Alternative Phosphorus Fertilisation with Bio-Based Pellet Fertilisers: A Case of Study on Ryegrass (Lollium perenne L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Experimental Design
2.2. OMF Production
2.3. Sampling and Analytical Methods
2.3.1. Soil Analysis
2.3.2. Plant Analysis
2.4. Statistical Analysis
3. Results
3.1. Soil Parameters
3.2. Crop Response to Fertiliser
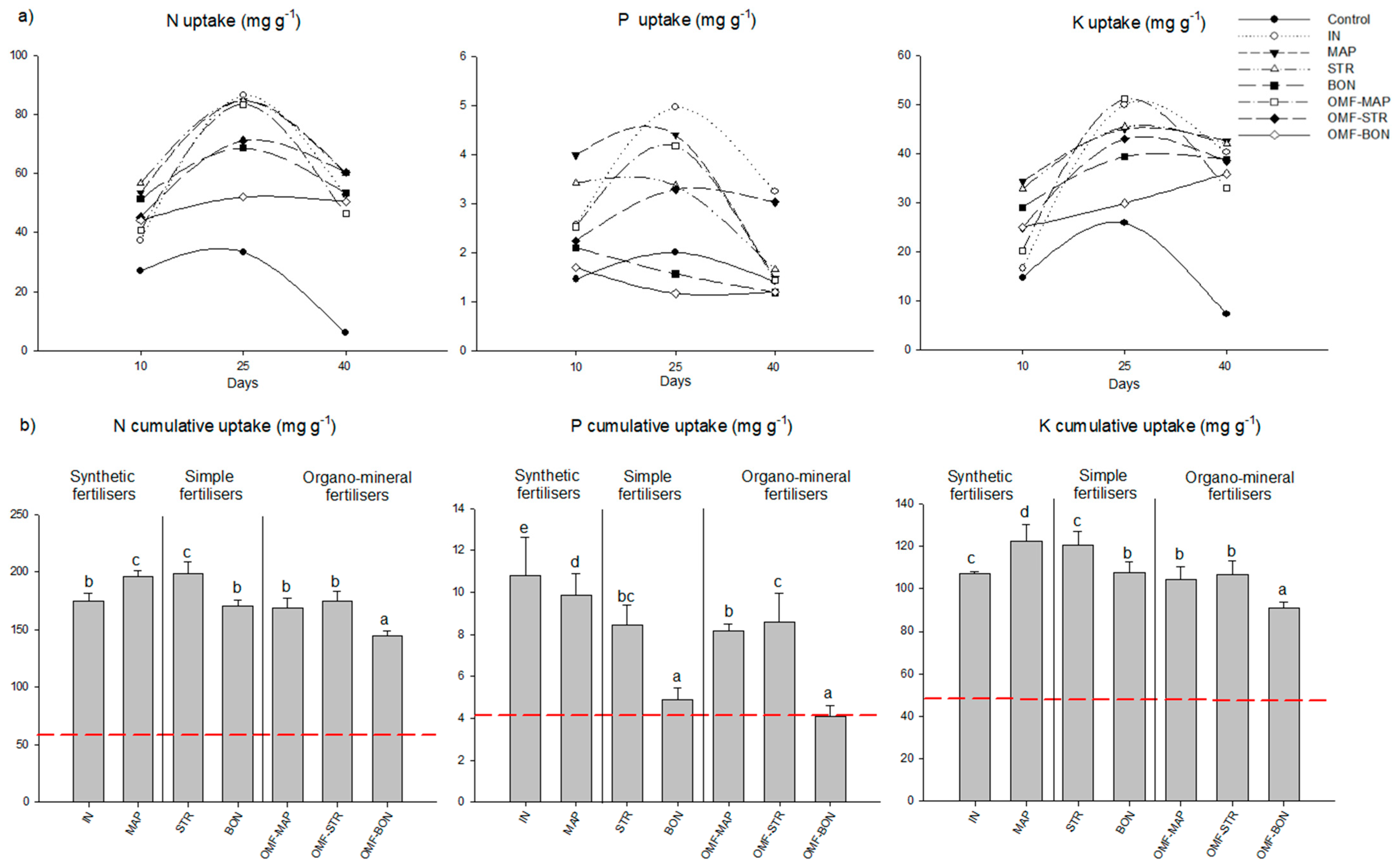
3.2.1. Nutrient Uptake
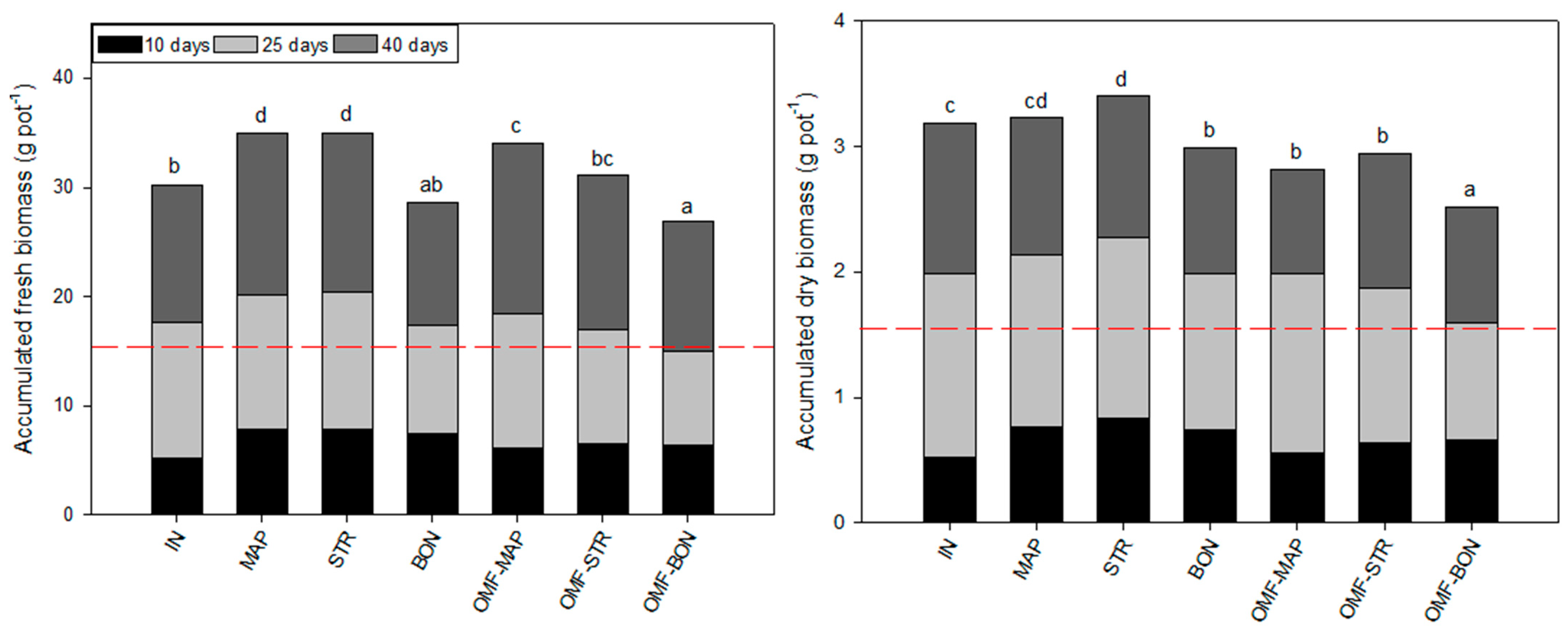
3.2.2. Yield and Nutrient Use Efficiency
4. Discussion
4.1. Effects of Fertilisation on Soil Properties
4.2. Effects of Fertilisation on Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hammond, D. Agronomy: Canola; AOCS Press: Urbana, IL, USA, 2011; pp. 93–117. ISBN 9780981893655. [Google Scholar]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A review of environment effects on nitrate accumulation in leafy vegetables grown in controlled environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Vaneeckhaute, C.; Ghekiere, G.; Michels, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Assessing Nutrient Use Efficiency and Environmental Pressure of Macronutrients in Biobased Mineral Fertilizers: A Review of Recent Advances and Best Practices at Field Scale. Adv. Agron. 2014, 128, 137–180. [Google Scholar]
- Nadarajan, S.; Sukumaran, S. Chemistry and toxicology behind chemical fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture, 2nd ed.; Lewu, F.B., Volova, T., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 195–229. [Google Scholar] [CrossRef]
- Eurostat. European Commission. Agri-Environmental Indicator Mineral Fertiliser Consumption. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?oldid=656189#Analysis_at_EU_level (accessed on 10 November 2024).
