Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Measurement and Calculation Methods
2.3. Soluble Protein Content Detection
2.4. Real-Time Quantitative PCR Analysis
2.5. Metabolites Extraction
2.6. UHPLC-MS/MS Analysis
2.7. Precision and Accuracy
3. Results
3.1. Changes of Nitrogen Accumulation of Soybean Plants Under Low-P Stress
3.2. 15N Abundance of Soybean Plants Under Low-P Stress
3.3. Changes in Nodule Nitrogen Fixation Accumulation and Ratio of Nodule Nitrogen Fixation of Soybean Plants Under Low-P Stress
3.4. Effect of Low-P Stress on Soluble Protein Content of Soybean
3.5. Effect of Low-P Stress on PTs of Soybean
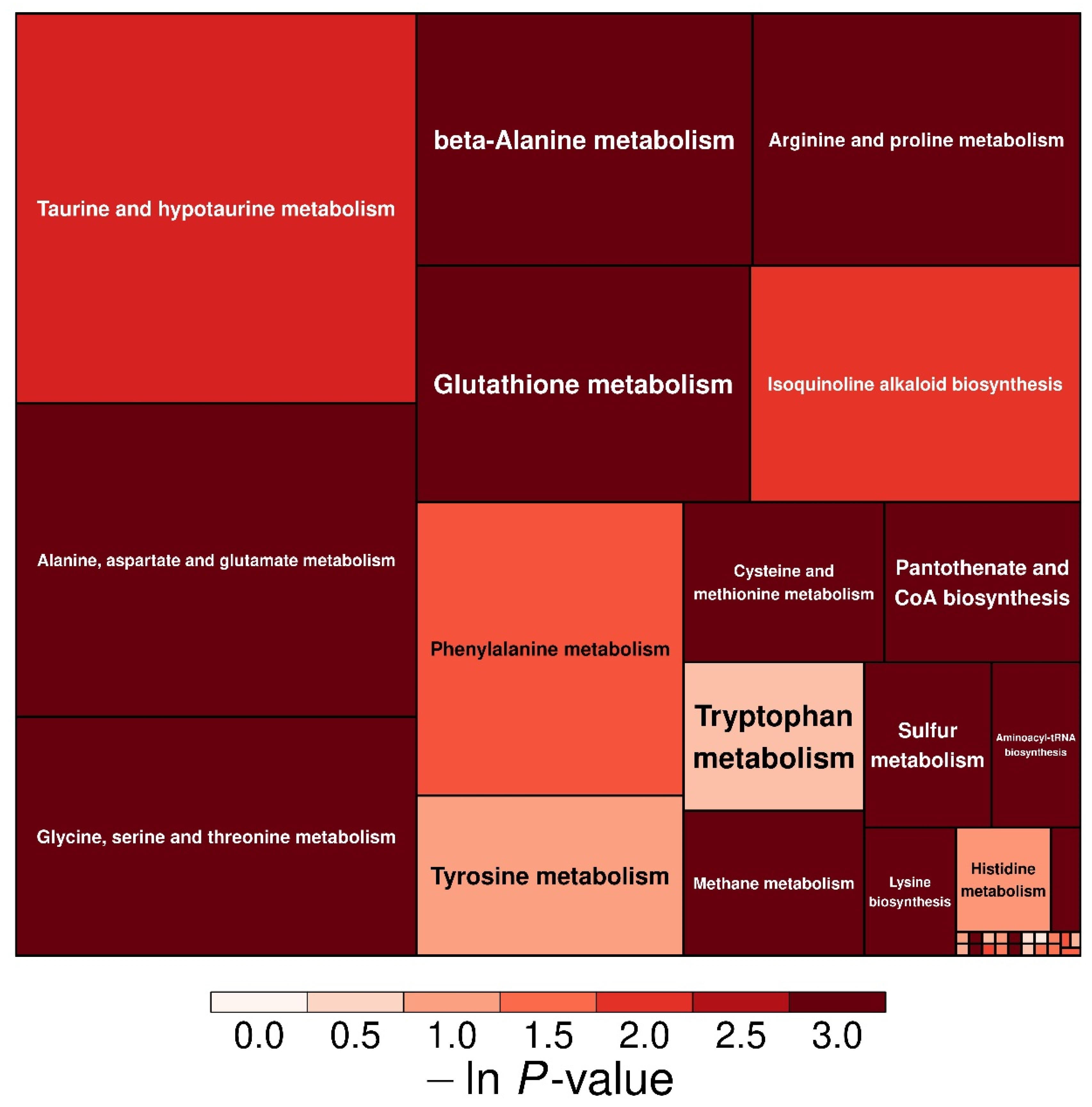
3.6. Effect of Low-P Stress on Amino Acid Metabolism in Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Z.B.; Zhang, M.; Liang, S.; Fan, L. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant Biotechnol. J. 2022, 20, 1807–1818. [Google Scholar] [CrossRef]
- Lu, J.L. Plant Nutrition; China Agricultural University: Beijing, China, 2003; pp. 35–36. [Google Scholar]
- Deng, L.; Zhang, C.; Yuan, K.; Gao, Y.; Pan, Y.W.; Ge, X.L.; He, Y.X.; Yuan, Y.; Lu, Y.; Zhang, X.X.; et al. Prioritizing natural-selection signals from the deep-sequencing genomic data suggests multi-variant adaptation in Tibetan highlanders. Natl. Sci. Rev. 2019, 6, 1201–1222. [Google Scholar] [CrossRef]
- King, K.E.; Lauter, N.; Lin, S.F.; Scott, M.P.; Shoemaker, R.C. Evaluation and QTL mapping of phosphorus concentration in soybean seed. Euphytica 2013, 189, 261–269. [Google Scholar] [CrossRef]
- Chen, L.; Liao, H. Engineering crop nutrient efficiency for sustainable agriculture. J. Integr. Plant Biol. 2017, 59, 710–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Gao, Z.; Zhou, H.W.; He, Y.; Liu, Y.X.; Lai, Y.L.; Zheng, J.K.; Li, X.X.; Liao, H. GmPTF1 modifies root architecture responses to phosphate starvation primarily through regulating GmEXPB2 expression in soybean. Plant J. 2021, 107, 525–543. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Efficiency of soil and fertilizer phosphorus use: Reconciling changing concepts of soil phosphorus behaviour with agronomic information. Exp. Agric. 2009, 45, 128–135. [Google Scholar] [CrossRef]
- Sun, M.; Li, P.C.; Wang, N.; Zheng, C.S.; Sun, X.Z.; Dong, H.L.; Han, H.M.; Feng, W.N.; Shao, J.J.; Zhang, Y.F. Soil available phosphorus deficiency reduces boll biomass and lint yield by affecting sucrose metabolism in cotton-boll subtending leaves. Agronomy 2022, 12, 1065. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Sulieman, S.; Van Ha, C.; Schulze, J.; Tran, L.S. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef]
