The Effects of Dried Apple Pomace on Fermentation Quality and Proteolysis of Alfalfa Silages
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Chemical and Microbial Composition Analysis
2.3. Nitrogen Distribution Analysis
2.4. Protease Activity Analysis
2.5. Biogenic Amine Content Analysis
2.6. Amino Acid Content Analysis
2.7. Statistical Analyses
3. Results
3.1. Enzymatic Activity, Nitrogen Distribution and Chemical and Microbial Compositions of Fresh Alfalfa and Dried Apple Pomace
3.2. Dry Matter and Fermentation Quality of Alfalfa Silages During Ensiling
3.3. Nitrogen Distribution of Alfalfa Silages During Ensiling
3.4. Protease Activities of Alfalfa Silages During Ensiling
3.5. The Biogenic Amine Content of Alfalfa Silage on Day 60 of Ensiling
3.6. The Amino Acid Content of Alfalfa Silages on Day 60 of Ensiling
3.7. Chemical Composition of Alfalfa Silages on Day 60 of Ensiling
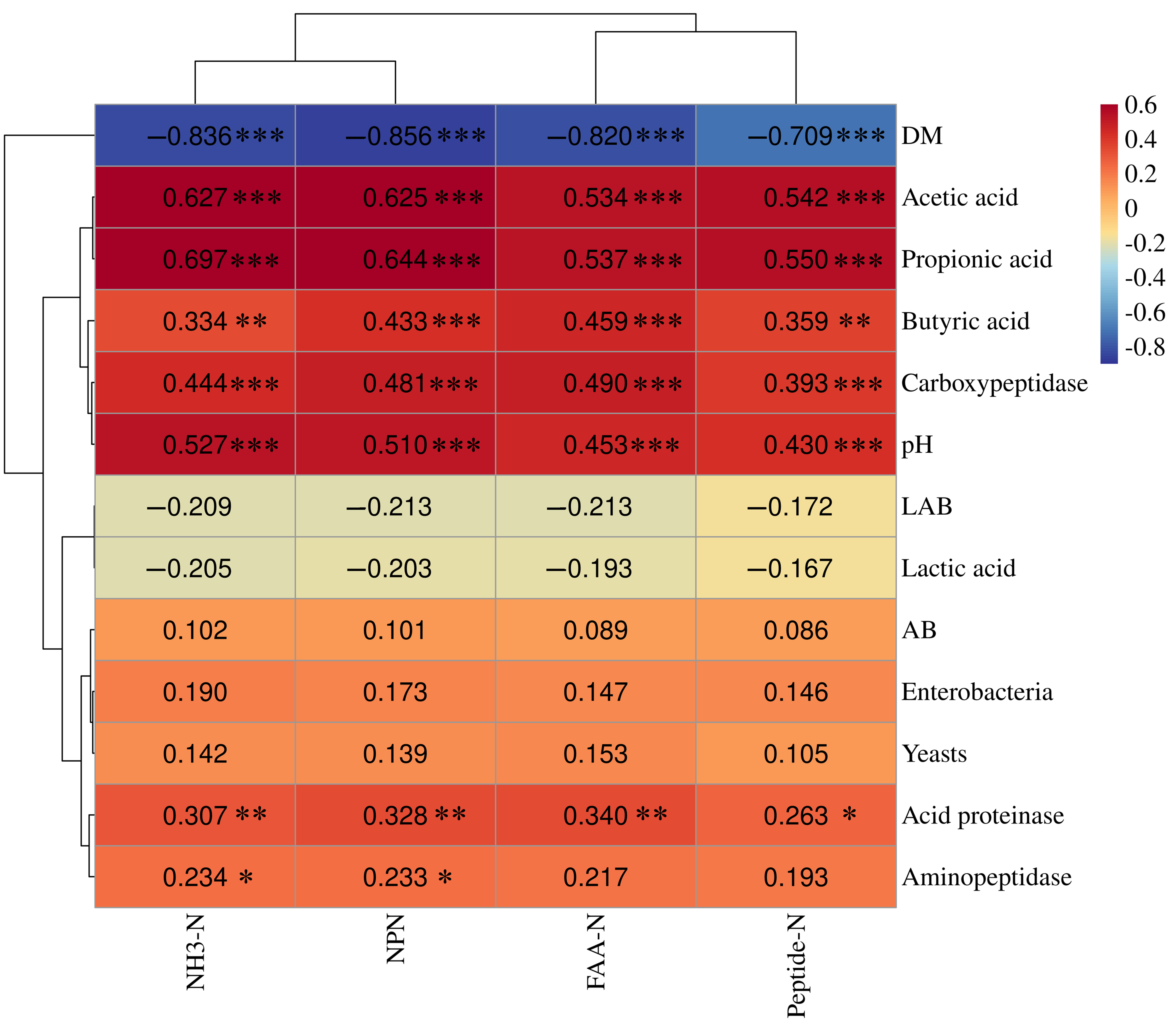
3.8. Correlation Relationship Between Fermentation Characteristics and Nitrogen Distribution
4. Discussion
4.1. Analysis of Raw Materials
4.2. Analysis of Fermentation Characteristics During Ensiling
4.3. Analysis of Nitrogen Distribution During Ensiling
4.4. Protease Activity During Ensiling
4.5. Analysis of Biogenic Amines on Day 60 of Ensiling
4.6. Analysis of Amino Acids on Day 60 of Ensiling
4.7. Analysis of Chemical Composition on Day 60 of Ensiling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Palmonari, A.; Federiconi, A.; Formigoni, A. Animal board invited review: The effect of diet on rumen microbial composition in dairy cows. Animal 2024, 18, 101319. [Google Scholar] [CrossRef]
- Phuntsok, T.; Froetschel, M.A.; Amos, H.E.; Zheng, M.; Huang, Y.W. Biogenic amines in silage, apparent postruminal passage, and the relationship between biogenic amines and digestive function and intake by steers. J. Dairy Sci. 1998, 81, 2193–2203. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef]
- Du, Y.; Huang, X.; Yuan, S.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W. Cold plasma and honey synergistically inhibit polyphenol oxidase to enhance fresh-cut apple preservation. Food Chem. 2025, 468, 142490. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of residual pesticides in fruit and vegetable waste (FVW) processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef]
- Korzeniowski, Ł.; Plata, M.; Świątek, K.; Olszewski, M.P.; Lewandowski, M.; Arauzo, P.J.; Maziarka, P.; Wądrzyk, M. Sweet-sour fate of saccharides during sequential processing from apple pomace through acidic extraction and hydrolysis. Food Bioprod. Process. 2025, 149, 337–352. [Google Scholar] [CrossRef]
