Inversion Study of Maize Leaf Physiological Information Under Light–Temperature Stress Using Visible–Near Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Methods
2.2. Experiment Data Collection and Processing
3. Results and Discussion
3.1. Response of Maize Leaf Physiological Parameters to Different Light and Temperature Environments
3.2. Spectral Characteristics of Maize Leaves in Visible and Near-Infrared Regions Under Different Light–Temperature Environments
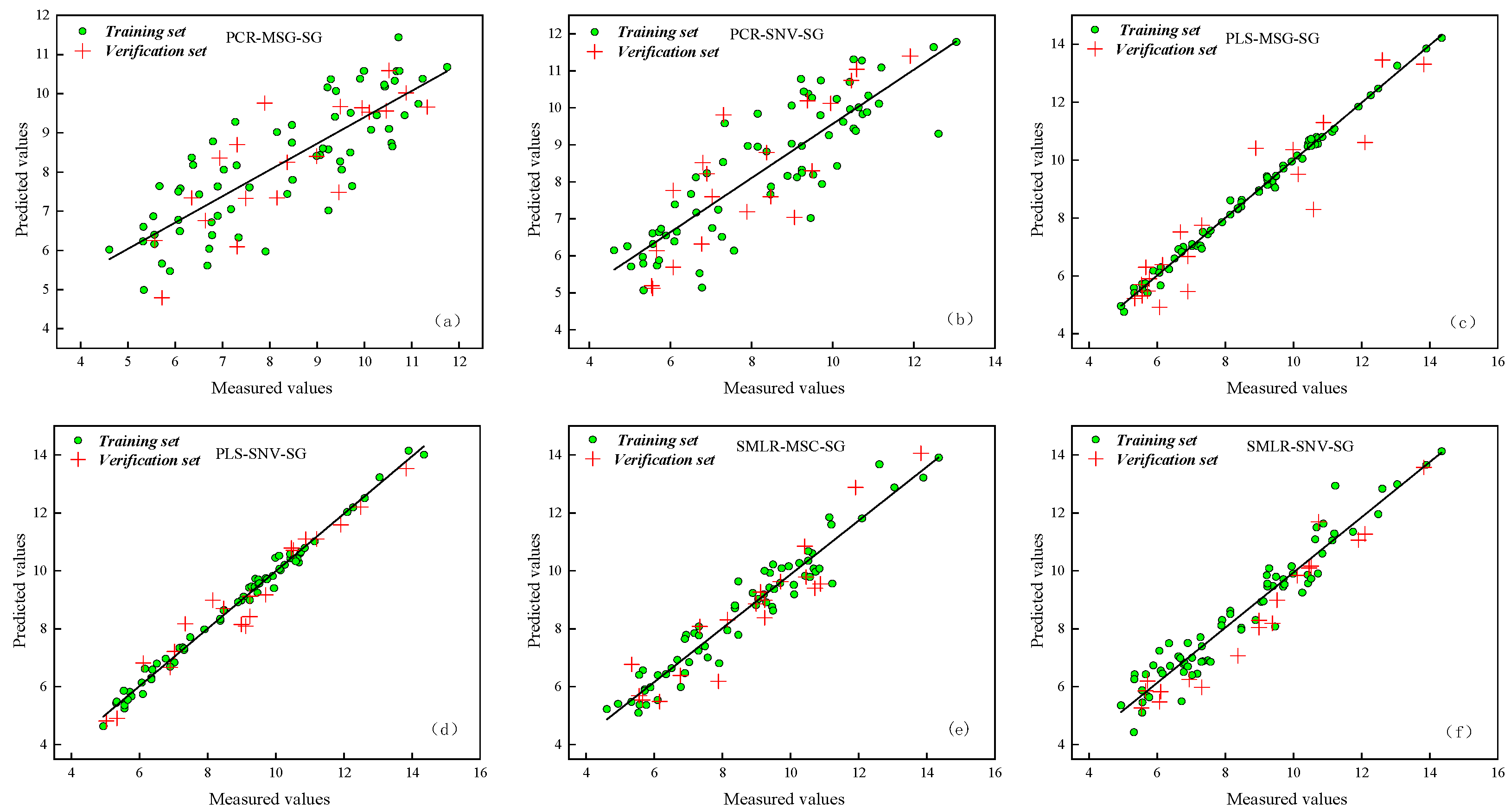
3.3. Establishment of the SPAD and Net Photosynthetic Rate Inversion Model for Maize Leaves
3.3.1. Selection of Sensitive Bands
3.3.2. Establishment and Comparison of Physiological Information Inversion Models in Maize Leaves
4. Conclusions
4.1. Main Conclusion
4.2. Potential Technology Development Path
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, M.H.; Yang, Y.; Zhang, Y.H.; Chen, C.; Tong, Y.X. The influence of continuous light supply with different blue light intensity on the morphology, photosynthetic characteristics and nutrient ion absorption of lettuce. Chin. J. Ecol. Agric. 2024, 32, 839–850, (In Chinese and English). [Google Scholar]
- Chen, G.L. Effects of light intensity on growth and photosynthesis in soybean seedling stage. Sichuan Agric. Sci. Technol. 2019, 9, 18–21. [Google Scholar]
