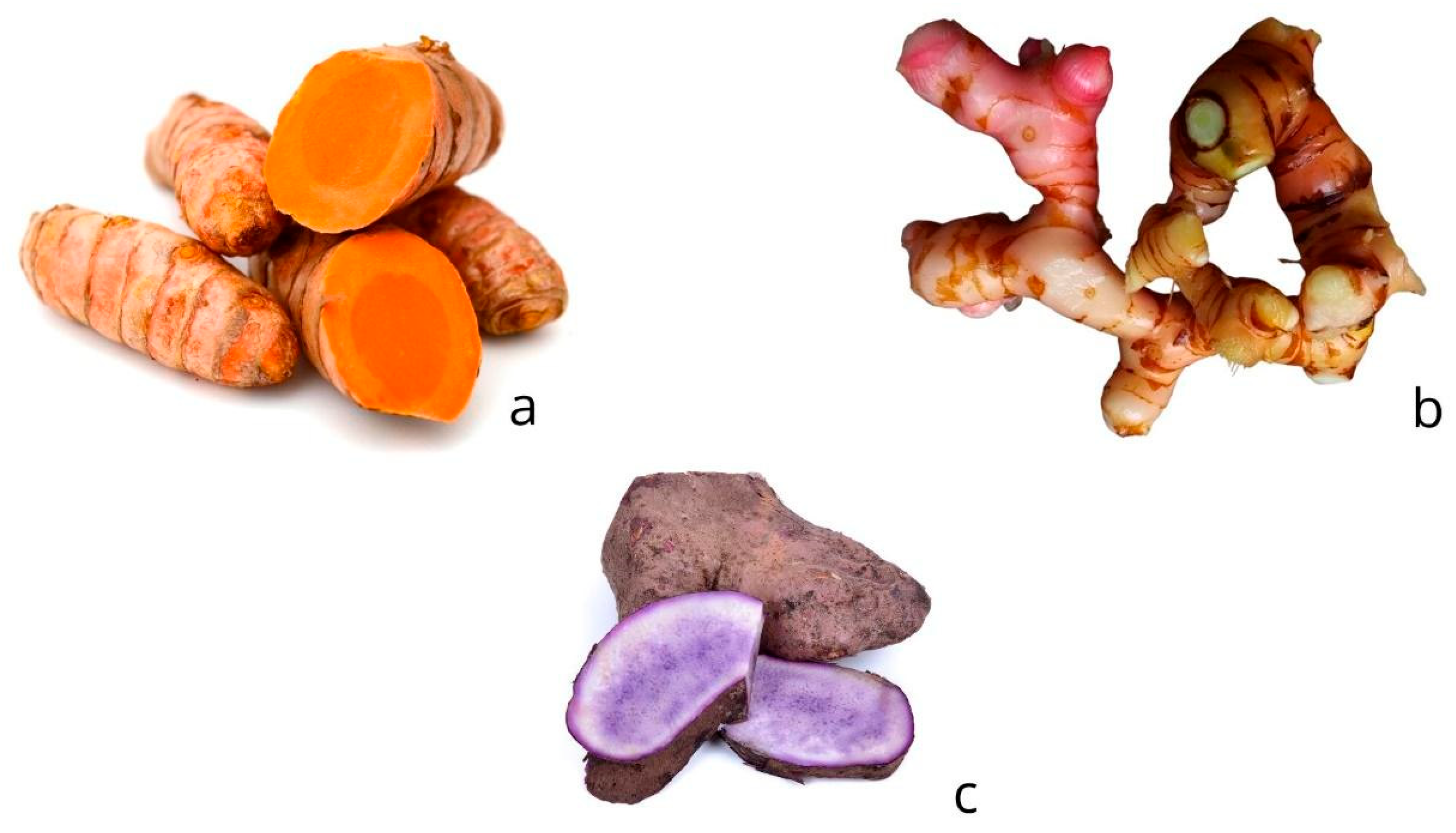
3.1. Roots
3.1.1. Dioscorea spp.
3.1.2. Alpinia officinarum Hance
3.1.3. Xanthosoma riedelianum Schott
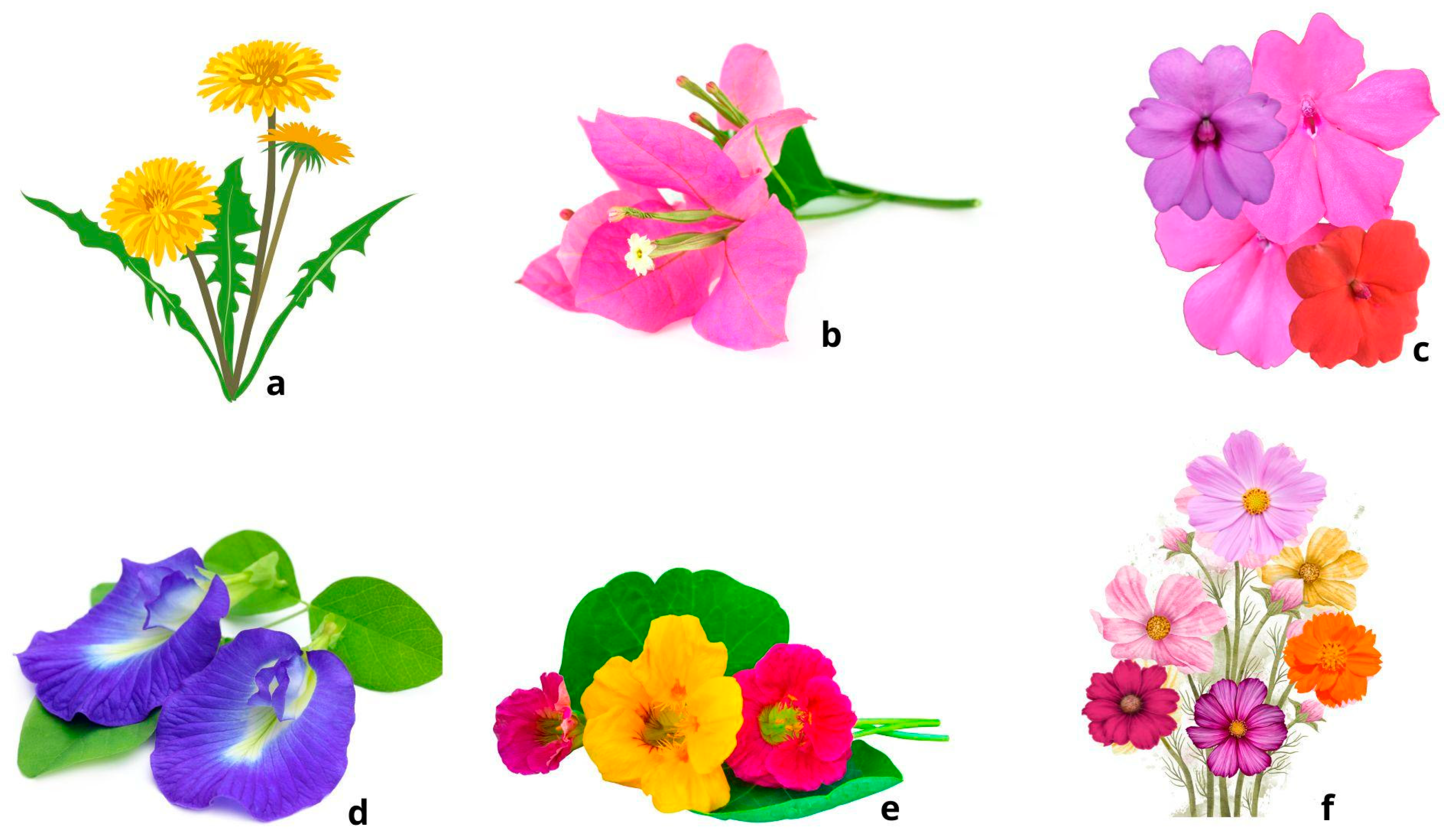
3.2. Flowers
3.2.1. Sonchus oleraceus L.
3.2.2. Bougainvillea glabra Choisy
3.2.3. Clitoria ternatea L.
3.2.4. Tropaeolum majus L.
3.2.5. Impatiens balsamina L.
3.2.6. Cosmos bipinnatus Cav.
3.3. Leaves
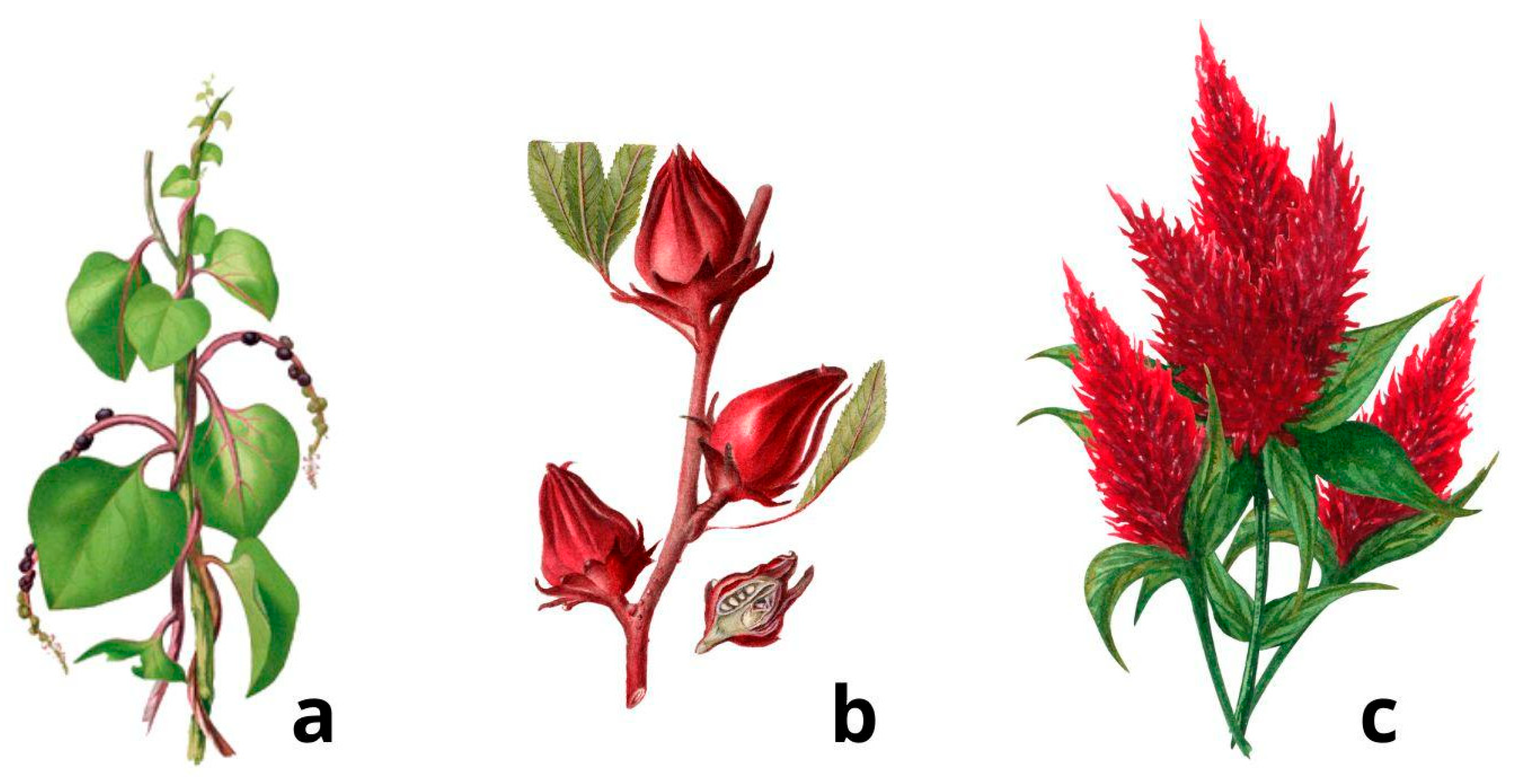
3.3.1. Basella rubra L.
3.3.2. Hibiscus sabdariffa L.
3.3.3. Celosia argentea L.
3.4. Fruits
3.4.1. Rubus rosifolius Smith
3.4.2. Syzygium jambos (L.) Alston
3.4.3. Solanum betaceum Cav.
3.4.4. Clidemia hirta (L.) D. Don
3.4.5. Genipa americana L.
3.4.6. Eugenia brasiliensis Lam.
4. Final Considerations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prabhu, K.H.; Bhute, A.S. Plant Based Natural Dyes and Mordnats: A Review. J. Nat. Prod. Plant Resour 2012, 2, 649–664. [Google Scholar]
- Hu, R.; Li, T.; Qin, Y.; Liu, Y.; Huang, Y. Ethnobotanical Study on Plants Used to Dye Traditional Costumes by the Baiku Yao Nationality of China. J. Ethnobiol. Ethnomed. 2022, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Andriamanantena, M.; Danthu, P.; Cardon, D.; Fawbush, F.R.; Raonizafinimanana, B.; Razafintsalama, V.E.; Rakotonandrasana, S.R.; Ethève, A.; Petit, T.; Caro, Y. Malagasy Dye Plant Species: A Promising Source of Novel Natural Colorants with Potential Applications—A Review. Chem. Biodivers. 2019, 16, e1900442. [Google Scholar] [CrossRef] [PubMed]
- Kaewsangsai, S.; Panyadee, P.; Panya, A.; Pandith, H.; Wangpakapattanawong, P.; Balslev, H.; Inta, A. Diversity of Plant Colorant Species in a Biodiversity Hotspot in Northern Thailand. Diversity 2024, 16, 194. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A.; Noor, N. Valorization of Natural Colors as Health-Promoting Bioactive Compounds: Phytochemical Profile, Extraction Techniques, and Pharmacological Perspectives. Food Chem. 2021, 362, 130141. [Google Scholar] [CrossRef]
- Martins, I.R.; da Silva Martins, L.H.; Chisté, R.C.; Picone, C.S.F.; Joele, M.R.S.P. Betalains from Vegetable Peels: Extraction Methods, Stability, and Applications as Natural Food Colorants. Food Res. Int. 2024, 195, 114956. [Google Scholar] [CrossRef]
- Yadav, S.; Tiwari, K.S.; Gupta, C.; Tiwari, M.K.; Khan, A.; Sonkar, S.P. A Brief Review on Natural Dyes, Pigments: Recent Advances and Future Perspectives. Results Chem. 2023, 5, 100733. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Chakraborty, R. Underutilized Plant Sources: A Hidden Treasure of Natural Colors. Food Biosci. 2023, 52, 102361. [Google Scholar] [CrossRef]
- Muthusamy, S.; Udhayabaskar, S.; Udayakumar, G.P.; Kirthikaa, G.B.; Sivarajasekar, N. Properties and Applications of Natural Pigments Produced from Different Biological Sources—A Concise Review. In Sustainable Development in Energy and Environment; Sivasubramanian, V., Pugazhendhi, A., Moorthy, I.G., Eds.; Springer: Singapore, 2020; pp. 105–119. [Google Scholar]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural Colorants from Vegetable Food Waste: Recovery, Regulatory Aspects, and Stability—A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Junior, O.V.; Costa, L.D.; Cuello, R.E.G.; Ramos, A.Q.; Otero, D.M. Innovation in Cacti Extraction: Evaluating Green Methods for Bioactive Compounds. Food Res. Int. 2024, 196, 115046. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of Active and Intelligent Packaging by Incorporating Betalains from Red Pitaya (Hylocereus polyrhizus) Peel into Starch/Polyvinyl Alcohol Films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B. Natural Pigments of Plant Origin: Classification, Extraction and Application in Foods. Food Chem. 2023, 398, 133908. [Google Scholar] [CrossRef] [PubMed]
- Memariani, Z.; Farzaei, M.H.; Ali, A.; Momtaz, S. Chapter Seven—Nutritional and Bioactive Characterization of Unexplored Food Rich in Phytonutrients. In Phytonutrients in Food; Woodhead Publishing Series in Food Science, Technology and Nutrition; Nabavi, S.M., Suntar, I., Barreca, D., Khan, H., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 157–175. ISBN 978-0-12-815354-3. [Google Scholar]
- Conceição, L.d.S.; Silva, L.C.e.; Coqueiro, J.M.; Costa, L.D.; Cardoso, P.d.S.; Zimmer, T.B.R.; Costa, I.H.d.L.; Otero, D.M. Unconventional Food Plants in Brazil: Knowledge and Consumption Analysis. Agroaliment. J.-Rev. Agroaliment. 2024, 29, 179–197. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-33892-7. [Google Scholar]
- Miranda, B.M.; Cruz, M.V.; de Campos, I.T.N.; Fernandes, K.F.; Silva, F.A. A Halochromic Film Containing Plinia Cauliflora Peel Anthocyanins Loaded into a Cashew Gum Polysaccharide-Polyvinyl Alcohol Matrix. Waste Biomass Valor. 2022, 13, 2565–2574. [Google Scholar] [CrossRef]