- FAO. Developments in International Fertilizer Markets. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/e379efe6-a955-4ffc-bb53-0c9a89f11f1a/content (accessed on 10 November 2024).
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Meers, E.; Michels, E.; Buysse, J.; Tack, F.M.G. Ecological and economic benefits of the application of bio-based mineral fertilizers in modern agriculture. Biomass Bioenerg. 2013, 49, 239–248. [Google Scholar] [CrossRef]
- European Union. Farm to Fork Strategy. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 10 November 2024).
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Dijkstra, A.F. Global analysis of phosphorus fertilizer use efficiency in cereal crops. Glob. Food Secur. 2021, 29, 100545. [Google Scholar] [CrossRef]
- Vico, A.; Sáez, J.A.; Pérez-Murcia, M.D.; Martinez-Tomé, J.; Andreu-Rodríguez, J.; Agulló, E.; Bustamante, M.A.; Sanz-Cobena, A.; Moral, R. Production of spinach in intensive Mediterranean horticultural systems can be sustained by organic-based fertilizers without yield penalties and with low environmental impacts. Agric. Syst. 2020, 178, 102765. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Martínez-Sabater, E.; Pérez-Murcia, M.D.; Andreu-Rodríguez, F.J.; Orden, L.; Agulló, E.; Sáez-Tovar, J.; Martínez-Tome, J.; Bustamante, M.Á.; Moral, R. Enhancing Sustainability in Intensive Dill Cropping: Comparative Effects of Biobased Fertilizers vs. Inorganic Commodities on Greenhouse Gas Emissions, Crop Yield, and Soil Properties. Agronomy 2022, 12, 2124. [Google Scholar] [CrossRef]
- Zajonc, O.; Frydrych, J.; Jezerska, L. Pelletization of Compost for Energy Utilization. IERI Procedia 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Stelte, W.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Ahrenfeldt, J.; Henriksen, U.B. A study of bonding and failure mechanisms in fuel pellets from different biomass resources. Biomass Bioenergy 2011, 35, 2–8. [Google Scholar] [CrossRef]
- Ilari, A.; Foppa-Pedretti, E.; De Francesco, C.; Duca, D. Pellet Production from Residual Biomass of Greenery Maintenance in a Small-Scale Company to Improve Sustainability. Resources 2021, 10, 122. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Meda, V.; Dalai, A.K. Densification of waste biomass for manufacturing solid biofuel pellets: A review. Environ. Chem. Lett. 2023, 21, 231–264. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yap, Y.J.; Ling, T.C.; Tao, Y.; Show, P.L. Densification of food waste compost: Effects of moisture content and dairy powder waste additives on pellet quality. Process Saf. Environ. Prot. 2018, 116, 780–786. [Google Scholar] [CrossRef]
- Alege, F.P.; Gu, X.; Tao, H.; Miito, G.J.; Ndegwa, P.M. Dairy manure compost pelleting process: A techno-economic analysis. J. Clean. Prod. 2021, 310, 127481. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Grunert, O.; Meers, E.; Jablonowski, N.D. Evaluating the Fertilising Potential of Blended Recovered Nutrients in Horticultural Growing Medium on Viola x wittrockiana L. Agronomy 2022, 12, 182. [Google Scholar] [CrossRef]