- Tsvetkova, G.E.; Georgiev, G.I. Effect of phosphorus nutrition on the nodulation, nitrogen fixation and nutrient-use efficiency of Bradyrhizobium Japonicum-soybean (Glycine max l. merr.) symbiosis. Bulg. J. Plant Physiol. 2003, 3, 315–335. [Google Scholar]
- Isaac, M.E.; Harmand, J.M.; Drevon, J.J. Growth and nitrogen acquisition strategies of Acacia senegal seedlings under exponential phosphorus additions. J. Plant Physiol. 2010, 168, 776–781. [Google Scholar] [CrossRef]
- Maistry, P.M.; Cramer, M.D.; Chimphango, S.B. N and P colimitation of N2-fixing and N-supplied fynbos legumes from the Cape Floristic Region. Plant Soil 2013, 373, 217–228. [Google Scholar] [CrossRef]
- Sharma, P.; Kumari, A. Approaches to Enhance Abiotic and Biotic Stress Tolerance in Leguminous Crops and Microgreens; Mathur, P., Gupta, A., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Qi, W.D. Functional Analysis of GmG3PT3 Involvement in Soybean Responses to Phosphorus Deficiency; South China Agricultural University: Guangzhou, China, 2017. [Google Scholar]
- Cai, B.Y.; Ge, J.P.; Zu, W. Soluble protein content in leaves and seeds of different soybean genotypes as affected by different phosphorus supplies. J. Plant Nutr. Fertil. 2007, 13, 1185–1188. [Google Scholar]
- Yang, L.X.; Wang, Y.L.; Huang, J.Y.; Zhu, J.G.; Yang, H.J.; Liu, G.; Dong, G.C.; Hu, J. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on phosphorus uptake and utilization of rice at three levels of nitrogen fertilization. Field Crops Res. 2007, 102, 141–150. [Google Scholar] [CrossRef]
- Chu, S.S.; Zhang, X.Q.; Yu, K.Y.; Lv, L.L.; Sun, C.Y.; Liu, X.Q.; Zhang, J.Y.; Jiao, Y.Q.; Zhang, D. Genome-wide analysis reveals dynamic epigenomic differences in soybean response to low-phosphorus stress. Int. J. Mol. Sci. 2020, 21, 6817. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Trigueros, M.; Rojas-Triana, M.; Fernández, M.; Albar, J.P.; Bustos, R.; Paz-Ares, J.; Rubio, V. Proteomics identifies ubiquitin-proteasome targets and new roles for chromatin-remodeling in the Arabidopsis response to phosphate starvation. J. Proteom. 2013, 94, 1–22. [Google Scholar] [CrossRef]
- Jiang, W.Z.; He, P.M.; Zhou, M.; Lu, X.; Chen, K.; Liang, C.Y.; Tian, J. Soybean responds to phosphate starvation through reversible protein phosphorylation. Plant Physiol. Biochem. 2021, 167, 222–234. [Google Scholar] [CrossRef]
