Identification and Expression of Laccase Gene Family in Potato (Solanum tuberosum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of StLAC Members in the Potato Genome
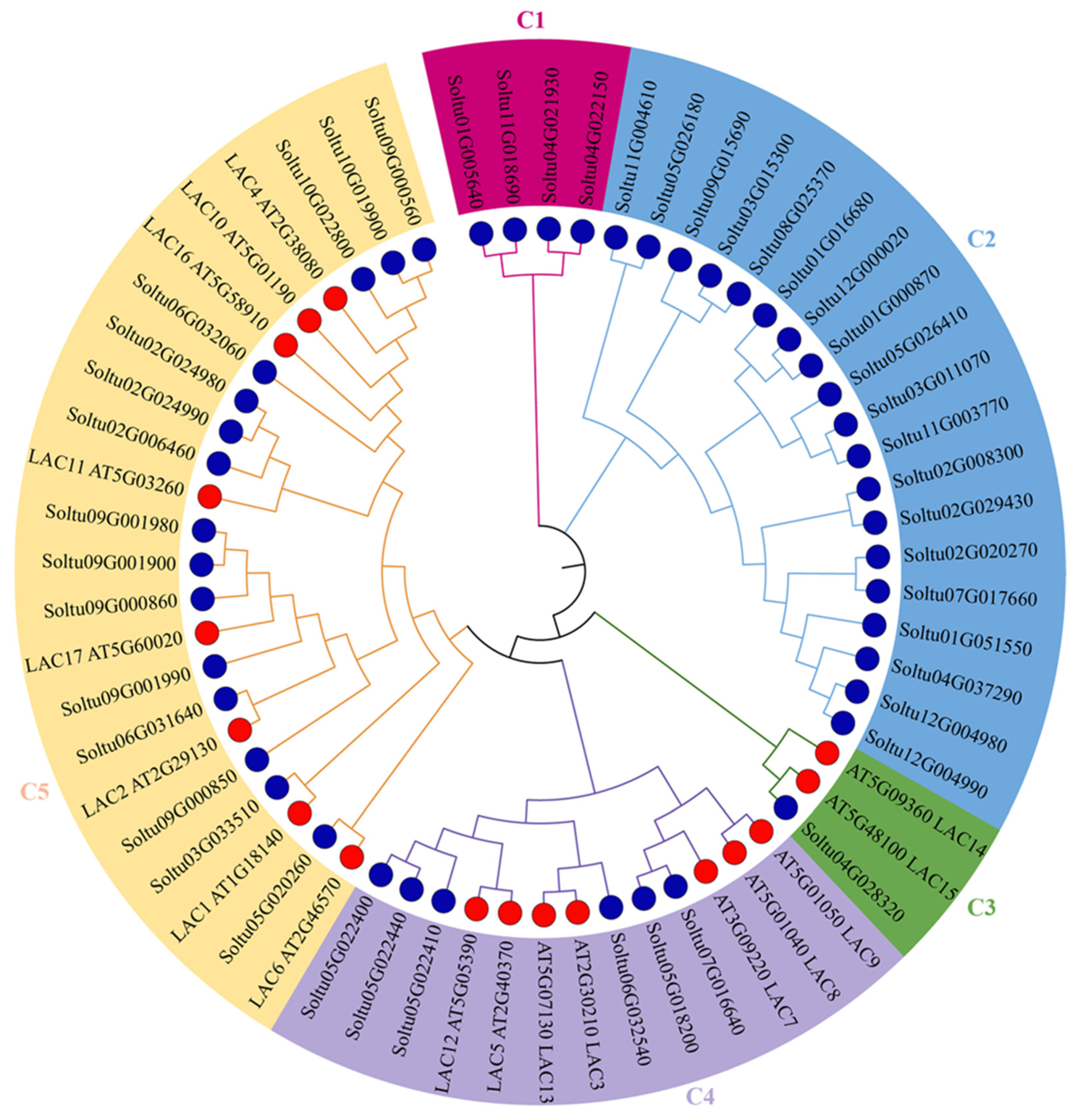
2.2. Evolutionary Analysis of StLACs
2.3. StLACs Sequence Analysis and Structural Characteristics
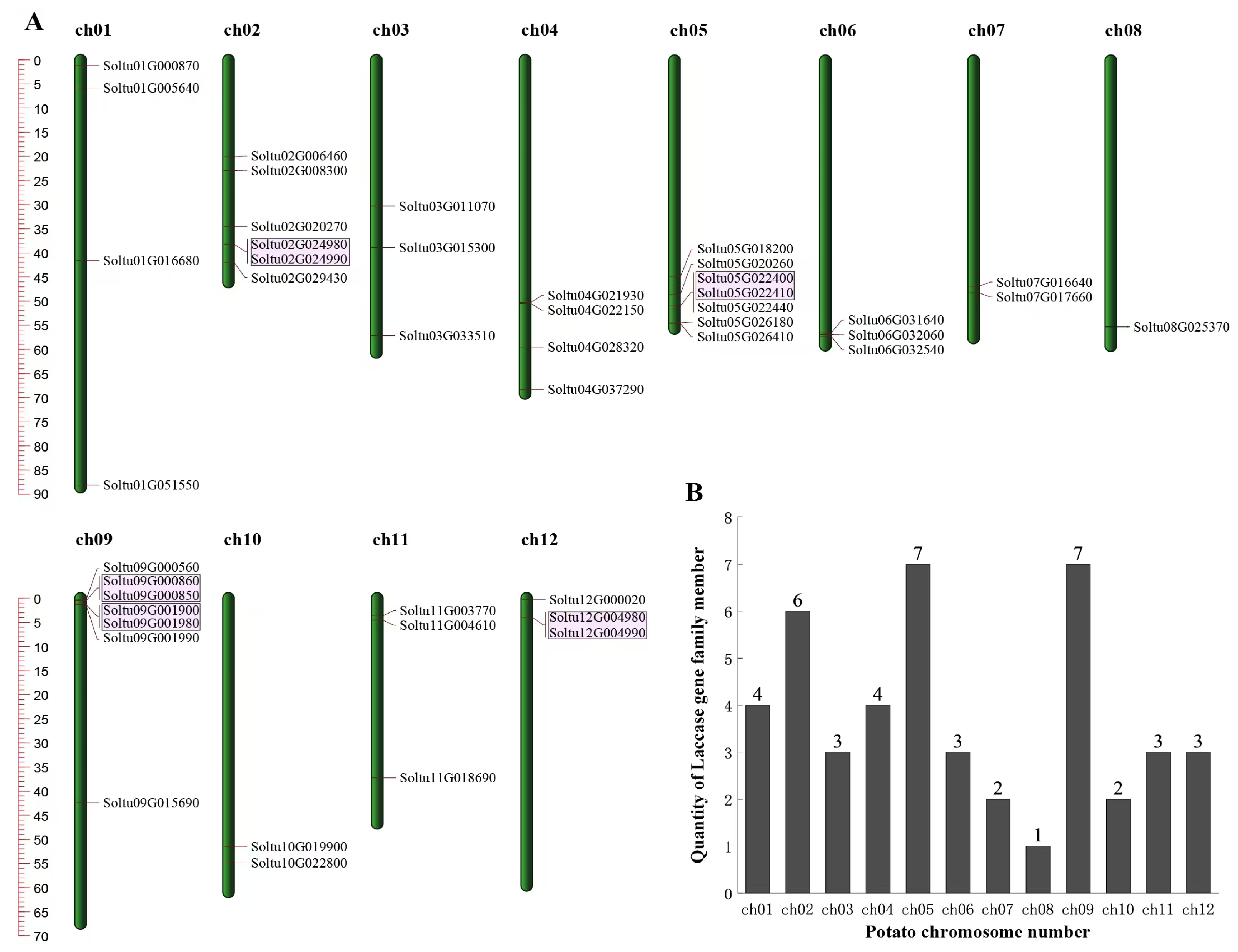
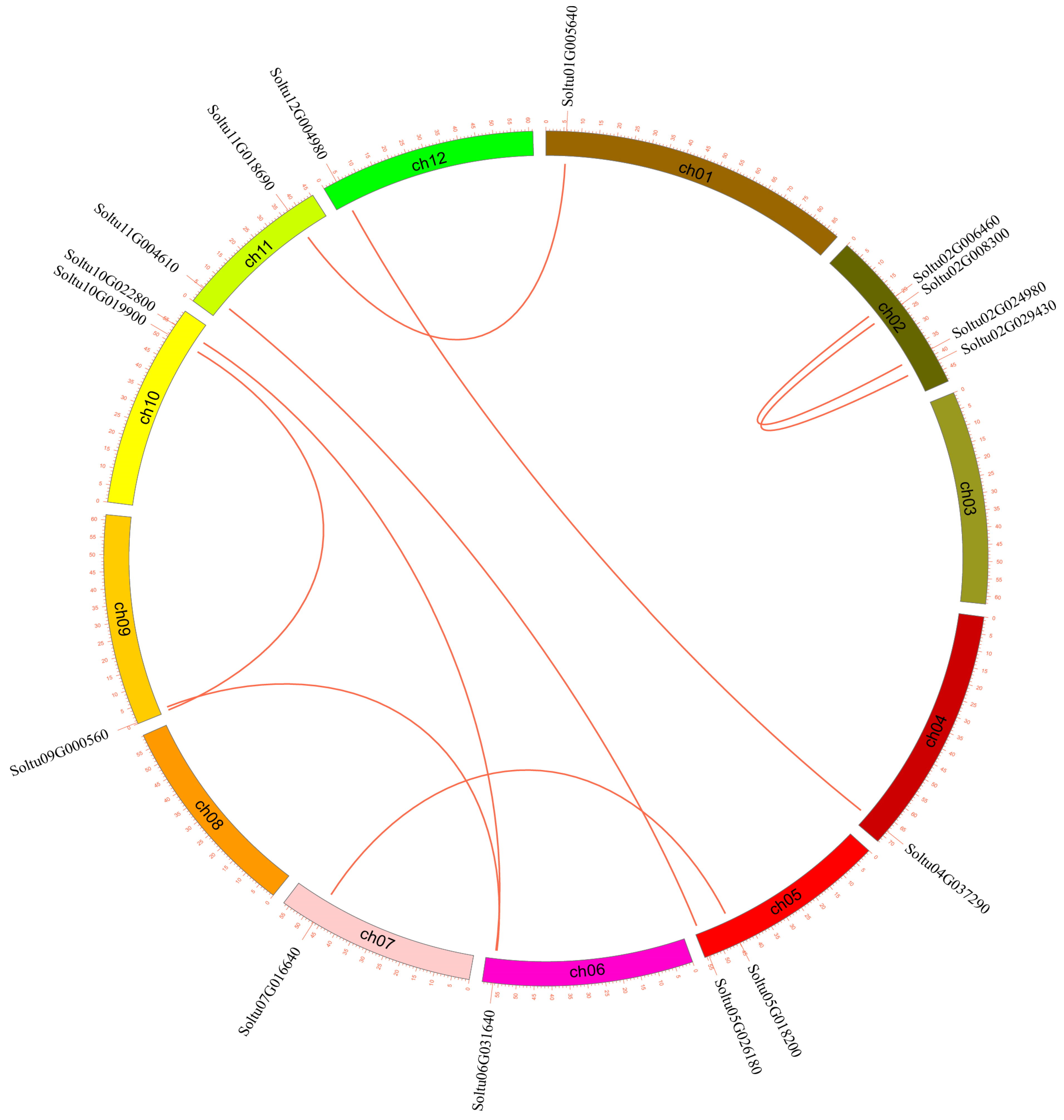
2.4. Duplication Events and Syntenic Patterns of LAC Genes
2.5. Functional Analysis of Genes in the Potato LAC Family
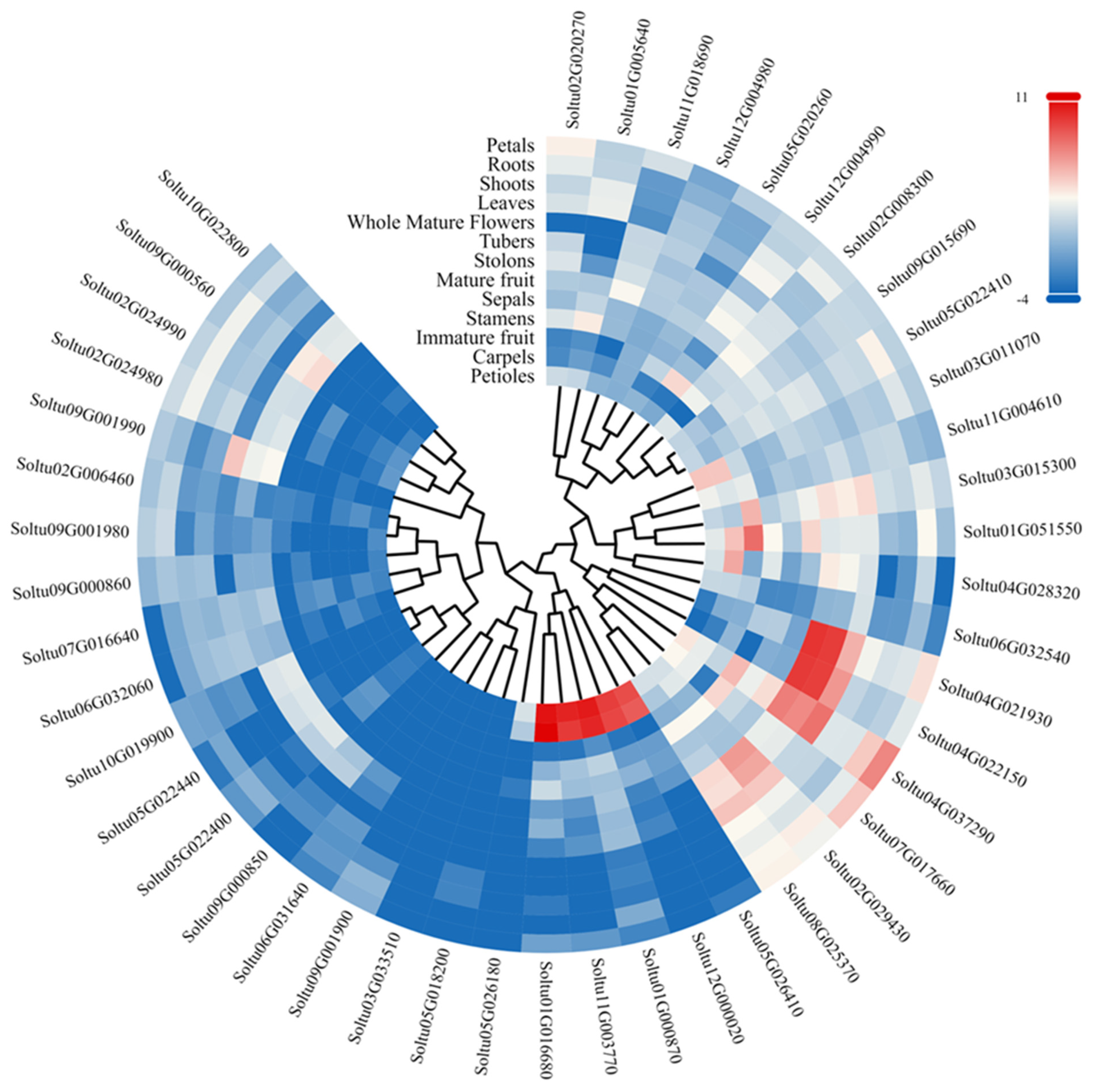
2.6. Expression Analysis of StLACs in Potato
2.7. Plant Materials and Treatments
2.8. RNA Extraction and qPCR
3. Results
3.1. Identification of StLACs and Characteristic Analysis of Corresponding Proteins
3.2. Phylogenetic Analysis of StLACs
3.3. Gene Structure, Motif Composition, and Conserved Domain Analysis of StLACs
3.4. Gene Replication Events of StLACs and Collinearity Analysis with Other Species
3.5. Analysis of StLAC Gene Expression in Different Tissues of DM Potato
3.6. KEGG and GO Enrichment Analysis of StLACs
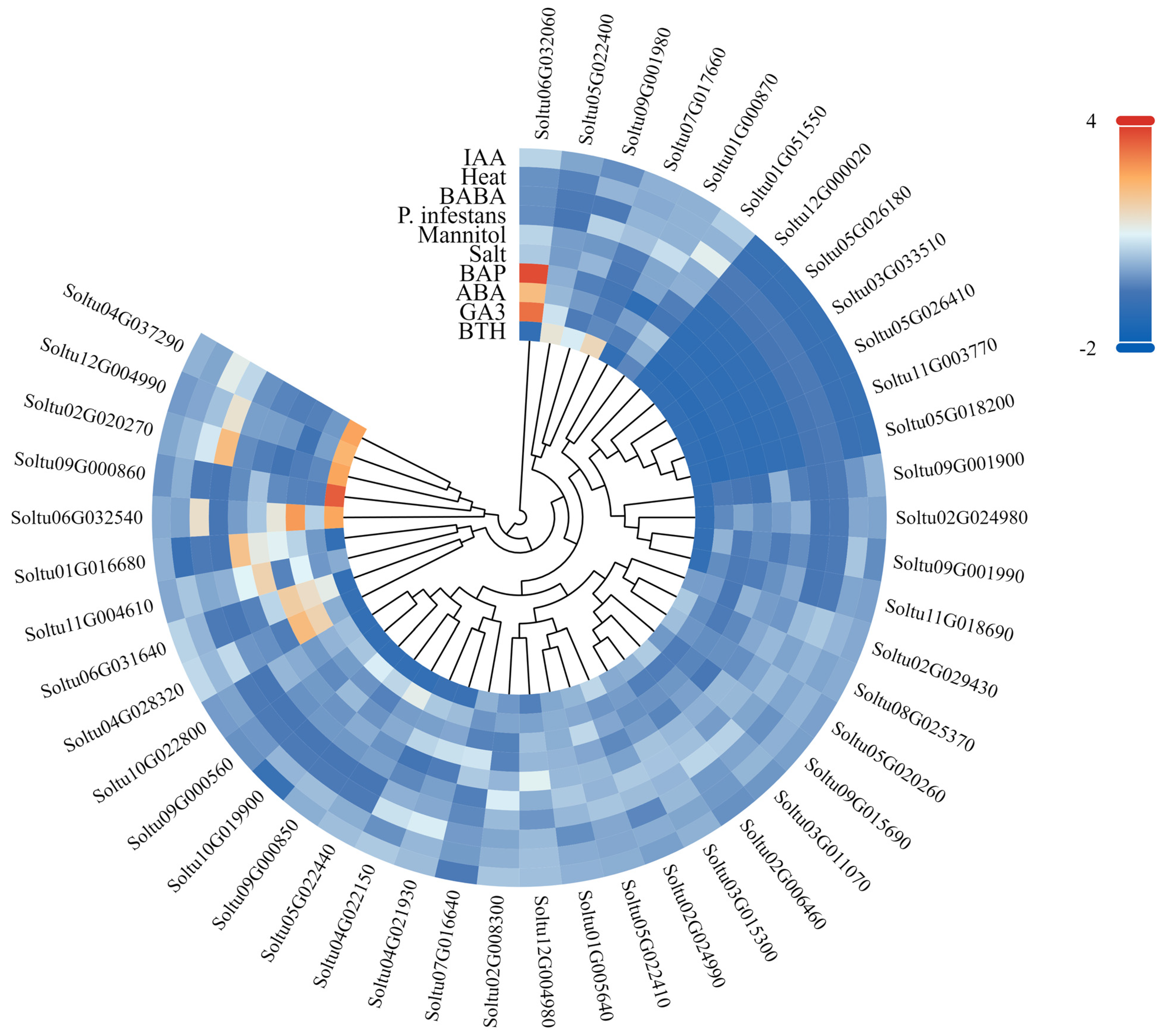
3.7. Expression Patterns of StLACs Under Different Treatments
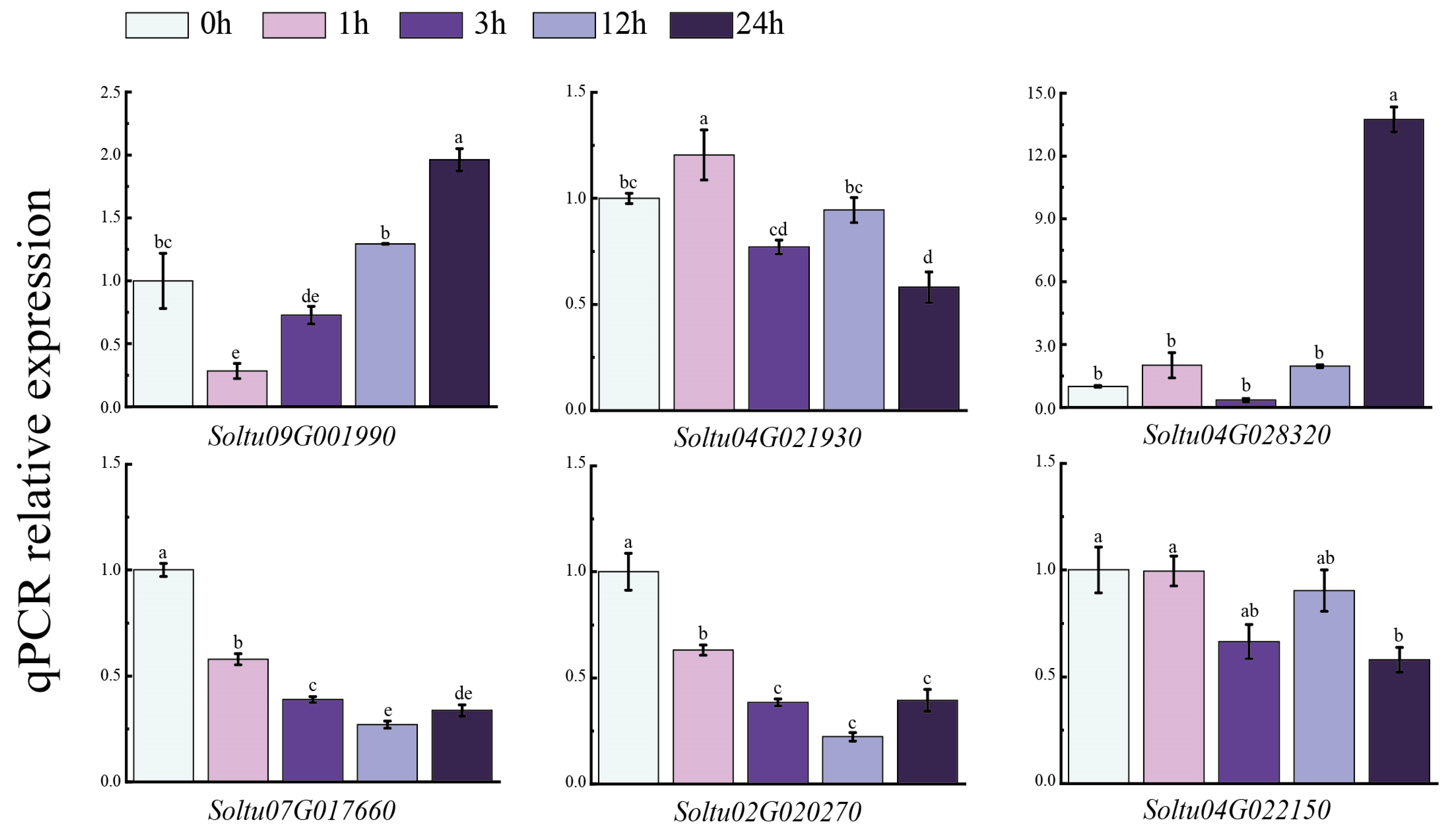