- Doyle, D.J.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.; Robles-Aguilar, A.A.; Liang, Q.; Pap, S.; Michels, E.; Meers, E. Substrate-Driven Phosphorus Bioavailability Dynamics of Novel Inorganic and Organic Fertilizing Products Recovered from Municipal Wastewater—Tests with Ryegrass. Agronomy 2022, 12, 292. [Google Scholar] [CrossRef]
- Clemente, R.; Sáez-Tovar, J.A.; Bernal, M.P. Extractability, distribution among different particle size fractions, and phytotoxicity of Cu and Zn in composts made with the separated solid fraction of pig slurry. Front. Sustain. Food Syst. 2020, 4, 2. [Google Scholar] [CrossRef]
- García-Rández, A.; Orden, L.; Marks, E.A.N.; Andreu-Rodríguez, F.J.; Franco-Luesma, S.; Martínez-Sabater, E.; Sáez-Tovar, J.A.; Pérez-Murcia, M.D.; Agulló, E.; Bustamante, M.A.; et al. Monitoring of greenhouse gas emissions and compost quality during olive mill waste co-composting at industrial scale: The effect of N and C sources. J. Waste Manag. 2025, 193, 33–43. [Google Scholar] [CrossRef]
- Paredes, C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Bustamante, M.A.; Moreno-Caselles, J. Recycling of two-phase olive-mill cake “Alperujo” by co-composting with animal manures. Commun. Soil Sci. Plant Anal. 2015, 46, 238–247. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Moreno-Caselles, J.; Pérez-Murcia, M.D.; Pérez- Espinosa, A.; Bernal, M.P. 2007. Co-composting of distillery and winery wastes with sewage sludge. Water Sci. Technol. 2007, 56, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 961–1010. ISBN 978-0-89118-866-7. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In USDA Circular Nr 939; United States Government Print Office: Washington, DC, USA, 1954. [Google Scholar]
- Bremmer, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium nitrate and nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Wang, J.; Dimech, A.M.; Spangenberg, G.; Smith, K.; Badenhorst, P. Rapid screening of nitrogen use efficiency in perennial ryegrass (Lolium perenne L.) using automated image-based phenotyping. Front. Plant Sci. 2020, 11, 565361. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- López-Bellido, L.; López-Bellido, J.; Redondo, R. Nitrogen efficiency in wheat under rainfed Mediterranean conditions as affected by split nitrogen application. Field Crop. Res. 2005, 94, 86–97. [Google Scholar] [CrossRef]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Version 2020; FCA Universidad Nacional de Córdoba: Córdoba, Argentina, 2020.
- Bouman, O.T.; Curtin, D.; Campbell, C.A.; Biederbeck, V.O.; Ukrainetz, H. Soil acidification from long-term use of anhydrous ammonia and urea. Soil Sci. Soc. Am. J. 1995, 59, 1488–1494. [Google Scholar] [CrossRef]
- Kavvadias, V.; Ioannou, Z.; Vavoulidou, E.; Paschalidis, C. Short term effects of chemical fertilizer, compost and zeolite on yield of lettuce, nutrient composition and soil properties. Agriculture 2023, 13, 1022. [Google Scholar] [CrossRef]
- Scotti, R.; Pane, C.; Spaccini, R.; Palese, A.M.; Piccolo, A.; Celano, G.; Zaccardelli, M. On-farm compost: A useful tool to improve soil quality under intensive farming systems. Appl. Soil Ecol. 2016, 107, 13–23. [Google Scholar] [CrossRef]
