Quantitative Trait Locus Mapping for Rapid Visco Analyzer Parameters in Wheat (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of RVA Parameters
2.3. RVA Analysis
2.4. QTL Analysis
3. Results
3.1. Variation Analysis of RVA Parameters
3.2. Correlation Analsysis Between RVA Parameters
3.3. QTL Mapping for the RVA Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RVA | Rapid visco analyzer |
| QTL | Quantitative trait loci |
| PV | Peak viscosity |
| TV | Trough viscosity |
| BD | Breakdown |
| FV | Final viscosity |
| SB | Setback |
| PeT | Peak time |
| PT | Pasting temperature |
References
- Mao, H.D.; Jiang, C.; Tang, C.L.; Nie, X.J.; Du, L.Y.; Liu, Y.L.; Cheng, P.; Wu, Y.F.; Liu, H.Q.; Kang, Z.S.; et al. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [PubMed]
- He, Z.; Zhuang, Q.; Cheng, S.; Yu, Z.; Zhao, Z.; Liu, X. Wheat production and technology improvement in China. J. Agric. 2018, 8, 107–114. [Google Scholar]
- Zhang, Y.; Ye, Y.; Liu, J.; Xiao, Y.; Sun, Q.; He, Z. The relationship between Chinese raw dumpling quality and flour characteristics of Shandong winter wheat cultivars. Agric. Sci. China 2011, 10, 1792–1800. [Google Scholar]
- Vijayendra, S.V.N.; Sreedhar, R. Production of buns, the bakery-based snack food, with reduced refined wheat flour content: Recent developments. J. Food Sci. Technol. 2023, 60, 2907–2915. [Google Scholar]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar]
- Ma, H.L.; Yang, Y.H.; Zhao, J.R.; Huang, X.L.; Yang, H.K.; Zheng, T.; Fan, G.Q. Relationship between the baking quality of wheat (Triticum aestivum L.) and the protein composition and structure after shading. Food Chem. 2024, 441, 138392. [Google Scholar] [PubMed]
- Cozzolino, D. The use of the rapid visco analyser (RVA) in breeding and selection of cereals. J. Cereal Sci. 2016, 70, 282–290. [Google Scholar]
- Zhang, Q.; Wang, F.; Hao, L.; Wang, S.; Zao, J.; Zhang, T. Research and analysis on the gelatinization characteristics of different types of wheat flour. Modern Flour Indus. 2013, 27, 26–31. [Google Scholar]
- Peng, Y.C.; Zhao, Y.; Yu, Z.T.; Zeng, J.B.; Xu, D.G.; Dong, J.; Ma, W.J. Wheat quality formation and its regulatory mechanisms. Front. Plant Sci. 2022, 13, 834654. [Google Scholar]
- Ma, M.M.; Li, Y.C.; Xue, C.; Xiong, W.; Peng, Z.P.; Han, X.; Ju, H.; He, Y. Current situation and key parameters for improving wheat quality in China. Front. Plant Sci. 2021, 12, 638525. [Google Scholar]
