Seasonal Variation in Nutritional Substances in Varieties of Leafy Chinese Kale (Brassica oleracea var. alboglabra): A Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Height, Plant Spreading, and Plant Weight
2.3. Reducing Sugars
2.4. Soluble Proteins
2.5. Soluble Solids
2.6. Chlorophyll and Carotenoids
2.7. Vitamin C
2.8. Total Phenols
2.9. Ferric Reducing Antioxidant Power (FRAP)
2.10. Glucosinolates
2.11. Statistical Analyses
3. Results
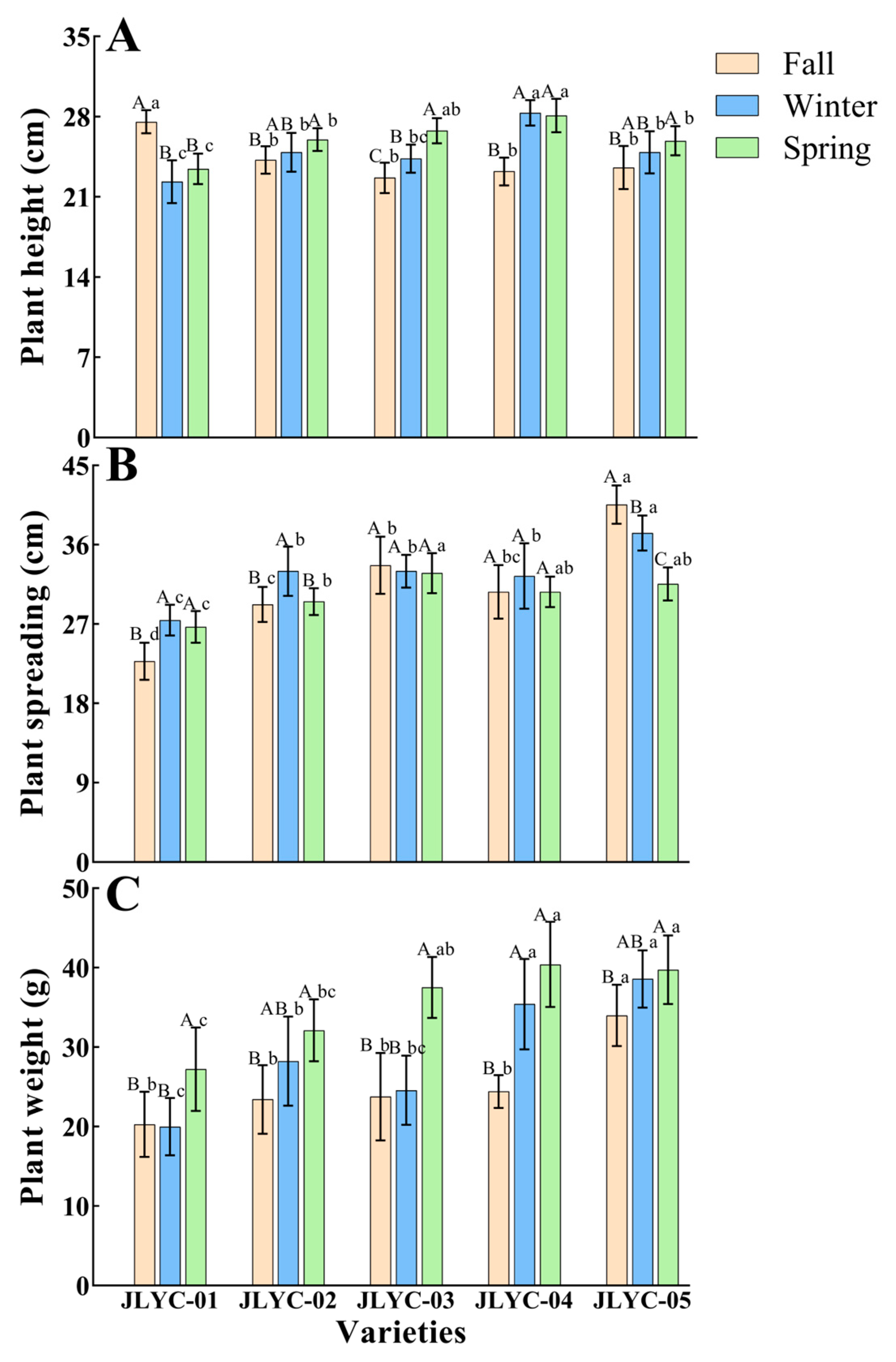
3.1. Plant Height, Plant Spreading, and Plant Weight
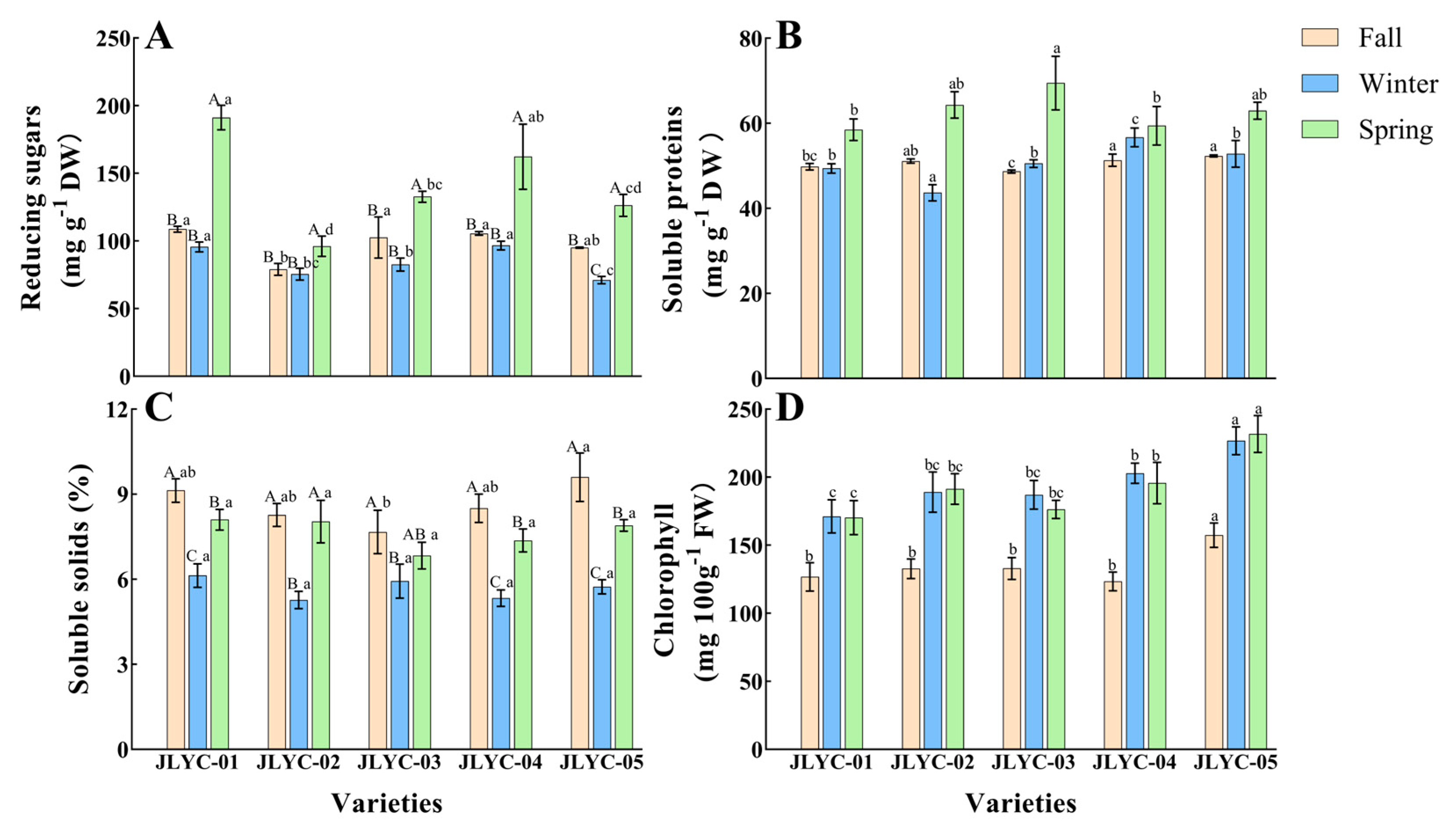
3.2. Reducing Sugars, Soluble Proteins, Soluble Solids, and Chlorophyll
3.3. Carotenoids, Vitamin C, Total Phenols, and Antioxidant Capacity
3.4. Glucosinolates
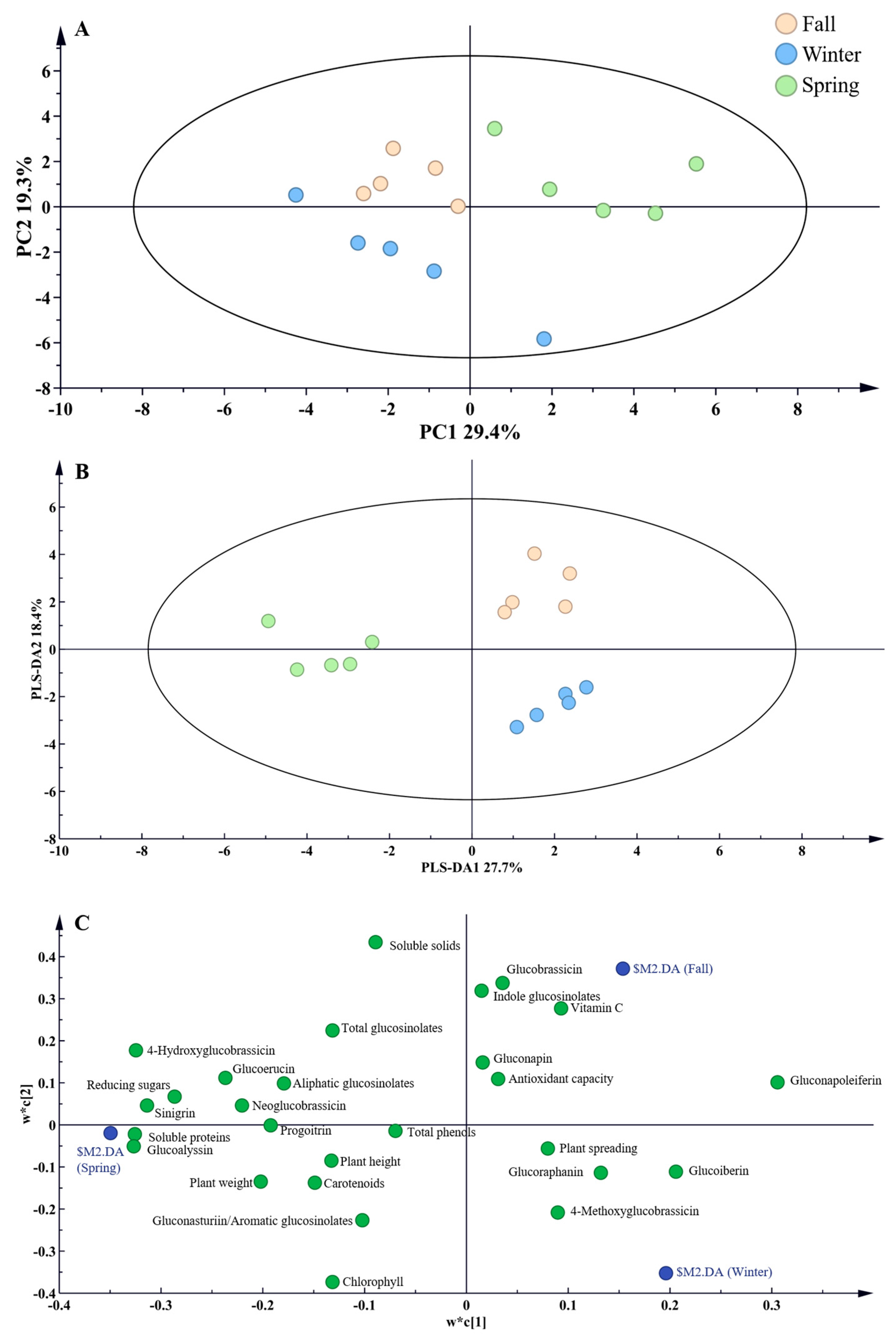
3.5. Principal Component Analysis
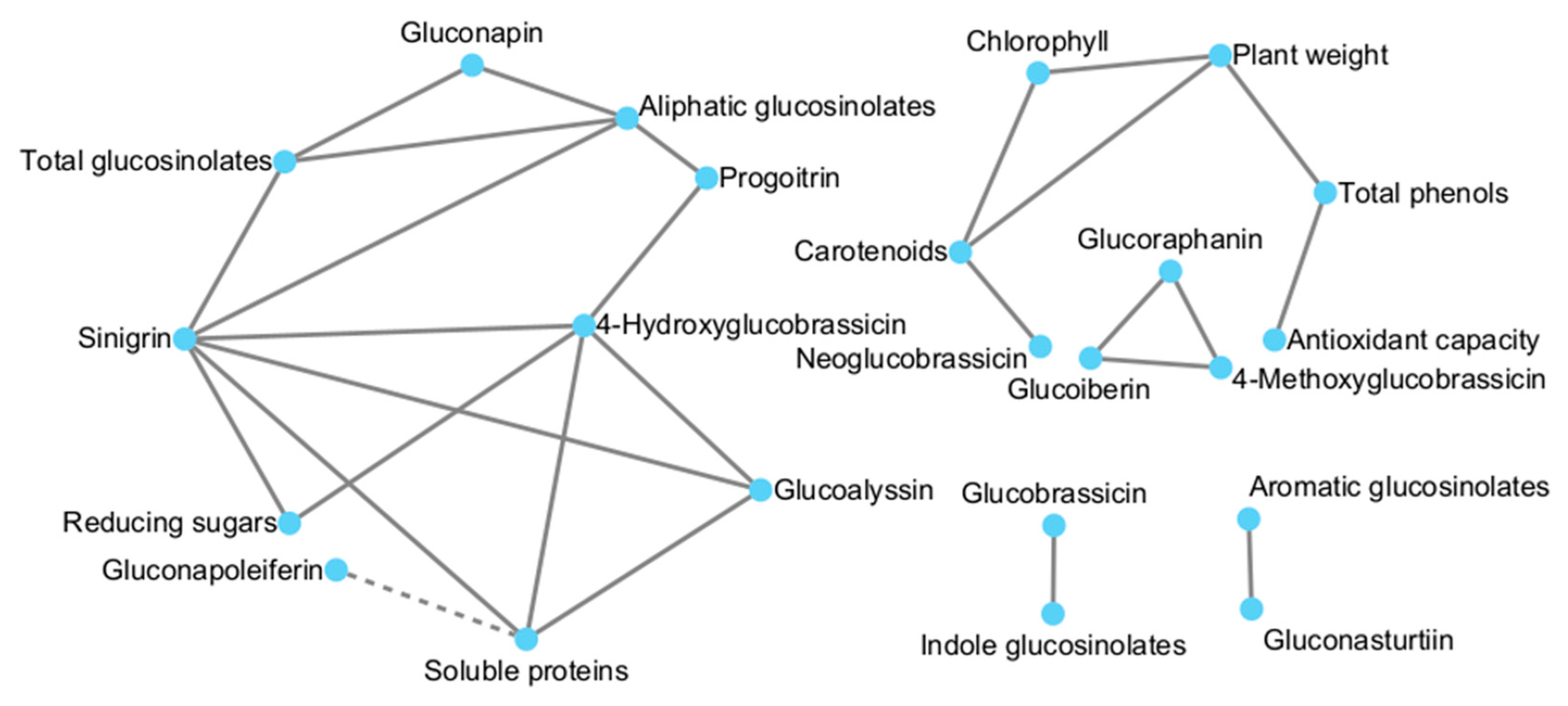
3.6. Correlation Analysis
3.7. Variance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lei, J.; Chen, G.; Chen, C.; Cao, B. Germplasm Diversity of Chinese Kale in China. Hortic. Plant J. 2017, 3, 101–104. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, Q.; Wang, Y.; Liang, S.; Huang, Z.; Li, H.; Escalona, V.H.; Yao, X.; Cheng, W.; Chen, Z.; et al. BoaBZR1.1 mediates brassinosteroid-induced carotenoid biosynthesis in Chinese kale. Hortic. Res. 2024, 11, uhae104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, L.; Liu, G.; Zhang, D.; He, H. Evaluation of the Nutritional Quality of Chinese Kale (Brassica alboglabra Bailey) Using UHPLC-Quadrupole-Orbitrap MS/MS-Based Metabolomics. Molecules 2017, 22, 1262. [Google Scholar] [CrossRef]
- Bell, L. The Biosynthesis of Glucosinolates: Insights, Inconsistencies, and Unknowns. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 969–1000. [Google Scholar]