3.8. Expression of Six StLAC Genes in Potato with NaCl Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshida, H.L. XIII.—Chemistry of lacquer (Urushi). Part I. Communication from the chemical Society of tokio. The United Kingdom of Great Britain and Northern Ireland. J. Chem. Soc. Trans. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Yao, W.; Liu, M.; Chen, M.; Liu, S.H.C. Genome-wide Identification and Expression Analysis of LAC Gene Family of Amaranthus tricolor L. Chin. Veg. 2024, 1, 48–56. [Google Scholar]
- Liu, N.; Jia, H.; Shen, K.; Cao, Z.; Dong, J. Fungal Laccase: Multi-biofunction and Complicated Natural Substrates. J. Agric. Biotechnol. 2020, 28, 333–341. (In Chinese) [Google Scholar]
- Turlapati, P.V.; Kim, K.W.; Davin, L.B.; Lewis, N.G. The laccase multigene family in Arabidopsis thaliana: Towards addressing the mystery of their gene function(s). Planta 2011, 233, 439–470. [Google Scholar] [CrossRef] [PubMed]
- Ranocha, P.; Chabannes, M.; Chamayou, S.; Danoun, S.; Jauneau, A.; Boudet, A.; Goffner, D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002, 129, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.L.; Zhu, M.L.; Yu, Y.; Zhang, Y.; Wei, Z. Generation and characterization of transgenic poplar plants overexpressing a cotton laccase gene. Plant Cell Tissue Organ Cult. 2008, 93, 303–310. [Google Scholar] [CrossRef]