- Battenfield, S.D.; Sheridan, J.L.; Silva, L.D.C.E.; Miclaus, K.J.; Dreisigacker, S.; Wolfinger, R.D.; Peña, R.J.; Singh, R.P.; Jackson, E.W.; Fritz, A.K.; et al. Breeding-assisted genomics: Applying meta-GWAS for milling and baking quality in CIMMYT wheat breeding program. PLoS ONE 2018, 13, e0204757. [Google Scholar]
- Rasheed, A.; Wen, W.E.; Gao, F.M.; Zhai, S.N.; Jin, H.; Liu, J.D.; Guo, Q.; Zhang, Y.J.; Dreisigacker, S.; Xia, X.C.; et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar]
- Zhang, N.; Tang, L.; Li, S.G.; Gao, M.J.; Wang, S.S.; Chen, D.Y.; Zhao, Y.C.; Zheng, R.Q.; Soleymaniniya, A.; Zhang, L.; et al. Integration of multi-omics data accelerates molecular analysis of common wheat traits. Nat. Commun. 2025, 16, 2200. [Google Scholar]
- Ullah, R.; Yin, M.; Li, S.; Israr, Y.; Wu, Z.; Wang, X.; Yu, J.; Li, B.; Ni, Z.; Liang, R. Genome-wide association study identifies loci and candidate genes for RVA parameters in wheat (Triticum aestivum L.). Front. Plant Sci. 2024, 15, 1421924. [Google Scholar]
- Tian, Y.S.; Liu, P.P.; Zhang, X.; Liu, Y.C.; Kong, D.Z.; Nie, Y.B.; Xu, H.J.; Han, X.N.; Sang, W.; Li, W.H. Genome-wide association study and KASP marker development for starch quality traits in wheat. Plant Genome 2024, 17, e20514. [Google Scholar] [PubMed]
- Deng, Z.Y.; Tian, J.C.; Chen, F.; Li, W.J.; Zheng, F.F.; Chen, J.S.; Shi, C.L.; Sun, C.L.; Wang, S.Y.; Zhang, Y.X. Genetic dissection on wheat flour quality traits in two related populations. Euphytica 2015, 203, 221–235. [Google Scholar]
- Jin, H.; Wen, W.; Liu, J.D.; Zhai, S.N.; Zhang, Y.; Yan, J.; Liu, Z.Y.; Xia, X.C.; He, Z.H. Genome-wide QTL mapping for wheat processing quality parameters in a Gaocheng 8901/Zhoumai 16 recombinant inbred line population. Front. Plant Sci. 2016, 7, 1032. [Google Scholar]
- Zhang, Y.; Wu, Y.; Xiao, Y.; Yan, J. QTL mapping for milling, gluten quality, and flour pasting properties in a recombinant inbred line population derived from a Chinese soft×hard wheat cross. Crop Pasture Sci. 2009, 60, 587–597. [Google Scholar]
- Niu, Y.A.; Chen, T.X.; Zhao, C.C.; Guo, C.; Zhou, M.X. Identification of QTL for stem traits in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 962253. [Google Scholar]