- Jena, B.; Padhan, B.; Pati, K.; Singh Chauhan, V.B. Critical Review on Nutra-Pharmaceutical Usage of Yams. Food Humanit. 2024, 2, 100273. [Google Scholar] [CrossRef]
- de Medeiros, P.M.; Dos Santos, G.M.C.; Barbosa, D.M.; Gomes, L.C.A.; Santos, É.M.d.C.; da Silva, R.R.V. Local Knowledge as a Tool for Prospecting Wild Food Plants: Experiences in Northeastern Brazil. Sci. Rep. 2021, 11, 594. [Google Scholar] [CrossRef]
- Lebot, V.; Lawac, F.; Legendre, L. The Greater Yam (Dioscorea alata L.): A Review of Its Phytochemical Content and Potential for Processed Products and Biofortification. J. Food Compos. Anal. 2023, 115, 104987. [Google Scholar] [CrossRef]
- Hornung, P.S.; Ávila, S.; Lazzarotto, M.; da Silveira Lazzarotto, S.R.; de Andrade de Siqueira, G.L.; Schnitzler, E.; Ribani, R.H. Enhancement of the Functional Properties of Dioscoreaceas Native Starches: Mixture as a Green Modification Process. Thermochim. Acta 2017, 649, 31–40. [Google Scholar] [CrossRef]
- Dey, P.; Ray, S.; Chaudhuri, T.K. Immunomodulatory Activities and Phytochemical Characterisation of the Methanolic Extract of Dioscorea alata Aerial Tuber. J. Funct. Foods 2016, 23, 315–328. [Google Scholar] [CrossRef]
- Jiang, S.; Cen, J.; Zhou, Y.; Wang, Y.; Wu, D.; Wang, Z.; Sun, J.; Shu, X. Physicochemical Characterizations of Five Dioscorea alata L. Starches from China. Int. J. Biol. Macromol. 2023, 237, 124225. [Google Scholar] [CrossRef]
- Santos, S.d.J.L.; Canto, H.K.F.; da Silva, L.H.M.; Rodrigues, A.M.d.C. Characterization and Properties of Purple Yam (Dioscorea trifida) Powder Obtained by Refractance Window Drying. Dry. Technol. 2022, 40, 1103–1113. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, R.K.; Srivastav, P.P. Exploring Novel Frontiers of Advancements in Purple Yam (Dioscorea alata L.) Starch Extraction, Modification, Characterization, Applications in Food and Other Industries. Meas. Food 2024, 15, 100196. [Google Scholar] [CrossRef]
- Ochoa, S.; Durango-Zuleta, M.M.; Felipe Osorio-Tobón, J. Techno-Economic Evaluation of the Extraction of Anthocyanins from Purple Yam (Dioscorea alata) Using Ultrasound-Assisted Extraction and Conventional Extraction Processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Alasmary, F.A.; Assirey, E.A.; El-Meligy, R.M.; Awaad, A.S.; El-Sawaf, L.A.; Allah, M.M.; Alqasoumi, S.I. Analysis of Alpina Officinarum Hance, Chemically and Biologically. Saudi Pharm. J. 2019, 27, 1107–1112. [Google Scholar] [CrossRef]
- Abd Rahman, I.Z.; Adam, S.H.; Hamid, A.A.; Mokhtar, M.H.; Mustafar, R.; Kashim, M.I.A.M.; Febriza, A.; Mansor, N.I. Potential Neuroprotective Effects of Alpinia Officinarum Hance (Galangal): A Review. Nutrients 2024, 16, 3378. [Google Scholar] [CrossRef]
- Lei, X.; Wang, J.; Zuo, K.; Xia, T.; Zhang, J.; Xu, X.; Liu, Q.; Li, X. Alpinia Officinarum Hance: A Comprehensive Review of Traditional Uses, Phytochemistry, Pharmacokinetic and Pharmacology. Front. Pharmacol. 2024, 15, 1414635. [Google Scholar] [CrossRef]
- Koçak, Ö.F.; Yılmaz, F. Use of Alpinia Officinarum Rhizome in Textile Dyeing and Gaining Simultaneous Antibacterial Properties. J. Nat. Fibers 2022, 19, 1925–1936. [Google Scholar] [CrossRef]
- Marchioni, I.; Gabriele, M.; Carmassi, G.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Najar, B. Phytochemical, Nutritional and Mineral Content of Four Edible Flowers. Foods 2024, 13, 939. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Whittall, J.B. Painting the Green Canvas: How Pigments Produce Flower Colours. Biochemist 2021, 43, 6–12. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers: Emerging Components in the Diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Anthocyanins From Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity, and Applications. Front. Plant Sci. 2021, 12, 792303. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, A.E.-N.G.; Mohamed, N.A.; Sarker, T.C.; Hassan, E.M.; Garaa, A.H.; Elshamy, A.I.; Abd-ElGawad, A.M. Chemical Composition, Antioxidant, and Cytotoxic Activity of Essential Oils in the Above-Ground Parts of Sonchus oleraceus L. Plants 2024, 13, 1712. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Kumar, J. Phytochemical Screening, Metal-Binding Studies and Applications of Floral Extract of Sonchus oleraceus as a Corrosion Inhibitor. J. Bio Tribo Corros. 2020, 6, 55. [Google Scholar] [CrossRef]
- Kumar, S.N.A.; Ritesh, S.K.; Sharmila, G.; Muthukumaran, C. Extraction Optimization and Characterization of Water Soluble Red Purple Pigment from Floral Bracts of Bougainvillea glabra. Arab. J. Chem. 2017, 10, S2145–S2150. [Google Scholar] [CrossRef]
- Damit, D.N.F.P.; Galappaththi, K.; Lim, A.; Petra, M.I.; Ekanayake, P. Formulation of Water to Ethanol Ratio as Extraction Solvents of Ixora Coccinea and Bougainvillea glabra and Their Effect on Dye Aggregation in Relation to DSSC Performance. Ionics 2017, 23, 485–495. [Google Scholar] [CrossRef]
