Abstract
The Satsuma mandarin, a prominent fresh citrus variety cultivated in Asia, is susceptible to fruit cracking, a physiological disorder that significantly impacts yield and economic efficiency. This phenomenon occurs during the fruit expansion phase. The present study sought to further elucidate the correlation between citrus fruit cracking and fruit peel development or mineral elements, as well as to propose efficacious management measures. The present experiment was conducted on Citrus unshiu Marc. cv. ‘Miyagawa Wase’ over two successive seasons—2022 and 2023. The dynamic changes in fruit morphology were recorded using calipers, and the peel strength was assessed via a Plus Texture Analyzer. Paraffin sectioning technology was used to observe the morphological structure of peel cells. At 10 days after full bloom (DAFB), the peel cells exhibited vigorous proliferation, and the fruit and peel thicknesses underwent rapid expansion. At 50–60 d after full bloom, the longitudinal and transverse diameters of the fruit exhibited a marked increase in the growth rate of the former over the latter. At 80 d after full bloom, both the peel thickness change and the fruit growth rate exhibited a marked deceleration, and the albedo layer cells began to show signs of perforation. The following two time points were preliminarily proposed as the key points for the control of citrus fruit cracking: key point one was 50–60 days after full bloom; and key point two was 80–90 days after full bloom. The nitrogen (N), phosphorus (P), and potassium (K) contents in the different orchards were measured via the semi-micro Kjeldahl nitrogen method, the molybdenum–antimony colorimetric method, and flame photometry, respectively. The determination of other mineral elements was conducted by means of inductively coupled plasma spectroscopy. Principal component analysis was employed to analyze the 21-parameter indices of mineral elements in soil and leaf samples from the three orchards with different levels of fruit cracking. The study found that high concentrations of leaf Fe, P, and soil Cu, as well as organic matter content, contributed negatively to the extent of fruit cracking. The impact of diverse control measures on the incidence of fruit cracking was subsequently observed, following the implementation of tree crown spray treatments. The application of 0.5% calcium superphosphate and 0.006% EDTA-Fe, in combination with 10 ppm GA3 sprayed during two critical periods, significantly reduced fruit cracking and did not adversely affect the internal or external quality of the fruits. The study emphasises the necessity of customising management measures according to the developmental characteristics of citrus fruits, given the observed varietal and regional distinctions in susceptibility to cracking. These findings are pivotal for advancing research in the field of fruit cracking and promoting the healthy development of the industry.
1. Introduction
Citrus fruits are recognised as one of the most widely cultivated fruits globally. They are characterised by their rich contents of carbohydrates, minerals, and vitamin C. The variety and flavour diversity of citrus fruits make them suitable for fresh consumption, juicing, and processing, attracting a broad consumer base. As of 2023, global citrus production has surpassed 150 million tons, with an average per capita consumption of approximately 20 kg. However, during the maturation process of citrus fruits, a phenomenon known as fruit cracking occurs, particularly affecting oranges [1], mandarins [2], and certain hybrid citrus varieties [3] to varying degrees. This phenomenon has been shown to have a substantial impact on both yield and economic returns.
As a physiological disorder, fruit cracking has been observed in various fruit species, including sweet cherry [4], pomegranate [5], jujube [6], tomato [7], melon [8], persimmon [9] and nectarine [10]. The factors influencing fruit cracking include both internal and external elements. Among the internal factors, the most notable are gene expression regulation [11], the differential growth rates of fruit skin and pulp [12], carbohydrate concentrations [13], mineral element concentrations [8], hormonal imbalances [14], and the mechanical properties of the epidermal layer [15]. Among the external factors that have been identified as contributing to fruit cracking are the aspects of cultivation management practices [16,17], fluctuations in water availability [18], exposure to intense light, and significant temperature variations [5]. However, most research has identified severe fluctuations in water availability as the primary cause of fruit cracking. The rapid absorption of water by the fruit’s pulp can generate internal pressure that the peel is unable to withstand, leading to rupture [6,19]. Consequently, numerous preventative measures against fruit cracking have been developed, with a focus on water management strategies in orchards to mitigate prolonged moisture stress on fruits. Specifically, the irrigation of Nova mandarins at 75% of the Class A pan evaporation measured has been shown to minimise the incidence of fruit cracking [20]. Irrigation frequency also significantly modified the tree water status, and the frequency of irrigation was reduced from 30 l tree−1 d−1 every day to 50 l tree−1 d−1 every other day thus increasing fruit cracking in the Nova mandarins [21]. Nevertheless, there is currently a paucity of widely recognised water management techniques that address alternative theories involved in fruit cracking and have the potential for practical application. In light of the aforementioned factors, the regulation of mineral elements and growth regulators may offer a more promising avenue for mitigating the incidence of fruit cracking.
Dynamic changes in mineral elements have been demonstrated to significantly affect fruit growth, and nutritional imbalances have been shown to lead to developmental and metabolic disorders in the peel. Potassium (K), calcium (Ca), boron (B), magnesium (Mg), manganese (Mn), phosphorus (P), and zinc (Zn) have been identified as playing a pivotal role in this process [8,22]. K can maintain high osmotic and turgor pressures, providing power for cell division and wall extension [23,24]. Studies have shown that the foliar application of K2SO4 reduced the incidence of lemon fruit cracking [25]. Ca is involved in cell wall metabolism and functions as a molecular signalling agent, playing a crucial role in maintaining the cellular water balance [26,27]. Foliar-Ca application during the young fruit growth stage was found to have a more pronounced effect on reducing fruit cracking in Bingtang oranges [28]. The application of organic fertiliser, in the form of a spray containing 0.2%Ca and 0.2%K2SO4, has been demonstrated to be the most effective method of preventing cracked fruits, regardless of whether they are oranges or mandarins [29]. However, contradictory studies have reported that there is no significant difference in calcium content between normal and cracked navel orange fruit peels [30]. Some studies have indicated that elevated K or Ca levels in citrus fruit peels may be detrimental, leading to poor fruit quality, along with an increase in rind thickness [31,32], whereas peel thickness itself does not significantly impact fruit cracking [33,34]. Additionally, research has reported conflicting results regarding the other elements related to fruit cracking, particularly B [5,35] and P [36,37]. The diverse citrus varieties and planting environments may be the main reason for this difference.
Plant growth regulators have been demonstrated to exert a pivotal role in the growth and development of plants [38]. Abscisic acid (ABA) [39], indole-3-acetic acid (IAA) [40], gibberellic acid (GA3) [6], chlorfenuron (CPPU) [41], 2,4-dichlorophenoxyacetic acid (2,4-D) [42], and ethylene [43] have been proven to be pivotal in fruit cracking. Of these, GA3 has been the subject of the most extensive research, with findings suggesting that it may reduce cracking by increasing the elasticity of the fruit peel rather than its thickness [33,34]. Nonetheless, the mechanisms by which GA3 controls cracking are complex, and the effects of cultivar, environmental factors, dosage, and timing of application remain largely unknown. The results are often contradictory within the same fruit type [4,44], influenced by cultivar characteristics [34,45], environmental differences [46], and the timing, concentration, and frequency of GA3 application [47,48]. Consequently, further investigation is required into the mechanisms by which nutrients and growth regulators influence fruit cracking.
It is evident that different fruit species exhibit variations in their growth and developmental processes. The developmental process of fruits can be generally divided into two types. The first type is characterised by a double-sigmoid pattern of growth, as observed in stone fruits such as cherries and peaches. The second type is mainly represented by pome and citrus fruits, which display only a phase of rapid growth (single-sigmoid pattern). The occurrence of fruit cracking is typically observed in the middle-to-late stages of cell expansion. When accounting for temperature and light influences, the rapid fruit growth phase can be considered a critical period for cracking [49]. The cracking of the lemon fruit has been found to be related to the phases of its development, and the significant changes in several parameters during the process of rapid fruit expansion are significant causes of cracking [50].
During the process of citrus fruit expansion, changes in the peel’s characteristics, including quality, thickness, and chemical components, are closely related to fruit cracking [28,50]. In general, the fruit peel is constituted by two primary layers, the cuticle and the epidermis. These layers are responsible for the mechanical properties of the peel, its thickness, and the pathways for water absorption [51,52]. However, it is notable that the peel of citrus fruits exhibits distinctive characteristics, including a thick albedo (mesocarp) layer situated beneath the epidermal cells. The spongy white internal layer constitutes the primary component of peel thickness and functions as the primary protective barrier of the flesh, playing a significant role in buffering the expansion pressure from the pulp [53]. Consequently, it is highly advantageous to investigate the characteristic changes in citrus peels to facilitate a comprehensive understanding of the mechanism of citrus fruit cracking.
The Satsuma mandarin is a key fresh fruit variety that has gained significant market acceptance in Asia. In China, it has historically been a prominent item in the premium segment of the citrus market, characterised by its thin skin, minimal residue, and rich flavour, and is also the main source of income for many Satsuma mandarin growers. The incidence of fruit cracking has been observed to occur primarily at 110 and 150 DAFB, resulting in yield losses that have been documented to range from approximately 5% in normal years to over 50% in years characterised by severe conditions. The onset of cracking typically occurs at the stylar ends, extending towards the equatorial zone, with occasional initiation occurring at the equatorial region. The propensity for cracking is generally higher in medium-sized fruits and is more prevalent at the periphery of the canopy. However, in severe cases of cracking, no positional difference in the affected fruit is observed. The occurrence of early cracking in fruit is associated with an increased propensity for fruit detachment. However, in the later stages of fruit cracking, fruit detachment is less likely to occur, and the fruit is more susceptible to infection by mould, which can lead to rotting and further deterioration in the fruit.
In this study, the focus was on Citrus unshiu Marc. cv. Miyagawa Wase, a variety of Satsuma mandarins that is susceptible to cracking. The investigation was conducted with the objective of identifying the factors that contribute to citrus fruit cracking and monitoring the developmental course of the fruit. The study endeavoured to emphasise the physicochemical factors and temporal information that influence citrus fruit cracking. It also sought to explore the unknown factors affecting Miyagawa Wase fruit cracking and establish measures to enhance the overall effectiveness of fruit cracking prevention through the management of mineral nutrients and GA3 sprays. The findings are expected to provide important references for increasing the income of citrus farmers and for research on the mechanisms of fruit cracking.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Experiments were conducted during the 2022–2023 growing seasons in the high-quality production area of Satsuma mandarins (Citrus unshiu Marc. cv. Miyagawa Wase). The test material was 15- to 20-year-old Miyagawa Wase root-grafted on Poncirus trifoliate (L.) Raf. The rootstock was planted on reddish-yellow loam soil, which is acidic, in a mountain orchard located in Yongquan Town, Linhai city (between 28°40′ and 29°04′ N latitude and 120°49′ and 121°41′ E longitude, Zhejiang Province, China). The alkali-hydrolysed nitrogen, available phosphorus, rapidly available potassium, and organic matter concentrations of the soil, as well as the pH, are 177.11 mg/kg, 304.03 mg/kg, 242.98 mg/kg, 35.61 g/kg, and 4.78, respectively. This region experienced a humid subtropical monsoon climate, with an average annual temperature of 17 °C, a cumulative temperature of 5370 °C, a frost-free period of 241 days, and an annual precipitation of 1550 mm. The average annual daily temperature, annual precipitation, and annual sunshine hours in 2022 were 18.7, 1332.5 mm, and 1998.2 h, respectively, and the corresponding values for 2023 were 18.9, 1148.7 mm, and 2150.5 h, respectively. The precipitation showed high seasonality and occurred mostly in the spring and summer.
2.2. Fruit Characteristics Observation
In 2022, the fruit samples were collected at the onset of full bloom and then at regular intervals until maturity. At 10-day intervals, 20 sound fruits of uniform quality were harvested at random from the middle layers of the outer canopy of trees. The fruit surfaces were meticulously washed under running water and dried. Subsequently, the horizontal and vertical diameters of each fruit were measured using a caliper. The peel thickness was subsequently measured at the equatorial region after a transverse cut had been made. Concurrently, small pieces of peel (0.5 cm × 1.0 cm) were excised from the equatorial region and fixed in FAA fixative (70% ethanol/glacial acetic acid/formalin = 90:5:5). These samples were then subjected to a vacuum for 15 min, stored at 4 °C for 24 h, and subsequently brought to room temperature for paraffin embedding. Sections were then stained with Sudan red and solid green, producing permanent slides with a thickness of 5 μm, which were then observed under a microscope, with the images captured by an EOS 500D camera. The procedures for paraffin sectioning and observation were executed according to Naifu et al. (2018) [54].
