Increasing Contribution of Microbial Residue Carbon to Soil Organic Carbon Accumulation in Degraded Grasslands
Abstract
:1. Introduction
2. Materials and Methods
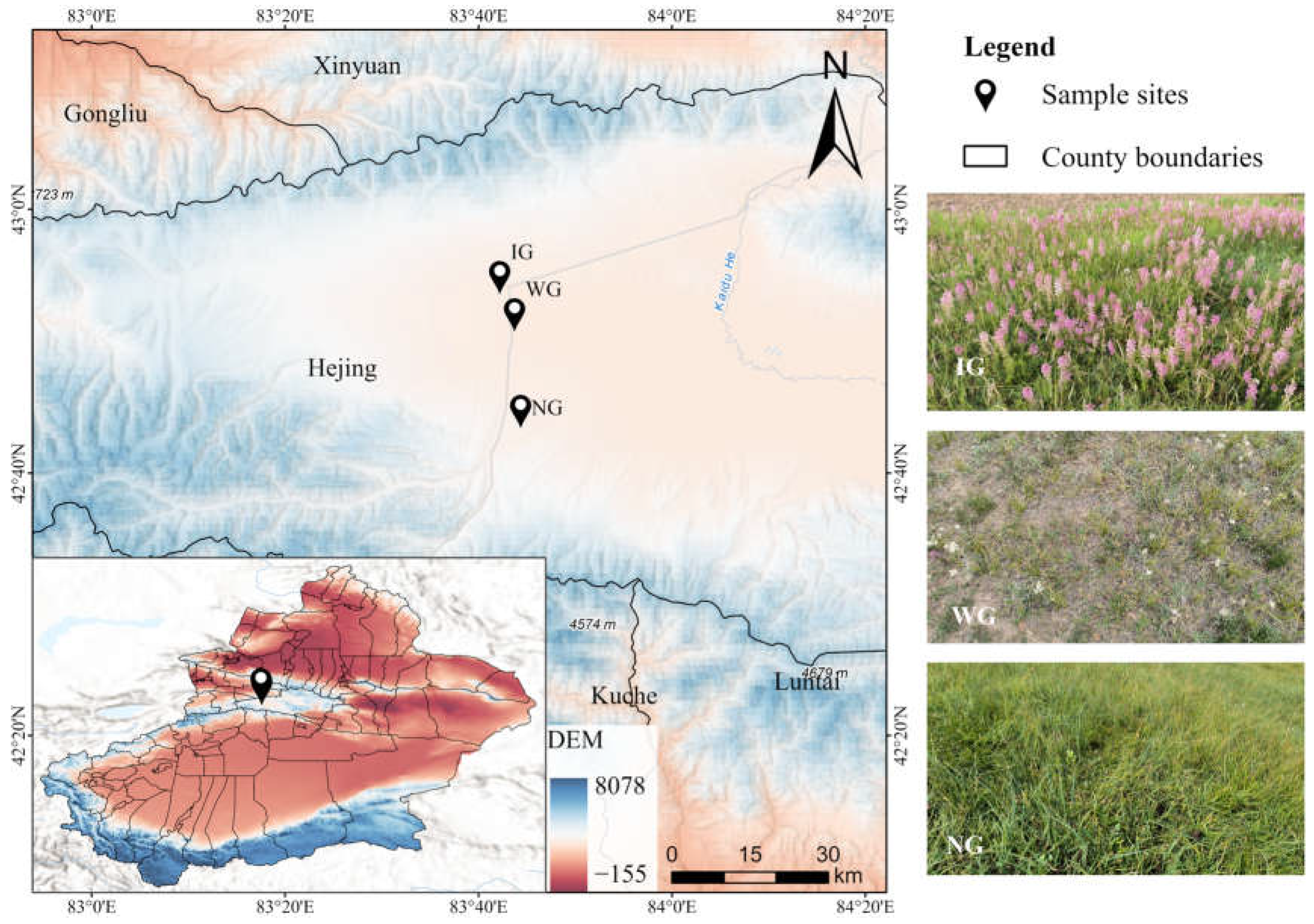
2.1. Study Sites and Soil Collection
2.2. Soil Physicochemical Property Analysis
2.3. Biomarker Analysis
2.4. Biomarker Proxy
2.5. Statistical Analysis
3. Results
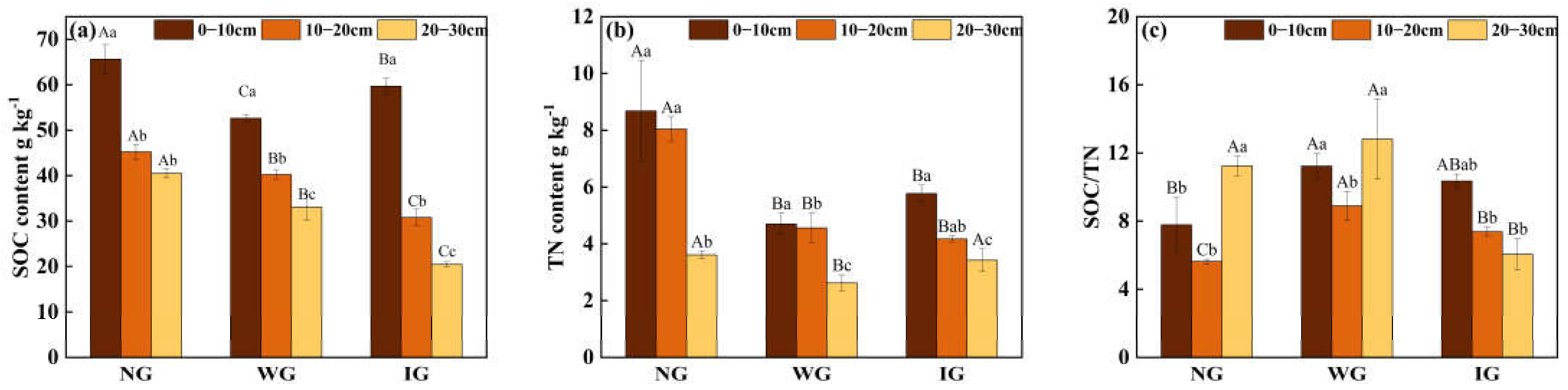
3.1. SOC and TN in Soil Profiles
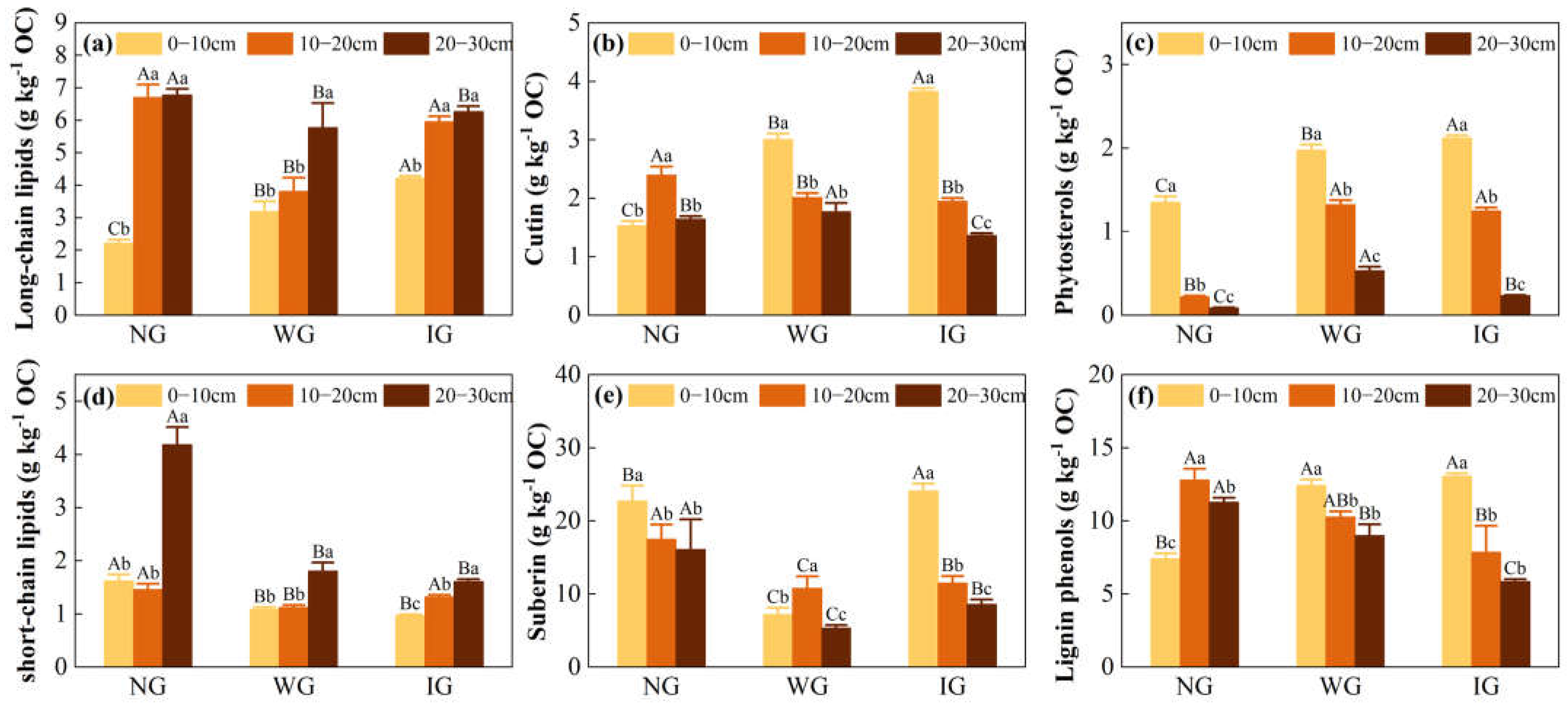
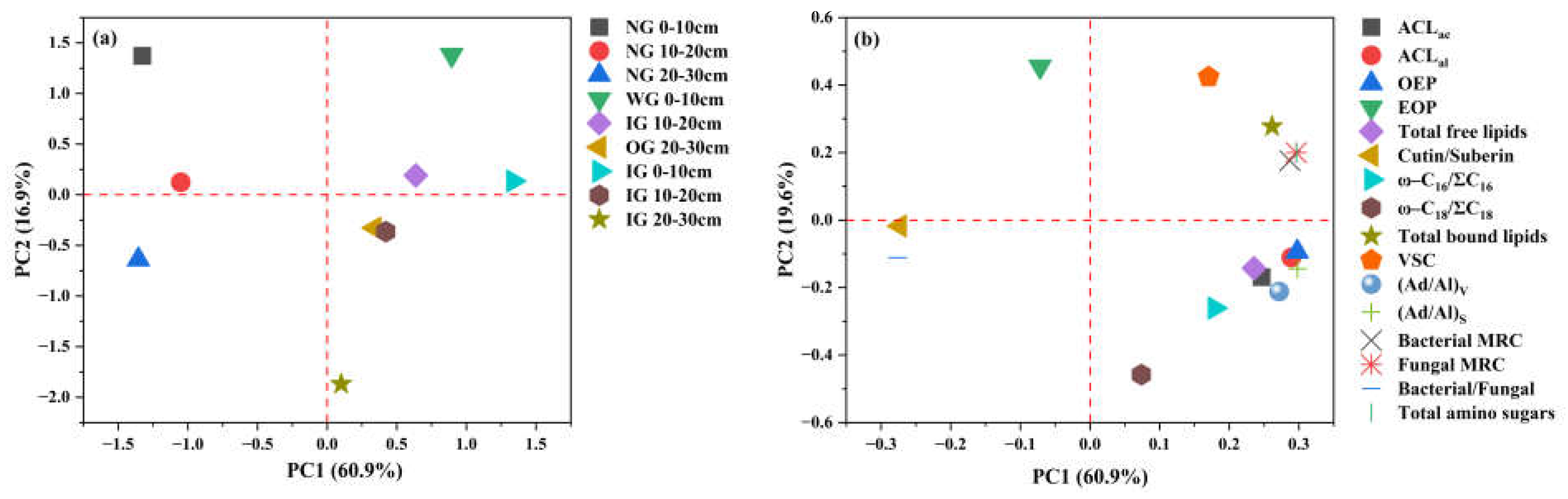
3.2. Abundance and Degradation Parameters of Biomarkers
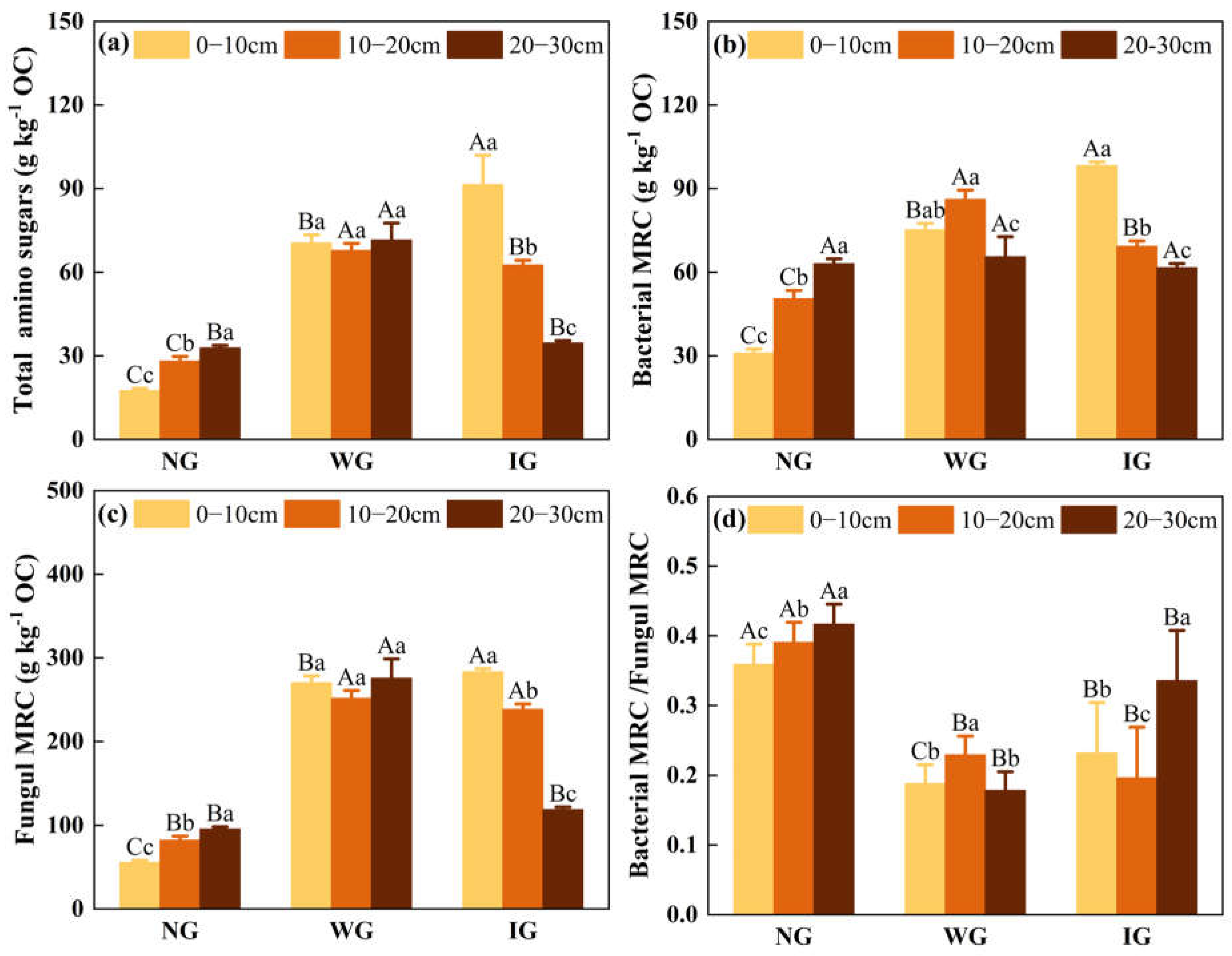
3.3. Bacterial and Fungal Residual Carbon
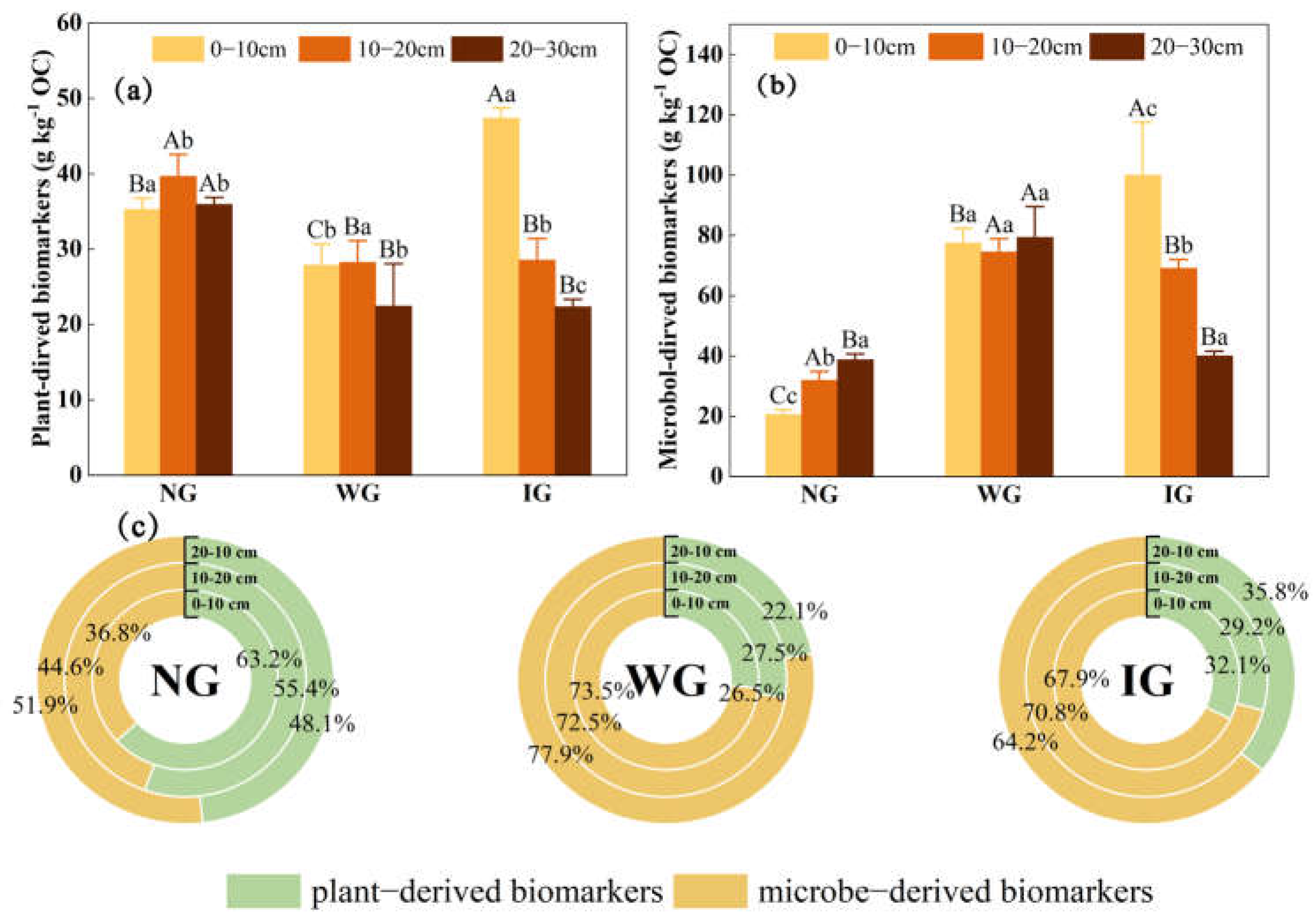
3.4. Plant- and Microbe-Derived Biomarkers
4. Discussion
4.1. Changes in Lipids and Lignin Phenols Under Grassland Community Degradation
4.2. Changes in Microbial Residues Under Grassland Community Degradation
4.3. Contributions of Plant and Microbial Residue Carbon to SOC Under Grassland Community Degradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimatti, M.; Chaplin-Kramer, R.; Di Marco, M. The Role of High-Biodiversity Regions in Preserving Nature’s Contributions to People. Nat. Sustain. 2023, 6, 1385–1393. [Google Scholar] [CrossRef]