- Abdelshafeek, K.A.; El-Shamy, A.M. Review on glucosinolates: Unveiling their potential applications as drug discovery leads in extraction, isolation, biosynthesis, biological activity, and corrosion protection. Food Biosci. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Ju, W.; Park, S.J.; Lee, M.; Park, S.; Min, S.; Ku, K.-M. Seasonal variation of metabolites in Kimchi cabbage: Utilizing metabolomics based machine learning for cultivation season and taste discrimination. Hortic. Environ. Biotechnol. 2024, 65, 981–996. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Zhao, X.; Li, J. Systemic effects of the vapor pressure deficit on the physiology and productivity of protected vegetables. Veg. Res. 2023, 3, 20. [Google Scholar] [CrossRef]
- Bhattarai, G.; Shi, A. Research advances and prospects of spinach breeding, genetics, and genomics. Veg. Res. 2021, 1, 9. [Google Scholar] [CrossRef]
- Shipman, E.N.; Yu, J.; Zhou, J.; Albornoz, K.; Beckles, D.M. Can gene editing reduce postharvest waste and loss of fruit, vegetables, and ornamentals? Hortic. Res. 2021, 8, 1. [Google Scholar] [CrossRef]
- Park, J.-E.; Kim, J.; Purevdorj, E.; Son, Y.-J.; Nho, C.W.; Yoo, G. Effects of long light exposure and drought stress on plant growth and glucosinolate production in pak choi (Brassica rapa subsp. chinensis). Food Chem. 2021, 340, 128167. [Google Scholar] [CrossRef]
- Chae, S.-H.; Min, S.G.; Moon, H.-W.; Jung, Y.B.; Park, S.H.; Seo, H.-Y.; Ku, K.-M. Kimchi cabbage (Brassica rapa subsp. pekinensis [Lour.]) Metabolic changes during growing seasons in the Republic of Korea. Hortic. Environ. Biotechnol. 2024, 65, 1–13. [Google Scholar] [CrossRef]
- Charron, C.S.; Sams, C.E. Glucosinolate Content and Myrosinase Activity in Rapid-cycling Brassica oleracea Grown in a Controlled Environment. J. Am. Soc. Hortic. Sci. 2004, 129, 321–330. [Google Scholar] [CrossRef]
- Hayata, Y.; Shinohara, Y.; Suzuki, Y. The Effect of High Temperature on the Growth and Endogenous Substances of Radish Root. J. Jpn. Soc. Hortic. Sci. 1986, 55, 51–55. [Google Scholar] [CrossRef]