- Hong, Y.; Zhang, M.N.; Zhu, J.; Zhang, Y.H.; Lv, C.; Guo, B.J.; Wang, F.F.; Xu, R.G. Genome-wide association studies reveal novel loci for grain size in two-rowed barley (Hordeum vulgare L.). Theor. Appl. Genet. 2024, 137, 58. [Google Scholar]
- Zhu, J.; Zhou, H.; Fan, Y.; Guo, Y.; Zhang, M.N.; Shabala, S.; Zhao, C.C.; Lv, C.; Guo, B.J.; Wang, F.F.; et al. HvNCX, a prime candidate gene for the novel qualitative locus qS7.1 associated with salinity tolerance in barley. Theor. Appl. Genet. 2023, 136, 9. [Google Scholar] [PubMed]
- Fan, Y.; Shabala, S.; Ma, Y.L.; Xu, R.G.; Zhou, M.X. Using QTL mapping to investigate the relationships between abiotic stress tolerance (drought and salinity) and agronomic and physiological traits. BMC Genom. 2015, 16, 43. [Google Scholar]
- Mourad, A.M.I.; Belamkar, V.; Baenziger, P.S. Molecular genetic analysis of spring wheat core collection using genetic diversity, population structure, and linkage disequilibrium. BMC Genom. 2020, 21, 434–456. [Google Scholar]
- Matus, I.A.; Hayes, P.M. Genetic diversity in three groups of barley germplasm assessed by simple sequence repeats. Genome 2002, 45, 1095–1106. [Google Scholar]
- Batey, I.L.; Hayden, M.J.; Cai, S.; Sharp, P.J.; Cornish, G.B.; Morell, M.K.; Appels, R. Genetic mapping of commercially significant starch characteristics in wheat crosses. Aust. J. Agric. Res. 2001, 52, 1287–1296. [Google Scholar]
- Ram, S.; Sharma, I. Allelic diversity in granule bound starch synthase genes in Indian wheats and their relationship with starch pasting properties. Cereal Res. Commun. 2013, 41, 141–149. [Google Scholar]
- Wu, Y.P.; Zhang, Y.L.; Xiao, Y.G.; Yan, J.; He, Z.H. QTL mapping for important quality traits in common wheat. Sci. Agric. Sin. 2008, 41, 331–339. [Google Scholar]
- Deng, Z.; Chen, F.; Hu, S.; Han, Q.; Chen, J.; Sun, C.; Zhang, S.; Wang, S.; Song, X.; Tian, J. Inheritance and QTL analysis of flour falling number using recombinant inbred lines derived from strong gluten wheat ‘Gaocheng 8901’ and waxy wheat ‘Nuomai 1’. Aust. J. Crop Sci. 2014, 8, 468–474. [Google Scholar]
- Zhao, L.; Zhang, K.; Liu, B.; Tian, J. Detection of quantitative trait loci for paste viscosity characteristics based on the doubled haploid progeny from a cross between two Chinese wheat varieties. Can. J. Plant Sci. 2009, 89, 837–844. [Google Scholar] [CrossRef]