- Liang, C.Y.; Tian, J.; Liao, H. Proteomics dissection of plant responses to mineral nutrient deficiency. Proteomics 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and Environmental Regulation of Root Development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qin, L.; Zhao, J.; Liao, H. Advances in Pht1 Phosphate Transporter Family Genes in Legumes. Soybean Sci. 2015, 34, 1057–1065. [Google Scholar]
- Song, H.N.; Yin, Z.T.; Chao, M.N.; Ning, L.H.; Zhang, D.; Yu, D.Y. Functional properties and expression quantitative trait loci for phosphate transporter GmPT1 in soybean. Plant Cell Environ. 2014, 37, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Wang, J.X.; Zhao, J.; Tian, J.; Liao, H. Control of phosphate homeostasis through gene regulation in crops. Curr. Opin. Plant Biol. 2014, 21, 59–66. [Google Scholar] [CrossRef]
- Mitsukawa, N.; Okumura, S.; Shirano, Y.; Sato, S.; Kato, T.; Harashima, S.; Shibata, D. Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc. Natl. Acad. Sci. USA 1997, 94, 7098–7102. [Google Scholar]
- Yang, X.J.; Finnegan, P.M. Regulation of phosphate starvation responses in higher plants. Ann. Bot. 2010, 105, 513–526. [Google Scholar] [CrossRef]
- Tian, J.; Venkatachalam, P.; Liao, H.; Yan, X.L.; Raghothama, K. Molecular cloning and characterization of phosphorus starvation responsive genes in common bean (Phaseolus vulgaris L.). Planta 2007, 227, 51–65. [Google Scholar] [CrossRef]
- Grønlund, M.; Albrechtsen, M.; Johansen, I.E.; Hammer, E.C.; Nielsen, T.H.; Jakobsen, I. The interplay between P uptake pathways in mycorrhizal peas: A combined physiologyogical and gene-silencing approach. Physiol. Plant. 2013, 149, 234–248. [Google Scholar] [CrossRef]
- Volpe, V.; Dellaglio, E.; Giovannetti, M.; Ruberti, C.; Costa, A.; Genre, A.; Guether, M.; Bonfante, P. An AM-induced, MYB-family gene of Lotusjaponicus (LjMAMI) affects root growth in an AM-independent manner. Plant J. 2013, 73, 442–455. [Google Scholar] [CrossRef]
- Wang, F.; Cui, P.J.; Tian, Y.; Huang, Y.; Wang, H.F.; Liu, F.; Chen, Y.F. Maize ZmPT7 regulates Pi uptake and redistribution which is modulated by phosphorylation. Plant Biotechnol. J. 2020, 18, 2406–2419. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Liang, R.S.; Shi, X.Y.; Chen, L.L.; Hu, X.; Wang, S.C.; Dai, X.L.; Qu, H.Y.; Li, H.H.; et al. OsWRKY21 and OsWRKY108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1;1 under phosphate-replete conditions. New Phytol. 2021, 229, 1598–1614. [Google Scholar] [CrossRef]