- Cheng, Y.J.; Chen, J.X.; Wang, Z.L.; Fan, Y.F.; Chen, S.Y.; Li, Z.L.; Liu, Q.L.; Li, Z.C.; Yang, F.; Yang, W.Y. Effect of light intensity and light quality on the morphology and photosynthetic characteristics of soybean seedlings. Agric. Sci. China 2018, 51, 2655–2663. [Google Scholar]
- Gao, Z.Y.; Zhang, Z.P.; Fan, L.; Yang, Y.H. Effects of light intensity and nitrogen levels on maize growth and development, photosynthetic physiology, and dry matter accumulation. J. Yunnan Agric. Univ. (Nat. Sci.) 2020, 35, 187–195. [Google Scholar]
- Zhang, S.H.; Zhang, X.H.; Yang, Z.R.; Huang, X.M.; Zhang, F.L. Effect of C. High temperature stress on seedling growth and fluorescence dynamic parameters of sand mustard and chlorophyll leaf plants. North. Hortic. 2024, 10, 1–8. [Google Scholar]
- Shi, S.B.; Shi, R.; Zhou, D.W.; Zhang, W. Effect of low temperature on the photochemical and non-photochemical energy dissipation characteristics of alpine songgrass leaves. J. Plant Ecol. 2023, 47, 1441–1452. [Google Scholar] [CrossRef]
- Li, X.F.; Shao, J.Y.; Yu, W.Z.; Liu, P.; Zhao, B.; Zhang, J.W.; Ren, B. Effect of high temperature and drought compound stress on the yield and photosynthetic properties of summer maize. Agric. Sci. China 2022, 55, 3516–3529. [Google Scholar]