- Sakalani, A.; Mwanga, S.; Cherupally, L.; Juluru, A. Evaluation of Absorbance for Crude and Purified Natural Dyes Using Senna Singueana, Bougainvillea glabra Bracts, and Ximenia Caffra on DSSC Performance Parameters. Energy Sources Part A: Recovery Util. Environ. Eff. 2022, 44, 379–392. [Google Scholar] [CrossRef]
- Rasool, W.; Adeel, S.; Batool, F.; Ahmad, S.A.; Mumtaz, S.; Hussaan, M. Environmental Friendly Silk and Cotton Dyeing Using Natural Colorant of Bougainvillea (Bougainvillea glabra) Flowers: The Sustainable Approach towards Textile Industry. Environ. Sci. Pollut. Res. Int. 2023, 30, 21863–21871. [Google Scholar] [CrossRef]
- Marpaung, A.M. Tinjauan Manfaat Bunga Telang (Clitoria ternatea L.) Bagi Kesehatan Manusia. J. Funct. Food Nutraceutical 2020, 1, 63–85. [Google Scholar] [CrossRef]
- Handayani, L.; Aprilia, S.; Arahman, N.; Bilad, M.R. Identification of the Anthocyanin Profile from Butterfly Pea (Clitoria ternatea L.) Flowers under Varying Extraction Conditions: Evaluating Its Potential as a Natural Blue Food Colorant and Its Application as a Colorimetric Indicator. S. Afr. J. Chem. Eng. 2024, 49, 151–161. [Google Scholar] [CrossRef]
- Shu, M.H.; Annamalai, K.K.; Idris, F.N.; Kamaruddin, A.H.; Nadzir, M.M. Dataset of Ultrasound-Assisted Extraction of Anthocyanin from the Petals of Clitoria ternatea Using Taguchi Method and Effect of Storage Conditions on the Anthocyanin Stability. Data Brief 2022, 40, 107803. [Google Scholar] [CrossRef]
- Caroline Paz Gonçalves, G.; Lizandra Gomes Rosas, A.; Carneiro de Sousa, R.; Regina Rodrigues Vieira, T.; César de Albuquerque Sousa, T.; Ramires, T.; Ferreira Ferreira da Silveira, T.; Barros, L.; Padilha da Silva, W.; Renato Guerra Dias, Á.; et al. A Green Method for Anthocyanin Extraction from Clitoria ternatea Flowers Cultivated in Southern Brazil: Characterization, in Vivo Toxicity, and Biological Activity. Food Chem. 2024, 435, 137575. [Google Scholar] [CrossRef] [PubMed]
- Netravati, N.; Gomez, S.; Pathrose, B.; N, M.R.; P, M.J.; Kuruvila, B. Comparative Evaluation of Anthocyanin Pigment Yield and Its Attributes from Butterfly Pea (Clitorea ternatea L.) Flowers as Prospective Food Colorant Using Different Extraction Methods. Future Foods 2022, 6, 100199. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Parvatam, G. Effect of Dehydration Methods on Pigment Characteristics, Bioactives Profile and Antioxidant Potential of Blue Petals of Clitoria ternatea L. Food Meas. 2024, 18, 3536–3546. [Google Scholar] [CrossRef]
- Kim, H.-J.; Roy, S.; Rhim, J.-W. Gelatin/Agar-Based Color-Indicator Film Integrated with Clitoria ternatea Flower Anthocyanin and Zinc Oxide Nanoparticles for Monitoring Freshness of Shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Goh, J.K.; Choo, W.S. Application of Anthocyanins from Blue Pea Flower in Yoghurt and Fermented Milk: An Alternate Natural Blue Colour to Spirulina. Int. J. Gastron. Food Sci. 2024, 37, 100957. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, I.C.; Sharma, S.; Kushwaha, S.P.S.; Srivastava, P.; Bahadur, L. Application of New Natural Dyes Extracted from Nasturtium Flowers (Tropaeolum majus) as Photosensitizer in Dye-Sensitized Solar Cells. Optik 2021, 243, 167331. [Google Scholar] [CrossRef]
- Barros, R.G.C.; Andrade, J.K.S.; Pereira, U.C.; de Oliveira, C.S.; Rafaella Ribeiro Santos Rezende, Y.; Oliveira Matos Silva, T.; Pedreira Nogueira, J.; Carvalho Gualberto, N.; Caroline Santos Araujo, H.; Narain, N. Phytochemicals Screening, Antioxidant Capacity and Chemometric Characterization of Four Edible Flowers from Brazil. Food Res Int. 2020, 130, 108899. [Google Scholar] [CrossRef]
- Demasi, S.; Mellano, M.G.; Falla, N.M.; Caser, M.; Scariot, V. Sensory Profile, Shelf Life, and Dynamics of Bioactive Compounds during Cold Storage of 17 Edible Flowers. Horticulturae 2021, 7, 166. [Google Scholar] [CrossRef]
- Qian, H.; Wang, B.; Ma, J.; Li, C.; Zhang, Q.; Zhao, Y. Impatiens balsamina: An Updated Review on the Ethnobotanical Uses, Phytochemistry, and Pharmacological Activity. J. Ethnopharmacol. 2023, 303, 115956. [Google Scholar] [CrossRef]
- Pires, E.d.O., Jr.; Pereira, E.; Carocho, M.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Garcia, C.C.; Ferreira, I.C.F.R.; et al. Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product. Int. J. Environ. Res. Public Health 2021, 18, 9062. [Google Scholar] [CrossRef]
- Teixeira, M.; Tao, W.; Fernandes, A.; Faria, A.; Ferreira, I.M.P.L.V.O.; He, J.; de Freitas, V.; Mateus, N.; Oliveira, H. Anthocyanin-Rich Edible Flowers, Current Understanding of a Potential New Trend in Dietary Patterns. Trends Food Sci. Technol. 2023, 138, 708–725. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Malheiro, R.; Rodrigues, N.; Saraiva, J.A.; Ramalhosa, E. Borage, Calendula, Cosmos, Johnny Jump up, and Pansy Flowers: Volatiles, Bioactive Compounds, and Sensory Perception. Eur. Food Res. Technol. 2019, 245, 593–606. [Google Scholar] [CrossRef]