During the period of maximum fruit cracking (25 August), 50 cracked fruits and 50 sound fruits were collected at random and assigned to two groups—the cracked fruit group and the normal fruit group. The dimensions of the fruit diameter (horizontal and vertical) and stem diameter were measured using calipers. The peel section samples were then taken from two opposing points in the centre region to measure peel strength, with the sampling point for the cracked fruits being chosen to avoid cracks. For the normal fruits, peel sections were also taken from the peduncle and stylar regions for strength measurement. The peel strength was then assessed via a texture analyser (TA.new plus Texture Analyser, Stable Micro Systems, Godalming, UK), which was equipped with a TA/2 probe and operated in compression mode. The pretest speed was set to 2 mm/s, and both the test and post-test speeds were set to 1 mm/s, with a trigger force of 5 gf. The peel was cut into 1.5 cm × 1.5 cm square pieces and secured onto a platform for puncture testing according to predetermined parameters.
2.3. Analysis of Orchard Nutrient Status
In 2022, three distinct orchards, exhibiting fruit cracking rates of 5.39%, 13.6%, and 28.57%, were selected for the purpose of determining their nutrient status. Subsequent to the maturation of the spring shoots (30 October), soil and leaf samples were collected. Each group of samples contained 4–6 sampling points (plants), arranged in an S-shaped configuration, with the leaf and soil samples being taken concurrently. The leaf samples were evenly collected around the tree crown at each sampling point. The leaves were collected from the second to third leaves (in a downward direction) at the top of the spring shoots of that year, with each composite sample comprising no fewer than 50 leaves. The soil samples were collected from the drip line down to a depth of 0–30 cm, with each tree being sampled from two points diagonally. The soil samples were then placed on a flat plate, the stones were removed, and the samples were crushed and thoroughly mixed. They were spread into a square and divided into four parts by drawing two diagonal lines. Two opposite parts were then combined into one part, where one combined section would be kept and the other discarded. If the obtained samples were still a lot, the above-mentioned steps were repeated until the mixed soil samples reached about 1 kg. Each orchard was sampled three times by this method, that is, three repetitions.
The soil pH was measured via the potentiometric method (soil–water ratio of 1:2.5), and the organic matter (SOM) content was determined via the potassium dichromate volumetric method with external heating. For mineral nutrient element determination, dried tissues were ground and sieved and then digested via the H2SO4–H2O2 method to prepare the test solutions. The nitrogen (N), phosphorus (P), and potassium (K) contents were measured via the semi–micro Kjeldahl nitrogen method, the molybdenum–antimony colorimetric method, and flame photometry, respectively, as described in detail in a previous study [55]. The calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), and boron (B) contents were determined via HNO3-HClO4 digestion, followed by inductively coupled plasma spectroscopy (HK-8100, Beijing Huake Yitong Analytical Instrument Co., Ltd., Beijing, China). All analyses were performed according to the method described by Li et al. (2021) [56].
2.4. Foliar Treatments
In 2023, a Miyagawa Wase orchard, known for past severe cracking events, was selected to conduct cracking prevention trials. A total of 36 uniform and healthy trees were arranged in a randomised complete block design. The experiment comprised six treatment groups, each consisting of six trees, with a single tree as a replicate (Table 1). All the experimental trees were subjected to the regular agricultural practices as recommended. The following treatments were applied via a backpack electric sprayer on June 10 and July 10.

Table 1.
Design of fruit cracking foliar treatments.
An equal volume of clear water was utilised as a control (CK). Each spray was applied either in the morning or afternoon on a day when it was sunny and guaranteed that no precipitation would fall within the following 24 h. All treatments employed Cu(OH)2 as opposed to mancozeb for comprehensive fungal disease management throughout the year.
The fruits were surveyed from seven days after the final spray application, and the total number of retained fruits per tree was recorded, followed by evaluation every five days, counting the number of cracked fruits until the peak cracking period ended (September 17). The cracking incidence (CI) was calculated as CI = (total number of cracked fruits/total number of retained fruits at first count) × 100%. The average cracking rate for each treatment was determined by removing the highest and lowest values among the remaining four measures. Quality assessments of the fruits collected on 20 November 2023, involved the random selection of 25 sound fruits from the outer canopy (at equal heights) for measuring peel thickness and strength. The total soluble solids (TSSs) and the titratable acidity (TA) content, as well as peel colour, were determined according to the protocol described by Qiu et al. (2021) [55].
2.5. Data Processing and Analysis
Statistical analyses were performed using the SPSS 17.0 software (IBM, Armonk, NY, USA). All treatment data were analysed for variance (ANOVA), and then the Independent Samples t-test, Student’s t-test, or Welch’s t-test were used to determine the differences between the treatments depending on the source of the data. p < 0.05, p < 0.01, or p < 0.001 were considered to indicate significance—significant, very significant, and extremely significant, respectively—between the groups. The values presented were the means ± SE.
The results of the statistical analyses were illustrated using the OriginPro 2021 software (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Fruit Growth and Changes in Peel Thickness
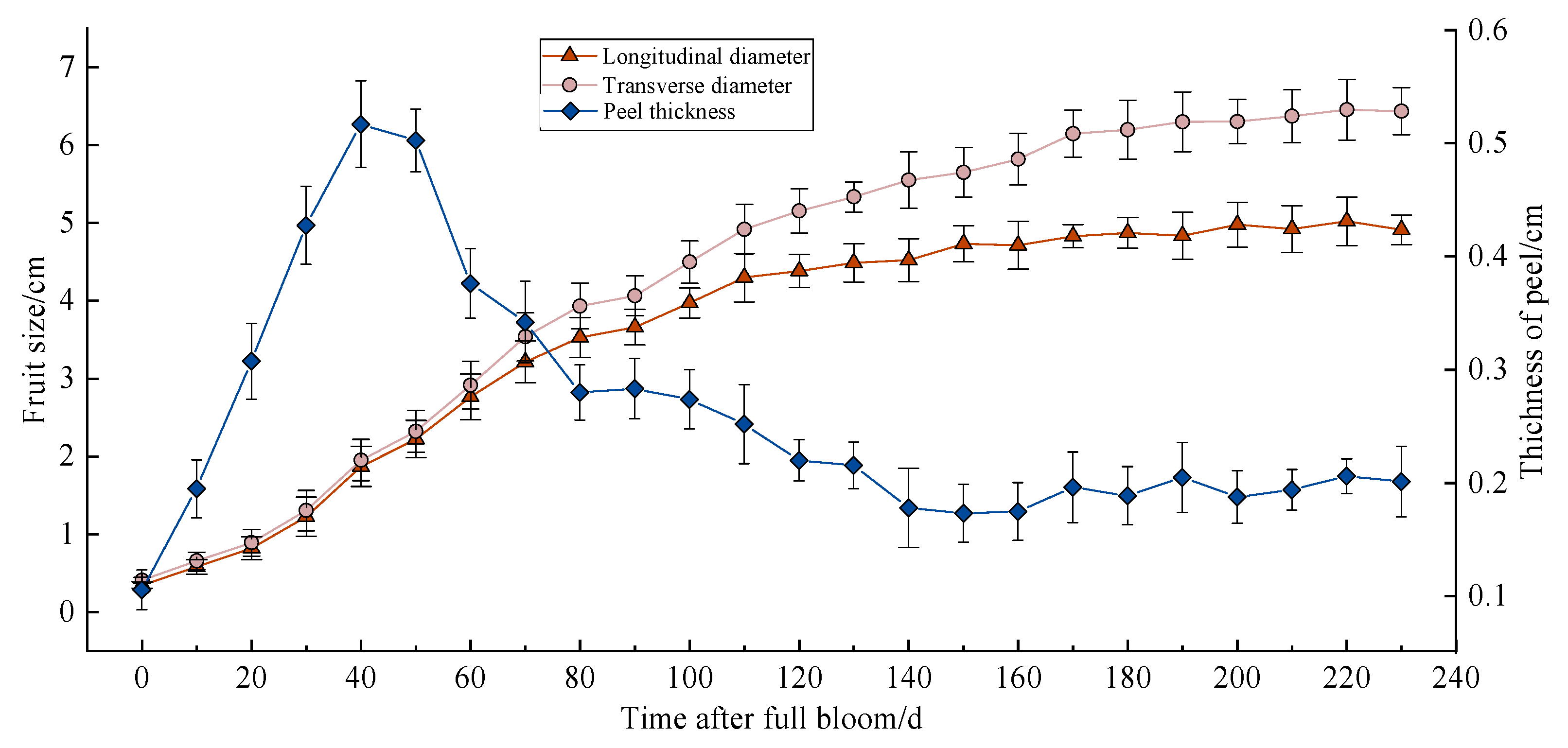
The annual growth process of Miyagawa Wase can be divided into the following three stages: the juvenile phase, the expansion phase, and the colour maturation phase. These stages are represented by a single sigmoid curve. As illustrated in Figure 1, the juvenile phase possesses the maximum growth rates at both diameters, maintaining a relatively consistent growth rate until the second physiological drop occurs, marking the transition to the expansion phase (60 days after full bloom (DAFB)). Subsequent to this transition, the growth rates in both dimensions decrease, with the radial growth rate surpassing the longitudinal growth rate, resulting in flattened spherical development. By the onset of colour maturation (170 DAFB), fruit enlargement ceases, with the shape index (length/width) stabilising at approximately 0.77. Figure 1 also displays the dynamic changes in peel thickness, which initially increase before decreasing. During the juvenile phase, increased cell division contributes to a rapid thickening of the peel, peaking at 0.52 cm at 40 DAFB (May 30). However, after mid-June (50 DAFB), the peel begins to thin significantly, reaching a minimum of 0.17 cm by 150 DAFB, and subsequently stabilizing at a relatively constant thickness. Significant decreases in both peel thickness and fruit growth rate were observed 80 days after full bloom. The overall rate of change in peel thickness exhibited a “fast–fast–slow–slow” trend. The onset of cracking occurred primarily between 110 and 150 DAFB, with the shape index ranging from 0.87 to 0.81 (1.14 to 1.23). At the peak of fruit cracking, the shape index was between 0.81 and 0.85 (1.18 to 1.23). This finding suggests a strong correlation between changes in the Miyagawa Wase fruit during development and its susceptibility to cracking.
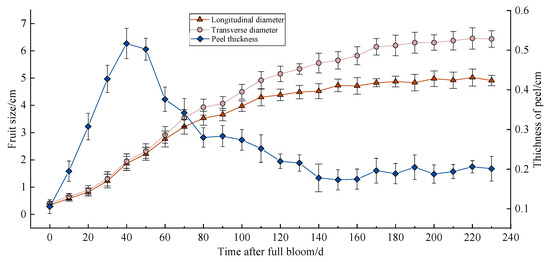
Figure 1.
Dynamic changes in Miyagawa Wase fruit development.
3.2. Relationships Among Cracking, Shape Index, Stem Diameter, and Peel Strength
A thicker fruit stem diameter implies more available conduits for moisture transport into the fruit, increasing instability in the fruit’s water status and its susceptibility to cracking [21]. In this study, no significant differences were noted in the fruit stem diameter between the cracked and normal fruits or in their respective fruit shape indices (Table 2). However, the peel strength of the normal citrus fruits was 20.31% greater than that of the cracked fruits, indicating that peel strength can serve as a critical reference for assessing crack resistance.

Table 2.
Differences in the fruit shape index, fruit stem diameter, and pericarp break force between cracked fruits and normal fruits.