- Shao, J.Y.; Li, X.F.; Yu, W.Z.; Liu, P.; Zhao, B.; Zhang, J.W.; Ren, B.C. Effect of heat and drought compound stress on yield and stalk microstructure in summer maize. Agric. Sci. China 2021, 54, 3623–3631. [Google Scholar]
- Newiton, D.S.T.; Paulo, C.C.; Cristiano, D.F.; Adriano, H.R.; Marina, P.S. Effects of drying temperature of corn from the center and extremities of the corncob on drying parameters, protein and starch properties, and carotenoid profile. Food Res. Int. 2023, 163, 112267. [Google Scholar]
- Han, Y.F.; Wu, C.X.; Wu, H.H.; He, Y.K.; Yang, B.H.; Yuan, Y.W. Fruit and vegetables quality detection method based on visible-near-infrared spectroscopy technology. Agric. Eng. 2024, 14, 95–101. [Google Scholar]
- Jiang, X.G.; Zhu, M.W.; Yao, J.L.; Li, B.; Liao, J.; Zhang, Y.X.; Liu, Y.D. Effect of apple size difference on the applicability of the sugar degree model based on NIRS technology. J. Huazhong Agric. Univ. 2024, 43, 242–248. [Google Scholar]
- Lu, Y.J.; Wang, W.J.; Zhang, J.D.; Wang, Z.Y.; Lu, S.H. Classification and identification of rice images at different growth stages in wheat based on NIR spectroscopy and ELM. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2023, 44, 104–111. [Google Scholar]
- Zhang, M.Y.; Hao, M.; Tian, H.Q. Nondestructive testing of pH values of silage corn feed based on hyperspectral imaging technology. J. Agric. Eng. 2023, 39, 239–247. [Google Scholar]