- Mazzilli, S.R.; Kemanian, A.R.; Ernst, O.R.; Jackson, R.B.; Piñeiro, G. Priming of soil organic carbon decomposition induced by corn compared to soybean crops. Soil Biol. Biochem. 2014, 75, 273–281. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1494. [Google Scholar] [CrossRef]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing soil fertility in organic farming systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef]
- Cordovil, C.D.S.; Coutinho, J.; Goss, M.; Cabral, F. Potentially mineralizable nitrogen from organic materials applied to a sandy soil: Fitting the one-pool exponential model. Soil Use Manag. 2005, 21, 65–72. [Google Scholar]
- Antille, D.L.; Sakrabani, R.; Godwin, R.J. Effects of biosolids-derived organomineral fertilizers, urea, and biosolids granules on crop and soil established with ryegrass (Lolium perenne L.). Commun. Soil Sci. Plant Anal. 2014, 45, 1605–1621. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Phosphorus use efficiency of bio-based fertilizers: Bioavailability and fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef]
- Castán, E.; Satti, P.; González-Polo, M.; Iglesias, M.C.; Mazzarino, M.J. Managing the value of compost as organic amendments and fertilizers. Agrosyst. Geosci. Environ. 2016, 224, 29–38. [Google Scholar] [CrossRef]
- Mumbach, G.L.; Gatiboni, L.C.; de Bona, F.D.; Schmitt, D.E.; Correa, J.C.; Gabriel, C.A.; Dall’Orsoletta, D.J.; Iochims, D.A. Agronomic efficiency of organomineral fertilizer in sequential grain crops in southern Brazil. Agron. J. 2020, 112, 3037–3049. [Google Scholar] [CrossRef]
- Sitzmann, T.J.; Alpigiano, A.; Lerda, C.; Moretti, B.; Zavattaro, L.; Grignani, C. Response of tomato to innovative organo-mineral fertilizers. Front. Sustain. Food Syst. 2024, 8, 1385828. [Google Scholar] [CrossRef]
- Sitzmann, T.J.; Sica, P.; Zavattaro, L.; Moretti, B.; Grignani, C.; Oberson, A. An isotope study on nitrogen and phosphorus use efficiency and movement in soil in a mimicked vermicompost-based organo-mineral fertilizer. Agrosyst. Geosci. Environ. 2024, 7, e20473. [Google Scholar] [CrossRef]
- Deeks, L.K.; Chaney, K.; Murray, C.; Sakrabani, R.; Gedara, S.; Le, M.S.; Tyrrel, S.; Pawlett, M.; Read, R.; Smith, G.H. A new sludge-derived organo-mineral fertilizer gives similar crop yields as conventional fertilizers. Agron. Sustain. Dev. 2013, 33, 539–549. [Google Scholar] [CrossRef]
- Florio, A.; Felici, B.; Migliore, M.; Dell’Abate, M.T.; Benedetti, A. Nitrogen losses, uptake and abundance of ammonia oxidizers in soil under mineral and organo-mineral fertilization regimes. J. Sci. Food Agric. 2016, 96, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Bian, R.; Li, L.; Lian, W.; Liu, X.; Zheng, J.; Cheng, K.; Zhang, X.; Drosos, M.; Joseph, S.; et al. Assessing the impacts of biochar-blended urea on nitrogen use efficiency and soil retention in wheat production. GCB Bioenergy 2021, 14, 65–83. [Google Scholar] [CrossRef]
- Uddin, M.K.; Yeasmin, S.; Mohiuddin, K.M.; Chowdhury, M.A.H.; Saha, B.K. Peat-Based Organo-Mineral Fertilizer Improves Nitrogen Use Efficiency, Soil Quality, and Yield of Baby Corn (Zea mays L.). Sustainability 2023, 15, 9086. [Google Scholar] [CrossRef]
- Richards, J.E.; Daigle, J.-Y.; LeBlanc, P.; Paulin, R.; Ghanem, I. Nitrogen availability and nitrate leaching from organo-mineral fertilizers. Can. J. Soil Sci. 1993, 73, 197–208. [Google Scholar] [CrossRef]