- Pascoalino, L.A.; Barros, L.; Barreira, J.C.; Oliveira, M.B.P.; Reis, F.S. Closing the loop: Exploring apple pomace as a source of bioactive compounds in the framework of circular economy. Sustain. Food Technol. 2025, 3, 81–95. [Google Scholar] [CrossRef]
- National Research Council, Committee on Animal Nutrition and Subcommittee on Dairy Cattle Nutrition. Nutrient Requirements of Dairy Cattle: 2001; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Arthur Thomas, T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agr. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Michalczyk, M.; Fiutak, G.; Tarko, T. Effect of hot water treatment of seeds on quality indicators of alfalfa sprouts. LWT 2019, 113, 108270. [Google Scholar] [CrossRef]
- Farouk, B.; Aref, N.; Rachid, C.; Mourad, L.; Emna, K.; Fethi, B.; Rania, B.; Wafa, N.; Kenza, B.; Boumediene, M.; et al. Characterization of three polyphenol oxidase isoforms in royal dates and inhibition of its enzymatic browning reaction by indole-3-acetic acid. Int. J. Biol. Macromol. 2020, 145, 894–903. [Google Scholar] [CrossRef]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agr. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy. Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed. Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Muck, R.E. Dry matter level effects on alfalfa silage quality I. Nitrogen transformations. Trans. ASAE 1987, 30, 7–14. [Google Scholar] [CrossRef]
- McKersie, B.D. Proteinases and peptidases of alfalfa herbage. Can. J. Plant Sci. 1981, 61, 53–59. [Google Scholar] [CrossRef]
- Jia, T.; Yun, Y.; Yu, Z. Propionic acid and sodium benzoate affected biogenic amine formation, microbial community, and quality of oat silage. Front. Microbiol. 2021, 12, 750920. [Google Scholar] [CrossRef]
- Semjon, B.; Bartkovský, M.; Očenáš, P.; Regecová, I.; Megyesy Eftimová, Z.; Výrostková, J.; Mesarčová, L.; Kováčová, M.; Várady, M.; Šuľáková, L.; et al. The Impact of Grape Maceration on Quality and Biogenic Amine Formation in Slovak Tokaj Wines: Examination of Microbial, Chemical and Sensory Properties. Fermentation 2025, 11, 27. [Google Scholar] [CrossRef]
- Zang, J.; Li, T.; Liu, K.; Wu, J.; Zhang, Z.; Liu, X.; Zhao, J.; Peng, C.; Li, Z. Correlations between microbiota succession and volatile profiles development and biogenic amine formation involved in the ripening of Chinese sour meat. LWT Food Sci. Technol. 2025, 215, 117238. [Google Scholar] [CrossRef]
- Mlejnkova, V.; Horky, P.; Kominkova, M.; Skladanka, J.; Hodulikova, L.; Adam, V.; Mlcek, J.; Jurikova, T.; Sochor, J. Biogenic amines and hygienic quality of lucerne silage. Open Life Sci. 2016, 11, 280–286. [Google Scholar] [CrossRef]