- Ortega-Medrano, R.J.; Ceja-Torres, L.F.; Vázquez-Sánchez, M.; Martínez-Ávila, G.C.G.; Medina-Medrano, J.R. Characterization of Cosmos Sulphureus Cav. (Asteraceae): Phytochemical Screening, Antioxidant Activity and Chromatography Analysis. Plants 2023, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Sarode, D.; Pagariya, M.; Jadhav, P.; Patil, S.; Devarumath, R.; Shingote, P.; Prasad, K.; Jain, S.; Penna, S.; Kawar, P. Edible Flowers: Biotechnological Interventions for Improving Bioactives of Food and Health Significance. J. Food Compos. Anal. 2024, 134, 106506. [Google Scholar] [CrossRef]
- Karangutkar, A.V.; Ananthanarayan, L. Evaluating the Effect of Additives on Stability of Betacyanin Pigments from Basella rubra in a Model Beverage System during Storage. J. Food Sci. Technol. 2021, 58, 1262–1273. [Google Scholar] [CrossRef]
- Kozioł, Ł.; Knap, M.; Sutor-Świeży, K.; Górska, R.; Dziedzic, E.; Bieniasz, M.; Mielczarek, P.; Popenda, Ł.; Tyszka-Czochara, M.; Wybraniec, S. Identification and Reactivity of Pigments in Prominent Vegetable Leaves of Basella alba L. Var. “Rubra” (Malabar spinach). Food Chem. 2024, 445, 138714. [Google Scholar] [CrossRef]
- Sravan Kumar, S.; Manoj, P.; Giridhar, P. A Method for Red-Violet Pigments Extraction from Fruits of Malabar spinach (Basella rubra) with Enhanced Antioxidant Potential under Fermentation. J. Food Sci. Technol. 2015, 52, 3037–3043. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Giridhar, P. Nutrition Facts and Functional Attributes of Foliage of Basella Spp. LWT—Food Sci. Technol. 2015, 64, 468–474. [Google Scholar] [CrossRef]
- Shende, A.S.; Joshi T, J.; Rao, P.S. Process Optimization of Microwave-Assisted Aqueous Extraction of Tannins and Saponins from Malabar spinach (Basella alba) Leaves Using ANN-GA and RSM Methodology. Meas. Food 2024, 13, 100117. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Nimisha, G.; Giridhar, P. Phytoconstituents and Stability of Betalains in Fruit Extracts of Malabar spinach (Basella rubra L.). J. Food Sci. Technol. 2016, 53, 4014–4022. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Natural Pigments Extraction from Basella rubra L. Fruits by Ultrasound-Assisted Extraction Combined with Box-Behnken Response Surface Design. Sep. Sci. Technol. 2015, 50, 1532–1540. [Google Scholar] [CrossRef]
- Sravan Kumar, S.; Singh Chauhan, A.; Giridhar, P. Nanoliposomal Encapsulation Mediated Enhancement of Betalain Stability: Characterisation, Storage Stability and Antioxidant Activity of Basella rubra L. Fruits for Its Applications in Vegan Gummy Candies. Food Chem. 2020, 333, 127442. [Google Scholar] [CrossRef] [PubMed]
- Karangutkar, A.V.; Ananthanarayan, L. Co-Crystallization of Basella rubra Extract with Sucrose: Characterization of Co-Crystals and Evaluating the Storage Stability of Betacyanin Pigments. J. Food Eng. 2020, 271, 109776. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a Source of Nutrients, Bioactive Compounds and Colouring Agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rodríguez, A.S.; Carrión, A.; Trejo, F.; Esparza-Ponce, H.E.; Nápoles-Duarte, J.M.; Ballinas-Casarrubias, M.L.; Fuentes-Cobas, L.E.; Salas, E.; Palomares-Báez, J.P.; Fuentes-Montero, M.E. Anthocyanins Stabilization of Hibiscus sabdariffa Extract with Sepiolite: Analytical and Reactive Force Fields Approaches. Sustain. Chem. Pharm. 2024, 42, 101831. [Google Scholar] [CrossRef]
- Nwuzor, I.C.; Adinoyi, B.J.; Okey-Onyesolu, C.F.; Oyeoka, H.C. Hibiscus sabdariffa Natural Dye Extraction Process with Central Composite Design for Optimal Extract Yield. Sustain. Chem. Environ. 2023, 2, 100008. [Google Scholar] [CrossRef]
- Li, N.; Simon, J.E.; Wu, Q. Development of a Scalable, High-Anthocyanin and Low-Acidity Natural Red Food Colorant from Hibiscus sabdariffa L. Food Chem. 2024, 461, 140782. [Google Scholar] [CrossRef]
- Villalobos-Vega, M.J.; Rodríguez-Rodríguez, G.; Armijo-Montes, O.; Jiménez-Bonilla, P.; Álvarez-Valverde, V. Optimization of the Extraction of Antioxidant Compounds from Roselle Hibiscus Calyxes (Hibiscus sabdariffa), as a Source of Nutraceutical Beverages. Molecules 2023, 28, 2628. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of Heat- and Ultrasound-Assisted Extraction of Anthocyanins from Hibiscus sabdariffa Calyces for Natural Food Colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef]
- Ngoc Nhon, H.T.; Diem My, N.T.; Tuong Vi, V.N.; Kim Lien, P.T.; Thao Minh, N.T.; Doan Duy, L.N.; Hong Anh, L.T.; Anh Dao, D.T. Enhancement of Extraction Effectiveness and Stability of Anthocyanin from Hibiscus sabdariffa L. J. Agric. Food Res. 2022, 10, 100408. [Google Scholar] [CrossRef]