- Pampuro, N.; Bertora, C.; Sacco, D.; Dinuccio, E.; Grignani, C.; Balsari, P.; Bernal, M.P. Fertilizer value and greenhouse gas emissions from solid fraction pig slurry compost pellets. J. Agric. Sci. 2017, 155, 1646–1658. [Google Scholar] [CrossRef]
- Gram, G.; Roobroeck, D.; Pypers, P.; Six, J.; Merckx, R.; Vanlauwe, B. Combining organic and mineral fertilizers as a climate-smart integrated soil fertility management practice in sub-Saharan Africa: A meta-analysis. PLoS ONE 2020, 15, e0239552. [Google Scholar] [CrossRef]
- Allam, M.; Radicetti, E.; Quintarelli, V.; Petroselli, V.; Marinari, S.; Mancinelli, R. Influence of organic and mineral fertilizers on soil organic carbon and crop productivity under different tillage systems: A meta-analysis. Agriculture 2022, 12, 464. [Google Scholar] [CrossRef]
- Antille, D.L.; Sakrabani, R.; Godwin, R.J. Nitrogen release characteristics from biosolids-derived organomineral fertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 1687–1698. [Google Scholar] [CrossRef]
- Valentine, J.; Charles, A.H. The association of dry-matter yield with nitrogen and soluble-carbohydrate concentration in perennial ryegrass (Lolium perenne L.). J. Agric. Sci. 1979, 93, 657–667. [Google Scholar] [CrossRef]
- Alvarez-Alonso, C.; Clemente, R.; Bernal, M.P. Carbon and nitrogen mineralisation in soils and nutrient efficiency of digestates from fruits and vegetable wastes. J. Soil Sci. Plant Nutr. 2022, 22, 4473–4486. [Google Scholar] [CrossRef]
- Yin, M.; Li, Y.; Hu, Q.; Yu, X.; Huang, M.; Zhao, J.; Dong, S.; Yuan, X.; Wen, Y. Potassium increases nitrogen and potassium utilization efficiency and yield in foxtail millet. Agronomy 2023, 13, 2200. [Google Scholar] [CrossRef]
| Fertiliser Class | Treatments | Acronym |
|---|---|---|
| Reference | Control | C |
| Synthetic | Complex (15-15-15) | IN |
| Synthetic | Monoammonium phosphate (11-61-0) | MAP |
| Simple mineral | Struvite (5-33-0) | STR |
| Simple organic | Bone meal (3-30-0) | BON |
| Pellet mineral | Monoammonium phosphate + compost | OMF-MAP |
| Pellet mineral | Struvite + compost | OMF-STR |
| Pellet organic | Bone meal + compost | OMF-BON |
| Parameter a | Unit | OMWC |
|---|---|---|
| pH | - | 8.86 ± 0.01 |
| Electrical conductivity | (dS m−1) | 3.42 ± 0.08 |
| Organic matter | (%) | 73.6 ± 0.8 |
| Total organic C | (g kg−1) | 419 ± 0.5 |
| Total N | (g kg−1) | 27.1 ± 0.1 |
| Total organic C/Total N | - | 15.5 ± 0.2 |
| Polyphenols | (mg kg−1) | 1456 ± 68 |
| P | (g kg−1) | 6.73 ± 0.82 |
| K | (g kg−1) | 26 ± 0.3 |
| Ca | (g kg−1) | 29.6 ± 0.2 |
| Mg | (mg kg−1) | 3.95 ± 0.04 |
| Na | (g kg−1) | 1.09 ± 0.01 |
| S | (g kg−1) | 3.55 ± 0.04 |
| Fe | (mg kg−1) | 2643 ± 338 |
| Mn | (mg kg−1) | 297 ± 33.0 |
| Cu | (mg kg−1) | 112 ± 9.0 |
| Zn | (mg kg−1) | 227 ± 23.0 |
| Parameter a | OMF-MAP | OMF-STR | OMF-BON |
|---|---|---|---|
| pH | 5.59 ± 1.61 | 6.68 ± 1.63 | 8.63 ± 1.70 |
| EC (dS m−1) | 3.02 ± 2.95 | 3.88 ± 1.78 | 1.88 ± 7.60 |
| TOC (g kg−1) | 216 ± 1.50 | 179 ± 0.40 | 294 ± 3.17 |
| TN (g kg−1) | 71 ± 0.60 | 37 ± 0.10 | 28 ± 0.20 |
| P (g kg−1) | 179 ± 5.10 | 73 ± 1.62 | 46 ± 4.31 |
| K (g kg−1) | 14.4 ± 0.23 | 11.9 ± 0.06 | 15.7 ± 1.60 |