- Liu, S.; Yu, H.Y.; Chen, M.C.; Yu, T.; Li, F.Q.W.; Sui, Y.Y. Hyperspectral analysis of different water and nitrogen stress response rules in soybean. Spectrosc. Spectrosc. Anal. 2020, 40, 1575–1580. [Google Scholar]
- Hetta, M.; Mussadiq, Z.; Wallsten, J.; Halling, M.; Swensson, C.; Geladi, P. Prediction of nutritive values, morphology and agronomic characteristics in forage maize using two applications of NIRS spectrometry. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 326–333. [Google Scholar]
- Xin, L.; Tian, J.; Wanli, D.; Sai, X. Dual-Channel Co-Spectroscopy–Based Non-Destructive Detection Method for Fruit Quality and Its Application to Fuji Apples. Agronomy 2025, 15, 484. [Google Scholar] [CrossRef]
- Li, J.C.; Gu, Y.; Liu, C.L.; Sun, X.R.; Zhang, S.Z. Asash content of wheat flour based on hyperspectroscopy. J. Food Saf. Qual. Test. 2023, 14, 60–67. [Google Scholar]
- Cai, X.; Li, X.X.; Wang, Q.; Zhang, X.; Feng, J.Y.; Li, J. Response of maize, wheat, and rape to different light intensity treatments. South. Agric. 2023, 17, 6–11. [Google Scholar]
- Wen, W.M. Key influencing factors and countermeasures of corn yield. Grain Oil Feed. Technol. 2024, 8, 93–95. [Google Scholar]