- Lyons, K.G.; Török, P.; Hermann, J.-M.; Kiehl, K.; Kirmer, A.; Kollmann, J.; Overbeck, G.E.; Tischew, S.; Allen, E.B.; Bakker, J.D.; et al. Challenges and Opportunities for Grassland Restoration: A Global Perspective of Best Practices in the Era of Climate Change. Glob. Ecol. Conserv. 2023, 46, e02612. [Google Scholar] [CrossRef]
- Renwick, A.; Jansson, T.; Verburg, P.H.; Revoredo-Giha, C.; Britz, W.; Gocht, A.; McCracken, D. Policy Reform and Agricultural Land Abandonment in the EU. Land Use Policy 2013, 30, 446–457. [Google Scholar] [CrossRef]
- Plieninger, T.; Hui, C.; Gaertner, M.; Huntsinger, L. The Impact of Land Abandonment on Species Richness and Abundance in the Mediterranean Basin: A Meta-Analysis. PLoS ONE 2014, 9, e98355. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting Global Grassland Degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Wang, Y.; Li, Y.; Duo, J. State-of-the-Art and Challenges in Global Grassland Degradation Studies. Geogr. Sustain. 2024, 6, 100229. [Google Scholar] [CrossRef]
- Rhodes, A.C.; Rutledge, J.; DuPont, B.; Plowes, R.M.; Gilbert, L.E. Targeted Grazing of an Invasive Grass Improves Outcomes for Native Plant Communities and Wildlife Habitat. Rangel. Ecol. Manag. 2021, 75, 41–50. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, C.; Dai, X.; Lang, L.; Li, X.; Chen, J.; Wang, R.; Cai, W.; Gao, Y. Toxic Nonpreferred Species Accelerate the Natural Restoration of Plant Productivity and Diversity in Degraded Grasslands. Sci. Total Environ. 2024, 935, 173391. [Google Scholar] [CrossRef]