- McCartney, C.A.; Somers, D.J.; Lukow, O.M.; Ames, N.P.; Noll, J.S.; Cloutier, S.; Humphreys, D.G.; McCallum, B.D. QTL analysis of quality traits in the spring wheat cross RL4452 × ‘AC Domain’. Plant Breed. 2006, 125, 565–575. [Google Scholar] [CrossRef]
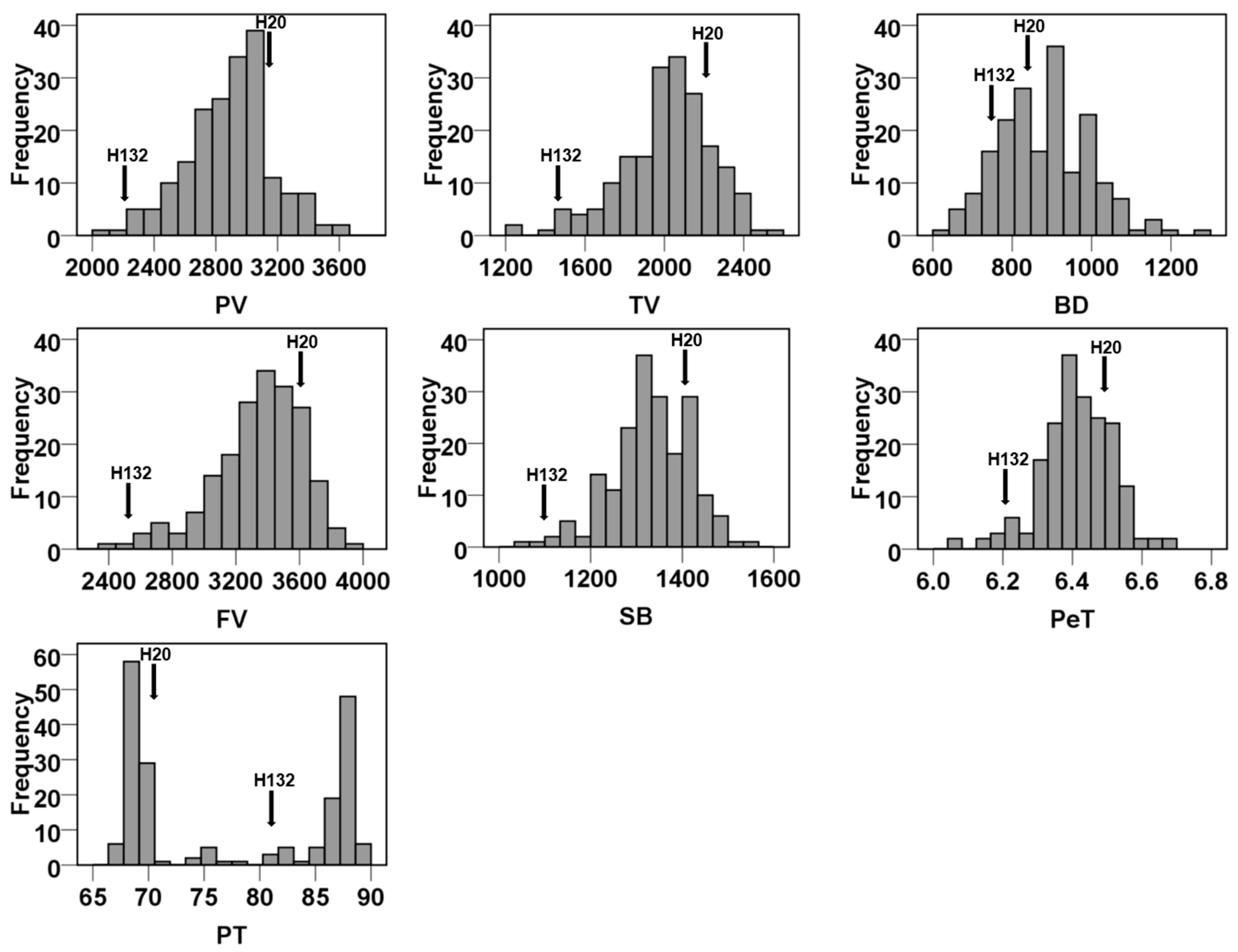
| RVA Parameters | H20 | H132 | Min. | Max. | Mean | Standard Deviation | CV (%) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|
| PV/cP | 3098.2 | 2172.7 | 2078.3 | 3650.0 | 2895.3 | 278.5 | 9.6 | −0.2 | 0.3 |
| TV/cP | 2212.0 | 1373.3 | 1248.3 | 2579.0 | 2015.1 | 232.5 | 11.5 | −0.6 | 0.6 |
| BD/cP | 886.2 | 799.3 | 628.0 | 1277.3 | 880.2 | 115.4 | 13.1 | 0.4 | 0.2 |
| FV/cP | 3613.3 | 2475.7 | 2361.0 | 3895.0 | 3346.5 | 278.7 | 8.3 | −0.8 | 0.8 |
| SB/cP | 1401.3 | 1102.3 | 1059.3 | 1549.7 | 1333.0 | 84.3 | 6.3 | −0.5 | 0.5 |
| PeT/min | 6.5 | 6.2 | 6.0 | 6.7 | 6.4 | 0.1 | 1.7 | −0.4 | 0.8 |
| PT/°C | 69.5 | 80.2 | 66.9 | 89.7 | 77.5 | 9.0 | 11.6 | 0.2 | −1.9 |
| RVA Parameters | PV | TV | BD | FV | SB | PeT | PT |
|---|---|---|---|---|---|---|---|
| PV | 1 | ||||||
| TV | 0.913 ** | 1 | |||||
| BD | 0.573 ** | 0.290 ** | 1 | ||||
| FV | 0.873 ** | 0.960 ** | 0.173 * | 1 | |||
| SB | 0.574 ** | 0.619 ** | 0.139 | 0.796 ** | 1 | ||
| PeT | 0.605 ** | 0.775 ** | −0.099 | 0.682 ** | 0.222 ** | 1 | |
| PT | 0.236 ** | 0.094 | 0.380 ** | −0.011 | −0.217 ** | 0.263 ** | 1 |
| Trait | Year | QTL | Chr. | Pos. (cM) | Locus | LOD | Expl. (%) | Additive |
|---|---|---|---|---|---|---|---|---|
| PV | 2021 | q.PV.2A | 2A | 107.50 | D55576755 | 7.2 | 15.0 | 126.31 |
| q.PV.5A | 5A | 73.16 | 3021302 | 3.4 | 6.7 | 85.65 | ||
| q.PV.6A | 6A | 101.82 | D7351888 | 3.5 | 6.9 | −88.04 | ||
| 2022 | q.PV.2A | 2A | 107.27 | D1401240 | 4.2 | 9.2 | 105.34 | |
| q.PV.2D | 2D | 325.53 | D2274461 | 3.2 | 6.9 | −103.85 | ||
| q.PV.6A | 6A | 105.51 | 3955024 | 4.0 | 8.7 | −103.27 | ||
| 2023 | q.PV.2A | 2A | 110.68 | D1094047 | 6.3 | 12.6 | 95.38 | |
| q.PV.6A | 6A | 106.08 | D1862824 | 7.5 | 15.3 | 104.95 | ||
| Average | q.PV.2A | 2A | 107.50 | D55576755 | 6.6 | 13.2 | 101.14 | |
| q.PV.6A | 6A | 106.08 | D1862824 | 5.6 | 11.0 | 92.51 | ||
| TV | 2021 | q.TV.2A | 2A | 107.50 | D55576755 | 7.9 | 16.4 | 115.57 |
| q.TV.6A | 6A | 101.82 | D7351888 | 3.3 | 6.4 | −73.56 | ||
| q.TV.7B | 7B | 152.75 | D3954877 | 4.7 | 9.4 | 87.13 | ||
| 2023 | q.TV.2A | 2A | 107.27 | D1401240 | 7.6 | 12.4 | 80.80 | |