- Lv, H.H.; Niu, Y.Y.; Zhang, M.; Li, H. Analysis of the trend of light intensity and air temperature and humidity in solar greenhouse. J. Agric. Mach. 2021, 52, 410–417. [Google Scholar]
- Wang, F.; Liu, L.; Wu, Y.Y.; Li, X.; Sun, Z.G.; Yin, F.; Jiao, N.Y.; Fu, G.Z. Mechanism of peanut intercropping. J. Plant Nutr. Fertil. 2020, 26, 901–913. [Google Scholar]
- Zhang, L.X.; Wu, X.D.; Cai, L.T.; Lu, C.P. Study on the effect of biodegradable plastic film mulching on fresh corn yield and soil temperature and humidity. Gard. Seedl. 2024, 44, 83–85. [Google Scholar]
- Jung, C.; Arar, M. Natural vs. Artificial Light: A Study on the Influence of Light Source on Chlorophyll Content and Photosynthetic Rates on Indoor Plants. Buildings 2023, 13, 1482. [Google Scholar] [CrossRef]
- Yao, J.F.; Liu, J. Design and implementation of crop growth environment information detection system based on DTU. Smart Agric. Guide 2022, 2, 11–13. [Google Scholar]
- Ji, H.; Zhang, M.; Huang, C.H.; Lin, W.; Lu, Y.; Wang, P.J.; Dong, B.; He, B.Z.; Wu, B.H.; Guo, L.J. The Impact of Piriformospora indica on plant heat and drought tolerance. Front. Plant Sci. 2024, 15, 1479561. [Google Scholar] [CrossRef]
- Wang, T.; Feng, J.L.; Zhang, C. Progress in the molecular mechanism of high temperature stress affecting corn growth and development. J. Bot. 2024, 59, 963–977. [Google Scholar]