- Wang, S.C.; Xu, T.T.; Chen, M.; Geng, L.Y.; Huang, Z.Y.; Dai, X.L.; Qu, H.Y.; Zhang, J.; Li, H.H.; Gu, M.; et al. The transcription factor OsWRKY10 inhibits phosphate uptake via suppressing OsPHT1;2 expression under phosphate-replete conditions in rice. J. Exp. Bot. 2022, 74, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.R.; Dai, X.L.; Qu, H.Y.; Men, Q.; Liu, J.Y.; Yu, L.; Gu, M.; Xu, G.H. The rice phosphate transporter OsPHT1;7 plays a dual role in phosphorus redistribution and anther development. Plant Physiol. 2022, 188, 2272–2288. [Google Scholar] [CrossRef]
- Noike, Y.; Okamoto, I.; Tada, Y. Root epidermis-specific expression of a phosphate transporter TaPT2 enhances the growth of transgenic Arabidopsis under Pi-replete and Pi-depleted conditions. Plant Sci. 2023, 327, 111540. [Google Scholar] [CrossRef]
- Wang, P.F.; Li, G.Z.; Li, G.W.; Yuan, S.S.; Wang, C.Y.; Xie, Y.X.; Guo, T.C.; Kang, G.Z.; Wang, D.W. TaPHT1;9-4B and its transcriptional regulator TaMYB4-7D contribute to phosphate uptake and plant growth in bread wheat. New Phytol. 2021, 231, 1968–1983. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Cao, H.R.; Zhao, J.; Bai, S.; Peng, W.T.; Li, J.; Sun, L.L.; Chen, L.Y.; Lin, Z.H.; Shi, C.; et al. A natural uORF variant confers phosphorus acquisition diversity in soybean. Nat. Commun. 2022, 13, 3796. [Google Scholar] [CrossRef]
- Luan, M.D.; Zhao, F.G.; Sun, G.F.; Xu, M.; Fu, A.; Lan, W.Z.; Luan, S. A SPX domain vacuolar transporter links phosphate sensing to homeostasis in Arabidopsis. Mol. Plant 2022, 15, 1590–1601. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, H.Y.; Chu, S.S.; Li, H.Y.; Chi, Y.J.; Triebwasser, F.D.; Lv, H.Y.; Yu, D.Y. Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol. Biol. 2017, 83, 137–150. [Google Scholar] [CrossRef]
- Medhi, A.K.; Dhar, S.; Roy, A. Effect of different growth regulators and phosphorus levels on nodulation, yield and quality components in green gram. Indian J. Plant Physiol. 2014, 19, 74–78. [Google Scholar] [CrossRef]
- Ran, Q.X.; Pang, J.Y.; Dong, R.; He, J. Enhanced seed yield, essential amino acids and unsaturated fatty acids in soybean seeds with phosphorus fertilizer supply. J. Food Compos. Anal. 2024, 125, 105813. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef]