| Na (g kg−1) | 0.61 ± 0.01 | 2.18 ± 0.00 | 2.80 ± 2.80 |
| S (g kg−1) | 2.62 ± 3.17 | 3.33 ± 0.01 | 3.32 ± 0.33 |
| Ca (g kg−1) | 19.8 ± 0.40 | 53.3 ± 0.07 | 115 ± 12.3 |
| Mg (g kg−1) | 2.25 ± 0.01 | 21.1 ± 3.42 | 4.05 ± 0.46 |
| Fe (g kg−1) | 1176 ± 13.0 | 4173 ± 78.0 | 1122 ± 17.0 |
| Mn (mg kg−1) | 158 ± 12.0 | 271 ± 3.00 | 165 ± 16.0 |
| Cu (mg kg−1) | 51 ± 3.30 | 51 ± 0.30 | 52 ± 5.20 |
| Zn (mg kg−1) | 119 ± 0.50 | 183 ± 2.10 | 167 ± 15 |
| Treatment | pH | EC | OM | C Stock | TN | NH4+-N | NO3−-N | Pext |
|---|---|---|---|---|---|---|---|---|
| (dS cm−1) | (%) | (kg ha−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | ||
| C | 7.85 b | 3.17 a | 0.60 a | 66.6 a | 0.36 a | 3.71 a | 3.34 a | 80 a |
| IN | 7.68 a | 3.19 a | 0.69 d | 78.2 e | 0.41 b | 8.43 b | 16.0 b | 127 b |
| MAP | 7.80 b | 3.39 c | 0.69 d | 75.9 e | 0.36 a | 13.8 c | 39.1 d | 178 c |
| STR | 7.84 b | 3.21 a | 0.62 ab | 68.7 ab | 0.35 a | 14.6 c | 30.1 c | 90 a |
| BON | 7.82 b | 3.39 c | 0.64 b | 70.5 bc | 0.33 a | 9.65 b | 42.9 de | 121 b |
| OMF-MAP | 7.84 b | 3.36 c | 0.67 cd | 74.2 de | 0.35 a | 13.8 c | 59.0 f | 177 c |
| OMF-STR | 7.84 b | 3.24 ab | 0.68 cd | 74.9 de | 0.36 a | 20.0 d | 59.4 f | 115 b |
| OMF-BON | 7.89 b | 3.33 bc | 0.65 bc | 72.2 cd | 0.36 a | 20.8 d | 44.7 e | 116 b |
| F-ANOVA | 3.53 * | 8.02 *** | 9.22 *** | 9.56 *** | 3.38 * | 32.7 *** | 240 *** | 11.7 *** |
| Treatment | NUE | PUE | KUE |
|---|---|---|---|
| (%) | (%) | (%) | |
| IN | 57.64 b | 3.96 b | 36.8 d |
| MAP | 68.80 c | 5.49 d | 13.3 c |
| STR | 70.23 c | 4.18 bc | 12.9 c |
| BON | 55.52 b | 0.42 a | 10.6 b |
| OMF-MAP | 54.37 b | 3.81 b | 9.90 b |
| OMF-STR | 57.59 b | 4.73 c | 10.1 b |
| OMF-BON | 41.71 a | 0.00 a | 7.39 a |
| F-ANOVA | 17 *** | 133 *** | 299 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Méndez, S.; Valverde-Vozmediano, L.; Orden, L.; Andreu-Rodríguez, F.J.; Sáez-Tovar, J.A.; Martínez-Sabater, E.; Bustamante, M.Á.; Moral, R. Alternative Phosphorus Fertilisation with Bio-Based Pellet Fertilisers: A Case of Study on Ryegrass (Lollium perenne L.). Agronomy 2025, 15, 579. https://doi.org/10.3390/agronomy15030579
Sánchez-Méndez S, Valverde-Vozmediano L, Orden L, Andreu-Rodríguez FJ, Sáez-Tovar JA, Martínez-Sabater E, Bustamante MÁ, Moral R. Alternative Phosphorus Fertilisation with Bio-Based Pellet Fertilisers: A Case of Study on Ryegrass (Lollium perenne L.). Agronomy. 2025; 15(3):579. https://doi.org/10.3390/agronomy15030579
Chicago/Turabian StyleSánchez-Méndez, Silvia, Lucía Valverde-Vozmediano, Luciano Orden, Francisco Javier Andreu-Rodríguez, José Antonio Sáez-Tovar, Encarnación Martínez-Sabater, María Ángeles Bustamante, and Raúl Moral. 2025. "Alternative Phosphorus Fertilisation with Bio-Based Pellet Fertilisers: A Case of Study on Ryegrass (Lollium perenne L.)" Agronomy 15, no. 3: 579. https://doi.org/10.3390/agronomy15030579
APA StyleSánchez-Méndez, S., Valverde-Vozmediano, L., Orden, L., Andreu-Rodríguez, F. J., Sáez-Tovar, J. A., Martínez-Sabater, E., Bustamante, M. Á., & Moral, R. (2025). Alternative Phosphorus Fertilisation with Bio-Based Pellet Fertilisers: A Case of Study on Ryegrass (Lollium perenne L.). Agronomy, 15(3), 579. https://doi.org/10.3390/agronomy15030579