- Barickman, T.C.; Ku, K.-M.; Sams, C.E. Differing precision irrigation thresholds for kale (Brassica oleracea L. var. acephala) induces changes in physiological performance, metabolites, and yield. Environ. Exp. Bot. 2020, 180, 104253. [Google Scholar] [CrossRef]
- Fang, K.; Xia, Z.; Li, H.; Jiang, X.; Qin, D.; Wang, Q.; Wang, Q.; Pan, C.; Li, B.; Wu, H. Genome-wide association analysis identified molecular markers associated with important tea flavor-related metabolites. Hortic. Res. 2021, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Maboud, M.M. Seasonal variations effect on antioxidant compounds and their role in the adaptation of some halophytes at Wadi Gharandal, Southwest Sinai. Ann. Agric. Sci. 2019, 64, 161–166. [Google Scholar] [CrossRef]
- Diljkan, M.; Škondrić, S.; Hasanagic, D.; Žabić, M.; Topalić-Trivunović, L.; Jimenez-Gallardo, C.; Kukavica, B. The antioxidant response of Hedera helix leaves to seasonal temperature variations. Bot. Serbica 2022, 46, 295–309. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, S.; Wang, Y.; Luo, S.; Yao, W.; He, H.; Tian, Y.; Li, H.; Zhang, F.; Sun, B. Variation of chlorophyll and carotenoids in different varieties and organs of Chinese kale. Qual. Assur. Saf. Crops Foods 2022, 14, 136–145. [Google Scholar] [CrossRef]
- Amri, Z.; Zaouay, F.; Lazreg-Aref, H.; Soltana, H.; Mneri, A.; Mars, M.; Hammami, M. Phytochemical content, Fatty acids composition and antioxidant potential of different pomegranate parts: Comparison between edible and non edible varieties grown in Tunisia. Int. J. Biol. Macromol. 2017, 104, 274–280. [Google Scholar] [CrossRef]
- Sun, B.; Liu, N.; Zhao, Y.; Yan, H.; Wang, Q. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dere, S.; Güneş, T.; Sivaci, R. Spectrophotometric Determination of Chlorophyll-A, B and Total Carotenoid Contents of Some Algae Species Using Different Solvents. Turk. J. Bot. 1998, 22, 13–17. [Google Scholar]