- Liang, M.; Davis, E.; Gardner, D.; Cai, X.; Wu, Y. Involvement of AtLAC15 in lignin synthesis in seeds and root elongation of Arabidopsis. Planta 2006, 224, 1185–1196. [Google Scholar] [CrossRef]
- Cesarino, I.; Araújo, P.; Sampaio Mayer, J.L.; Vicentini, R.; Berthet, S.; Demedts, B.; Vanholme, B.; Boerjan, W.; Mazzafera, P. Expression of SofLAC, a new laccase in sugarcane, restores lignin content but not S: G ratio of Arabidopsis lac17 mutant. J. Exp. Bot. 2013, 64, 1769–1781. [Google Scholar] [CrossRef]
- Wang, Y.X.; Tao, J.L.; Zhu, H.S.; Xu, T.; Zhang, Y.F.; Cen, H.F. Heterologous expression of miR397-5p from Medicago sativa cv. ‘Pianguan’ improves the drought tolerance of tobacco. Acta Prataculturae Sin. 2024, 33, 123–134. [Google Scholar]
- Cho, H.Y.; Lee, C.; Hwang, S.G.; Park, Y.C.; Lim, H.L.; Jang, C.S. Overexpression of the OsChI1 gene, encoding a putative laccase precursor, increases tolerance to drought and salinity stress in transgenic Arabidopsis. Gene 2014, 552, 98–105. [Google Scholar] [CrossRef]