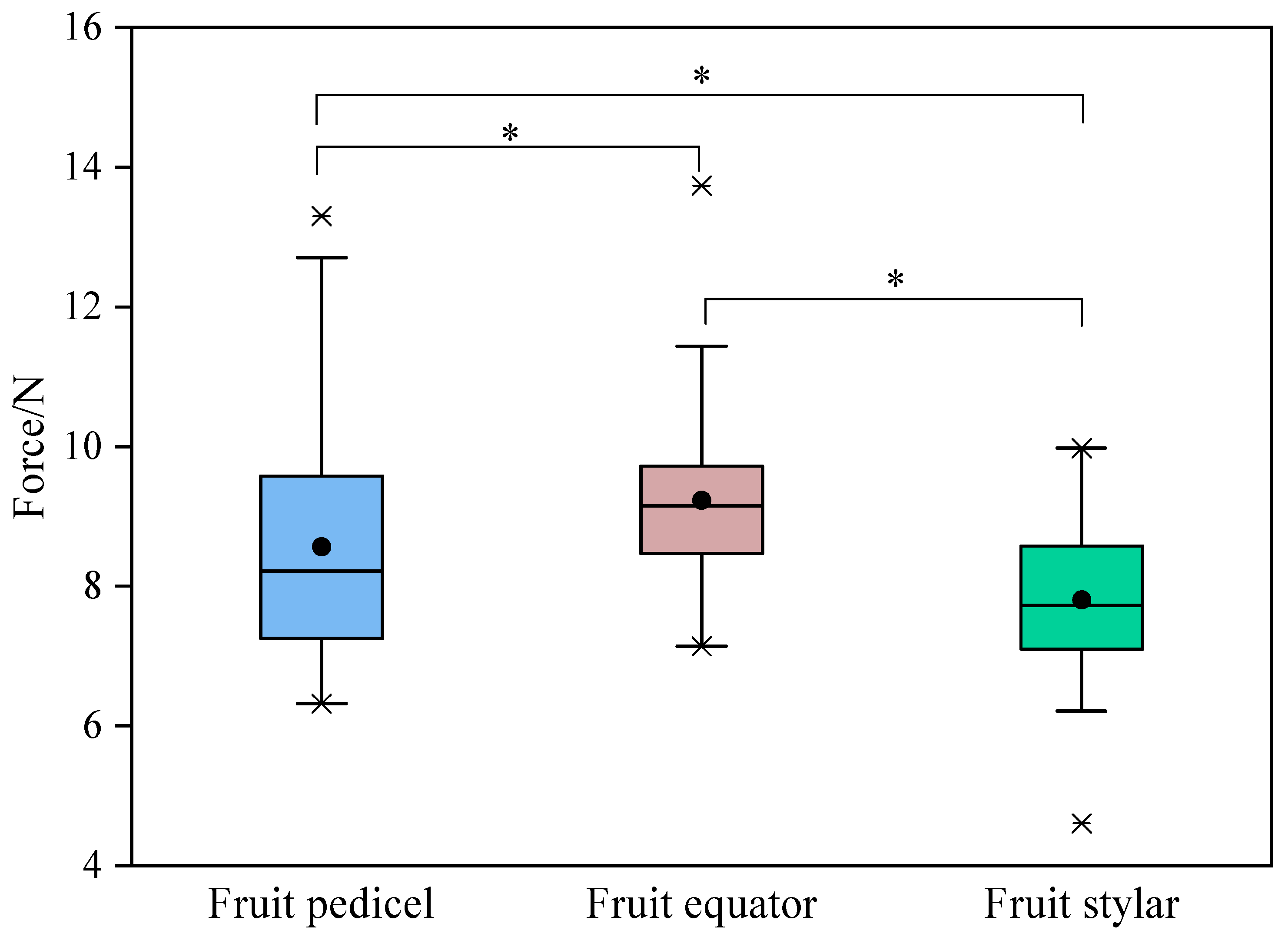
As demonstrated in Figure 2, there is a clear variation in peel strength across the different regions of the Miyagawa Wase fruit. It is noteworthy that the equatorial region exhibited the highest level of strength, followed by the fruit pedicel end, with significant disparities observed between these regions. The hypothesis derived from these findings is that the rapid lateral development of the pulp during the expansion phase imposes continuous stress on the peel, thereby compressing its thickness. This process may increase peel strength or toughness within a certain range. It is further hypothesised that variations in the management conditions and the positioning could lead to differences in the peel strength. As the expansion process continues, the accumulated stress may exceed the critical threshold for relatively weak peeling, resulting in cracking. It is observed that a significant proportion of cracks in Miyagawa Wase originate from the stylar end, with some also originating from the equatorial region, extending either longitudinally or laterally. Given that the peel thickness in the equatorial region of citrus fruits is significantly lower than those of the fruit pedicel and stylar ends, it is essential to implement the appropriate measures at the opportune time to regulate peel growth, with the aim of increasing peel strength to accommodate the increasing pulp swelling. The findings of this study suggest that subtle changes in peel strength may be more significant in inducing Miyagawa Wase fruit cracking compared to changes in fruit shape and fruit water potential.
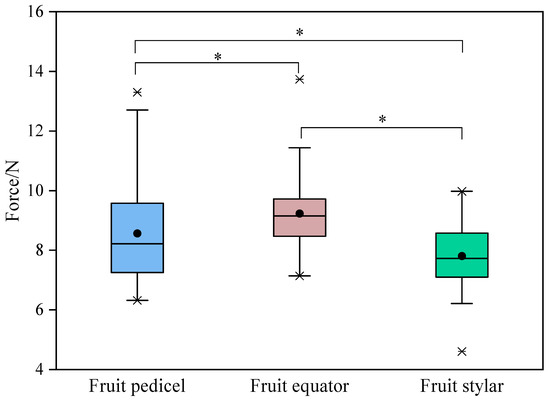
Figure 2.
Changes in peel strength in different regions of Miyagawa Wase fruit. *, indicate significant differences (p < 0.05) by Student’s t-test.
3.3. Changes in the Anatomical Structure of the Peel
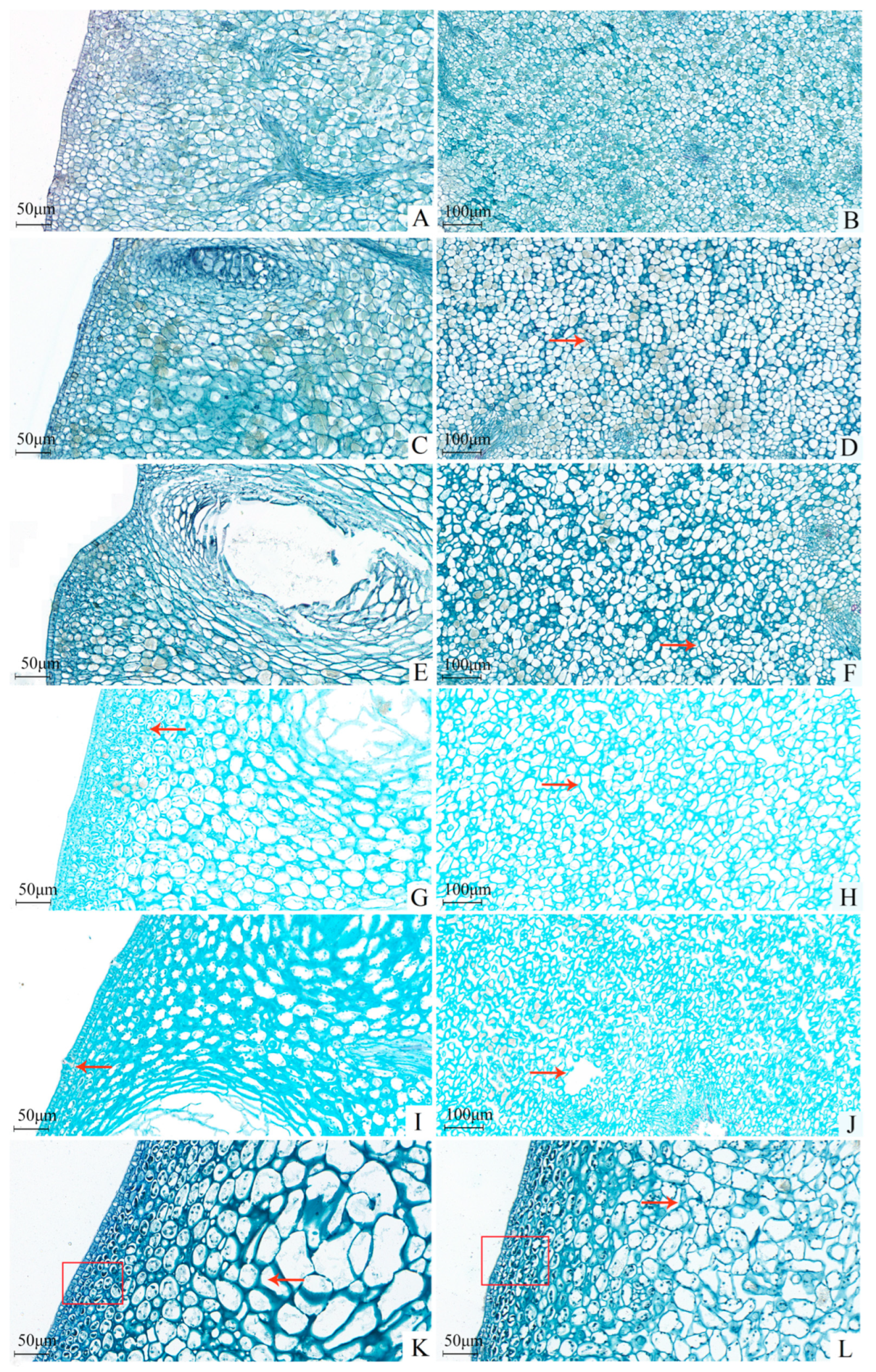
At 10 DAFB, the cells within the peel were actively dividing, resulting in tightly packed, corn-like albedo cells with minimal morphological variation (Figure 3). By 30 DAFB, cell division had significantly diminished, leading to microgaps among the albedo cells, although the connectivity remained high. At 40 DAFB, cell division had largely ceased, with cells retaining their structural integrity but showing an increasing trend in intercellular spaces. By 50 DAFB, the gaps among the albedo cells had begun to enlarge and connect, disrupting the previous uniformity and causing a loosening of the arrangement, thereby reducing the toughness of the spongy layer. The initial compactness of the epidermal cell layer had also begun to decrease. At approximately 90 DAFB, holes appeared in the albedo layer, resulting in cellular rupture and cytoplasmic leakage, further diminishing toughness and increasing susceptibility to cracking. Concurrently, the connections between epidermal layer cells were also compromised.
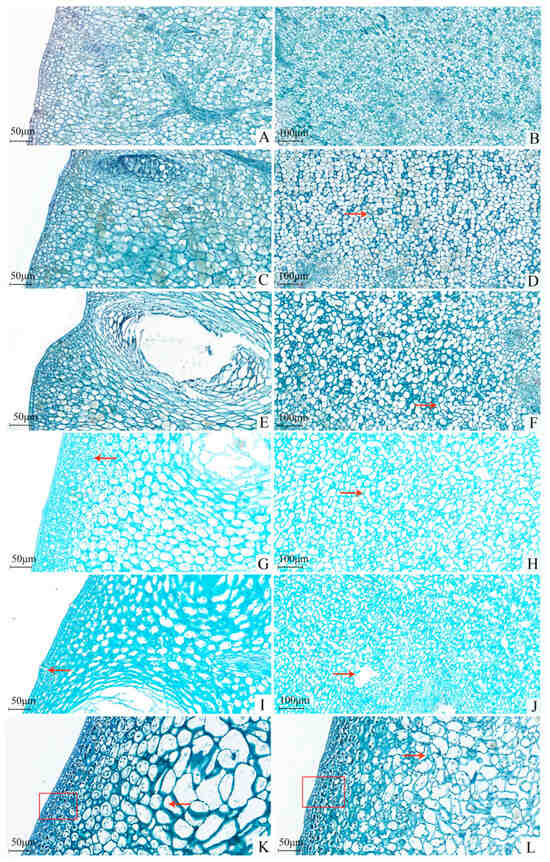
Figure 3.
The histological structure of the rind of Miyagawa Wase fruit. (A,C,E,G,I,K,L) Microstructure of the cuticular layer on the 10th, 30th, 40th, 50th, 90th and 120th day after full bloom (×200); (B,D,F,H,J) Microstructure of the albedo layer on the 10th, 30th, 40th, 50th, and 90th day after full bloom (×100). Red arrows or box marks indicate where the difference occurs.
In summary, the occurrence of cracking in Miyagawa Wase primarily arises from the asynchronous development of albedo cells relative to the overall fruit growth, resulting in reduced toughness. In the case of abnormal turgor pressure from the internal pulp, the albedo layer may tear, leading to voids and visible cracks on the peel surface.