- Yuan, M.; Jin, Z.X.; Sun, Z.S.; Chen, C. Effect of different light intensities on the photosynthetic properties of long fruit scale hammer trees. Fujian For. Sci. Technol. 2024, 51, 24–31. [Google Scholar]
- Stepanova, N.; Tarakhovskaya, E.; Soboleva, A.; Orlova, A.; Basnet, A.; Smolenskaya, A.; Frolova, N.; Bilova, T.; Kamionskaya, A.; Folov, A.; et al. Green Light Drives Embryonic Photosynthesis and Protein Accumulation in Cotyledons of Developing Pea (Pisum sativum L.) Seeds. Agronomy 2024, 14, 2367. [Google Scholar] [CrossRef]
- Almoujahed, M.B.; Apolo-Apolo, O.E.; Alhussein, M.; Kazlauskas, M.; Kriaučiūnienė, Z.; Šarauskis, E.; Mouazen, A.M. Prediction of Deoxynivalenol contamination in wheat kernels and flour based on visible near-infrared spectroscopy, feature selection and machine learning modelling. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2025, 330, 125718. [Google Scholar]
- Yang, H.; Ming, B.; Nie, C.; Xue, B.; Xin, J.; Lu, X.; Xue, J.; Hou, P.; Xie, R.; Wang, K.; et al. Maize Canopy and Leaf Chlorophyll Content Assessment from Leaf Spectral Reflectance: Estimation and Uncertainty Analysis across Growth Stages and Vertical Distribution. Remote Sens. 2022, 14, 2115. [Google Scholar] [CrossRef]
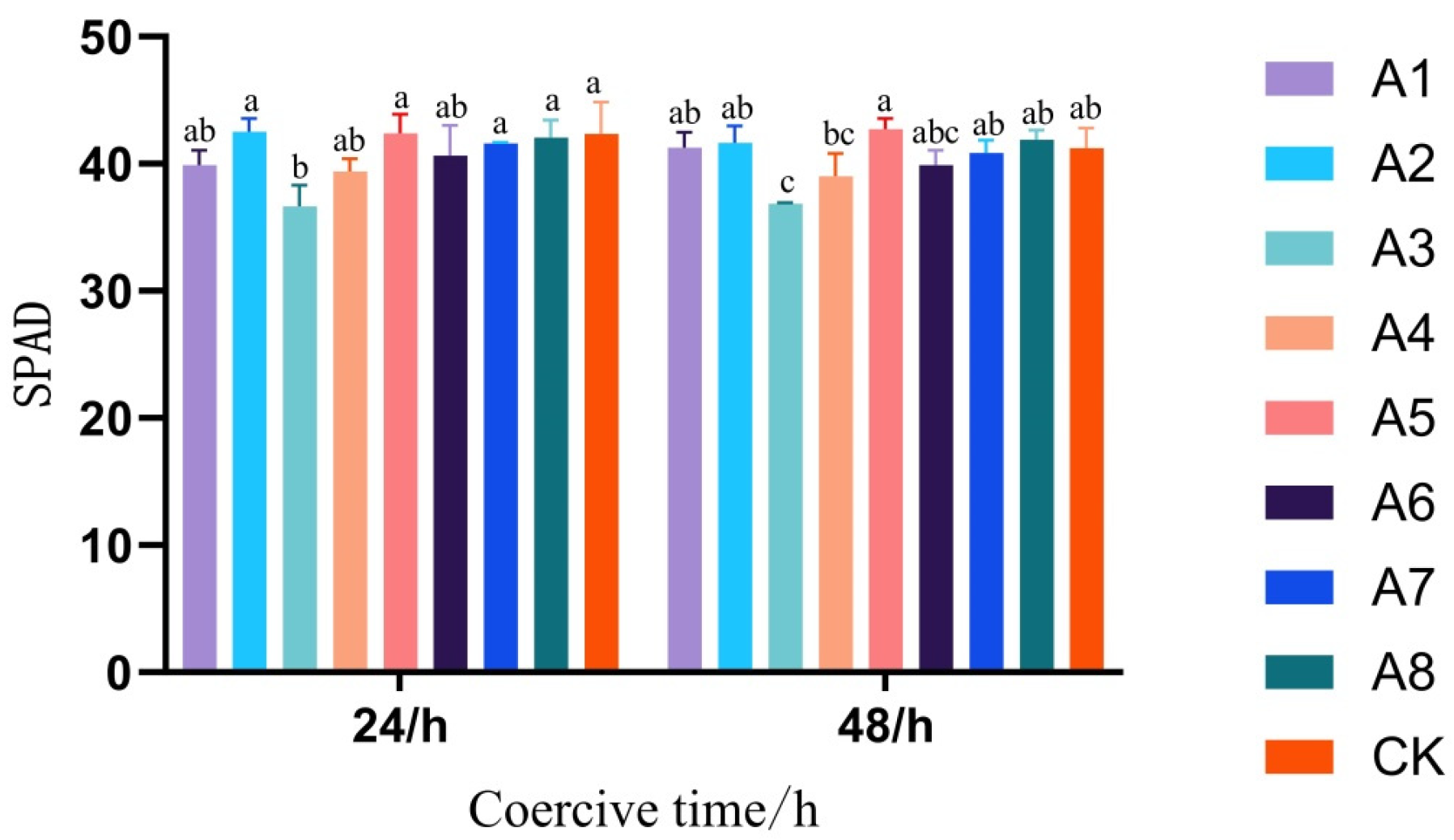
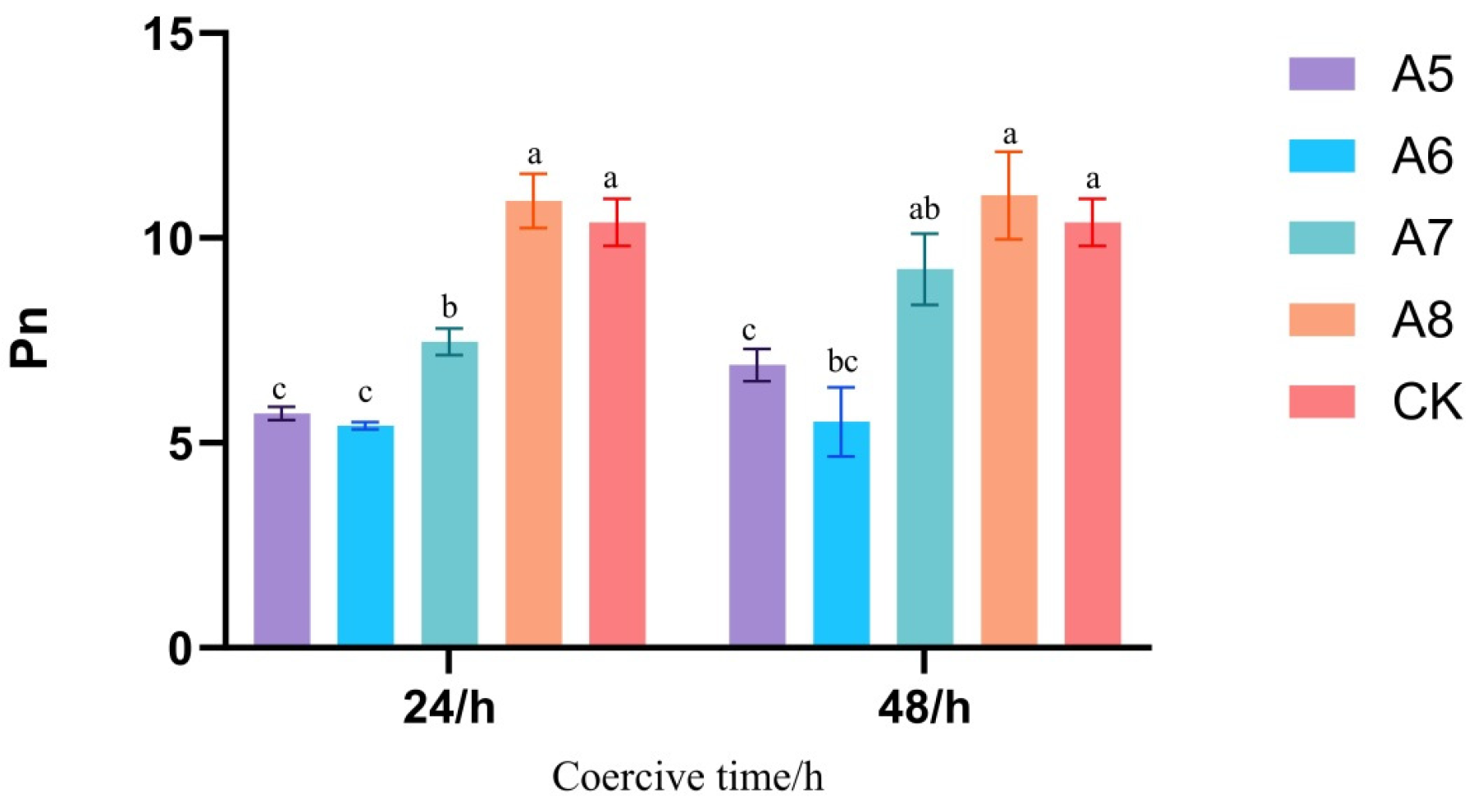
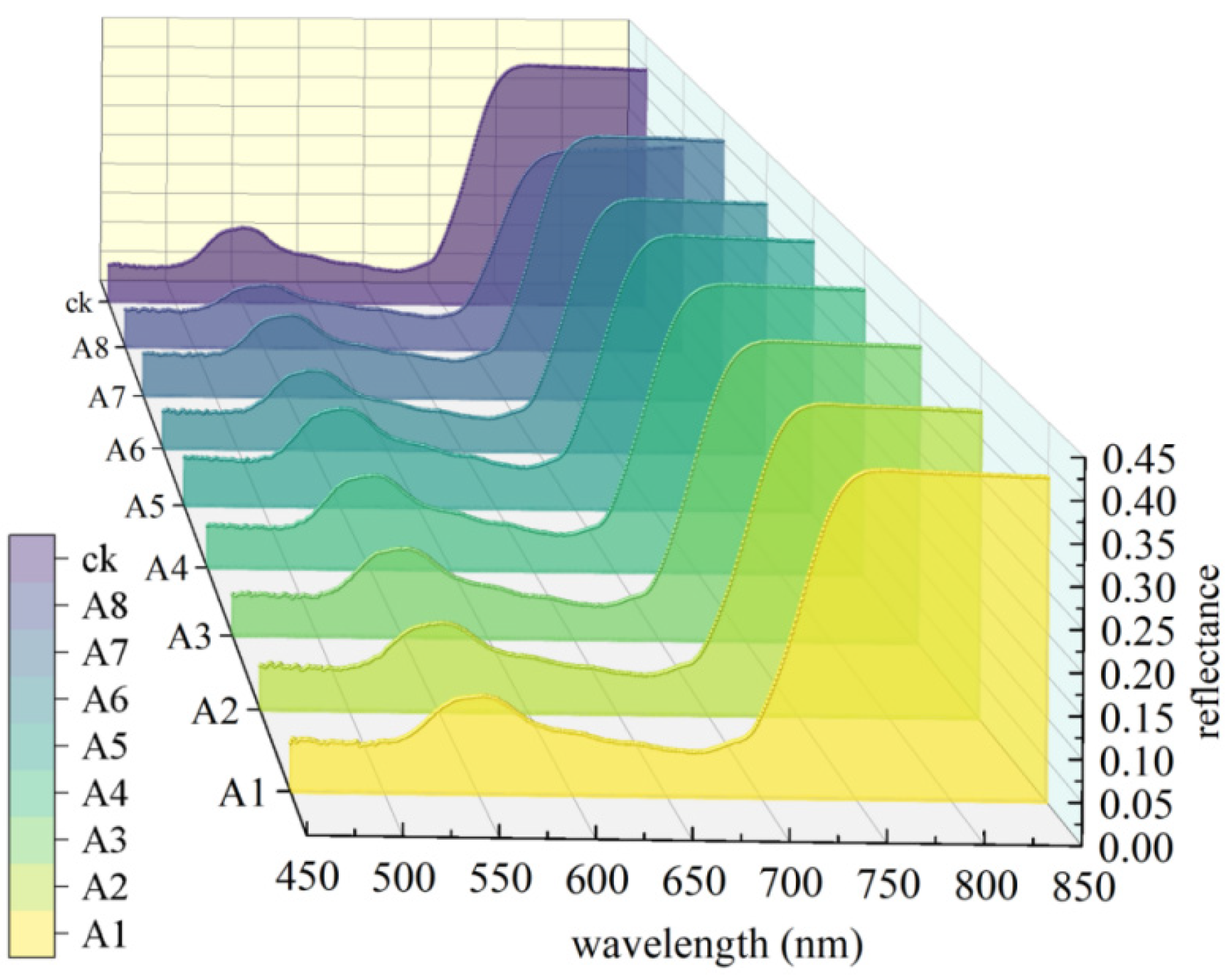