- Nisperos-Carriedo, M.O.; Buslig, B.S.; Shaw, P.E. Simultaneous detection of dehydroascorbic, ascorbic, and some organic acids in fruits and vegetables by HPLC. J. Agric. Food Chem. 1992, 40, 1127–1130. [Google Scholar] [CrossRef]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of Glucosinolates in Vegetable Crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Starzyńska, A.; Rożek, S. Antioxidant ability of broccoli flower buds during short-term storage. Food Chem. 2001, 72, 219–222. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Potential bioactive compounds in health promotion from broccoli cultivars grown in Spain. J. Sci. Food Agric. 2002, 82, 1293–1297. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; de Haro, A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate–myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Rosa, E.; Heaney, R. Seasonal variation in protein, mineral and glucosinolate composition of Portuguese cabbages and kale. Anim. Feed Sci. Technol. 1996, 57, 111–127. [Google Scholar] [CrossRef]
- Rosa, E.A.S.; Rodrigues, A.S. Total and Individual Glucosinolate Content in 11 Broccoli Cultivars Grown in Early and Late Seasons. HortScience 2001, 36, 56–59. [Google Scholar] [CrossRef]
- Kleinhenz, M.D.; Wszelaki, A. Yield and Relationships among Head Traits in Cabbage as Influenced by Planting Date and Cultivar. I. Fresh Market. HortScience 2003, 38, 1349–1354. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Mølmann, J.; Bengtsson, G.B.; Johansen, T.J. Influence of Day Length and Temperature on the Content of Health-Related Compounds in Broccoli (Brassica oleracea L. var. italica). J. Agric. Food Chem. 2013, 61, 10779–10786. [Google Scholar] [CrossRef]
- Liu, K.; Gao, M.; Jiang, H.; Ou, S.; Li, X.; He, R.; Li, Y.; Liu, H. Light Intensity and Photoperiod Affect Growth and Nutritional Quality of Brassica Microgreens. Molecules 2022, 27, 883. [Google Scholar] [CrossRef]