- Guo, X.S.; Ding, W.R.; Han, J.G.; Zhou, H. Characterization of protein fractions and amino acids in ensiled alfalfa treated with different chemical additives. Anim. Feed. Sci. Tech. 2008, 142, 89–98. [Google Scholar] [CrossRef]
- Du, E.; Mao, N.; Liu, S.; Zhang, H.; Fan, M.; Sun, H.; Zheng, Y.; Cheng, Q.; Wang, C.; Li, P.; et al. Effects of different wet distillers’ grains ratios on fermentation quality, nitrogen fractions and bacterial communities of total mixed ration silage. BMC Microbiol. 2025, 25, 31. [Google Scholar] [CrossRef]
- Grøseth, M.; Karlsson, L.; Steinshamn, H.; Johansen, M.; Kidane, A.; Prestløkken, E. Effects of dry matter concentration in grass silage on milk production of dairy cows fed concentrates high or low in metabolizable protein concentration. Livest. Sci. 2025, 291, 105611. [Google Scholar] [CrossRef]
- Jung, J.S.; Wong, J.W.; Soundharrajan, I.; Lee, K.W.; Park, H.S.; Kim, D.; Choi, K.C.; Chang, S.W.; Ravindran, B. Changes in microbial dynamics and fermentation characteristics of alfalfa silage: A potent approach to mitigate greenhouse gas emission through high-quality forage silage. Chemosphere 2024, 362, 142920. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Dai, T.; Dong, D.; Wang, J.; Yin, X.; Zong, C.; Jia, Y.; Shao, T. Effects of wet brewers grains on fermentation quality and in vitro ruminal digestibility of mixed silage prepared with corn stalk, sweet potato peel and dried apple pomace in southeast China. J. Anim. Physiol. Anim. Nutr. 2023, 107, 340–349. [Google Scholar] [CrossRef]
- Khejornsart, P.; Juntanam, T.; Meenongyai, W.; Wanapat, M. Effects of cassava pulp fermentation with traditional starter media on rumen fermentation, nutrients digestibility in beef cattle. Ital. J. Anim. Sci. 2025, 24, 182–192. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Yu, F.; Huang, Y.; Xie, T.; Bian, H.; Lv, L.; Hu, Y.; Tao, R.; Fan, C.; et al. Effects of Different Molasses Levels and Slow-Release Urea Combinations on Growth Performance, Serum Biochemistry, Rumen Fermentation, and Microflora of Holstein Fattening Bulls. Agriculture 2025, 15, 183. [Google Scholar] [CrossRef]
- Fijałkowska, M.; Pysera, B.; Lipiński, K.; Strusińska, D. Changes of nitrogen compounds during ensiling of high protein herbages-a review. Ann. Anim. Sci. 2015, 15, 289–305. [Google Scholar] [CrossRef]
- Tao, L.; Zhou, H.; Guo, X.S.; Long, R.J.; Zhu, Y.; Cheng, W. Contribution of exopeptidases to formation of nonprotein nitrogen during ensiling of alfalfa. J. Dairy Sci. 2011, 94, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wen, A.; Desta, S.T.; Dong, Z.; Shao, T. Effects of four short-chain fatty acids or salts on the dynamics of nitrogen transformations and intrinsic protease activity of alfalfa silage. J. Sci. Food Agr. 2017, 97, 2759–2766. [Google Scholar] [CrossRef]