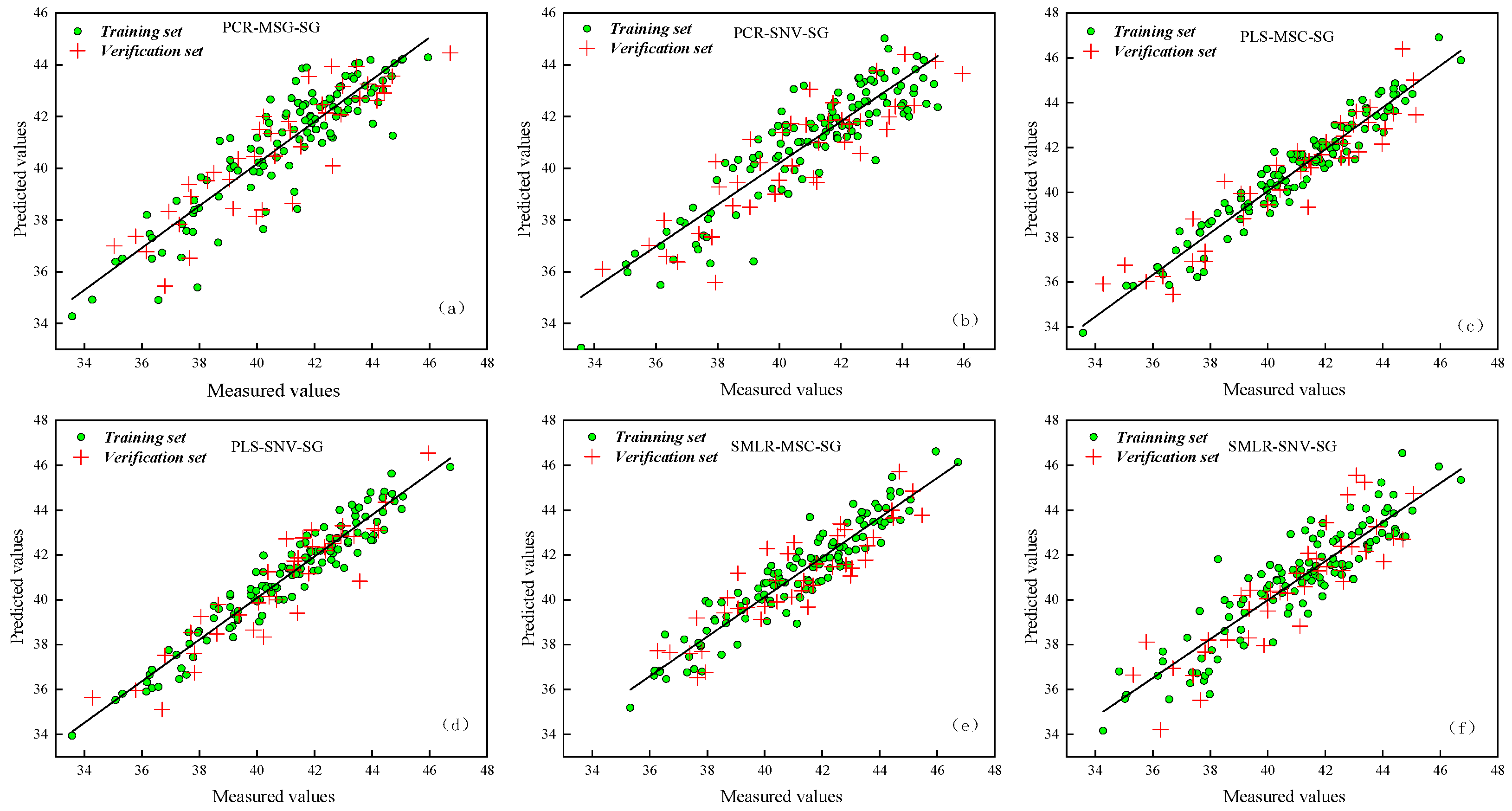
| Experimental Grouping | Treatment Number | Treatment Combination Description | Day/Night Temperature (°C) | Light Intensity (lux) |
|---|---|---|---|---|
| Single-Factor Experiment | A1 | Low-light intensity stress environment | 20/15 | 4400 |
| A2 | High-light intensity stress environment | 20/15 | 22000 | |
| A3 | Low-temperature stress environment | 14/5 | 8800 | |
| A4 | High-temperature stress environment | 34/22 | 8800 | |
| Light and Temperature Factors Experiment | A5 | Photothermal environment 1 | 25/15 | 12,000 |
| A6 | Photothermal environment 2 | 34/22 | 12,000 | |
| A7 | Photothermal environment 3 | 34/22 | 22,000 | |
| A8 | Photothermal environment 4 | 25/15 | 22,000 |
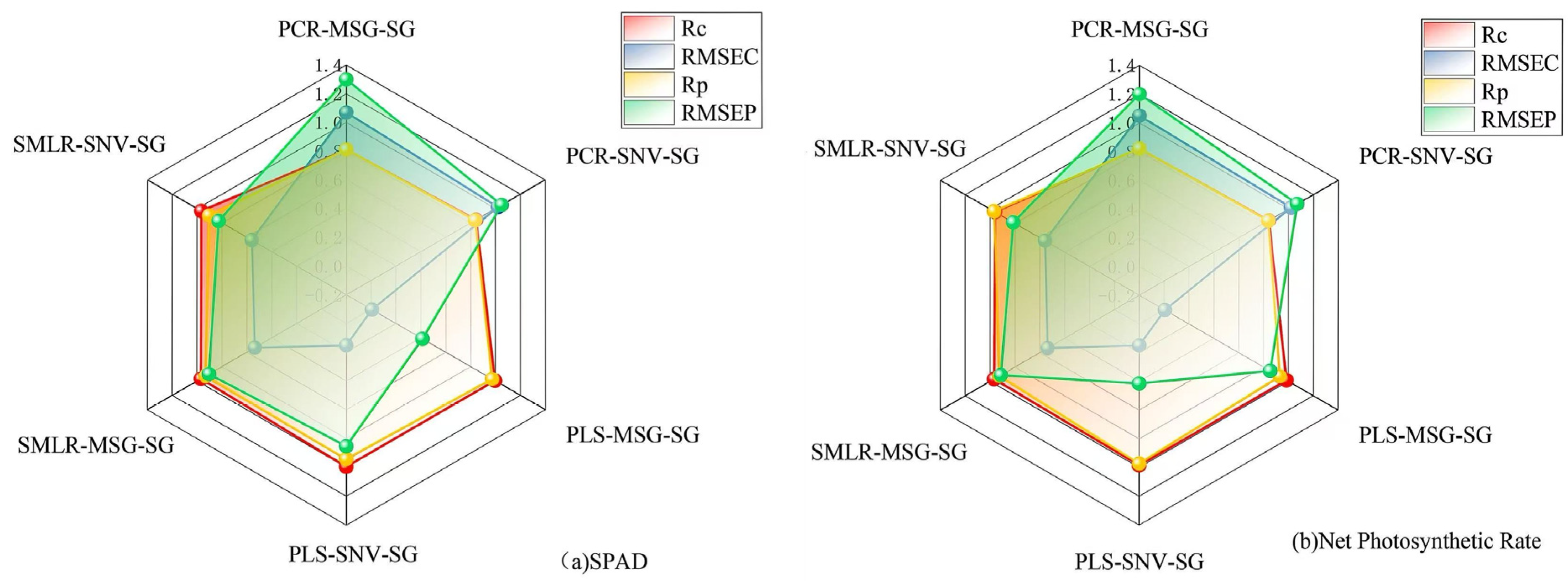
| Physiological Information | Spectral Combination Method | Rc | RMSEC | Rp | RMSEP |
|---|---|---|---|---|---|
| SPAD | PCR-MSG-SG | 0.8104 | 1.0700 | 0.8118 | 1.300 |
| PCR-SNV-SG | 0.8450 | 1.0200 | 0.8367 | 1.050 | |
| PLS-MSG-SG | 0.9960 | 0.0064 | 0.9743 | 0.413 | |
| PLS-SNV-SG | 0.9949 | 0.1520 | 0.9428 | 0.853 | |
| SMLR-MSG-SG | 0.9694 | 0.5360 | 0.9321 | 0.904 | |
| SMLR-SNV-SG | 0.9674 | 0.5610 | 0.9040 | 0.827 | |
| Net Photosynthetic Rate | PCR-MSG-SG | 0.8203 | 1.0500 | 0.8214 | 1.200 |
| PCR-SNV-SG | 0.8414 | 1.0200 | 0.8374 | 1.070 | |