- Rosa, E.; David, M.; Gomes, M.H. Glucose, fructose and sucrose content in broccoli, white cabbage and Portuguese cabbage grown in early and late seasons. J. Sci. Food Agric. 2001, 81, 1145–1149. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Sherudilo, E.G.; Rubaeva, A.A.; Titov, A.F. Continuous LED Lighting Enhances Yield and Nutritional Value of Four Genotypes of Brassicaceae Microgreens. Plants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef] [PubMed]


| Season | Variety | GNA (μmol g−1 DW) | SIN (μmol g−1 DW) | PRO (nmol g−1 DW) | GRA (nmol g−1 DW) | GIB (nmol g−1 DW) | GNL (nmol g−1 DW) | GAL (nmol g−1 DW) | GER (nmol g−1 DW) | AGS (μmol g−1 DW) | GBS (μmol g−1 DW) | 4-OMGBS (nmol g−1 DW) | NGBS (nmol g−1 DW) | 4-OHGBS (nmol g−1 DW) | IGS (μmol g−1 DW) | GST/RGS (nmol g−1 DW) | GS (μmol g−1 DW) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fall | JLYC-01 | 2.63 ± 0.87 Aab | 0.89 ± 0.22 Bab | 97.5 ± 11.0 Aa | 33.9 ± 12.3 Aa | 5.9 ± 1.5 Ab | 24.8 ± 10.8 Aa | 11.9 ± 3.3 Ba | 17.7 ± 16.5 Aa | 3.71 ± 0.98 Aab | 0.86 ± 0.05 Ac | 67.9 ± 8.2 Aa | 92.0 ± 18.4 Bbc | 10.5 ± 5.6 ABa | 1.03 ± 0.06 Ac | 22.3 ± 20.9 Aa | 4.76 ± 1.05 Aab |
| JLYC-02 | 1.96 ± 0.24 Ab | 0.59 ± 0.16 Bab | 34.6 ± 1.2 Bb | 71.4 ± 42.1 Aa | 10.8 ± 2.5 Bb | 13.0 ± 7.4 Aab | 8.9 ± 3.9 Ba | 3.4 ± 3.5 Ba | 2.68 ± 0.45 Ab | 2.52 ± 0.40 Aa | 89.5 ± 15.0 Aa | 135.3 ± 9.3 Aa | 7.7 ± 5.0 ABa | 2.75 ± 0.42 Aa | 19.4 ± 8.4 Aa | 5.45 ± 0.35 Aab | |
| JLYC-03 | 1.46 ± 0.28 Ab | 0.50 ± 0.10 Bb | 20.6 ± 2.5 Bb | 41.2 ± 1.9 Aa | 7.0 ± 1.3 Ab | 7.4 ± 1.3 Ab | 6.4 ± 3.2 Ba | 10.2 ± 4.6 ABa | 2.05 ± 0.39 Bb | 1.83 ± 0.28 Aab | 62.5 ± 18.0 Ba | 35.7 ± 19.9 Bd | 7.9 ± 2.9 ABa | 1.93 ± 0.30 Aab | 35.2 ± 7.6 Ba | 4.02 ± 0.69 Abb | |