| q.TV.3D | 3D | 253.58 | D1258529 | 5.4 | 8.7 | 68.08 | ||
| q.TV.5B | 5B | 65.50 | D4991852 | 3.0 | 4.7 | −49.67 | ||
| q.TV.6A | 6A | 106.08 | D1862824 | 8.3 | 13.7 | 84.62 | ||
| q.TV.6D | 6D | 135.12 | D5325771 | 3.0 | 4.7 | 50.22 | ||
| Average | q.TV.2A | 2A | 100.44 | D1252842 | 5.7 | 11.5 | 79.08 | |
| q.TV.6A | 6A | 105.51 | 3955024 | 5.5 | 11.4 | −79.59 | ||
| BD | 2021 | q.BD.3A1 | 3A | 118.59 | 1147712 | 5.8 | 10.0 | 38.66 |
| q.BD.5A | 5A | 73.59 | 7337511 | 3.4 | 5.7 | 29.45 | ||
| q.BD.5D | 5D | 26.26 | D1087040 | 8.5 | 15.1 | −47.17 | ||
| q.BD.6D | 6D | 149.80 | D3027538 | 3.1 | 5.1 | 27.35 | ||
| q.BD.7B | 7B | 140.38 | D3385231 | 4.8 | 8.1 | −34.15 | ||
| 2022 | q.BD.2A | 2A | 114.33 | D7331301 | 5.3 | 9.7 | 45.53 | |
| q.BD.2D | 2D | 149.35 | D4993099 | 4.6 | 8.3 | −41.70 | ||
| q.BD.5D | 5D | 27.56 | D7352280 | 6.1 | 11.3 | −49.76 | ||
| 2023 | q.BD.2D | 2D | 152.56 | D4991532 | 3.5 | 5.2 | −26.47 | |
| q.BD.3A2 | 3A | 271.46 | D4993402 | 3.3 | 4.8 | 25.17 | ||
| q.BD.4D | 4D | 73.73 | D5328849 | 4.5 | 6.7 | −29.30 | ||
| q.BD.5B | 5B | 244.23 | 3064727 | 5.9 | 8.9 | 34.03 | ||
| q.BD.5D | 5D | 26.26 | D1087040 | 8.4 | 13.1 | −41.21 | ||
| Average | q.BD.2A | 2A | 115.01 | 3064474 | 3.1 | 4.8 | 25.78 | |
| q.BD.2D | 2D | 152.56 | D4991532 | 3.6 | 5.6 | −28.28 | ||
| q.BD.4D | 4D | 45.98 | D1102564 | 3.1 | 4.9 | −26.19 | ||
| q.BD.5B | 5B | 244.23 | 3064727 | 4.6 | 7.4 | 31.77 | ||
| q.BD.5D | 5D | 26.26 | D1087040 | 5.8 | 10.5 | −36.45 | ||
| FV | 2021 | q.FV.2A | 2A | 102.89 | D11913233 | 3.5 | 6.6 | 88.79 |
| q.FV.2B | 2B | 240.41 | D4003760 | 3.2 | 6.0 | −85.68 | ||
| q.FV.5A | 5A | 73.16 | 3021302 | 3.8 | 7.3 | 95.02 | ||
| q.FV.7B1 | 7B | 152.75 | D3954877 | 5.7 | 11.1 | 116.09 | ||
| 2023 | q.FV.3B | 3B | 217.73 | D1161423 | 3.7 | 7.1 | 69.70 | |
| q.FV.3D | 3D | 252.46 | D4909411 | 3.8 | 7.2 | 70.08 | ||
| q.FV.5B | 5B | 65.69 | D7333589 | 3.1 | 5.8 | −62.46 | ||
| q.FV.6A | 6A | 106.08 | D1862824 | 3.6 | 6.8 | 67.56 | ||
| Average | q.FV.1D | 1D | 166.38 | D4910014 | 3.3 | 6.5 | −71.52 | |
| q.FV.5A | 5A | 73.16 | 3021302 | 5.0 | 10.2 | 91.26 | ||
| q.FV.7B2 | 7B | 65.61 | D2303265 | 4.1 | 8.1 | 79.54 | ||
| SB | 2021 | q.SB.5A | 5A | 77.82 | D4408148 | 8.2 | 17.5 | 44.94 |
| q.SB.7B | 7B | 31.92 | 1082004 | 4.3 | 8.7 | 31.27 | ||
| 2022 | q.SB.1A | 1A | 112.35 | D4539577 | 4.6 | 10.6 | −35.36 | |