3.4. Influence of Mineral Element Concentrations on Citrus Fruit Cracking
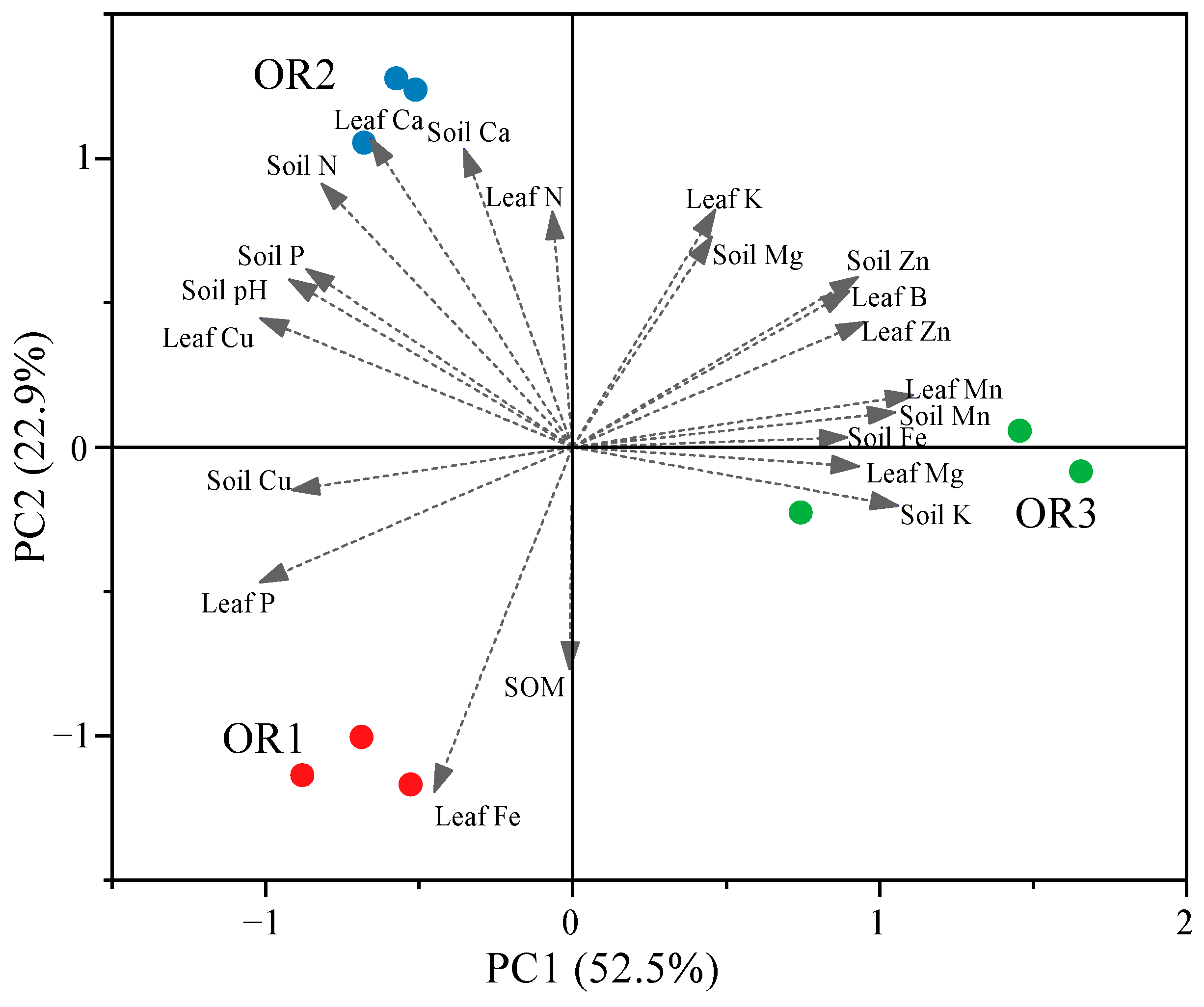
Principal component analysis (PCA) was used to determine the important mineral elements of the orchards with different degrees of fruit cracking. A criterion of eigenvalues (OAVs) ≥ 1 was applied to assess 21 measured parameters from the soil and leaf samples for PCA. The resulting biplot of this analysis is depicted in Figure 4.
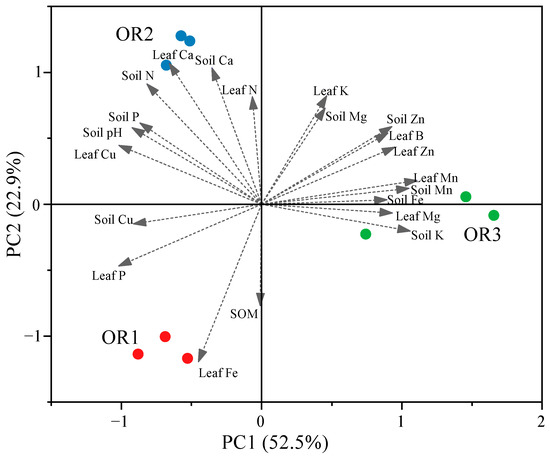
Figure 4.
Principal component analysis (PCA) biplot of the nutrient content properties in orchards with different degrees of cracked fruit of Miyagawa Wase. The arrows are the vectors and show the direction in which shows the correlation between different nutrient factors and the distribution of the highest levels of nutrient content. OR1, OR2, and OR3 scores are differentiated to indicate the level of fruit cracking rates of 5.39%, 13.6%, and 28.57%.
Orchard 1 (OR1), with the lowest fruit cracking rate, was located on the same side as the leaf Fe and P and soil Cu and organic matter (SOM) concentrations, indicating that these characteristics may make a greater contribution to preventing fruit cracking than those characteristics located near Orchard 3 (OR3), which had severe fruit cracking. On the other hand, the leaf and soil Mn and Zn, as well as the soil Fe and K, concentrations demonstrated higher contributions to Orchard 3, indicating that these characteristics were positively correlated to fruit cracking, as were leaf B and leaf Mg. Therefore, the spatial distribution of mineral elements plays a critical role in the process of citrus fruit cracking. We speculate that the mineral elements which play an important role in citrus fruit cracking regulation may be richer or more complex.
3.5. Effects of Different Measures on Cracking Incidence and Fruit Quality
Based on the significant changes observed in fruit morphology and peel characteristics during development, two key periods of interest were identified. The first of these key periods occurred around mid-June (50–60 DAFB), shortly after cell division in the albedo layer ceased and when peel thickness reached its peak before beginning to thin. During this period, the growth rate of the fruit’s horizontal diameter surpassed that of its vertical diameter. This coincided with the second physiological fruit drop peak period of the Satsuma mandarins. Key point two, occurring around mid-July (80–90 DAFB), marked a period of rapid fruit expansion and carbohydrate accumulation, characterised by the continuous widening of fissures between the albedo cells, potentially leading to void formation. This period was characterised by high temperatures and low rainfall, and the changes in the diameter of the fruit and the thickness of the peel underwent slow yet transient changes. This was shown to exacerbate the negative effects on the physicochemical properties of the peel. The second critical period was characterised by the rapid accumulation of total soluble solids in the Satsuma mandarins, coinciding with the cessation of summer shoot growth and physiological fruit drop.
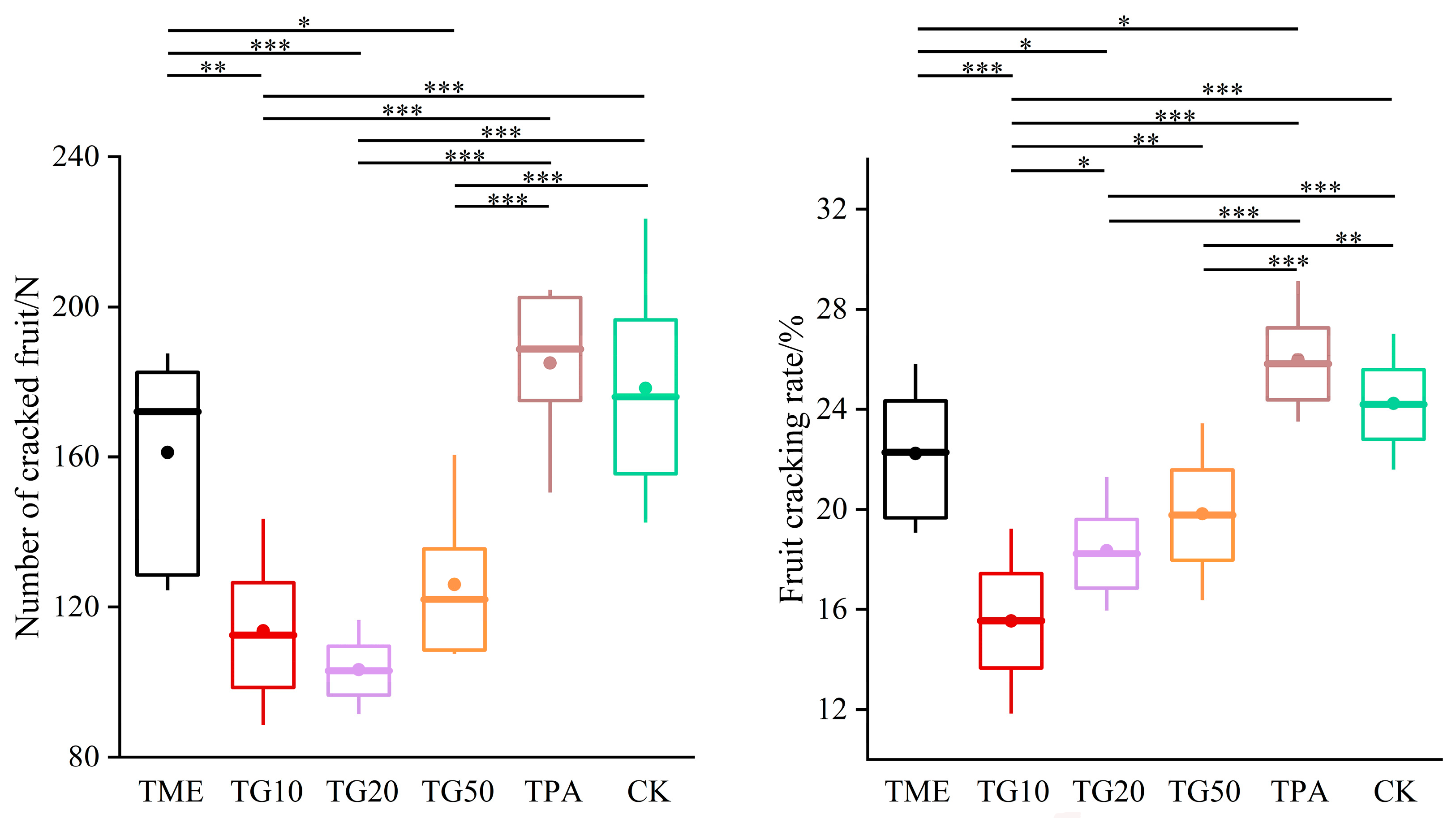
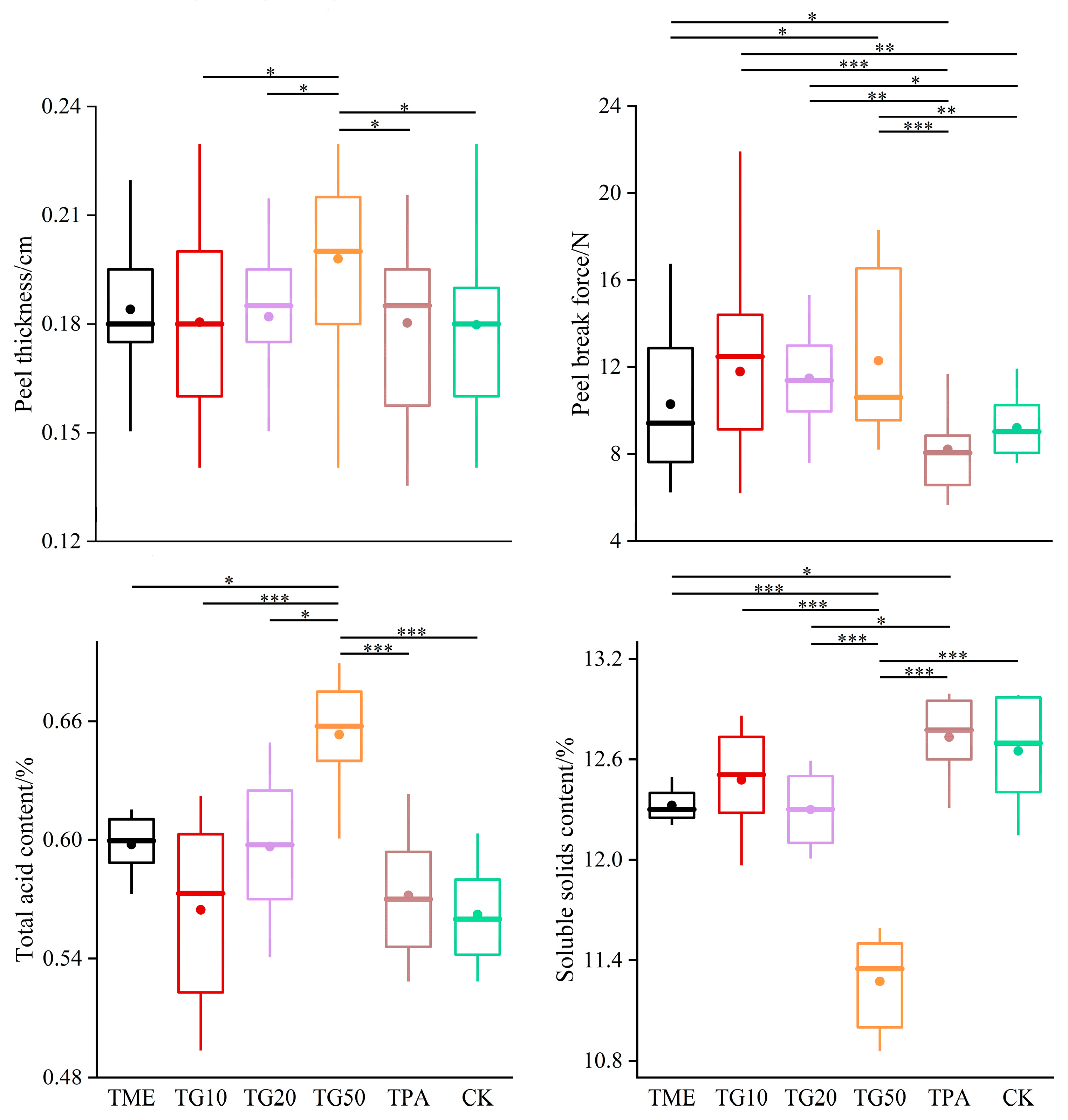
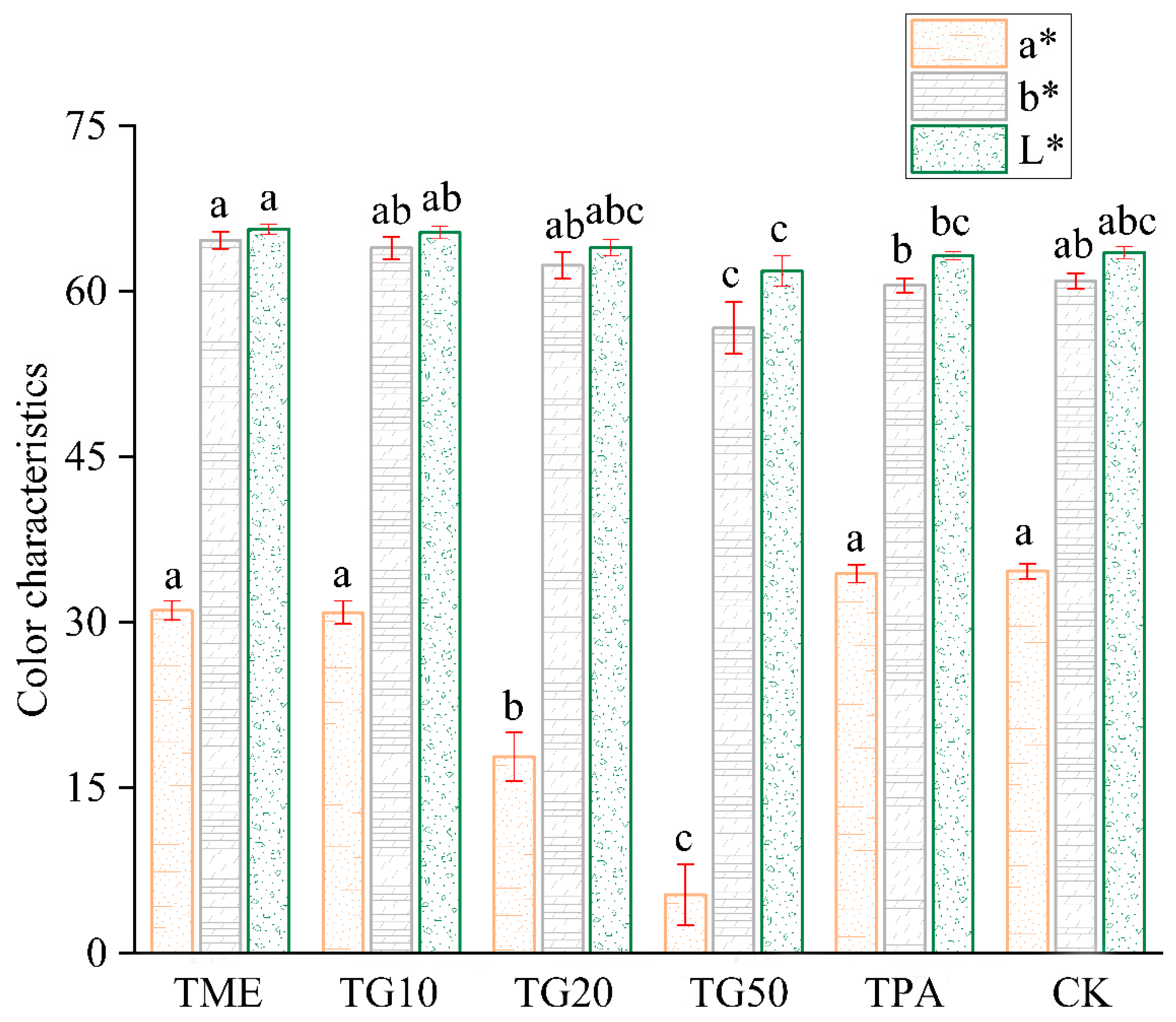
In the two periods, several treatments were established in order to investigate their impact on cracking incidence and fruit quality. The results are presented in Figure 5. The application of mineral element management (TME) resulted in a reduction in the number of cracked fruits and a decrease in the cracking rate. The differences observed in comparison to the control (CK) were not statistically significant. However, the combination of TME with the varying concentrations of GA3 (10 ppm, 20 ppm, and 50 ppm) exhibited a significant influence on the cracking rates, with the rates decreasing to 35.87%, 24.23%, and 18.19%, respectively, compared to the CK. It is also notable that variations in the GA3 concentration produced discrepancies in the outcomes. The impact of GA3 on citrus cracking can be attributed to the alterations in peel strength, with TG10, TG20, and TG50 increasing the peel strength by 28.1%, 24.75%, and 33.5%, respectively (Figure 6). Furthermore, TG50 significantly altered peel thickness, while no significant differences in peel strength were detected among the TG10, TG20, and TG50 treatments. This finding indicates that, within certain limits, there is no clear correlation between peel thickness and strength. Higher concentrations of GA3 (20 ppm, 50 ppm) adversely affected TA reduction and SSC accumulation in the fruit and affected chlorophyll degradation and anthocyanin production (Figure 7). Overall, the TG10 treatment resulted in the most effective control of cracking incidence. Notably, the application of an antitranspirant (pinolene) had no significant effect on either the incidence of cracking or the internal or external quality of the fruit, possibly due to the inappropriate timing of application.