- Le roux, M.R.; Khan, S.; Vapentine, A.J. Nitrogen and carbon costs of soybean and lupin root systems during phosphate starvation. Symbiosis 2009, 48, 102–109. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yuan, H.M.; Wu, G.W.; Yan, J.; Zhao, D.S.; Chen, S.; Kang, Q.H.; Ma, C.M.; Gong, Z.P. Nitrogen fixation capacity and metabolite responses to phosphorus in soybean nodules. Symbiosis 2022, 88, 21–35. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yuan, H.M.; Wu, G.W.; Ma, C.M.; Gong, Z.P. Proteome Analysis of the Soybean Nodule Phosphorus Response Mechanism and Characterization of Stress-Induced Ribosome Structural and Protein Expression Changes. Front. Plant Sci. 2022, 13, 908889. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, S.N.; Mo, X.H.; Guo, Q.; Li, Y.X.; Tian, J.; Liang, C.Y. Proteomic analysis dissects molecular mechanisms underlying plant responses to phosphorus deficiency. Cells 2022, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Lazali, M.; Bargaz, A.; Carlsson, G.; Ounane, S.M.; Drevon, J.J. Discrimination against 15N among recombinant inbred lines of Phaseolus vulgaris L. contrasting in phosphorus use efficiency for nitrogen fixation. J. Plant Physiol. 2014, 171, 199–204. [Google Scholar] [CrossRef]
- Høgh-Jensen, H.; Schjoerring, J.K.; Soussana, J.F. The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Ann. Bot. 2002, 90, 745–753. [Google Scholar] [CrossRef]
- Desnos, T. Root branching responses to phosphate and nitrate. Curr. Opin. Plant Biol. 2007, 11, 82–87. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qin, L.; Zhou, L.L.; Li, X.X.; Chen, Z.C.; Liao, H. A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 2019, 221, 2013–2025. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yang, A.H.; Kong, L.J.; Wang, H.Y.; Ao, X. Proteome characterization of two contrasting soybean genotypes in response to different phosphorus treatments. AoB Plants 2021, 13, lab019. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, X.M.; Dong, S.K. Biochemical characterization and metabolic reprogramming of amino acids in Soybean roots under drought stress. Physiol. Plant. 2024, 176, e14319. [Google Scholar] [CrossRef] [PubMed]
- Tawaraya, K.; Horie, R.; Shinano, T.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci. Plant Nutr. 2014, 60, 679–694. [Google Scholar]
- Zhang, L. Root exudates and soil fertility. Bull. Biol. 2000, 35, 17. [Google Scholar] [CrossRef]