- Hu, Q.; Min, L.; Yang, X.Y.; Jin, S.; Zhang, L.; Li, Y.; Ma, Y.; Qi, X.; Li, D.; Liu, H.; et al. Laccase GhLac1 modulates broad-spectrum biotic stress tolerance via manipulating phenylpropanoid pathway and jasmonic acid synthesis. Plant Physiol. 2018, 176, 1808–1823. [Google Scholar] [CrossRef]
- Stokstad, E. The new potato. Science 2019, 363, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Liu, Z.H.; Zeng, Y.T.; Li, Z.T.; Chen, L.M.; Li, H.Y. Genome-wide identification of potato (Solanum tuberosum L.) PAL gene family and its expression analysis in abiotic stress and tuber anthocyanin synthesis. Acta Mater. Sin. 2023, 49, 2978–2990. [Google Scholar]
- Kong, J.J.; Xiong, R.; Qiu, K.L.; Lin, X.; Li, D.; Lu, L.; Zhou, J.; Zhu, S.; Liu, M.; Sun, Q. Genome-Wide Identification and Characterization of the Laccase Gene Family in Fragaria vesca and Its Potential Roles in Response to Salt and Drought Stresses. Plants 2024, 13, 3366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Ren, C.; Xu, J.; Wang, H.; Wen, B.; Zhao, Q.; Zhang, W.; Yu, G.; Zhang, Y. Genome-wide analysis of the common bean (Phaseolus vulgaris) laccase gene family and its functions in response to abiotic stress. BMC Plant Biol. 2024, 24, 688. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhou, Y.P.; Wang, B.; Li, D.; Yue, W.; Li, L.; Yue, Z.H.; Wei, W.K. Genome-wide identification and characterization of the laccase gene family in Citrus sinensis. Gene 2019, 689, 114–123. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Tan, M.; Wang, X.; Wang, G.-L.; Qi, M.; Liu, B.; Gao, J.; Pan, Y.; Wang, Y. Genome-wide identification and characterization of laccase family members in eggplant (Solanum melongena L.). PeerJ 2022, 10, e12922. [Google Scholar] [CrossRef] [PubMed]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, F.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Wang, J.K. Prediction of protein subcellular localization. Proteins: Structure. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Liang, M.; Kong, W.; Liu, J. The Citrus Laccase Gene CsLAC18 Contributes to Cold Tolerance. Int. J. Mol. Sci. 2022, 23, 14509. [Google Scholar] [CrossRef]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Bailey, T.L.; Mikael, B.; Buske, F.A.; Martin, F.; Grant, C.E.; Luca, C.; Ren, J.Y.; Li, W.F.; William, S.N. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.P.; Guo, A.Y. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. Tbtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, N.; Si, H.J.; Caideron, A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods 2017, 13, 85. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cézard, L.; Le-Bris, P.; Borrega, N.; Hervé, J.; Blondet, E.; Balzergue, S.; et al. Disruption of Laccase4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef]
- Sedbrook, J.C.; Carroll, K.L.; Hung, K.F.; Massonance, P.H.; Somerville, C.R. The arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol-anchored glycoprotein involved in directional root growth. Plant Cell 2002, 14, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Kerhoas, L.; Caboche, M.; Lepiniec, L.; Debeaujon, I. TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in the oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 2005, 17, 2966–2980. [Google Scholar] [CrossRef]
- Wang, T.H.; Liu, Y.; Zou, K.L. The Analysis, Description, and Examination of the Maize LAC Gene Family’s Reaction to Abiotic and Biotic Stress. Genes 2024, 15, 749. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Feng, J.J.; Jia, W.; Fan, P.; Bao, H.; Li, Y. Genome-wide Identification of Sorghum bicolor laccases reveals potential targets for lignin