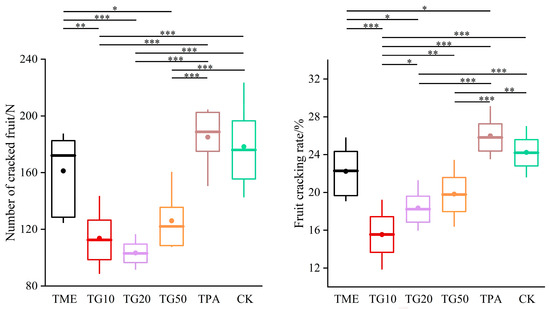
Figure 5.
Effect of different treatments on Miyagawa Wase fruit cracking. The values are the means ± SE of 4 individual analyses. Significance indicated by the asterisks (unpaired two-sided Welch’s t-test. p value: *, <0.05; **, <0.01; ***, <0.001). p < 0.05, p < 0.01 or p < 0.001 were considered to indicate significance, very significant, and extremely significant between groups.
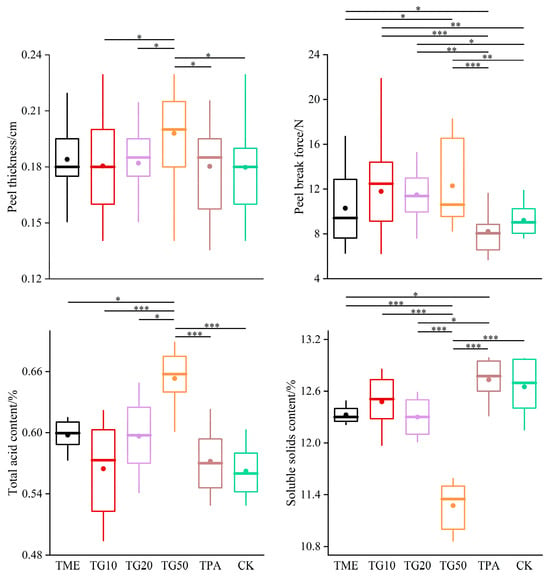
Figure 6.
Effect of different treatments on Miyagawa Wase fruit quality. Significance indicated by the asterisks (unpaired two-sided Welch’s t-test. p value: *, <0.05; **, <0.01; ***, <0.001). p < 0.05, p < 0.01 or p < 0.001 were considered to indicate significance, very significant, and extremely significant between groups.
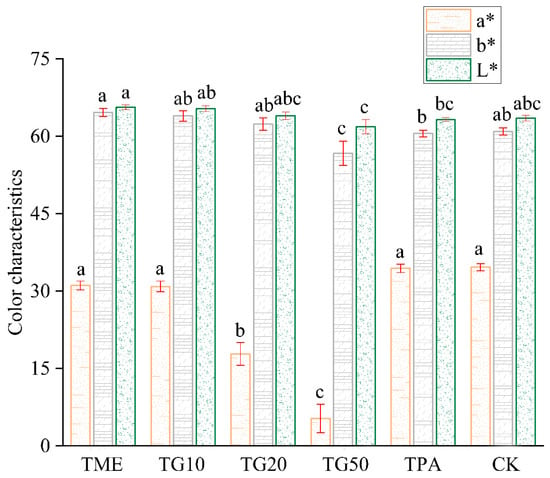
Figure 7.
Effect of different treatments on Miyagawa Wase fruit colour characteristics. Different lower-case letters within columns indicate significant characteristic differences and the same letter indicates no significant difference according to Student’s t-test (p < 0.05).
A comparison of the CK (Figure 3L) and TG10 treatments (Figure 3K) revealed that TG10 moderately enhanced peel toughness by increasing the volume of albedo cells and reducing the number of irregular cells, thereby increasing the intercellular contact area. The layering of cells near the epidermis became more pronounced, exhibiting improved uniformity and denser arrangements, indicative of enhanced intercellular connectivity and compactness. These findings further validate that GA3 treatment can increase peel strength, with the epidermal layer being the primary contributor to peel strength [7,57], and Ca, P, and Fe also play a positive role.
4. Discussion
Citrus fruit cracking is recognised as a major physiological disorder, leading to substantial economic losses. Miyagawa Wase, a variety of Satsuma mandarins, is particularly susceptible to this disorder, with the onset of cracking occurring at an earlier stage compared to other citrus varieties [58]. The occurrence of cracking is primarily observed during the phase of rapid fruit expansion, particularly following prolonged periods of high temperatures and drought, which are subsequently interrupted by sudden rainfall events that induce widespread fruit cracking. Other potential internal factors, including hormonal imbalances, mineral element deficiencies, and fruit developmental processes, also contribute to the incidence of citrus cracking. To explore effective measures for preventing fruit cracking, this study investigated the mechanisms behind cracking in Miyagawa Wase from multiple angles and established the strategies to alleviate cracking through enhancing peel strength.
4.1. Correlation Between Citrus Peel Characteristics and Water Transport
It is widely believed that the influx of water through xylem vessels into the fruit is a primary factor leading to citrus fruit cracking [18]. However, the results of this study indicate that the diameter of the fruit stem is not the main reason for the rapid entry of large amounts of water into the fruit, which results in peel cracking. We propose that direct absorption of water through the peel into the fruit may be a significant contributing factor for citrus cracking, which is aligned with the findings of a previous study on cherry fruit cracking, in which direct absorption of water directly from the fruit surface resulted in the blockage of cuticle membrane extension, which in turn led to fruit cracking [4,59].
Water movement into the fruit is influenced by water potential gradients. Unlike other fruits, citrus comprises both peel and juice segments; the peel consists of the flavedo and the albedo, whereas the pulp consists of 9–13 juice segments and 16–24 layers of segment membranes, each composed of 14–20 cell layers with cell wall thicknesses ranging from 1.6 to 3.6 μm [60]. This thickness exceeds that of both the fruit granules [61] and the typical fruit cell walls, which generally average approximately 1 μm [62,63]. Compared with the other structural components of the pulp, cell walls pose the greatest barrier to water transport [64]. Therefore, the segment membranes in citrus significantly impede rapid water transport, making it challenging for water entering from the peduncle to quickly establish a large water potential gradient.