- Mo, X.H. Phosphate Starvation Responsive Protein Phosphatase GmHAD1-2 Regulates Flavonol Biosynthesis and Lateral Root Development in Soybean; South China Agricultural University: Guangzhou, China, 2019. [Google Scholar] [CrossRef]
- Shi, G.Y. Physiological Mechanism and Proteomics of Soybean Root Response to Low Phosphorus Stress; Guizhou University: Guiyang, China, 2021. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yuan, H.M.; Liu, D.D.; Cheng, L.L. Response of soybean root exudates and related metabolic pathways to low phosphorus stress. PLoS ONE 2024, 19, e0314256. [Google Scholar] [CrossRef]
- Keitaro, T.; Ryota, H.; Saki, S.; Tadao, W.; Kazuki, S.; Akira, O. Metabolite profiling of root exudates of common bean under phosphorus deficiency. Metabolites 2014, 4, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Valdés-López, O.; Ramírez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.K.; et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238. [Google Scholar] [CrossRef]
- Lundberg, P.; Lundquist, P.O. Primary metabolism in N2 fixing Alnus incanaFrankia symbiotic root nodules studied with 15N and 31P nuclear magnetic resonance spectroscopy. Planta 2004, 219, 661–672. [Google Scholar]
- Yao, Y.B. Multi-omics analysis of carbon and nitrogen metabolism pathways in soybean nodules under phosphorus stress. Soybean Sci. 2024, 43, 624–631. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, M.; Yang, G.; Sun, M.; Yang, A.; Sun, C.; Zhao, H.; Ao, X. Root morphology, nitrogen metabolism and amino acid metabolism in soybean under low phosphorus stress. Sci. Rep. 2024, 14, 28583. [Google Scholar] [CrossRef]


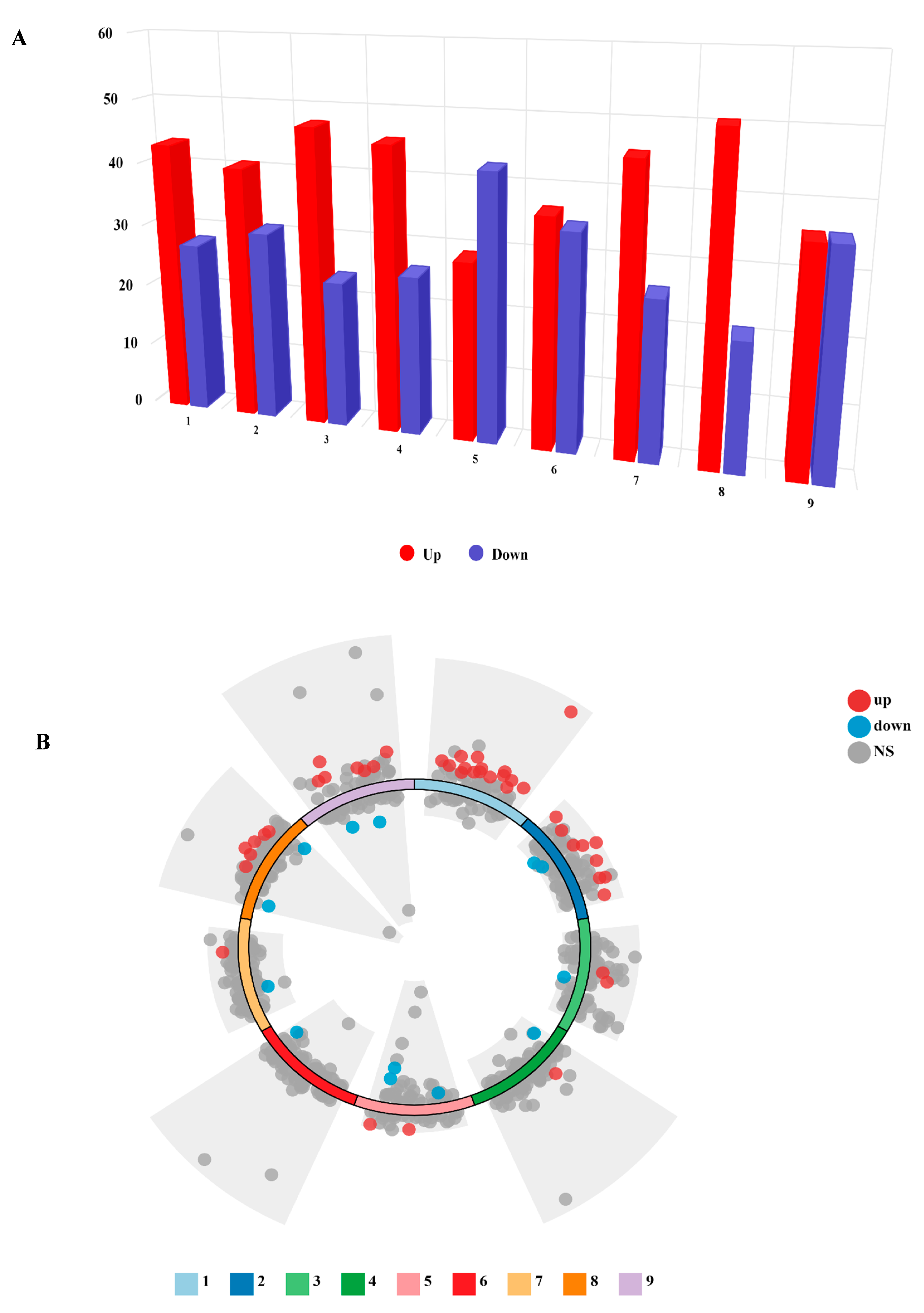

| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 23.98 ± 0.21 * | 31.78 ± 1.67 * | 9.53 ± 0.10 * | 9.54 ± 0.10 * | 1.22 ± 0.00 * | 1.19 ± 0.08 * |
| P31 | 39.49 ± 0.47 | 66.90 ± 1.82 | 10.73 ± 0.16 | 10.73 ± 0.16 | 5.39 ± 0.12 | 8.82 ± 0.34 | |
| 14 days | P1 | 29.78 ± 0.49 * | 47.68 ± 1.02 * | 16.74 ± 0.01 * | 9.94 ± 0.24 * | 1.53 ± 0.00 * | 2.56 ± 0.05 * |
| P31 | 90.78 ± 2.70 | 92.42 ± 1.97 | 19.60 ± 0.15 | 14.17 ± 0.13 | 11.20 ± 0.46 | 8.49 ± 0.06 | |
| 21 days | P1 | 48.26 ± 3.11 * | 46.48 ± 0.75 * | 18.96 ± 0.96 * | 17.09 ± 0.21 * | 1.23 ± 0.12 * | 2.67 ± 0.14 * |
| P31 | 143.23 ± 7.02 | 225.48 ± 3.62 | 37.99 ± 0.08 | 44.90 ± 2.57 | 26.82 ± 0.21 | 24.64 ± 0.30 | |
| 28 days | P1 | 75.03 ± 2.98 * | 63.15 ± 0.12 * | 25.38 ± 1.12 * | 20.02 ± 1.04 * | 2.23 ± 0.04 * | 2.67 ± 0.17 * |