- Idham, Z.; Putra, N.R.; Aziz, A.H.A.; Zaini, A.S.; Rasidek, N.A.M.; Mili, N.; Yunus, M.A.C. Improvement of Extraction and Stability of Anthocyanins, the Natural Red Pigment from Roselle Calyces Using Supercritical Carbon Dioxide Extraction. J. CO2 Util. 2022, 56, 101839. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Pimentel-Mora, S.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. A Novel Sustainable Approach for the Extraction of Value-Added Compounds from Hibiscus sabdariffa L. Calyces by Natural Deep Eutectic Solvents. Food Res. Int. 2020, 137, 109646. [Google Scholar] [CrossRef]
- Millinia, B.L.; Mashithah, D.; Nawatila, R.; Kartini, K. Microencapsulation of Roselle (Hibiscus sabdariffa L.) Anthocyanins: Effects of Maltodextrin and Trehalose Matrix on Selected Physicochemical Properties and Antioxidant Activities of Spray-Dried Powder. Future Foods 2024, 9, 100300. [Google Scholar] [CrossRef]
- Pereira, A.R.; Fernandes, V.C.; Delerue-Matos, C.; de Freitas, V.; Mateus, N.; Oliveira, J. Exploring Acylated Anthocyanin-Based Extracts as a Natural Alternative to Synthetic Food Dyes: Stability and Application Insights. Food Chem. 2024, 461, 140945. [Google Scholar] [CrossRef]
- Teixeira, V.M.C.; da Silva, R.F.G.; Gonçalves, O.H.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R.; Bona, E.; Leimann, F.V. Chemometric Approaches to Evaluate the Substitution of Synthetic Food Dyes by Natural Compounds: The Case of Nanoencapsulated Curcumin, Spirulina, and Hibiscus Extracts. LWT 2022, 154, 112786. [Google Scholar] [CrossRef]
- Hoang, N.T.N.; Nguyen, N.N.K.; Nguyen, L.T.K.; Le, A.T.H.; Dong, D.T.A. Research on Optimization of Spray Drying Conditions, Characteristics of Anthocyanins Extracted from Hibiscus sabdariffa L. Flower, and Application to Marshmallows. Food Sci. Nutr. 2024, 12, 2003–2015. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Phan-Thi, H.; Pham-Hoang, B.-N.; Ho, P.-T.; Tran, T.T.T.; Waché, Y. Encapsulation of Hibiscus sabdariffa L. Anthocyanins as Natural Colours in Yeast. Food Res. Int. 2018, 107, 275–280. [Google Scholar] [CrossRef]
- Salami, S.O.; Afolayan, A.J. Suitability of Roselle-Hibiscus sabdariffa L. as Raw Material for Soft Drink Production. J. Food Qual. 2020, 2020, 8864142. [Google Scholar] [CrossRef]
- Radhouane, M.F.; da Silveira, T.F.F.; Ribeiro, J.; Rodrigues, P.; Guimarães, R.; Calhelha, R.; Mandim, F.; Charfi, I.; Ferreira, I.C.F.R.; Alves, M.J.; et al. Development, Characterization and Stability of a Novel Sport Drink Based on Thermal Water, Apple Juice and Hibiscus. Food Chem. Adv. 2024, 5, 100823. [Google Scholar] [CrossRef]
- Halim, Y.; Evelyne, C.; Rosa, D.; Ramli, S. Development of Roselle (Hibiscus sabdariffa L.) Calyx Jelly Candy. J. Sustain. Agric. 2022, 37, 357–372. [Google Scholar] [CrossRef]
- Zuluaga-Vega, J.; Fernández-Fernández, J.; Santana-Fuentes, N.; Arteaga-Márquez, M.; De Paula, C.; Simanca-Sotelo, M.; Durango-Villadiego, A.; Pastrana-Puche, Y.; Álvarez-Badel, B.; Bustamante-Vargas, C.; et al. Effect of pH and Temperature on the Stability of the Natural Dye from the Roselle Flower (Hibiscus sabdariffa L.) and Its Application in Flavored Milk. J. Food Sci. Technol. 2024, 62, 178–184. [Google Scholar] [CrossRef]
- Sankaralingam, B.; Balan, L.; Chandrasekaran, S.; Muthu Selvam, A. Anthocyanin: A Natural Dye Extracted from Hibiscus sabdariffa (L.) for Textile and Dye Industries. Appl. Biochem. Biotechnol. 2023, 195, 2648–2663. [Google Scholar] [CrossRef]
- Mansour, R.; Ben Ali, H. Investigating the Use of Chitosan: Toward Improving the Dyeability of Cotton Fabrics Dyed with Roselle (Hibiscus sabdariffa L.). J. Nat. Fibers 2021, 18, 1007–1016. [Google Scholar] [CrossRef]
- Nansu, W.; Ross, S.; Waisarikit, A.; Ross, G.M.; Charoensit, P.; Suphrom, N.; Mahasaranon, S. Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(Butylene Succinate) for Biodegradable Packaging Films. Polymers 2023, 15, 4193. [Google Scholar] [CrossRef]
- Nansu, W.; Chaiwut, P.; Ross, S.; Ross, G.; Suphrom, N.; Mahasaranon, S. Developments of Biodegradable Polymer Based on Polylactic Acid (PLA) with Natural Color Extracts for Packaging Film Applications. J. Met. Mater. Miner. 2021, 31, 127–133. [Google Scholar] [CrossRef]
- Adegbaju, O.D.; Otunola, G.A.; Afolayan, A.J. Proximate, Mineral, Vitamin and Anti-Nutrient Content of Celosia argentea at Three Stages of Maturity. S. Afr. J. Bot. 2019, 124, 372–379. [Google Scholar] [CrossRef]
- Shahzadi, T.; Zaib, M.; Riaz, T.; Shehzadi, S.; Abbasi, M.A.; Shahid, M. Synthesis of Eco-Friendly Cobalt Nanoparticles Using Celosia argentea Plant Extract and Their Efficacy Studies as Antioxidant, Antibacterial, Hemolytic and Catalytical Agent. Arab. J. Sci. Eng. 2019, 44, 6435–6444. [Google Scholar] [CrossRef]
- Guadarrama-Flores, B.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; García-Carmona, F.; Gandía-Herrero, F. Production of Dihydroxylated Betalains and Dopamine in Cell Suspension Cultures of Celosia argentea Var. Plumosa. J. Agric. Food Chem. 2015, 63, 2741–2749. [Google Scholar] [CrossRef]