The structural development of the peel is also of crucial importance in studies of fruit cracking. The peel can be likened to a semipermeable membrane that regulates the rate of water movement in or out [65,66], with the albedo tissue acting as a water reservoir [49]. Direct water entry through the peel into the pulp could be a significant cause of cracking [67]. Peet (1992) [68] hypothesised that water influx into fruit frequently coincides with alterations in peel strength, and that measures aimed at enhancing peel strength can serve to mitigate the occurrence of cracking. The findings of this study demonstrate that the onset of cracking in Miyagawa Wase typically occurs in the least robust regions of the peel, specifically at the fruit apex. Consequently, the study of citrus fruit cracking should focus more on the process of fruit water absorption through the peel and the changes in peel characteristics that occur during this process; strengthening the peel appears to be a more important approach in mitigating cracking in Miyagawa Satsuma mandarins.
4.2. Relationship Between Cracking in Miyagawa Wase and Fruit Development Processes
The occurrence of cracking in fruit is closely linked to the morphological changes that take place during the process of fruit development. The rate of fruit growth has been identified as a significant factor influencing the susceptibility to cracking [37]. During development, if the absolute growth rate of the horizontal diameter exceeds that of the vertical diameter, the mechanical stress imposed on the peel increases and its distribution is altered, leading to crack formation in areas experiencing maximal stress [7,68,69]. The present study also revealed that cracking in Miyagawa Wase predominantly occurred between 110 and 150 DAFB, with the shape index shifting from 0.87 to 0.81. During the middle to later stages of fruit expansion, the absolute growth rate of the transverse diameter surpassed that of the longitudinal diameter, thereby imposing maximum stress on the fruit stylar area with the lowest peel strength, rendering it more susceptible to cracking. This explains why most cracks in Miyagawa Wase initiate from the stylar end. However, some cracks originate from the equatorial region, extending laterally or longitudinally toward the apex because the equatorial region is subjected to stress from the pulp first during the elliptical growth of the fruit.
Furthermore, the investigation revealed that there was no significant difference in the fruit shape index between the cracked and normal fruits, which is in contrast with the findings reported in the Clementina and Nova mandarin varieties [42]. One potential explanation for this phenomenon is that the rapid uptake of water by the fruit during a brief period may result in alterations to its shape. However, the fruit shape index is known to recover once the volume of the pulp exceeds that of the peel.
During the fruit enlargement process, abnormalities in the structural development of peel cells (including the epidermal layer and albedo layer) arose. For example, at approximately 30 DAFB, microfractures had begun to form between the albedo cells during the middle phase of cell division. At this stage, the majority of albedo cells were formed, determining the structural integrity of the peel. As development continued, the gaps expanded and even connected, leading to the formation of cracks in the albedo layer. At 50 days after full bloom, the volume of epidermal cells increased, causing them to lose their original compactness, which significantly altered the mechanical properties and crack resistance of both the albedo and fruit peel [7,70]. Research has indicated that the emergence of cracks in the albedo layer results from internal stress exerted by rapidly growing pulp, suggesting that stress during Stage I of fruit development is a critical determinant of orchard susceptibility to fruit cracking [19]. Therefore, on the basis of data regarding changes in fruit shape, we propose that prevention measures against cracking in Miyagawa Satsuma mandarins should begin early, ideally at the peak onset of the second physiological fruit drop (50–60 DAFB) or, even sooner, during the first physiological fruit drop (later phase of Stage I fruit development). Another key time point may occur between July 10 and July 20 (80–90 DAFB), during which the fruit diameter and the peel thickness experience gradual changes, often coinciding with brief periods of high temperatures and drought, potentially subjecting the peel to significant physical stress.
4.3. Differences in Mineral Element Concentrations Affecting Cracking
Research has indicated that several mineral elements, such as B, Ca, Mg, K, and Zn, are associated with fruit cracking [8,19]. Calcium applications have been shown to inhibit the expression of cell wall degradation genes, thereby increasing peel resistance [26]. Spraying Ca(NO3)2 has been found to reduce cracking in ‘Hongjiang’ oranges. Furthermore, the increased application of K, Zn, Mg, B, and Mn fertilisers has been shown to reduce fruit cracking or increase yield in ‘Page’ mandarins [71], ‘Terigas’ mandarins [72], Clementine mandarins [73], Shogun mandarins [74], and sweet oranges [75], although the extent of this effect varies. However, our findings indicate that the elevated concentrations of Mn, Zn, soil Fe, and K, along with leaf Mg and B, are positively correlated with increased cracking rates. Conversely, the high levels of SOM, Cu, leaf Fe, and P are negatively correlated with cracking incidence. These findings present significant discrepancies with those of the previous studies. The observed discrepancies may be attributed to variations in the concentrations of related elements attributable to divergent management practices.
K is essential for maintaining high osmotic and turgor pressures, supplying energy for cell division, wall extension, and growth. High potassium levels promote fruit enlargement and yield thicker, smoother peels [23]. Leaf potassium concentrations between 0.62% and 0.78% show no significant linear correlation with cracking, yet a concentration near 0.71% corresponds to the lowest cracking rates [76]. In this study, the leaf K levels ranged from 0.81% to 1.0%, indicating a positive correlation with cracking incidence. Premature potassium applications may increase setting rates and competition among fruits, leading to increased cracking susceptibility [77]. Furthermore, excessive potassium can contribute to rough peels and poor flavours [32]. Therefore, the time and concentration of potassium need to be determined.
P plays a crucial role in the composition of proteins and nucleic acids, influences nitrogen metabolism and carbohydrate transport [78], is also involved in signal transduction and photosynthesis in plants, and plays an important role in the process of plant stress resistance [79]. Higher phosphorus levels result in thinner fruit peels, which are positively correlated with increased cracking rates [22]. Conversely, our study indicates that leaf phosphorus contents of 0.23%, 0.16%, and 0.06% corresponded negatively with the cracking rate. According to the reports of Mongi Zekri et al. [80], the appropriate P content of citrus leaves ranges from 0.09 to 0.3%, which means that P is deficient, suggesting that phosphorus deficiency may exacerbate cracking fruit.
Mg is vital for photosynthesis and protein synthesis and contributes to the cell wall structure as a key cofactor for numerous enzymes [81,82]. Thus, Mg affects cell wall formation and hence fruit cracking. In this sense, we found that the Mg content was approximately 0.24% in all the orchards with low fruit cracking and only 0.31% in the orchards with severe fruit cracking. The optimum concentration of Mg in citrus leaves is generally in the range of 0.26–0.6% [83]. Thus, Mg as a single element does not significantly affect citrus fruit cracking, but there are strong and moderate correlations with some element, such as K, Ca, or Mn [8], which act together to affect fruit cracking.
Mn influences lignin biosynthesis and amino acid synthesis [84], impacting rind hardness; excessive levels may render the rind too rigid and inflexible, whereas deficiencies can reduce hardness. In our findings, the Mn concentration increased from 171.6 mg/kg to 556.94 mg/kg with increasing cracking rates, indicating a positive correlation. Excessive levels of Mn affect the lignin content of the peel, making the peel too stiff and inelastic thus increasing the incidence of cracking [8], and Mn2+ leads to an increase in the activity of indoleacetic acid (IAA) oxidase, the breakdown and reduction of IAA, and the blockage of cell wall elongation [22].
Zn is essential for membrane integrity, phospholipid levels, and ion transport systems [85,86] thus influencing water absorption and damage prevention. Leaf applications of chelated zinc during early fruit development stimulate cell expansion and accelerate growth [87]. In this study, the leaf Zn concentration gradually increased from 23.2 mg/kg with a low fruit cracking rate to 34.56 mg/kg with high fruit cracking rate, with consistent trends observed at the soil zinc level. These findings indicate that the concentration of Zn in fruits with a high fruit cracking rate was relatively high, which could stimulate fruit expansion and water absorption, exacerbating cracking.
B is critical for cell wall biosynthesis and the development of new tissues [32]. The apiosyl residue of rhamnogalacturonan-II (RGII) is associated with two monomers of borate cross-links [33,34], providing cell wall elasticity [15]. In addition to its structural function, B affects membrane permeability and integrity by increasing potassium permeability and calcium binding to the membrane. Our data revealed B levels of 39.53 mg/kg, 54.35 mg/kg, and 70.11 mg/kg for varying degrees of cracking severity, falling within the optimal nutrient concentration range for citrus trees [88]. Tariq et al. (2007) [75] also reported that spraying boron on leaves may soften fruit peels, potentially exacerbating cracking. Therefore, the application of B in citrus fruit cracking needs to be treated with caution.
There is a paucity of direct research linking Cu and Fe to fruit cracking. In the present study, the citrus plants exhibited leaf concentrations of Cu at 5.99 mg/kg and Fe at 150 mg/kg in the low-cracking orchards, which decreased to 3.01 mg/kg and 83.94 mg/kg, respectively, in high-cracking orchards. The concentrations of Fe in the present study were both within the moderate range, whereas the concentration of Cu in the leaves of orchards with severe occurrence of cracked fruits was within the borderline of deficiency [89]. According to the previous reports, mild Cu deficiency has been shown to result in cell lignification, leading to the stylar end cracking of orange fruits [89,90]. Insufficient Fe levels may arise from excessive Mn [22]. Conversely, the application of CuCl2 and FeCl3 has been observed to impede the ingress of water into the fruit interior, thereby mitigating the subsequent surge in fruit turgor pressure [91]. The findings suggest that the judicious application of Cu and Fe can contribute to the mitigation of citrus fruit cracking. The management of nutrients in citrus orchards is a complex process, necessitating a comprehensive assessment of the overall nutrient status to address cracking effectively.
4.4. Feasible Measures for Reducing Cracking in Miyagawa Wase
A substantial body of research has been dedicated to investigating strategies for mitigating fruit cracking, frequently involving the application of plant growth regulators to the foliage. However, discrepancies in research outcomes are frequently ascribed to the variations in target species, cultivation environments, application timing, and concentration of reagent. The timing of application may hold greater significance than the concentration itself [92]. The present study utilised the widely recognised gibberellic acid (GA3) as a growth regulator, in combination with SSP and EDTA-Fe for foliar applications. The application of 10 ppm GA3, alongside 0.5% SSP and 0.006% EDTA-Fe, during the critical periods of 50–60 d and 80–90 DAFB improved peel strength by changing the structural characteristics of peel cells, significantly reducing the fruit cracking rate by 35.87%, and it did not affect fruit quality characteristics.
The previous results achieved by models comparing growth regulators alone or in combination with mineral elements demonstrated a reduced cracking/splitting rate of 6% for Nova hybrid mandarins [42], 16% for Nova mandarins [93], 11.41% for Eureka lemons [76], 20.8% for lemons [94], 9.84% for Daisy mandarins [95], and 23% for Page mandarins [71]. The performance of GA3+P and Fe spraying achieved in this study was superior to the aforementioned results, validating the feasibility of this approach for preventing cracking in citrus fruits.
However, the results of this study are also slightly inferior or similar to those obtained by Habibi et al. (2021) [96], who employed 100 mg L−1 GA3 and 20 mg∙L−1 2, 4-D for Thompson navel oranges, and Stander et al. (2013, 2014) [97,98], who employed 10 mg∙L−1 2, 4-D for ‘Marisol’ and ‘Mor’ mandarins; however, the rinds in these results are relatively coarse, which is unfavourable for the production and sale of the fine fruit of Miyagawa Wase.
In agricultural production practice, the frequency of management has a significant impact on cost savings and management timeliness, etc., which can be constrained by labour and the weather. The two spray managements employed in our study were better than the following three studies: Nirmala (2019) [99] implemented K, Ca, and Mg for Mandarin cv. Terigas and Tangerine cv. Pontianak, Odemis et al. (2014) [20] implemented NPK+ Ca(NO3)2 for Nova mandarin, and Habibi et al. (2021) [96] implemented GA3 for Thompson navel oranges, while the application of higher concentrations of calcium sulphate (0.75%)and GA3 (20 ppm) were employed in Eureka lemons [76] and Nova mandarins [100], respectively. Compared with the negative impact of another widely used 2,4-D growth regulator on the internal quality of the fruit [98], the GA3 used in this study did not reduce the internal quality of the fruit. In short, our study takes into account both the control of fruit cracking and the management of fruit quality for Miyagawa Wase. Citrus fruit cracking has obvious varieties and regional characteristics, and management time has priority when establishing management measures.