| q.SB.7B | 7B | 35.08 | D2275229 | 4.3 | 9.9 | 33.93 | ||
| 2023 | q.SB.5A | 5A | 77.36 | D3023377 | 3.9 | 7.7 | 21.71 | |
| q.SB.6A | 6A | 117.05 | 3064745 | 3.1 | 6.0 | −19.19 | ||
| q.SB.7B | 7B | 26.21 | 2258137 | 5.8 | 11.8 | 26.58 | ||
| Average | q.SB.5A | 5A | 75.87 | D3022143 | 5.7 | 11.4 | 28.74 | |
| q.SB.7B | 7B | 31.92 | 1082004 | 5.9 | 11.8 | 29.02 | ||
| PeT | 2021 | q.PeT.3D | 3D | 76.71 | D4733545 | 4.6 | 10.8 | 0.04 |
| q.PeT.6A1 | 6A | 48.10 | D1104248 | 3.1 | 7.2 | −0.03 | ||
| 2023 | q.PeT.1B | 1B | 199.23 | D3944391 | 3.6 | 8.7 | −0.03 | |
| Average | q.PeT.3D | 3D | 76.71 | D4733545 | 6.3 | 12.4 | 0.04 | |
| q.PeT.6A2 | 6A | 104.26 | D4405997 | 3.6 | 6.7 | −0.03 | ||
| q.PeT.7D | 7D | 195.33 | D994906 | 4.1 | 7.9 | 0.03 |
| QTL | Chr. | Pos. (cM) | RVA Parameters (Year) | Expl. (%) |
|---|---|---|---|---|
| q.RVA.2A | 2A | 102.89–114.33 | PV (2021, 2022, 2023), TV (2021, 2023), BD (2022), FV (2021) | 9.2–16.4 |
| q.RVA.3D | 3D | 252.46–253.58 | TV (2023), FV (2023) | 7.2–8.7 |
| q.RVA.5A | 5A | 73.16–77.36 | PV (2021), BD (2021), FV (2021), SB (2021, 2023) | 5.7–17.5 |
| q.RVA.5B | 5B | 65.50–65.69 | TV (2023), FV (2023) | 4.7–5.8 |
| q.RVA.6A | 6A | 101.82–117.05 | PV (2021, 2022, 2023), TV (2021, 2023), FV (2023), SB (2023) | 6.4–15.3 |
| q.RVA.6D | 6D | 135.12–149.80 | TV (2023), BD (2021) | 4.7–5.1 |
| q.RVA.7B | 7B | 140.38–152.75 | TV (2021), BD (2021), FV (2021) | 8.1–11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, J.; Xu, K.; Cao, F.; Zhang, P. Quantitative Trait Locus Mapping for Rapid Visco Analyzer Parameters in Wheat (Triticum aestivum L.). Agronomy 2025, 15, 790. https://doi.org/10.3390/agronomy15040790
Fan X, Zhang J, Xu K, Cao F, Zhang P. Quantitative Trait Locus Mapping for Rapid Visco Analyzer Parameters in Wheat (Triticum aestivum L.). Agronomy. 2025; 15(4):790. https://doi.org/10.3390/agronomy15040790
Chicago/Turabian StyleFan, Xiangyun, Jinrui Zhang, Kewen Xu, Fangbin Cao, and Peng Zhang. 2025. "Quantitative Trait Locus Mapping for Rapid Visco Analyzer Parameters in Wheat (Triticum aestivum L.)" Agronomy 15, no. 4: 790. https://doi.org/10.3390/agronomy15040790
APA StyleFan, X., Zhang, J., Xu, K., Cao, F., & Zhang, P. (2025). Quantitative Trait Locus Mapping for Rapid Visco Analyzer Parameters in Wheat (Triticum aestivum L.). Agronomy, 15(4), 790. https://doi.org/10.3390/agronomy15040790