- Mao, K.; Franco, M.; Xu, Y.; Chai, H.; Wang, J.; Huang, S.; Wang, Z.; Xun, W.; Liang, Z.; Yu, Z.; et al. Fermentation Parameters, Amino Acids Profile, Biogenic Amines Formation, and Bacterial Community of Ensiled Stylo Treated with Formic Acid or Sugar. Animals 2024, 14, 2397. [Google Scholar] [CrossRef] [PubMed]
- Nishino, N.; Hattori, H.; Wada, H.; Touno, E. Biogenic amine production in grass, maize and total mixed ration silages inoculated with Lactobacillus casei or Lactobacillus buchneri. J. Appl. Microbiol. 2007, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Pobednov, Y.A.; Kosolapov, V.M. Biology of alfalfa silage making. Sel’skokhozyaistvennaya Biol. 2018, 53, 258–269. [Google Scholar] [CrossRef]
- Kruczek, M.; Gumul, D.; IvaniÅ, E.; GambuÅ, H. Industrial apple pomace by-products as a potential source of pro-health compounds in functional food. J. Microb. Biotech. Food Sci. 2017, 7, 22–26. [Google Scholar] [CrossRef]
- Malenica, D.; Maciel, L.S.; Herodes, K.; Kass, M.; Bhat, R. Optimization of Ultrasonic-Assisted Extraction of Antioxidants in Apple Pomace (var. Belorusskoje malinovoje) Using Response Surface Methodology: Scope and Opportunity to Develop as a Potential Feed Supplement or Feed Ingredient. Sustainability 2024, 16, 2765. [Google Scholar] [CrossRef]
- Zheng, B.; Jiang, J.; Wang, L.; Huang, M.; Zhou, Q.; Cai, J.; Wang, X.; Dai, T.; Jiang, D. Reducing nitrogen rate and increasing plant density accomplished high yields with satisfied grain quality of soft wheat via modifying the free amino acid supply and storage protein gene expression. J. Agric. Food Chem. 2022, 70, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
| Items | Alfalfa | Dried Apple Pomace | p Value |
|---|---|---|---|
| DM (g/kg FW) | 307.18 | 951.76 | <0.001 |
| pH | 6.05 | 3.76 | <0.001 |
| BC (mEq/kg DM) | 358.69 | 72.29 | <0.001 |
| NDF (g/kg DM) | 487.15 | 458.11 | NS |
| ADF (g/kg DM) | 314.12 | 324.69 | NS |
| HC (g/kg DM) | 173.03 | 133.42 | 0.013 |
| WSCs (g/kg DM) | 32.69 | 157.06 | <0.001 |
| CP (g/kg DM) | 193.13 | 53.44 | <0.001 |
| TPC (mg GAE/g DM) | 3.75 | 6.13 | 0.038 |
| LAB (log10 cfu/g FW) | 5.86 | ND | - |
| AB (log10 cfu/g FW) | 7.29 | 1.20 | 0.007 |
| Yeast (log10 cfu/g FW) | 6.82 | 0.87 | 0.007 |
| Enterobacteria (log10 cfu/g FW) | 7.14 | ND | - |
| TN (g/kg DM) | 30.90 | 8.55 | <0.001 |
| NPN (g/kg TN) | 34.29 | 36.85 | NS |
| Peptide-N (g/kg TN) | 8.48 | 21.84 | NS |
| FAA-N (g/kg TN) | 22.99 | 13.71 | 0.004 |
| NH3-N (g/kg TN) | 2.82 | 1.31 | 0.036 |
| Carboxypeptidase activity (μmol/g of free amino acids released/h·g DM) | 40.52 | 2.19 | <0.001 |