modification. Front. Plant Sci. 2017, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.P.; Lin, J.S.; Dong, F.; Ma, Q.; Ming, R. Genomic and allelic analyses of laccase genes in sugarcane (Saccharum spontaneum L.). Trop. Plant Biol. 2019, 12, 219–229. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Luo, L.; Wang, X.X.; Shen, Z.; Zheng, L. Comprehensive Analysis of Rice Laccase Gene (OsLAC) Family and Ectopic Expression of OsLAC10 Enhances Tolerance to Copper Stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, G.; Zheng, K.J.; Zhu, X.; Ma, J.; Wang, D.; Tang, K.; Feng, X.; Leng, J.; Yu, H.; et al. The soybean laccase gene family: Evolution and possible roles in plant defense and stem strength selection. Genes 2019, 10, 701. [Google Scholar] [CrossRef]
- Yadav, S.; Chattopadhyay, D. Lignin: The Building Block of Defense Responses to Stress in Plants. J. Plant Growth Regul. 2023, 42, 6652–6666. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Lise, J.; Serge, B.; Julien, M. Identification of laccases involved in lignin polymerization and strategies to deregulate their expression in order to modify lignin content in Arabidopsis and poplar. BMC Proc. 2011, 5, O39. [Google Scholar] [CrossRef]