| JLYC-04 | 3.59 ± 0.71 Aa | 0.96 ± 0.14 Ba | 47.7 ± 28.9 Bb | 39.9 ± 7.1 Ba | 18.9 ± 4.3 Bb | 10.5 ± 5.8 ABab | 9.2 ± 5.0 Ba | 3.4 ± 1.2 Aa | 4.68 ± 0.80 Aa | 1.89 ± 0.43 Aab | 74.9 ± 8.7 Ba | 65.5 ± 11.8 Bcd | 8.8 ± 4.2 Ba | 2.04 ± 0.41 Aab | 18.1 ± 3.9 Ba | 6.74 ± 1.18 Aa | |
| JLYC-05 | 2.35 ± 0.22 Aab | 0.87 ± 0.14 Bab | 104.0 ± 19.6 Ba | 43.6 ± 13.4 Aa | 43.8 ± 19.8 Aa | 14.1 ± 0.7 Aab | 13.0 ± 3.2 Aa | 10.8 ± 6.9 Ba | 3.44 ± 0.40 ABab | 1.54 ± 0.11 Abc | 68.6 ± 4.4 Ba | 108.0 ± 10.5 Bab | 11.9 ± 1.1 Ba | 1.73 ± 0.13 Abc | 35.2 ± 11.6 Aa | 5.21 ± 0.27 Aab | |
| Winter | JLYC-01 | 1.47 ± 0.51 Ab | 0.33 ± 0.13 Cb | 20.7 ± 7.8 Bb | 32.6 ± 8.6 Ab | 3.8 ± 1.5 Ac | 9.6 ± 2.6 ABa | 8.5 ± 5.2 Bab | 3.7 ± 2.1 Aa | 1.88 ± 0.65 Bb | 0.62 ± 0.19 Aba | 72.2 ± 48.1 Ab | 62.5 ± 0.8 Bab | 2.0 ± 0.4 Ba | 0.76 ± 0.24 Aa | 25.5 ± 4.6 Aa | 2.67 ± 0.45 Bb |
| JLYC-02 | 1.84 ± 0.45 Ab | 0.69 ± 0.07 Bb | 62.6 ± 7.5 Ba | 60.9 ± 17.6 Aab | 23.9 ± 3.4 Abc | 13.5 ± 2.1 Aa | 21.0 ± 10.0 Ba | 5.5 ± 1.8 Ba | 2.72 ± 0.51 Ab | 0.85 ± 0.25 Ba | 109.6 ± 50.7 Ab | 57.2 ± 14.0 Bb | 5.7 ± 3.4 Ba | 1.02 ± 0.30 Ba | 38.8 ± 35.5 Aa | 3.77 ± 0.83 Bb | |
| JLYC-03 | 1.20 ± 0.28 Ab | 0.43 ± 0.13 Bb | 26.9 ± 5.3 Bb | 45.2 ± 18.7 Ab | 9.0 ± 0.7 Abc | 7.8 ± 2.8 Aa | 12.9 ± 2.2 Bab | 5.9 ± 1.8 Ba | 1.74 ± 0.39 Bb | 0.80 ± 0.27 Ba | 97.0 ± 9.5 Aa | 38.8 ± 15.5 Bb | 1.8 ± 0.8 Ba | 0.93 ± 0.29 Ba | 44.4 ± 15.2 ABa | 2.71 ± 0.57 Bb | |
| JLYC-04 | 2.94 ± 0.40 Aa | 1.18 ± 0.15 Aa | 77.9 ± 8.0 Ba | 118.4 ± 37.9 Aa | 73.5 ± 24.3 Aa | 16.5 ± 5.3 Aa | 13.3 ± 1.8 Bab | 4.9 ± 0.7 Aa | 4.43 ± 0.52 Aa | 0.94 ± 0.24 Ba | 267.8 ± 78.9 Aa | 75.9 ± 16.9 Bab | 4.8 ± 0.9 Ba | 1.28 ± 0.34 Aa | 76.3 ± 37.4 Aa | 5.79 ± 0.83 Aa | |
| JLYC-05 | 1.98 ± 0.34 Aab | 0.41 ± 0.27 Bb | 27.7 ± 3.6 Cb | 39.5 ± 15.2 Ab | 40.2 ± 15.4 Aab | 7.5 ± 3.3 Ba | 3.8 ± 1.0 Bb | 4.0 ± 1.1 Ba | 2.51 ± 0.04 Bb | 1.05 ± 0.31 Ba | 106.1 ± 41.5 Ab | 106.4 ± 29.9 Ba | 3.1 ± 1.4 Ba | 1.26 ± 0.38 Aa | 38.2 ± 17.1 Aa | 3.81 ± 0.36 Bb | |