- Thiyajai, P.; Koyama, T. Binary Ethanol-Water Solvents Affect Betalain Contents and Health-Promoting Properties of Red Celosia argentea Inflorescence Extracts. Int. Food Res. J. 2022, 29, 67–77. [Google Scholar] [CrossRef]
- Talukder, S.; Mendiratta, S.K.; Biswas, A.K.; Sen, A.R.; Devadason, I.P.; Chand, S.; Ahmad, T.; Kumar, D.; Agrawal, R.; Tarafdar, A. Natural Dye-Based Sensor for Monitoring Temperature Variation in Storage for Chicken Patties. Food Biosci. 2024, 60, 104425. [Google Scholar] [CrossRef]
- Kaba, B.; Zannou, O.; Ali Redha, A.; Koca, I. Enhancing Extraction of Betalains from Beetroot (Beta vulgaris L.) Using Deep Eutectic Solvents: Optimization, Bioaccessibility and Stability. Food Prod. Process. Nutr. 2024, 6, 38. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, P.; Henarejos-Escudero, P.; Pagán-López, D.J.; Hernández-García, S.; Guerrero-Rubio, M.A.; Gómez-Pando, L.R.; Gandía-Herrero, F. Dopamine-Derived Pigments in Nature: Identification of Decarboxybetalains in Amaranthaceae Species. Plant Physiol. 2024, 196, 446–460. [Google Scholar] [CrossRef]
- Sang A Roon, T.; Klanrit, P.; Klanrit, P.; Thanonkeo, P.; Apiraksakorn, J.; Thanonkeo, S.; Klanrit, P. Establishment of Betalain-Producing Cell Line and Optimization of Pigment Production in Cell Suspension Cultures of Celosia argentea Var. Plumosa. Plants 2024, 13, 3225. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.S.; Quintero-Lira, A.; Gómez-Aldapa, C.A.; Alanís-García, E.; Cervantes-Elizarrarás, A.; Güemes-Vera, N.; Ramírez-Moreno, E. Application of Ultrasound in a Closed System: Optimum Condition for Antioxidants Extraction of Blackberry (Rubus fructicosus) Residues. Molecules 2016, 21, 950. [Google Scholar] [CrossRef]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of Anthocyanins, Polyphenols, and Antioxidant Capacities among Raspberry, Blackberry, and Japanese Wild Rubus Species. Sci. Hortic. 2021, 285, 110204. [Google Scholar] [CrossRef]
- Mohammadi, N.; Franchin, M.; Girotto Pressete, C.; Maria Greggi Antunes, L.; Granato, D. Green Recovery and Application of Berry Anthocyanins in Functional Gummies: Stability Study, Plasma and Cellular Antioxidant and Anti-Inflammatory Activity. Food Res. Int. 2024, 196, 115128. [Google Scholar] [CrossRef]
- Moreno-Medina, B.L.; Casierra-Posada, F.; Medina-Vargas, O.J. Phenolic Profile and Antioxidant Capacity of Blackberry Fruits (Rubus Spp.) Grown in Colombia. Erwerbs-Obstbau 2023, 65, 1047–1056. [Google Scholar] [CrossRef]
- Moraes, D.P.; Lozano-Sánchez, J.; Machado, M.L.; Vizzotto, M.; Lazzaretti, M.; Leyva-Jimenez, F.J.J.; da Silveira, T.L.; Ries, E.F.; Barcia, M.T. Characterization of a New Blackberry Cultivar BRS Xingu: Chemical Composition, Phenolic Compounds, and Antioxidant Capacity in Vitro and in Vivo. Food Chem. 2020, 322, 126783. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of Anthocyanins from Residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by Ultrasound Assisted Extraction, Pressurized Liquid Extraction and Their Combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an Anthocyanin-Rich Blackberry (Rubus Spp.) by-Product Extract by Freeze-Drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Repon, M.R.; Dev, B.; Rahman, M.A.; Jurkonienė, S.; Haji, A.; Alim, M.A.; Kumpikaitė, E. Textile Dyeing Using Natural Mordants and Dyes: A Review. Environ. Chem. Lett. 2024, 22, 1473–1520. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; Neri-Numa, I.A.; de Araújo, F.F.; Pastore, G.M. A Critical Review of Some Fruit Trees from the Myrtaceae Family as Promising Sources for Food Applications with Functional Claims. Food Chem. 2020, 306, 125630. [Google Scholar] [CrossRef]
- Gavillán-Suárez, J.; Aguilar-Perez, A.; Rivera-Ortiz, N.; Rodríguez-Tirado, K.; Figueroa-Cuilan, W.; Morales-Santiago, L.; Maldonado-Martínez, G.; Cubano, L.A.; Martínez-Montemayor, M.M. Chemical Profile and in Vivo Hypoglycemic Effects of Syzygium Jambos, Costus Speciosus and Tapeinochilos Ananassae Plant Extracts Used as Diabetes Adjuvants in Puerto Rico. BMC Complement. Altern. Med. 2015, 15, 244. [Google Scholar] [CrossRef]
- Tamiello, C.S.; Adami, E.R.; de Oliveira, N.M.T.; Acco, A.; Iacomini, M.; Cordeiro, L.M.C. Structural Features of Polysaccharides from Edible Jambo (Syzygium jambos) Fruits and Antitumor Activity of Extracted Pectins. Int. J. Biol. Macromol. 2018, 118, 1414–1421. [Google Scholar] [CrossRef]
- Wang, H.; Gang, H.; Chen, J.; Liu, J.; Zhang, X.; Fu, C.; Shao, K.; Wang, X.; Qin, D.; Huo, J. Transcriptomic and Metabolomic Analyses Reveal Molecular and Metabolic Regulation of Anthocyanin Biosynthesis in Three Varieties of Currant. Food Res. Int. 2024, 196, 115056. [Google Scholar] [CrossRef]