- Costa, D.P.D.; Araujo, A.S.F.; Pereira, A.P.D.A.; Mendes, L.W.; França, R.F.D.; Silva, T.D.G.E.D.; Oliveira, J.B.D.; Araujo, J.S.; Duda, G.P.; Menezes, R.S.C.; et al. Forest-to-Pasture Conversion Modifies the Soil Bacterial Community in Brazilian Dry Forest Caatinga. Sci. Total Environ. 2022, 810, 151943. [Google Scholar] [CrossRef]
- DiTomaso, J.M. Invasive Weeds in Rangelands: Species, Impacts, and Management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef]
- Tamura, M.; Tharayil, N. Plant Litter Chemistry and Microbial Priming Regulate the Accrual, Composition and Stability of Soil Carbon in Invaded Ecosystems. New Phytol. 2014, 203, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Li, X.; Dang, D.; Dou, H.; Xuan, X.; Liu, S.; Li, M.; Gong, J. A New Method for Grassland Degradation Monitoring by Vegetation Species Composition Using Hyperspectral Remote Sensing. Ecol. Indic. 2020, 114, 106310. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland Soil Carbon Sequestration: Current Understanding, Challenges, and Solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-Associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Wu, J.; Hu, H.; Chen, Q.; Fang, H.; Tao, K. Effects of Alpine Grassland Degradation on Soil Microbial Community Structure and Metabolic Activity in the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2024, 200, 105458. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, L.; Degen, A.A.; Rafiq, M.K.; Kuzyakov, Y.; Zhao, J.; Zhang, R.; Zhang, T.; Wang, W.; Li, X.; et al. Long-term Active Restoration of Extremely Degraded Alpine Grassland Accelerated Turnover and Increased Stability of Soil Carbon. Glob. Change Biol. 2020, 26, 7217–7228. [Google Scholar] [CrossRef]
- Häring, V.; Fischer, H.; Cadisch, G.; Stahr, K. Implication of Erosion on the Assessment of Decomposition and Humification of Soil Organic Carbon after Land Use Change in Tropical Agricultural Systems. Soil Biol. Biochem. 2013, 65, 158–167. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Hu, C.; Xu, X.; Aziz, T.; Wu, L.; Waqas, M.A.; Farooq, M.; Hu, X.; Zhang, W.; Xu, M. Long-Term Manure Application Increased Soil Organic Carbon and Nitrogen Mineralization through Accumulation of Unprotected and Physically Protected Carbon Fractions. Pedosphere 2023, 33, 343–354. [Google Scholar] [CrossRef]
- Otto, A.; Shunthirasingham, C.; Simpson, M.J. A Comparison of Plant and Microbial Biomarkers in Grassland Soils from the Prairie Ecozone of Canada. Org. Geochem. 2005, 36, 425–448. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, S.; Wang, Z.; Chen, D.; Dai, G.; Feng, B.; Su, X.; Hu, H.; Li, K.; Han, W.; et al. Divergent Accumulation of Microbial Necromass and Plant Lignin Components in Grassland Soils. Nat. Commun. 2018, 9, 3480. [Google Scholar] [CrossRef]
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased Contribution of Root Exudates to Soil Carbon Input during Grassland Degradation. Soil Biol. Biochem. 2020, 146, 107817. [Google Scholar] [CrossRef]
- Tang, Q.; Li, W.; Dai, W.; Wang, J.; Zhang, F.; Daniell, T.J.; Cheng, Y.; Wang, S.; Yin, W.; Wang, X. Patterns and Determinants of Microbial- and Plant-Derived Carbon Contributions to Soil Organic Carbon in Tea Plantation Chronosequence. Plant Soil 2024, 505, 811–825. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Chen, X.; Ni, X.; Zheng, G.; Hu, M.; Chen, H.Y.H. Changes in Plant Lignin Components and Microbial Necromass Matter with Subtropical Forest Restoration. Geoderma 2024, 445, 116875. [Google Scholar] [CrossRef]
- Zhang, Z.; Kaye, J.P.; Bradley, B.A.; Amsili, J.P.; Suseela, V. Cover Crop Functional Types Differentially Alter the Content and Composition of Soil Organic Carbon in Particulate and Mineral-associated Fractions. Glob. Change Biol. 2022, 28, 5831–5848. [Google Scholar]