| Aminopeptidase activity (units/h·g DM) | 24.75 | 0.65 | <0.001 |
| Acid proteinase activity (units/h·g DM) | 21.25 | 0.68 | <0.001 |
| PPO (U/mL·min) | ND | 400.00 | - |
| Items | Alfalfa |
|---|---|
| Tryptamine (mg/kg DM) | 4.18 |
| Phenylethylamine (mg/kg DM) | 30.28 |
| Putrescine (mg/kg DM) | 3.98 |
| Cadaverine (mg/kg DM) | 15.08 |
| Histamine (mg/kg DM) | 37.74 |
| Tyramine (mg/kg DM) | 11.84 |
| Spermidine (mg/kg DM) | 20.95 |
| Spermine (mg/kg DM) | 10.63 |
| Total biogenic amines (mg/kg DM) | 134.66 |
| Threonine (g/kg DM) | 9.20 |
| Valine (g/kg DM) | 10.15 |
| Methionine (g/kg DM) | 0.51 |
| Isoleucine (g/kg DM) | 8.15 |
| Leucine (g/kg DM) | 15.50 |
| Phenylalanine (g/kg DM) | 10.71 |
| Lysine (g/kg DM) | 12.55 |
| Histidine (g/kg DM) | 4.73 |
| Arginine (g/kg DM) | 9.19 |
| Aspartic acid (g/kg DM) | 24.29 |
| Serine (g/kg DM) | 9.00 |
| Glutamic (g/kg DM) | 19.37 |
| Glycine (g/kg DM) | 9.60 |
| Alanine (g/kg DM) | 11.38 |
| Cysteine (g/kg DM) | 1.29 |
| Tyrosine (g/kg DM) | 6.06 |
| Proline (g/kg DM) | 8.60 |
| Total amino acids (g/kg DM) | 170.27 |
| Items | Ensiling Days | Treatments | SEM | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5% DAP | 10% DAP | 15% DAP | T | D | T × D | A-L | A-Q | |||
| DM (g/kg FW) | 1 | 299.21 c | 349.65 Ab | 378.43 Aab | 415.35 Aa | 4.632 | <0.001 | <0.001 | NS | <0.001 | 0.013 |
| 3 | 296.06 d | 341.16 ABc | 369.13 ABb | 403.77 ABa | |||||||
| 7 | 295.33 d | 340.18 ABc | 367.65 ABb | 395.01 ABa | |||||||
| 14 | 294.79 d | 336.87 ABc | 366.55 ABb | 391.53 ABa | |||||||
| 30 | 291.02 d | 328.75 Bc | 362.91 ABb | 389.55 ABa | |||||||
| 60 | 289.83 d | 327.93 Bc | 353.40 Bb | 379.82 Ba | |||||||
| pH | 1 | 6.11 Aa | 5.66 Ab | 5.57 Ab | 5.20 Ac | 0.066 | <0.001 | <0.001 | 0.034 | <0.001 | NS |
| 3 | 5.75 Ba | 5.51 Ab | 5.35 ABbc | 5.06 ABc | |||||||
| 7 | 5.64 BCa | 5.32 ABb | 5.16 Bb | 4.96 ABb | |||||||
| 14 | 5.48 Ca | 4.95 BCb | 4.79 Cb | 4.56 BCb | |||||||
| 30 | 5.21 Da | 4.78 Cb | 4.49 CDc | 4.31 Cc | |||||||
| 60 | 5.18 Da | 4.20 Db | 4.14 Db | 4.07 Cb | |||||||
| LA (g/kg DM) | 1 | 1.40 Eb | 1.46 Db | 1.80 Fab | 3.67 Da | 4.626 | <0.001 | <0.001 | 0.001 | 0.045 | NS |
| 3 | 24.67 Dc | 29.33 Cb | 31.08 Eab | 33.05 Ca | |||||||
| 7 | 45.52 Cb | 53.71 Ba | 54.49 Da | 58.12 BCa | |||||||
| 14 | 56.37 BCb | 63.87 Ba | 71.00 Ca | 95.20 ABa | |||||||
| 30 | 67.10 Bb | 90.01 Aa | 100.72 Ba | 116.86 Aa | |||||||
| 60 | 88.65 Ab | 100.44 Aa | 112.64 Aa | 133.31 Aa | |||||||
| AA (g/kg DM) | 1 | 4.53 Ca | 2.72 Eb | 1.55 Ec | 0.72 Cd | 0.674 | <0.001 | <0.001 | <0.001 | <0.001 | NS |
| 3 | 12.49 Ba | 6.36 Db | 4.95 Db | 2.68 BCc | |||||||
| 7 | 15.61 Ba | 8.94 Cb | 7.81 Cb | 5.04 ABc | |||||||