| P31 | 248.56 ± 14.83 | 325.61 ± 8.05 | 66.41 ± 1.26 | 76.50 ± 2.38 | 33.59 ± 1.18 | 24.87 ± 1.04 | |
| 35 days | P1 | 102.52 ± 0.27 * | 97.70 ± 3.49 * | 41.52 ± 1.04 * | 22.74 ± 1.28 * | 3.65 ± 0.35 * | 2.69 ± 0.23 * |
| P31 | 403.80 ± 14.93 | 476.66 ± 2.02 | 92.52 ± 2.31 | 90.20 ± 1.73 | 37.02 ± 0.39 | 36.22 ± 0.68 | |
| 42 days | P1 | 186.66 ± 0.59 * | 175.45 ± 1.29 * | 53.08 ± 1.68 * | 58.64 ± 0.16 * | 6.69 ± 0.04 * | 2.92 ± 0.21 * |
| P31 | 425.70 ± 2.87 | 464.67 ± 5.73 | 89.55 ± 6.04 | 140.15 ± 1.13 | 40.09 ± 0.65 | 30.95 ± 1.67 | |
| 49 days | P1 | 302.11 ± 0.88 * | 271.70 ± 5.51 * | 92.58 ± 0.19 * | 87.96 ± 4.19 * | 11.23 ± 0.07 * | 5.58 ± 0.23 * |
| P31 | 446.68 ± 1.03 | 684.25 ± 7.95 | 117.42 ± 1.36 | 108.26 ± 2.85 | 46.79 ± 0.77 | 25.94 ± 1.39 | |
| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 1.89 ± 0.01 | 1.91 ± 0.00 | 2.11 ± 0.04 * | 2.02 ± 0.01 | 0.91 ± 0.00 * | 1.02 ± 0.02 * |
| P31 | 1.91 ± 0.02 | 1.94 ± 0.02 | 1.80 ± 0.00 | 1.81 ± 0.05 | 1.21 ± 0.01 | 0.78 ± 0.00 | |
| 14 days | P1 | 2.08 ± 0.00 * | 2.17 ± 0.02 | 2.22 ± 0.02 | 2.33 ± 0.05 | 1.00 ± 0.03 * | 1.18 ± 0.05 * |
| P31 | 1.84 ± 0.01 | 2.00 ± 0.04 | 2.12 ± 0.04 | 2.13 ± 0.01 | 0.62 ± 0.00 | 0.78 ± 0.03 | |
| 21 days | P1 | 2.31 ± 0.00 * | 2.28 ± 0.04 * | 2.29 ± 0.03 * | 2.23 ± 0.03 * | 1.01 ± 0.04 * | 1.21 ± 0.00 * |
| P31 | 1.33 ± 0.06 | 1.30 ± 0.03 | 1.71 ± 0.02 | 1.76 ± 0.01 | 0.51 ± 0.03 | 0.44 ± 0.00 | |
| 28 days | P1 | 2.42 ± 0.00 * | 2.31 ± 0.03 * | 2.40 ± 0.00 * | 2.35 ± 0.03 * | 1.28 ± 0.02 * | 1.06 ± 0.01 * |
| P31 | 1.17 ± 0.02 | 1.55 ± 0.03 | 1.61 ± 0.01 | 2.00 ± 0.00 | 0.50 ± 0.00 | 0.53 ± 0.02 | |
| 35 days | P1 | 2.45 ± 0.02 * | 2.43 ± 0.01 * | 2.44 ± 0.01 * | 2.44 ± 0.03 * | 1.00 ± 0.03 * | 1.40 ± 0.01 * |
| P31 | 1.20 ± 0.01 | 1.60 ± 0.00 | 1.75 ± 0.00 | 1.98 ± 0.03 | 0.57 ± 0.01 | 0.59 ± 0.04 | |
| 42 days | P1 | 2.27 ± 0.08 * | 2.58 ± 0.02 * | 2.47 ± 0.05 * | 2.63 ± 0.00 * | 0.80 ± 0.01 * | 1.08 ± 0.04 * |
| P31 | 1.23 ± 0.00 | 1.72 ± 0.01 | 1.68 ± 0.00 | 2.07 ± 0.00 | 0.55 ± 0.00 | 0.53 ± 0.00 | |
| 49 days | P1 | 2.20 ± 0.05 * | 2.38 ± 0.06 * | 2.41 ± 0.03 * | 2.49 ± 0.04 * | 0.77 ± 0.03 * | 0.89 ± 0.08 * |
| P31 | 1.18 ± 0.01 | 1.72 ± 0.06 | 1.66 ± 0.02 | 2.06 ± 0.06 | 0.46 ± 0.00 | 0.53 ± 0.03 | |
| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 10.26 ± 0.17 * | 11.76 ± 0.00 * | 5.86 ± 0.11 | 3.18 ± 0.02 * | 0.89 ± 0.00 * | 0.79 ± 0.06 * |
| P31 | 16.67 ± 0.51 | 24.11 ± 0.15 | 5.42 ± 0.02 | 4.33 ± 0.13 | 3.42 ± 0.06 | 6.54 ± 0.26 | |