- Shi, J.; Simal-Gandara, J.; Mei, J.; Ma, W.; Peng, Q.; Shi, Y.; Xu, Q.; Lin, Z.; Lv, H. Insight into the Pigmented Anthocyanins and the Major Potential Co-Pigmented Flavonoids in Purple-Coloured Leaf Teas. Food Chem. 2021, 363, 130278. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Tamarillo (Solanum betaceum): Chemical Composition, Biological Properties, and Product Innovation. Trends Food Sci. Technol. 2020, 95, 45–58. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Adzahan, N.M.; Hamzah, M.Y.; Mustafa, S.; Muhammad, K. Physicochemical Properties of Tamarillo (Solanum betaceum Cav.) Hydrocolloid Fractions. Food Chem. 2015, 182, 292–301. [Google Scholar] [CrossRef]
- Bakić, M.T.; Pedisić, S.; Zorić, Z.; Dragović-Uzelac, V.; Grassino, A.N. Effect of Microwave-Assisted Extraction on Polyphenols Recovery from Tomato Peel Waste. Acta Chim. Slov. 2019, 66, 367–377. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Ferreira, T.A.P.C.; Celli, G.B.; Brooks, M.S. Optimization of Lycopene Extraction from Tomato Processing Waste Using an Eco-Friendly Ethyl Lactate–Ethyl Acetate Solvent: A Green Valorization Approach. Waste Biomass Valor. 2019, 10, 2851–2861. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef]
- Osorio, C.; Hurtado, N.; Dawid, C.; Hofmann, T.; Heredia-Mira, F.J.; Morales, A.L. Chemical Characterisation of Anthocyanins in Tamarillo (Solanum betaceum Cav.) and Andes Berry (Rubus glaucus Benth.) Fruits. Food Chem. 2012, 132, 1915–1921. [Google Scholar] [CrossRef]
- Susanti, D.; Nafi, M.; Purwaningsih, H.; Fajarin, R.; Kusuma, G.E. The Preparation of Dye Sensitized Solar Cell (DSSC) from TiO2 and Tamarillo Extract. Procedia Chem. 2014, 9, 3–10. [Google Scholar] [CrossRef]
- Yudaputra, A.; Rahardjo, P. Short Communication: Plant Species Richness and Diversity in Karangsambung-Karangbolong National Geopark, Indonesia. Biodivers. J. Biol. Divers. 2020, 21, 1735–1742. [Google Scholar] [CrossRef]
- Larasati, G.A.; Kartawiria, I.S.; Marpaung, A.M. Anthocyanin Extraction from Clidemia hirta (L.) D. Don Fruit and Its Stability During Storage. In Proceedings of the 6th International Conference of Food, Agriculture, and Natural Resource (IC-FANRES 2021); Atlantis Press: Amsterdam, The Netherlands, 2022; pp. 150–154. [Google Scholar]
- Bomfim, E.M.S.; Coelho, A.A.O.P.; Silva, M.C.; Marques, E.J.; Vale, V.L.C. Phytochemical Composition and Biological Activities of Extracts from Ten Species of the Family Melastomataceae Juss. Braz. J. Biol. 2021, 82, e242112. [Google Scholar] [CrossRef]
- Lopez, T.; Corbin, C.; Falguieres, A.; Doussot, J.; Montguillon, J.; Hagège, D.; Hano, C.; Lainé, É. Secondary Metabolite Accumulation, Antibacterial and Antioxidant Properties of in Vitro Propagated Clidemia hirta L. Extracts Are Influenced by the Basal Culture Medium. C. R. Chim. 2016, 19, 1071–1076. [Google Scholar] [CrossRef]
- Assunção-Júnior, S.O.; Rodrigues, L.S.I.; Raposo, D.S.; Rodrigues, J.G.C.; de Lima, E.J.S.P.; da Silva, F.M.A.; Scudeller, V.V.; Corrêa, A.L.; Lima, E.S.; Albuquerque, P.M.; et al. Amazonian Melastomataceae Blueberries: Determination of Phenolic Content, Nutritional Composition, and Antioxidant and Anti-Glycation Activities. Food Res. Int. 2022, 158, 111519. [Google Scholar] [CrossRef]
- Mar, J.M.; Silva, L.S.; Rabelo, M.d.S.; Muniz, M.P.; Nunomura, S.M.; Correa, R.F.; Kinupp, V.F.; Campelo, P.H.; Bezerra, J.d.A.; Sanches, E.A. Encapsulation of Amazonian Blueberry Juices: Evaluation of Bioactive Compounds and Stability. LWT 2020, 124, 109152. [Google Scholar] [CrossRef]
- Santos, C.S.; Dalmolin, Â.C.; dos Santos, M.S.; dos Santos, R.B.; Lima, T.M.; Pérez-Molina, J.P.; Mielke, M.S. Morphometry of the Fruits of Genipa americana (Rubiaceae): A Case Study from the Southern Coast of Bahia, Brazil. Rodriguésia 2021, 72, e00652020. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Arreola-Enríquez, J.; Leyva-Trinidad, D. Evaluation of the Genipa americana L./Heliconia stricta Huber agroforestry system and its effects on soil fertility. Agro Product. 2023, 16, 159–166. [Google Scholar] [CrossRef]
- Colmenares, L.B.H.; Nejati, M.; Fang, Y.; Guo, B.; Jiménez-Quero, A.; Capezza, A.J.; Sabino, M.A. New Sources of Genipin-Rich Substances for Crosslinking Future Manufactured Bio-Based Materials. RSC Sustain. 2024, 2, 125–138. [Google Scholar] [CrossRef]
- Codignoto, P.S.C.; de AraÃojo, S.B.; Bastos, N.M.; de Oliveira Fernandes, T.; Barbosa, T.A.S.; Igidio, C.E.D.; Faustino, F.; Fernandes, M.J.B.; da Conceição, A.O. In Vitro Cytotoxicity and Biological Activities of Genipa Americana (Rubiaceae) Ethanolic Extracts. AJMR 2017, 11, 385–390. [Google Scholar] [CrossRef]
- Ahmed, R.; ul ain Hira, N.; Wang, M.; Iqbal, S.; Yi, J.; Hemar, Y. Genipin, a Natural Blue Colorant Precursor: Source, Extraction, Properties, and Applications. Food Chem. 2024, 434, 137498. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Meireles, M.A.A. Extraction of Natural Blue Colorant from Genipa americana L. Using Green Technologies: Techno-Economic Evaluation. Food Bioprod. Process. 2019, 114, 132–143. [Google Scholar] [CrossRef]