| 14 | 15.78 Ba | 10.84 Bb | 8.28 BCb | 6.53 Ab | |||||||
| 30 | 19.61 Aa | 10.92 Bb | 9.76 Bb | 6.48 Ac | |||||||
| 60 | 23.38 Aa | 15.18 Ab | 12.07 Ac | 6.70 Ad | |||||||
| PA (g/kg DM) | 1 | ND | ND | ND | ND | 0.371 | <0.001 | <0.001 | <0.001 | <0.001 | 0.019 |
| 3 | 4.28 | 1.32 | 1.61 | ND | |||||||
| 7 | 7.81 | 1.89 | 1.91 | ND | |||||||
| 14 | 8.19 | 2.03 | 2.08 | ND | |||||||
| 30 | 8.24 a | 2.43 b | 2.23 b | 0.83 c | |||||||
| 60 | 9.60 a | 4.48 b | 3.73 bc | 0.84 c | |||||||
| BA (g/kg DM) | 1 | 0.19 B | ND | ND | ND | 0.093 | <0.001 | <0.001 | NS | <0.001 | NS |
| 3 | 1.36 AB | 0.80 | 0.08 | 0.05 | |||||||
| 7 | 1.79 Aa | 1.59 ab | 1.21 b | 0.61 c | |||||||
| 14 | 1.80 Aa | 1.62 ab | 1.20 b | 0.59 c | |||||||
| 30 | 1.84 Aa | 1.72 ab | 1.58 ab | 0.90 b | |||||||
| 60 | 2.02 Aa | 1.83 a | 1.75 a | 1.03 b | |||||||
| Items | Ensiling Days | Treatments | SEM | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5% DAP | 10% DAP | 15% DAP | T | D | T × D | A-L | A-Q | |||
| NPN | 1 | 245.28 Ea | 214.07 Db | 209.13 Db | 192.58 Cb | 12.412 | <0.001 | <0.001 | <0.001 | <0.001 | NS |
| 3 | 379.46 Da | 346.80 Cb | 321.47 Cbc | 275.25 Bc | |||||||
| 7 | 442.42 Ca | 400.12 BCb | 378.26 BCbc | 334.36 Ac | |||||||
| 14 | 506.96 Ba | 424.45 Bb | 396.54 ABb | 338.96 Ac | |||||||
| 30 | 564.74 ABa | 443.70 ABb | 407.75 ABbc | 354.58 Ac | |||||||
| 60 | 593.61 Aa | 499.08 Ab | 442.99 Abc | 376.25 Ac | |||||||
| Peptide-N | 1 | 190.06 Da | 168.11 Cb | 166.89 Cb | 161.10 Cb | 5.991 | <0.001 | <0.001 | NS | <0.001 | NS |
| 3 | 266.24 C | 261.35 B | 248.32 B | 220.36 B | |||||||
| 7 | 292.46 BC | 287.07 A | 278.76 A | 254.89 A | |||||||
| 14 | 322.13 ABa | 298.08 Aab | 280.56 Ab | 243.59 ABb | |||||||
| 30 | 348.52 Aa | 296.15 Aab | 275.02 Ab | 249.55 ABb | |||||||
| 60 | 275.24 BCa | 255.63 Bab | 236.98 Bb | 211.38 Bb | |||||||
| FAA-N | 1 | 42.42 Ea | 40.04 Ea | 36.93 Ea | 27.48 Eb | 5.126 | <0.001 | <0.001 | <0.001 | 0.025 | NS |
| 3 | 60.59 DEa | 58.80 DEab | 54.92 Dab | 44.24 Db | |||||||
| 7 | 75.73 CDa | 69.93 CDab | 64.22 CDab | 57.92 Cb | |||||||
| 14 | 97.47 BCa | 80.21 BCb | 77.13 BCb | 63.49 BCc | |||||||
| 30 | 111.47 Ba | 91.48 Bb | 87.94 Bb | 71.22 Bc | |||||||
| 60 | 200.91 Aa | 171.36 Ab | 155.68 Abc | 129.48 Ac | |||||||
| NH3-N | 1 | 12.68 Ea | 5.94 Eb | 5.70 Eb | 4.00 Db | 3.559 | <0.001 | <0.001 | <0.001 | <0.001 | 0.034 |
| 3 | 52.62 Da | 26.65 Db | 18.23 Dc | 10.65 Cd | |||||||
| 7 | 74.22 Ca | 43.12 Cb | 35.28 Cb | 21.54 Bc | |||||||
| 14 | 87.35 Ba | 46.16 BCb | 38.85 BCbc | 31.88 Ac | |||||||
| 30 | 104.75 Aa | 56.07 Bb | 44.79 ABc | 33.82 Ac | |||||||
| 60 | 117.46 Aa | 72.09 Ab | 50.33 Ac | 35.39 Ad | |||||||