- Wang, W.; Tang, J.; Zhang, N.; Wang, Y.; Xu, X.; Zhang, A. Spatiotemporal Pattern of Invasive Pedicularis in the Bayinbuluke Land, China, during 2019–2021: An Analysis Based on PlanetScope and Sentinel-2 Data. Remote Sens. 2023, 15, 4383. [Google Scholar] [CrossRef]
- Kou, Y.; Li, J.; Wang, Y.; Li, C.; Tu, B.; Yao, M.; Li, X. Scale-Dependent Key Drivers Controlling Methane Oxidation Potential in Chinese Grassland Soils. Soil Biol. Biochem. 2017, 111, 104–114. [Google Scholar]
- Hu, J.; Li, K.; Deng, C.; Gong, Y.; Liu, Y.; Wang, L. Seed Germination Ecology of Semiparasitic Weed Pedicularis Kansuensis in Alpine Grasslands. Plants 2022, 11, 1777. [Google Scholar] [CrossRef]
- Otto, A.; Simpson, M.J. Sources and Composition of Hydrolysable Aliphatic Lipids and Phenols in Soils from Western Canada. Org. Geochem. 2006, 37, 385–407. [Google Scholar] [CrossRef]
- Jin, X.; Wang, L.; Zhang, J.; Fang, Y.; Wang, X.; Xiaoyang, D.; Ma, L.; Li, H.; Zhang, L.; Liu, Y.; et al. Two Decades of No Tillage Divergently Accumulate Plant Lignin and Microbial Necromass in the Top and Sublayers. Soil Tillage Res. 2024, 244, 106211. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas Chromatographic Determination of Muramic Acid, Glucosamine, Mannosamine, and Galactosamine in Soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- NIST23; NIST Standard Reference Database 1A. NIST: Gaithersburg, MA, USA, 2014.