- Liu, M.Y.; Dong, H.; Wang, M. Evolutionary divergence of function and expression of laccase genes in plants. J. Genet. 2020, 99, 23. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Liu, Z.; Zhu, J.; Li, Z.; Qiu, X.; Wang, W.; Gao, C.; Qi, J.; Bao, M.; Liu, Y. Identification and Expression of Laccase Gene Family in Potato (Solanum tuberosum). Agronomy 2025, 15, 585. https://doi.org/10.3390/agronomy15030585
Luo H, Liu Z, Zhu J, Li Z, Qiu X, Wang W, Gao C, Qi J, Bao M, Liu Y. Identification and Expression of Laccase Gene Family in Potato (Solanum tuberosum). Agronomy. 2025; 15(3):585. https://doi.org/10.3390/agronomy15030585
Chicago/Turabian StyleLuo, Hongyu, Zhen Liu, Jinyong Zhu, Zhitao Li, Xiaoqiang Qiu, Weilu Wang, Chengwei Gao, Jiangpeng Qi, Minmin Bao, and Yuhui Liu. 2025. "Identification and Expression of Laccase Gene Family in Potato (Solanum tuberosum)" Agronomy 15, no. 3: 585. https://doi.org/10.3390/agronomy15030585
APA StyleLuo, H., Liu, Z., Zhu, J., Li, Z., Qiu, X., Wang, W., Gao, C., Qi, J., Bao, M., & Liu, Y. (2025). Identification and Expression of Laccase Gene Family in Potato (Solanum tuberosum). Agronomy, 15(3), 585. https://doi.org/10.3390/agronomy15030585