| Items | Ensiling Days | Treatments | SEM | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5% DAP | 10% DAP | 15% DAP | T | D | T × D | A-L | A-Q | |||
| Carboxypeptidase activity (μmol/g of free amino acids released/h·g DM) | 1 | 33.05 Aa | 32.40 Aa | 20.75 Ab | 17.15 Ab | 1.254 | <0.001 | <0.001 | <0.001 | <0.001 | NS |
| 3 | 32.90 Aa | 30.33 Ab | 19.57 Ab | 14.74 Bb | |||||||
| 7 | 31.63 ABa | 29.59 Aa | 12.99 Bb | 11.02 Cb | |||||||
| 14 | 24.30 Ba | 19.89 Bb | 10.12 Bc | 8.68 Dc | |||||||
| 30 | 13.61 Ca | 8.67 Cb | 3.96 Cc | 1.84 Ec | |||||||
| 60 | 10.48 Ca | 6.70 Cb | 2.97 Cbc | 1.15 Ec | |||||||
| Aminopeptidase activity (Units/h·g DM) | 1 | 22.82 Aa | 19.89 Ab | 18.18 Ac | 16.42 Ad | 0.748 | <0.001 | <0.001 | <0.001 | 0.034 | NS |
| 3 | 16.38 Ba | 14.09 Bb | 12.77 Bbc | 11.53 Bc | |||||||
| 7 | 12.68 Ca | 11.09 Cb | 10.78 Bb | 10.09 Bb | |||||||
| 14 | 11.22 Ca | 4.86 Db | 4.55 Cb | 2.53 Cb | |||||||
| 30 | 4.93 Da | 3.88 Db | 2.77 Cbc | 2.39 Cc | |||||||
| 60 | 3.67 Da | 2.95 Db | 2.26 Cbc | 1.94 Cc | |||||||
| Acid proteinase activity (Units/h·g DM) | 1 | 17.73 Aa | 16.10 Aab | 13.72 Abc | 11.71 Ac | 0.580 | <0.001 | <0.001 | <0.001 | 0.001 | NS |
| 3 | 16.06 Aa | 15.44 Aa | 12.53 Ab | 11.24 Ab | |||||||
| 7 | 15.33 Aa | 14.82 Aa | 8.27 Bb | 6.85 Bc | |||||||
| 14 | 8.04 Ba | 7.44 Bab | 6.16 BCb | 5.54 Bb | |||||||
| 30 | 6.98 Ba | 5.39 Cb | 4.05 CDbc | 2.86 Cc | |||||||
| 60 | 5.95 Ba | 4.25 Cb | 2.95 Dbc | 2.24 Cc | |||||||
| Items | Treatments | SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 5% DAP | 10% DAP | 15% DAP | T | A-L | A-Q | ||
| Tryptamine | 124.25 a | 34.58 b | 28.73 b | 5.56 b | 14.191 | 0.001 | <0.001 | 0.007 |
| Phenylethylamine | 917.42 a | 384.40 b | 276.41 b | 117.08 b | 94.599 | <0.001 | <0.001 | 0.019 |
| Putrescine | 822.94 a | 391.42 b | 264.78 bc | 109.18 c | 81.636 | <0.001 | <0.001 | 0.007 |
| Cadaverine | 1601.88 a | 736.05 b | 490.45 bc | 247.75 c | 156.886 | <0.001 | <0.001 | 0.002 |
| Histamine | 366.82 a | 120.75 b | 36.68 b | 24.61 b | 46.681 | 0.004 | 0.001 | 0.048 |
| Tyramine | 1128.91 a | 882.07 ab | 743.08 b | 653.32 b | 60.022 | 0.003 | <0.001 | NS |
| Spermidine | 25.88 a | 20.96 b | 13.56 c | 10.00 d | 1.895 | <0.001 | <0.001 | NS |
| Spermine | 22.66 a | 18.27 b | 15.05 c | 10.34 d | 1.373 | <0.001 | <0.001 | NS |
| Total biogenic amines | 5010.76 a | 2588.51 b | 1868.74 c | 1177.85 c | 440.901 | <0.001 | <0.001 | 0.001 |
| Items | Treatments | SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 5% DAP | 10% DAP | 15% DAP | T | A-L | A-Q | ||
| Threonine | 2.65 b | 5.20 a | 5.57 a | 5.85 a | 0.422 | 0.002 | 0.001 | 0.026 |
| Valine | 8.22 c | 8.63 bc | 9.99 ab | 11.47 a | 0.418 | 0.001 | <0.001 | NS |