| Spring | JLYC-01 | 2.70 ± 0.20 Aa | 1.29 ± 0.09 Aa | 71.7 ± 21.8 Ab | 37.3 ± 10.7 Aa | 3.7 ± 1.8 Aa | ND | 39.9 ± 8.6 Aab | 11.6 ± 2.9 Acd | 4.16 ± 0.27 Aab | 0.51 ± 0.07 Bb | 40.7 ± 6.8 Ac | 130.5 ± 14.7 Ac | 13.7 ± 3.3 Aa | 0.69 ± 0.09 Ab | 24.0 ± 5.4 Ac | 4.87 ± 0.35 Abc |
| JLYC-02 | 1.15 ± 0.06 Bc | 1.11 ± 0.10 Aa | 154.2 ± 53.8 Ab | 36.9 ± 7.7 Aa | ND | ND | 48.1 ± 10.2 Ba | 24.3 ± 3.9 Aab | 2.53 ± 0.17 Ab | 1.23 ± 0.13 Ba | 72.3 ± 7.4 Ab | 78.9 ± 4.9 Bd | 18.3 ± 4.6 Aa | 1.40 ± 0.15 Ba | 49.8 ± 9.0 Ab | 3.97 ± 0.26 Bc | |
| JLYC-03 | 1.58 ± 0.19 Abc | 1.72 ± 0.46 Aa | 122.7 ± 14.8 Ab | 41.6 ± 12.6 Aa | ND | ND | 33.8 ± 6.8 Aab | 15.5 ± 1.5 Abc | 3.51 ± 0.64 Ab | 1.44 ± 0.18 Aa | 51.8 ± 8.2 Bbc | 86.1 ± 13.6 Ad | 13.3 ± 5.2 Aa | 1.59 ± 0.20 ABa | 67.0 ± 2.4 Aa | 5.17 ± 0.44 Ab | |
| JLYC-04 | 2.75 ± 0.30 Aa | 1.63 ± 0.30 Aa | 1012.0 ± 171.6 Aa | 39.2 ± 16.2 Ba | ND | ND | 43.5 ± 15.2 Aab | 5.1 ± 1.8 Ad | 6.00 ± 0.77 Aa | 1.29 ± 0.08 ABa | 71.0 ± 10.5 Bb | 181.4 ± 13.1 Ab | 21.4 ± 1.5 Aa | 1.56 ± 0.09 Aa | 32.4 ± 1.3 Abc | 7.07 ± 0.19 Aa | |
| JLYC-05 | 2.08 ± 0.13 Ab | 1.51 ± 0.26 Aa | 175.9 ± 8.9 Ab | 32.6 ± 6.8 Aa | ND | ND | 18.3 ± 4.9 Ab | 21.6 ± 1.5 Aab | 4.43 ± 1.22 Aab | 1.20 ± 0.05 ABa | 154.5 ± 8.7 Aa | 281.2 ± 25.7 Aa | 16.3 ± 4.7 Aa | 1.65 ± 0.06 Aa | 57.3 ± 8.4 Aab | 5.55 ± 0.41 Ab |
| Parameter | Vs/Vp | Vv/Vp | Vsv/Vp |
|---|---|---|---|
| Plant height | 0.103 ** | 0.110 ** | 0.459 ** |
| Plant spreading | 0.0441 ** | 0.577 ** | 0.156 ** |
| Plant weight | 0.265 ** | 0.390 ** | 0.0790 ** |
| Reducing sugars | 0.557 ** | 0.273 ** | 0.123 ** |
| Soluble proteins | 0.667 ** | 0.234 ** | 0.0340 |
| Soluble solids | 0.776 ** | 0.0762 ** | 0.0609 * |
| Chlorophyll | 0.667 ** | 0.234 ** | 0.034 |
| Carotenoids | 0.288 ** | 0.477 ** | 0.0930 * |
| Vitamin C | 0.331 ** | 0.380 ** | 0.130 * |
| Total phenols | 0.0273 | 0.714 ** | 0.0402 |
| Antioxidant capacity | 0.0546 ** | 0.669 ** | 0.197 ** |
| Gluconapin | 0.0794 ** | 0.592 ** | 0.139 * |