| PLS-MSG-SG | 0.9860 | 0.0059 | 0.9328 | 0.854 | |
| PLS-SNV-SG | 0.9848 | 0.1490 | 0.9738 | 0.415 | |
| SMLR-MSG-SG | 0.9684 | 0.5374 | 0.9248 | 0.913 | |
| SMLR-SNV-SG | 0.9592 | 0.5590 | 0.9679 | 0.814 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Qiao, J.; Zhou, L.; Liu, S.; Chen, L.; Yu, Y.; Kong, L. Inversion Study of Maize Leaf Physiological Information Under Light–Temperature Stress Using Visible–Near Infrared Spectroscopy. Agronomy 2025, 15, 828. https://doi.org/10.3390/agronomy15040828
Gao S, Qiao J, Zhou L, Liu S, Chen L, Yu Y, Kong L. Inversion Study of Maize Leaf Physiological Information Under Light–Temperature Stress Using Visible–Near Infrared Spectroscopy. Agronomy. 2025; 15(4):828. https://doi.org/10.3390/agronomy15040828
Chicago/Turabian StyleGao, Siyao, Jianlei Qiao, Lina Zhou, Shuang Liu, Limei Chen, Yue Yu, and Lijuan Kong. 2025. "Inversion Study of Maize Leaf Physiological Information Under Light–Temperature Stress Using Visible–Near Infrared Spectroscopy" Agronomy 15, no. 4: 828. https://doi.org/10.3390/agronomy15040828
APA StyleGao, S., Qiao, J., Zhou, L., Liu, S., Chen, L., Yu, Y., & Kong, L. (2025). Inversion Study of Maize Leaf Physiological Information Under Light–Temperature Stress Using Visible–Near Infrared Spectroscopy. Agronomy, 15(4), 828. https://doi.org/10.3390/agronomy15040828