| Methionine | 0.61 | 0.69 | 0.34 | 0.57 | 0.083 | NS | NS | NS |
| Isoleucine | 6.70 c | 7.04 c | 8.40 b | 9.27 a | 0.356 | 0.006 | 0.001 | NS |
| Leucine | 11.31 c | 12.03 bc | 14.18 ab | 15.51 a | 0.573 | 0.005 | 0.001 | NS |
| Phenylalanine | 5.24 b | 6.64 a | 6.75 a | 6.98 a | 0.233 | 0.005 | 0.002 | NS |
| Lysine | 5.27 b | 7.75 ab | 7.94 a | 8.03 a | 0.419 | 0.022 | 0.009 | NS |
| Histidine | 2.29 b | 3.11 a | 3.18 a | 3.62 a | 0.159 | 0.002 | <0.001 | NS |
| Arginine | 1.44 c | 2.03 bc | 2.41 ab | 2.81 a | 0.168 | 0.002 | <0.001 | NS |
| Aspartic acid | 8.88 b | 16.29 a | 16.60 a | 17.41 a | 1.098 | <0.001 | <0.001 | 0.004 |
| Serine | 2.66 c | 4.03 b | 4.81 ab | 5.23 a | 0.313 | <0.001 | <0.001 | NS |
| Glutamic | 10.31 c | 10.62 bc | 11.45 b | 12.61 a | 0.285 | <0.001 | <0.001 | NS |
| Glycine | 8.80 a | 8.19 ab | 7.05 b | 6.60 b | 0.305 | 0.008 | 0.001 | NS |
| Alanine | 12.84 a | 12.12 ab | 10.09 bc | 9.06 c | 0.508 | 0.003 | <0.001 | NS |
| Cysteine | 11.71 a | 6.30 b | 3.44 bc | 1.53 c | 1.218 | <0.001 | <0.001 | NS |
| Tyrosine | 1.54 | 2.16 | 2.37 | 2.44 | 0.140 | NS | 0.016 | NS |
| Proline | 8.64 a | 8.02 ab | 6.92 bc | 6.41 c | 0.302 | 0.006 | 0.001 | NS |
| Total amino acids | 109.12 b | 120.85 ab | 121.50 ab | 125.40 a | 2.256 | 0.028 | 0.007 | NS |
| Items | Treatments | SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 5% DAP | 10% DAP | 15% DAP | T | A-L | A-Q | ||
| NDF | 467.75 | 464.45 | 462.14 | 438.90 | 4.708 | NS | 0.030 | NS |
| ADF | 333.61 | 3331.86 | 331.79 | 329.29 | 4.596 | NS | NS | NS |
| HC | 134.14 | 132.59 | 130.35 | 109.61 | 5.018 | NS | NS | NS |
| WSCs | 12.45 c | 13.81 bc | 18.47 ab | 23.72 a | 1.428 | 0.001 | <0.001 | NS |
| CP | 163.14 c | 171.01 bc | 178.49 ab | 184.09 a | 2.609 | 0.002 | <0.001 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, T.; Long, J.; Zhang, G.; Yuan, X.; Dong, Z. The Effects of Dried Apple Pomace on Fermentation Quality and Proteolysis of Alfalfa Silages. Agronomy 2025, 15, 438. https://doi.org/10.3390/agronomy15020438
Dai T, Long J, Zhang G, Yuan X, Dong Z. The Effects of Dried Apple Pomace on Fermentation Quality and Proteolysis of Alfalfa Silages. Agronomy. 2025; 15(2):438. https://doi.org/10.3390/agronomy15020438
Chicago/Turabian StyleDai, Tongtong, Jiangyu Long, Guanjun Zhang, Xianjun Yuan, and Zhihao Dong. 2025. "The Effects of Dried Apple Pomace on Fermentation Quality and Proteolysis of Alfalfa Silages" Agronomy 15, no. 2: 438. https://doi.org/10.3390/agronomy15020438
APA StyleDai, T., Long, J., Zhang, G., Yuan, X., & Dong, Z. (2025). The Effects of Dried Apple Pomace on Fermentation Quality and Proteolysis of Alfalfa Silages. Agronomy, 15(2), 438. https://doi.org/10.3390/agronomy15020438