| Sinigrin | 0.602 ** | 0.123 ** | 0.147 ** |
| Progoitrin | 0.249 ** | 0.255 ** | 0.470 ** |
| Glucoraphanin | 0.116 * | 0.199 ** | 0.354 ** |
| Glucoiberin | 0.312 ** | 0.00912 | 0.563 ** |
| Gluconapoleiferin | 0.552 ** | 0.0690 * | 0.172 ** |
| Glucoalyssin | 0.650 ** | 0.097 ** | 0.117 ** |
| Glucoerucin | 0.321 ** | 0.107 * | 0.286 ** |
| Glucobrassicin | 0.433 ** | 0.291 ** | 0.158 ** |
| 4-Methoxyglucobrassicin | 0.195 ** | 0.222 ** | 0.405 ** |
| Neoglucobrassicin | 0.330 ** | 0.340 ** | 0.285 ** |
| 4-Hydroxyglucobrassicin | 0.684 ** | 0.0526 | 0.0526 |
| Aliphatic glucosinolates | 0.270 ** | 0.0320 | 0.541 * |
| Indole glucosinolates | 0.374 ** | 0.313 ** | 0.174 ** |
| Aromatic glucosinolates/ Gluconasturtiin | 0.182 ** | 0.158 * | 0.273 * |
| Total glucosinolates | 0.233 ** | 0.0423 ** | 0.584 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Miao, H.; Zhang, F.; Sun, B.; Wang, Q. Seasonal Variation in Nutritional Substances in Varieties of Leafy Chinese Kale (Brassica oleracea var. alboglabra): A Pilot Trial. Agronomy 2025, 15, 671. https://doi.org/10.3390/agronomy15030671
Wang Y, Miao H, Zhang F, Sun B, Wang Q. Seasonal Variation in Nutritional Substances in Varieties of Leafy Chinese Kale (Brassica oleracea var. alboglabra): A Pilot Trial. Agronomy. 2025; 15(3):671. https://doi.org/10.3390/agronomy15030671
Chicago/Turabian StyleWang, Yating, Huiying Miao, Fen Zhang, Bo Sun, and Qiaomei Wang. 2025. "Seasonal Variation in Nutritional Substances in Varieties of Leafy Chinese Kale (Brassica oleracea var. alboglabra): A Pilot Trial" Agronomy 15, no. 3: 671. https://doi.org/10.3390/agronomy15030671
APA StyleWang, Y., Miao, H., Zhang, F., Sun, B., & Wang, Q. (2025). Seasonal Variation in Nutritional Substances in Varieties of Leafy Chinese Kale (Brassica oleracea var. alboglabra): A Pilot Trial. Agronomy, 15(3), 671. https://doi.org/10.3390/agronomy15030671