- Inthamat, P.; Boonsiriwit, A.; Lee, Y.S.; Siripatrawan, U. Effects of Genipin as Natural Crosslinker on Barrier and Mechanical Properties of Chitosan-Astaxanthin Film. J. Food Process. Preserv. 2022, 46, e15707. [Google Scholar] [CrossRef]
- Ishimoto, C.K.; Paulino, B.N.; Neri-Numa, I.A.; Bicas, J.L. The Blue Palette of Life: A Comprehensive Review of Natural Bluish Colorants with Potential Commercial Applications. Food Res. Int. 2024, 196, 115082. [Google Scholar] [CrossRef]
- Brauch, J.E.; Zapata-Porras, S.P.; Buchweitz, M.; Aschoff, J.K.; Carle, R. Jagua Blue Derived from Genipa americana L. Fruit: A Natural Alternative to Commonly Used Blue Food Colorants? Food Res. Int. 2016, 89, 391–398. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Underutilized Plants of the Cactaceae Family: Nutritional Aspects and Technological Applications. Food Chem. 2021, 362, 130196. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Nogueira, L.A.; Silva, M.R.; do Carmo Mazzinghy, A.C.; Mariano, A.P.X.; de Albuquerque Rodrigues, T.N.; de Paula, A.C.C.F.F.; de Melo, A.C.; Augusti, R.; de Araújo, R.L.B.; et al. Chemical Approach to the Optimization of Conditions Using HS-SPME/GC–MS for Characterization of Volatile Compounds in Eugenia Brasiliensis Fruit. Molecules 2022, 27, 4955. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Finimundy, T.C.; Pinela, J.; Pires, T.C.S.P.; Mandim, F.; Vaz, J.; Corrêa, R.C.G.; Oliveira, M.B.P.P.; Barros, L. Brazilian Berry Waste as a Source of Bioactive Compounds: Grumixama (Eugenia brasiliensis Lam.) as a Case Study. Food Funct. 2023, 14, 3994–4005. [Google Scholar] [CrossRef]
- Modesto Junior, E.N.; Martins, M.G.; Pereira, G.A.; Chisté, R.C.; Pena, R.d.S. Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments. Foods 2023, 12, 565. [Google Scholar] [CrossRef]

| First Part of the Search | Second Part of the Search | |
|---|---|---|
| (Composition OR Dye OR Colorants OR Pigment) | AND | Xanthosoma riedelianum Schott |
| Alpinia officinarum Hance | ||
| Dioscorea trifida L.f. | ||
| Tropaeolum majus L. | ||
| Bougainvillea glabra Choisy | ||
| Clitoria ternatea L. | ||
| Cosmos bipinnatus Cav. | ||
| Impatiens balsamina L. | ||
| Sonchus oleraceus L. | ||
| Basella rubra L. | ||
| Hibiscus sabdariffa L. | ||
| Celosia argentea L. Rubus rosifolius Sm. | ||
| Syzygium jambos (L.) Alston | ||
| Solanum betaceum Cav. | ||
| Clidemia hirta (L.) D. Don | ||
| Genipa americana L. | ||
| Eugenia brasiliensis Lam. |
| Vegetable | Database | ||
|---|---|---|---|
| ScienceDirect | SpringerLink | Web of Science | |
| Xanthosoma riedelianum Schott | 0 | 0 | 0 |
| Alpinia officinarum Hance | 129 | 0 | 18 |
| Dioscorea trifida L.f. | 55 | 0 | 6 |
| Tropaeolum majus L. | 208 | 123 | 26 |
| Bougainvillea glabra Choisy | 183 | 79 | 37 |
| Clitoria ternatea L. | 501 | 224 | 98 |
| Cosmos bipinnatus Cav. | 65 | 50 | 12 |
| Impatiens balsamina L. | 155 | 122 | 18 |
| Sonchus oleraceus L. | 326 | 250 | 42 |
| Basella rubra L. | 154 | 154 | 23 |
| Hibiscus sabdariffa L. | 11 | 11 | 220 |
| Celosia argentea L. Rubus rosifolius Sm. | 88 | 88 | 20 |
| 228 | 657 | 0 | |
| Syzygium jambos (L.) Alston | 246 | 140 | 41 |
| Solanum betaceum Cav. | 72 | 34 | 31 |
| Clidemia hirta (L.) D. Don | 63 | 47 | 15 |
| Genipa americana L. | 249 | 100 | 46 |
| Eugenia brasiliensis Lam. | 208 | 126 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo Miranda, B.; Vilela Junior, O.; Santos Fernandes, S.; Mendes Lemos, G.R.; Schwan, C.L.; Aliaño-González, M.J.; Fernández Barbero, G.; Murowaniecki Otero, D. Potential of New Plant Sources as Raw Materials for Obtaining Natural Pigments/Dyes. Agronomy 2025, 15, 405. https://doi.org/10.3390/agronomy15020405
Melo Miranda B, Vilela Junior O, Santos Fernandes S, Mendes Lemos GR, Schwan CL, Aliaño-González MJ, Fernández Barbero G, Murowaniecki Otero D. Potential of New Plant Sources as Raw Materials for Obtaining Natural Pigments/Dyes. Agronomy. 2025; 15(2):405. https://doi.org/10.3390/agronomy15020405
Chicago/Turabian StyleMelo Miranda, Bruna, Orlando Vilela Junior, Sibele Santos Fernandes, Gabriela R. Mendes Lemos, Carla Luisa Schwan, María José Aliaño-González, Gerardo Fernández Barbero, and Deborah Murowaniecki Otero. 2025. "Potential of New Plant Sources as Raw Materials for Obtaining Natural Pigments/Dyes" Agronomy 15, no. 2: 405. https://doi.org/10.3390/agronomy15020405
APA StyleMelo Miranda, B., Vilela Junior, O., Santos Fernandes, S., Mendes Lemos, G. R., Schwan, C. L., Aliaño-González, M. J., Fernández Barbero, G., & Murowaniecki Otero, D. (2025). Potential of New Plant Sources as Raw Materials for Obtaining Natural Pigments/Dyes. Agronomy, 15(2), 405. https://doi.org/10.3390/agronomy15020405