5. Conclusions
Fruit cracking is a physiological disease that has been observed to occur in a variety of fruits and has been identified as a significant factor contributing to a decline in citrus yield. In this study, we initiated our research by monitoring the growth and development of Miyagawa Wase fruits, demonstrating that certain changes in fruit characteristics during development are closely related to fruit cracking. Through this monitoring, we identified two critical periods of 50–60 and 80–90 DAFB, which are crucial for the prevention of fruit cracking. The monitoring results of nutrient status in citrus orchards with different severities of fruit cracking revealed that high concentrations of Mn, Zn, soil Fe, K, and leaf Mg and B were conducive to the occurrence of fruit cracking, whereas high concentrations of soil organic matter, Cu, leaf Fe and P were not conducive to the occurrence of fruit cracking. A single application of 10 ppm GA3 with 0.5% SSP and 0.006% EDTA-Fe at each of the above-mentioned two critical periods significantly improved fruit cracking in Miyagawase Wase. It is important to acknowledge the limitations of the study, which are primarily attributed to the unique characteristics of the research subjects and the planting environment. Further research is necessary to evaluate the applicability of the other study subjects and the potential negative impact of long-term use of the control measure proposed in this paper. In future research, the optimisation of the management measures and the exploration of the mechanism of some elements involved in citrus fruit cracking will be a priority.
Author Contributions
Conceptualisation, Y.L. and M.W.; methodology, G.J., M.W. and Y.Z.; formal analysis, G.J. and Y.Z.; investigation, X.Z. and Y.L.; data curation, M.W.; writing—original draft preparation, Y.L.; writing—review and editing, G.J. and M.W.; funding acquisition, M.W. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31972991), the Key Research and Development Project of South-west University Pilot Plan (SWU-XDZD22004), and the Agricultural Major Technology Collaborative Promotion Programme of Zhejiang Provincial (2022XTTGP03), the Soil health evaluation and cultivation technology of cultivated land in Kecheng District, Quzhou City, Zhejiang Province (ZJHWCG2024028).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Bower, J.P.; Gilfillan, I.M.; Skinner, H. Fruit splitting in ‘Valencia’ and its relationship to the pectin status of the rind. Proc. Int. Soc. Citric. 1992, 1, 511–514. [Google Scholar]
- Goldschmidt, E.E.; Galili, D. Fruit splitting in ‘Murcott’ tangerines: Control by reduced water supply. Proc. Int. Soc. Citric. 1992, 2, 657–660. [Google Scholar]
- Barry, G.H.; Bower, J.P. Manipulation of fruit set and stylar-end fruit split in ‘Nova’ mandarin hybrid. Sci. Hortic. 1997, 70, 243–250. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, A.K.; Meghwal, P.R. Fruit cracking in pomegranate: Extent, cause, and management—A Review. Int. J. Fruit Sci. 2020, 20 (Suppl. S3), S1234–S1253. [Google Scholar] [CrossRef]
- Ozturk, B.; Bektas, E.; Aglar, E.; Karakaya, O.; Gun, S. Cracking and quality attributes of jujube fruits as affected by covering and pre-harvest Parka and GA3 treatments. Sci. Hortic. 2018, 240, 65–71. [Google Scholar] [CrossRef]
- Yang, Z.E.; Wu, Z.; Zhang, C.; Hu, E.; Zhou, R.; Jiang, F. The composition of pericarp, cell aging, and changes in water absorption in two tomato genotypes: Mechanism, factors, and potential role in fruit cracking. Acta Physiol. Plant 2016, 38, 215. [Google Scholar] [CrossRef]
- Lopez-Zaplana, A.; Bárzana, G.; Agudelo, A.; Carvajal, M. Foliar mineral treatments for the reduction of melon (Cucumis melo L.) fruit cracking. Agronomy 2020, 10, 1815. [Google Scholar] [CrossRef]
- Yamada, M.; Ikeda, I.; Yamane, H.; Hirabayashi, T. Inheritance of fruit cracking at the calyx end and stylar end in Japanese persimmon (Diospyros kaki Thunb.). J. Jpn. Soc. Hortic. Sci. 1988, 57, 8–16. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Dai, S.; Xie, N.; Tang, Y.; Pi, S.; Zhu, M. PpAmy1 Plays a Role in Fruit-Cracking by Regulating Mesocarp Starch Hydrolysis of Nectarines. J. Agric. Food Chem. 2024, 72, 2667–2677. [Google Scholar] [CrossRef]
- Quero-García, J.; Letourmy, P.; Campoy, J.A.; Branchereau, C.; Malchev, S.; Barreneche, T.; Dirlewanger, E. Multi-year analyses on three populations reveal the first stable QTLs for tolerance to rain-induced fruit cracking in sweet cherry (Prunus avium L.). Hortic. Res. 2021, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Bal, J.S. Response of lemon (Citrus limon (L.) Burm.) cv. Baramasi to irrigation scheduling and mulching. Progress. Hortic. 2014, 46, 232–235. [Google Scholar]
- Huang, S.; Yang, X.; Wang, T.; Li, H.; Deng, L.; Bi, X.; Wang, Z. Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities. Plants 2024, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- García-Luis, A.; Duarte, A.M.M.; Porras, I.; García-Lidón, A.; Guardiola, J.L. Fruit splitting in ‘Nova’hybrid mandarin in relation to the anatomy of the fruit and fruit set treatments. Sci. Hortic. 1994, 57, 215–231. [Google Scholar] [CrossRef]
- Macnee, N.C.; Rebstock, R.; Hallett, I.C.; Schaffer, R.J.; Bulley, S.M. A review of current knowledge about the formation of native peridermal exocarp in fruit. Funct. Plant Biol. 2020, 47, 1019–1031. [Google Scholar] [CrossRef]
- Cronje, P.J.R. Postharvest rind disorders of ‘Nadorcott’ mandarin are affected by rootstock in addition to postharvest treatments. Acta Hortic. 2013, 1007, 111–117. [Google Scholar] [CrossRef]
- Ikram, S.; Shafqat, W.; Qureshi, M.A.; Din, S.; Rehman, S.; Mehmood, A.; Sajjad, Y.; Nafees, M. Causes and control of fruit cracking in pomegranate: A review. J. Glob. Innov. Agric. Soc. Sci. 2020, 8, 183–190. [Google Scholar] [CrossRef]
- Gonzalo, R.; Eduardo, F.; Cory, W.; Eike, L.; Italo, F.C. Adapting sweet cherry orchards to extreme weather events-Decision Analysis in support of farmers’ investments in Central Chile. Agric. Syst. 2021, 187, 103031. [Google Scholar]
- Cronje, P.J.; Stander, O.P.; Theron, K.I. Fruit splitting in citrus. Hortic. Rev. 2013, 41, 177–200. [Google Scholar]
- Odemis, B.; Turhan, S.; Buyuktas, D. The effects of irrigation and fertilizer applications on yield, pomological characteristics and fruit cracking in Nova mandarin. Agric. Water Manag. 2014, 135, 54–60. [Google Scholar] [CrossRef]
- Mesejo, C.; Reig, C.; Martínez-Fuentes, A.; Gambetta, G.; Gravina, A.; Agustí, M. Tree water status influences fruit splitting in Citrus. Sci. Hortic. 2016, 209, 96–104. [Google Scholar] [CrossRef]
- Li, J.; Chen, J. Citrus fruit-cracking: Causes and occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Alva, A.K.; Mattos, J.D.; Paramasivam, S.; Patil, B.; Dou, H. Potassium management for optimizing citrus production and quality. Int. J. Fruit Sci. 2006, 6, 3–43. [Google Scholar] [CrossRef]
- Mohamed, A.K.; Abdel-Galil, H.A.; Galal, N. Effect of some nutrients and amino acids spraying on yield and fruit quality of Manfalouty pomegranate. SVU-Int. J. Agric. Sci. 2020, 2, 18–29. [Google Scholar] [CrossRef]
- Sandhu, S.; Bal, J.S. Quality improvement in lemon (Citrus limon (L.) Burm.) through integrated management of fruit cracking. Afr. J. Agric. Res. 2013, 8, 3552–3557. [Google Scholar]
- Huai, B.; Wu, Y.; Liang, C.; Tu, P.; Mei, T.; Guan, A.; Chen, J. Effects of calcium on cell wall metabolism enzymes and expression of related genes associated with peel creasing in Citrus fruits. PeerJ 2022, 10, e14574. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, X.J.; Liu, X.M.; Srivastava, A.K.; Shi, X.J.; Zhang, Y.Q.; Hu, C.X.; Zhang, F.S. Calcium application regulates fruit cracking by cross-linking of fruit peel pectin during young fruit growth stage of citrus. Sci. Hortic. 2025, 340, 113922. [Google Scholar] [CrossRef]
- Shi, X.; Wen, M.; Dong, Z.; Zhang, J.Z.; Srivastava, A.K.; Moussa, M.G.; Zhang, Y.Q. Periodicity of Fruit Cracking in Orange Fruit and Integrated Management Intervention. Plants 2025, 14, 389. [Google Scholar] [CrossRef]
- Erickson, L.C. Compositional differences between normal and split Washington Navel oranges. Proc. Am. Soc. Hort. Sci. 1957, 70, 257–260. [Google Scholar]
- Wang, T.; Tan, L.; Chen, Z.F.; Yang, Y.T.; Yuan, Y.; Zheng, Z.D.; Deng, L.J.; Zhang, M.F.; Sun, G.C.; He, S.Y.; et al. Mitigating citrus fruit cracking: The efficacy of chelated calcium or silicon foliar fertilizers in ‘Okitsu no. 58’citrus fruit. Front. Plant Sci. 2024, 15, 1402945. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.T.; Rouse, R.E.; Roka, F.M.; Futich, S.H.; Zekri, M. Leaf and fruit mineral content and peel thickness of ‘Hamlin’ orange. Proc. Fla. State Hort. Soc. 2005, 118, 19–21. [Google Scholar]
- Sharifi, H.; Sepahi, A. Effect of gibberellic acid on fruit cracking in Meykhosh pomegranate. Iran Agric. Res. 1984, 3, 149–155. [Google Scholar]
- Fidelibus, M.W.; Teixeira, A.A.; Davies, F.S. Mechanical properties of orange peel and fruit treated pre–harvest with gibberellic acid. Trans. ASAE 2002, 45, 1057. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M. Effect of boron fertilization on sweet cherry tree yield and fruit quality. J. Plant Nutr. 2006, 29, 1755–1766. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, B.; Gu, M.; Lee, U.Y.; Kim, M.S.; Jung, S.K.; Choi, H.S. Course of Fruit Cracking in ‘Whansan’ Pears. Hortic. Environ. Biotechnol. 2020, 61, 51–59. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2015, 37, 1718. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Li, Y.; Jones, L.; McQueen-Mason, S. Expansins and cell growth. Curr. Opin. Plant Biol. 2003, 6, 603–610. [Google Scholar] [CrossRef]
- Yilmaz, C.E.A.A.P.; Ozguven, A.I. Hormone physiology of preharvest fruit cracking in pomegranate (Punica granatum L.). Acta Hortic. 2006, 727, 545–549. [Google Scholar] [CrossRef]
- Prativa, S.; Narender, S. Fruit cracking and quality of pomegranate (Punica granatum L.) cv. Kandhari as influenced by CPPU and boron. J. Pharmacogn. Phytochem. 2019, 8, 2644–2648. [Google Scholar]
- Garcia-Luis, A.; Duarte, A.M.M.; Kanduser, M.; Guardiola, J.L. The anatomy of the fruit in relation to the propensity of citrus species to split. Sci. Hortic. 2001, 87, 33–52. [Google Scholar] [CrossRef]
- Peschel, S.; Franke, R.; Schreiber, L.; Knoche, M. Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 2007, 68, 1017–1025. [Google Scholar] [CrossRef]
- Cline, J.A.; Trought, M. Effect of gibberellic acid on fruit cracking and quality of Bing and Sam sweet cherries. Can. J. Plant Sci. 2007, 87, 545–550. [Google Scholar] [CrossRef]
- Rabe, E.; Van Rensburg, P.J.J. Gibberellic acid sprays, girdling, flower thinning and potassium applications affect fruit splitting and yield in the ‘Ellendale’ tangor. J. Hort. Sci. 1996, 71, 195–203. [Google Scholar] [CrossRef]