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of Plant-Derived Carbon and Stability of Organic Matter in Soil: Implications for Global Change. Glob. Change Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Dai, G.; Zhu, S.; Cai, Y.; Zhu, E.; Jia, Y.; Ji, C.; Tang, Z.; Fang, J.; Feng, X. Plant-Derived Lipids Play a Crucial Role in Forest Soil Carbon Accumulation. Soil Biol. Biochem. 2022, 168, 108645. [Google Scholar]
- Li, T.; Cheng, H.; Li, Y.; Mou, Z.; Zhu, X.; Wu, W.; Zhang, J.; Kuang, L.; Wang, J.; Hui, D.; et al. Divergent Accumulation of Amino Sugars and Lignins Mediated by Soil Functional Carbon Pools under Tropical Forest Conversion. Sci. Total Environ. 2023, 881, 163204. [Google Scholar] [CrossRef]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in Amino Sugar and Ergosterol Contents after Addition of Sucrose and Cellulose to Soil. Soil Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Karimi Nezhad, M.T.; Šamonil, P.; Daněk, P.; Jaroš, J.; Hájek, M.; Hájková, P.; Jabinski, S.; Meador, T.B.; Roleček, J. Lipid Biomarkers and Stable Isotopes Uncover Paleovegetation Changes in Extremely Species-Rich Forest-Steppe Ecosystems, Central Europe. Environ. Res. 2024, 259, 119564. [Google Scholar] [CrossRef]
- Li, X.; Anderson, B.J.; Vogeler, I.; Schwendenmann, L. Long-Chain n-Alkane and n-Fatty Acid Characteristics in Plants and Soil—Potential to Separate Plant Growth Forms, Primary and Secondary Grasslands? Sci. Total Environ. 2018, 645, 1567–1578. [Google Scholar] [CrossRef]
- Man, M.; Gregorich, E.G.; Beare, M.H.; Ellert, B.H.; Simpson, M.J. Distinct Dynamics of Plant- and Microbial-Derived Soil Organic Matter in Relation to Varying Climate and Soil Properties in Temperate Agroecosystems. Geochim. Cosmochim. Acta 2023, 361, 276–287. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Leide, J.; Riedel, M.; Zemke, J.; Kögel-Knabner, I. Distribution of Cutin and Suberin Biomarkers under Forest Trees with Different Root Systems. Plant Soil 2014, 381, 95–110. [Google Scholar] [CrossRef]
- Afifi, M.; Swanton, C. Early Physiological Mechanisms of Weed Competition. Weed Sci. 2012, 60, 542–551. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, A.J.; Wilson, K.P.; Dudley Williams, D.; Simpson, M.J. Increased Cuticular Carbon Sequestration and Lignin Oxidation in Response to Soil Warming. Nat. Geosci. 2008, 1, 836–839. [Google Scholar] [CrossRef]
- Li, J.; He, J.-Z.; Liu, M.; Yan, Z.-Q.; Xu, X.-L.; Kuzyakov, Y. Invasive Plant Competitivity Is Mediated by Nitrogen Use Strategies and Rhizosphere Microbiome. Soil Biol. Biochem. 2024, 192, 109361. [Google Scholar] [CrossRef]
- Liu, X.; Bol, R.; An, T.; Liu, Y.; Xu, Y.; Li, S.; Wang, J. Divergent Accumulation of Microbial Necromass and Plant Lignin Phenol Induced by Adding Maize Straw to Fertilized Soils. Soil Tillage Res. 2024, 243, 106177. [Google Scholar] [CrossRef]
- Zou, Z.; Ma, L.; Wang, X.; Chen, R.; Jones, D.L.; Bol, R.; Wu, D.; Du, Z. Decadal Application of Mineral Fertilizers Alters the Molecular Composition and Origins of Organic Matter in Particulate and Mineral-Associated Fractions. Soil Biol. Biochem. 2023, 182, 109042. [Google Scholar] [CrossRef]
- Sanaullah, M.; Rumpel, C.; Charrier, X.; Chabbi, A. How Does Drought Stress Influence the Decomposition of Plant Litter with Contrasting Quality in a Grassland Ecosystem? Plant Soil 2012, 352, 277–288. [Google Scholar] [CrossRef]