- Horvitz, S.; Godoy, C.; López Camelo, A.F.; Yommi, A. Application of gibberellic acid to ‘Sweetheart ’sweet cherries: Effects on fruit quality at harvest and during cold storage. In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture, Toronto, ON, Canada, 11–17 August 2002; Volume 628, pp. 311–316. [Google Scholar]
- Usenik, V.; Kastelec, D.; Štampar, F. Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
- Suran, P.; Vavra, R.; Zeleny, L. Effectiveness of potential products to reduce rain cracking of cherry fruit. Acta Hortic. 2016, 1137, 183–186. [Google Scholar] [CrossRef]
- Pedro, R.; Galindo, A.; Jacinta, C.G.; Medina, S.; Corell, M.; Memmi, H.; Goron, I.F.; Centeno, A.; Martin-Palomo, M.L.; Cruz, Z.N. Fruit response to water-scarcity scenarios. water relations and biochemical changes-sciencedirect. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Academic Press: Cambridge, MA, USA, 2018; pp. 349–375. [Google Scholar]
- Kaur, R.; Kaur, N.; Singh, H. Fruit cracking in lemon cv. Punjab Baramasi in relation to developmental physiology. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 561–568. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, W.; Wang, H.; Li, J.; Huang, H.; Shi, L.; Jinhua, Y. Linking cracking resistance and fruit dessication rate to pericarp structure in litchi (Litchi chinensis Sonn.). J. Hortic. Sci. Biotechnol. 2004, 79, 897–905. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S. Studies on water transport through the sweet cherry fruit surface: VI. Effect of Hydrostatic pressure on water uptake. J. Hortic. Sci. Biotechnol. 2002, 77, 609–614. [Google Scholar] [CrossRef]
- Monselise, S.P. CRC Handbook of Fruit Set and Development; CRC Press, Inc.: Boca Raton, FL, USA, 1986. [Google Scholar]
- Zhou, N.F.; Zhao, J.P.; Liu, H.; Zha, W.W.; Pei, D. New protocols for paraffin sections of heterogeneous tissues of woody plants. Chin. Bull. Bot. 2018, 53, 653–660. [Google Scholar]
- Qiu, F.; Liu, W.; Chen, L.; Wang, Y.; Lyu, Q.; Yi, S.L.; Zheng, Y.Q. Bacillus subtilis biofertilizer application reduces chemical fertilization and improves fruit quality in fertigated Tarocco blood orange groves. Sci. Hortic. 2021, 281, 110004. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Sun, S.; Zhao, C. Effects of Tree Species and Soil Enzyme Activities on Soil Nutrients in Dryland Plantations. Forests 2021, 12, 1153. [Google Scholar] [CrossRef]
- Bargel, H.; Neinhuis, C. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Yamaga, I.; Iwata, M.; Asama, M.; Emoto, Y. Calcium carbonate treatments affect cultivation environment around the fruit surface and mitigate sunburn formation and rind puffing of satsuma mandarin fruits. Hortic. Environ. Biotechnol. 2024, 1–11. [Google Scholar] [CrossRef]
- Winkler, A.; Blumenberg, I.; Schürmann, L.; Knoche, M. Rain cracking in sweet cherries is caused by surface wetness, not by water uptake. Sci. Hortic. 2020, 269, 109400. [Google Scholar] [CrossRef]
- Blanke, M.M. Fine structure and elemental composition of segment membranes of Valencia orange fruit and their possible role in raggyness. J. Appl. Bot. 2003, 77, 28–31. [Google Scholar]
- Zhang, A.; Liu, Y.Z.; Liu, Y.; Zheng, F.X.; Xie, X.J.; Ye, Z.Z.; Cheng, J.N.; Den, Y.Y.; Zeng, X.X.; Yun, L. Investigation of chromoplast ultrastructure and tissue-specific accumulation of carotenoids in citrus flesh. Sci. Hortic. 2019, 256, 108547. [Google Scholar] [CrossRef]
- Zdunek, A.; Kurenda, A. Determination of the elastic properties of tomato fruit cells with an atomic force microscope. Sensors 2013, 13, 12175–12191. [Google Scholar] [CrossRef]
- Kevin, V.; Cédric, G.; Camille, R.; Siret, R.; Lahaye, M. Cryo-laser scanning confocal microscopy of diffusible plant compounds. Plant Methods 2018, 14, 89. [Google Scholar]
- Solomon, W.F.; Metadel, K.A.; Quang, T.H.; Pieter, V.; Jan, C.; Bart, M.N. Microscale modeling of water transport in fruit tissue. J. Food Eng. 2013, 118, 229–237. [Google Scholar]
- Measham, P. Rain-Induced Fruit Cracking in Sweet Cherry (Prunus avium L.). Ph.D. Thesis, School of Agricultural Science, University of Tasmania, Hobart, Australia, 2011; 170p. [Google Scholar]
- Simon, G. Review on rain induced fruit cracking of sweet cherries (Prunus avium L.), its causes and the possibilities of prevention. Int. J. Hortic. Sci. 2006, 12, 27–35. [Google Scholar] [CrossRef]
- Sekse, L. Fruit cracking in sweet cherries (Prunus avium L.). Some physiological aspects—A mini review. Sci. Hortic. 1995, 63, 135–141. [Google Scholar] [CrossRef]
- Peet, M. Fruit cracking in tomato. Horttechnology 1992, 2, 216–223. [Google Scholar] [CrossRef]
- Considine, J.; Brown, K. Physical aspects of fruit growth. Theoretical analysis of distribution of surface growth forces in relation to cracking and splitting. Plant Physiol. 1981, 68, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Sato, I.; Ishiguro, M. Influences of epidermal cell sizes and flesh firmness on cracking susceptibility in sweet cherry (Prunus avium L.) cultivars and selections. J. Jpn. Soc. Hortic. Sci. 2002, 71, 738–746. [Google Scholar] [CrossRef]
- Alikhani, M.; Babakhani, B.; Golein, B.; Asadi, M.; Rahdari, P. Foliar application of potassium nitrate and 2, 4-dichlorophenoxyacetic acid affect some fruit splitting related characteristics and biochemical traits of mandarin cv.‘page’. EurAsian J. Biosci. 2020, 14, 4251–4260. [Google Scholar]
- Devy, N.F.; Dwiastuti, M.E.; Sugiyatno, A.; Ashari, H.; Endarto, O.; Triwiratno, A.; Martasari, C. The fruit peel anatomy, leaf nutrient content, and fruit quality of ‘Terigas’ mandarin in relation to fruit cracking. Emir. J. Food Agric. (EJFA) 2022, T34, 12. [Google Scholar]
- Chabbal, M.D.; Yfran-Elvira, M.D.L.M.; Giménez, L.I.; Martínez, G.C.; Llarens-Beyer, L.A.; Rodríguez, V.A. Control of fruit cracking in clementino mandarin plants. Cultiv. Trop. 2020, 41, e06. [Google Scholar]
- Sdoodee, S.; Rawee, C. Fruit splitting occurrence of Shogun mandarin (Citrus reticulata Blanco cv. Shogun) in southern Thailand and alleviation by calcium and boron sprays. Songklanakarin J. Sci. Technol. 2005, 27, 719–730. [Google Scholar]
- Tariq, M.; Sharif, M.; Shah, Z.; Khan, R. Effect of foliar application of micronutrients on the yield and quality of sweet orange (Citrus sinensis L.). Pak. J. Biol. Sci. 2007, 10, 1823–1828. [Google Scholar] [CrossRef]
- Devi, K.; Kumar, R.; Wali, V.K.; Bakshi, P.; Sharma, N.; Arya, V.M. Effect of foliar nutrition and growth regulators on nutrient status and fruit quality of Eureka lemon (Citrus limon). Indian J. Agric. Sci. 2018, 88, 704–708. [Google Scholar] [CrossRef]
- Rabe, E.; van Rensburg, P.; van der Walt, H.; Bower, J. Factors influencing preharvest fruit splitting in Ellendale (C. reticulata). HortScience 1990, 25, 1163–1183. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Ann. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Graham, J.H.; Syvertsen, J.P. Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol. 1985, 101, 667–676. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T. Phosphorus (P) for Citrus Trees: SL379/SS581, 7/2013. EDIS 2013, 2013, 1–4. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Cowan, J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. BioMetals 2002, 15, 225–235. [Google Scholar] [CrossRef]
- Conell, J. Citrus Nutrition; University of California, Cooperative Extension, Agriculture & Natural Resources Central Valley Region: Berkeley, CA, USA, 2018. [Google Scholar]
- Chen, Z.; Yan, W.; Sun, L.; Tian, J.; Liao, H. Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. J. Proteom. 2016, 143, 151–160. [Google Scholar] [CrossRef]
- Kasim, W.A. Physiological consequences of structural and ultra-structural changes induced by Zn stress in Phaseolus vulgaris. I. Growth and photosynthetic apparatus. Int. J. Bot. 2007, 3, 15–22. [Google Scholar] [CrossRef]
- Dang, H.K.; Li, R.Q.; Sun, Y.H.; Zhang, X.W.; Li, Y.M. Absorption, accumulation and distribution of Zinc in highly-yielding winter wheat. Agric. Sci. China 2010, 9, 965–973. [Google Scholar] [CrossRef]
- Eman, A.A.; El Migeed, M.A.; Omayma, M.M. GA3 and zinc sprays for improving yield and fruit quality of Washington Navel orange trees grown under sandy soil conditions. Res. J. Agric. Bio Sci. 2007, 3, 498–503. [Google Scholar]
- Srivastava, A.K.; Singh, S. Boron nutrition in citrus-current status and future strategies—A review. Agric. Rev. 2005, 26, 173–186. [Google Scholar]
- Kumar, D.; Das, K.K.; Kumar, S. IMPORTANCE OF IRON (Fe) AND COPPER (Cu) FOR CITRUS. Marumegh 2017, 2, 12–15. [Google Scholar]
- Hippler, F.W.R.; Boaretto, R.M.; Dovis, V.L.; Quaggio, J.A.; Azevedo, R.A.; Mattos, D. Oxidative stress induced by Cu nutritional disorders in Citrus depends on nitrogen and calcium availability. Sci. Rep. 2018, 8, 1641. [Google Scholar] [CrossRef]
- Weichert, H.; von Jagemann, C.; Peschel, S.; Knoche, M.; Neumann, D.; Erfurth, W. Studies on water transport through the sweet cherry fruit surface: VIII. Effect of selected cations on water uptake and fruit cracking. J.-Am. Soc. Hortic. Sci. 2004, 129, 781–788. [Google Scholar] [CrossRef]
- Krajewski, A.; Ebert, T.; Schumann, A.; Waldo, L. Pre-Harvest Fruit Splitting of Citrus. Agronomy 2022, 12, 1505. [Google Scholar] [CrossRef]
- Greenberg, J.; Kaplan, I.; Fainzack, M.; Egozi, Y.; Giladi, B. Effects of auxin sprays on yield, fruit size, fruit splitting and the incidence of creasing of ‘Nova’ mandarin. Acta Hortic. 2006, 727, 249–254. [Google Scholar] [CrossRef]
- Sandhu, S. Improving lemon [Citrus limon (L.) Burm.] quality using growth regulators. J. Hortic. Sci. 2013, 8, 88–90. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, M.; Rattanpal, H.S.; Chahal, T.S.; Singh, G. Impact of foliar application of growth regulators on fruit splitting, yield and quality of daisy mandarin (Citrus reticulata). Indian J. Agric. Sci. 2024, 94, 181–186. [Google Scholar] [CrossRef]
- Habibi, S.; Ebadi, A.; Ladanmoghadam, A.R.; Rayatpanah, S. Effect of Plant Growth Regulators on Fruit Splinting in Thompson Navel Orange. Acta Sci. Pol. Hortorum Cultus 2021, 20, 83–92. [Google Scholar] [CrossRef]
- Stander, O.P.J.; Theron, K.I.; Cronje, P.J.R. Foliar 2, 4-dichlorophenoxy acetic acid (2, 4-d) application after physiological fruit drop reduces fruit splitting and increases fruit size in mandarin. In Proceedings of the XII International Symposium on Plant Bioregulators in Fruit Production 1042, Orlando, FL, USA, 28 July–1 August 2013; pp. 43–50. [Google Scholar]
- Stander, O.P.J.; Theron, K.I.; Cronjé, P.J. Foliar 2, 4-D application after physiological fruit drop reduces fruit splitting of mandarin. HortTechnology 2014, 24, 717–723. [Google Scholar] [CrossRef]
- Nirmala, F.D. Application of K, Ca, and Mg on peel thickness and fruit cracking incidence of citrus. Russ. J. Agric. Socio-Econ. Sci. 2019, 87, 45–56. [Google Scholar]
- Aliviela, V.; Zaragoza, S.; Primo-Millo, E.; Agusti, M. Hormonal control of splitting in ‘Nova’ mandarin fruit. J. Hortic. Sci. 1994, 69, 969–973. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).