- Zhu, S.; Dai, G.; Ma, T.; Chen, L.; Chen, D.; Lü, X.; Wang, X.; Zhu, J.; Zhang, Y.; Bai, Y.; et al. Distribution of Lignin Phenols in Comparison with Plant-Derived Lipids in the Alpine versus Temperate Grassland Soils. Plant Soil 2019, 439, 325–338. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, L.; Fang, Y.; Zhang, A.; Li, G.; Wang, J.; Wu, D.; Wu, W.; Du, Z. Conservation Tillage for 17 Years Alters the Molecular Composition of Organic Matter in Soil Profile. Sci. Total Environ. 2021, 762, 143116. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Yuan, Z.-Q.; Li, F.-M. Alfalfa-Livestock System Promotes the Accumulation of Soil Organic Carbon in a Semi-Arid Marginal Land. Agric. Ecosyst. Environ. 2024, 375, 109200. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased Microbial Growth, Biomass, and Turnover Drive Soil Organic Carbon Accumulation at Higher Plant Diversity. Glob. Change Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef]
- Ottoy, S.; Van Meerbeek, K.; Sindayihebura, A.; Hermy, M.; Van Orshoven, J. Assessing Top- and Subsoil Organic Carbon Stocks of Low-Input High-Diversity Systems Using Soil and Vegetation Characteristics. Sci. Total Environ. 2017, 589, 153–164. [Google Scholar] [CrossRef]
- Almagro, M.; Ruiz-Navarro, A.; Díaz-Pereira, E.; Albaladejo, J.; Martínez-Mena, M. Plant Residue Chemical Quality Modulates the Soil Microbial Response Related to Decomposition and Soil Organic Carbon and Nitrogen Stabilization in a Rainfed Mediterranean Agroecosystem. Soil Biol. Biochem. 2021, 156, 108198. [Google Scholar] [CrossRef]
- Xue, Z.; Qu, T.; Li, X.; Chen, Q.; Zhou, Z.; Wang, B.; Lv, X. Different Contributing Processes in Bacterial vs. Fungal Necromass Affect Soil Carbon Fractions during Plant Residue Transformation. Plant Soil 2024, 494, 301–319. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Wang, Y.; Liang, C.; An, S.; Soromotin, A.; Kuzyakov, Y. Increasing Contribution of Microbial Residues to Soil Organic Carbon in Grassland Restoration Chronosequence. Soil Biol. Biochem. 2022, 170, 108688. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the Primacy of Living Root Inputs, Not Root or Shoot Litter, in Forming Soil Organic Carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Šarūnaitė, L.; Arlauskienė, A.; Jablonskytė-Raščė, D. Effect of Plant Edges Strips on the Conservation Soil Properties in Modern Farming Field. PLoS ONE 2024, 19, e0299104. [Google Scholar] [CrossRef]
- Liao, J.; Dou, Y.; Yang, X.; An, S. Soil Microbial Community and Their Functional Genes during Grassland Restoration. J. Environ. Manag. 2023, 325, 116488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, G.; Liu, H.; Li, Y.; Ma, X.; Fan, L.; Mao, J. Increasing Contribution of Microbial Residue Carbon to Soil Organic Carbon Accumulation in Degraded Grasslands. Agronomy 2025, 15, 810. https://doi.org/10.3390/agronomy15040810
Zhang W, Wang G, Liu H, Li Y, Ma X, Fan L, Mao J. Increasing Contribution of Microbial Residue Carbon to Soil Organic Carbon Accumulation in Degraded Grasslands. Agronomy. 2025; 15(4):810. https://doi.org/10.3390/agronomy15040810
Chicago/Turabian StyleZhang, Wenbo, Guangyu Wang, Haoyu Liu, Yaoming Li, Xuexi Ma, Lianlian Fan, and Jiefei Mao. 2025. "Increasing Contribution of Microbial Residue Carbon to Soil Organic Carbon Accumulation in Degraded Grasslands" Agronomy 15, no. 4: 810. https://doi.org/10.3390/agronomy15040810
APA StyleZhang, W., Wang, G., Liu, H., Li, Y., Ma, X., Fan, L., & Mao, J. (2025). Increasing Contribution of Microbial Residue Carbon to Soil Organic Carbon Accumulation in Degraded Grasslands. Agronomy, 15(4), 810. https://doi.org/10.3390/agronomy15040810