| 14 days | P1 | 10.99 ± 0.11 * | 13.54 ± 0.71 * | 5.50 ± 0.09 * | 2.31 ± 0.11 * | 1.07 ± 0.01 * | 1.57 ± 0.01 * |
| P31 | 40.34 ± 1.59 | 31.33 ± 0.79 | 7.01 ± 0.22 | 4.22 ± 0.09 | 9.10 ± 0.37 | 6.32 ± 0.15 | |
| 21 days | P1 | 14.56 ± 0.79 * | 11.49 ± 0.50 * | 5.81 ± 0.11 * | 4.53 ± 0.11 * | 0.86 ± 0.10 * | 1.61 ± 0.08 * |
| P31 | 85.36 ± 1.55 | 128.9 ± 0.50 | 18.31 ± 0.28 | 18.81 ± 0.86 | 22.70 ± 0.43 | 21.08 ± 0.19 | |
| 28 days | P1 | 20.06 ± 0.71 * | 15.03 ± 0.75 * | 6.94 ± 0.29 * | 4.50 ± 0.03 * | 1.37 ± 0.04 * | 1.73 ± 0.12 * |
| P31 | 160.14 ± 7.94 | 159.62 ± 7.75 | 34.13 ± 0.87 | 26.14 ± 0.80 | 28.49 ± 0.97 | 20.55 ± 1.02 | |
| 35 days | P1 | 26.56 ± 0.75 * | 19.61 ± 1.10 * | 10.80 ± 0.82 * | 4.46 ± 0.02 * | 2.54 ± 0.21 * | 1.44 ± 0.11 * |
| P31 | 256.88 ± 7.70 | 225.39 ± 2.39 | 43.48 ± 1.31 | 31.36 ± 1.50 | 30.63 ± 0.50 | 29.12 ± 0.02 | |
| 42 days | P1 | 58.44 ± 1.28 * | 26.11 ± 1.54 * | 13.38 ± 0.37 * | 7.83 ± 0.82 * | 5.07 ± 0.00 * | 1.88 ± 0.09 * |
| P31 | 267.45 ± 1.61 | 200.22 ± 4.51 | 44.03 ± 1.14 | 44.77 ± 0.50 | 33.43 ± 0.59 | 25.55 ± 1.31 | |
| 49 days | P1 | 101.24 ± 4.32 * | 58.84 ± 6.80 * | 24.97 ± 1.04 * | 15.85 ± 0.32 * | 8.62 ± 0.05 * | 3.96 ± 0.31 * |
| P31 | 287.07 ± 2.21 | 295.93 ± 11.39 | 58.63 ± 1.56 | 34.78 ± 1.55 | 40.28 ± 0.54 | 21.48 ± 1.43 | |
| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 42.79 ± 0.32 | 37.01 ± 0.14 | 36.05 ± 1.49 * | 33.37 ± 0.56 * | 72.41 ± 0.16 * | 66.49 ± 0.56 * |
| P31 | 42.18 ± 0.78 | 36.09 ± 0.74 | 45.57 ± 0.16 | 40.40 ± 1.88 | 63.53 ± 0.34 | 74.16 ± 0.08 | |
| 14 days | P1 | 36.93 ± 0.21 * | 28.35 ± 0.88 * | 32.85 ± 0.61 | 23.36 ± 1.77 * | 69.86 ± 1.05 * | 61.27 ± 1.67 * |
| P31 | 44.42 ± 0.42 | 33.97 ± 1.59 | 35.80 ± 1.43 | 29.72 ± 0.25 | 81.29 ± 0.06 | 74.39 ± 1.30 | |
| 21 days | P1 | 30.20 ± 0.03 * | 24.76 ± 1.47 * | 30.76 ± 0.94 * | 26.54 ± 1.02 * | 69.54 ± 1.26 * | 60.23 ± 0.13 * |
| P31 | 59.78 ± 1.87 | 57.20 ± 1.14 | 48.20 ± 0.84 | 41.95 ± 0.48 | 84.63 ± 0.93 | 85.57 ± 0.26 | |
| 28 days | P1 | 26.75 ± 0.11 * | 23.80 ± 1.15 * | 27.35 ± 0.05 * | 22.60 ± 1.02 * | 61.31 ± 0.77 * | 64.94 ± 0.61 * |
| P31 | 64.50 ± 0.65 | 48.96 ± 1.16 | 51.38 ± 0.34 | 34.17 ± 0.01 | 84.83 ± 0.07 | 82.56 ± 0.67 | |
| 35 days | P1 | 25.90 ± 0.70 * | 20.04 ± 0.41 * | 26.13 ± 0.56 * | 19.75 ± 1.02 * | 69.88 ± 0.93 * | 53.78 ± 0.57 * |
| P31 | 63.65 ± 0.44 | 47.44 ± 0.31 | 46.98 ± 0.24 | 34.72 ± 0.99 | 82.73 ± 0.48 | 80.45 ± 1.45 | |
| 42 days | P1 | 31.32 ± 0.24 * | 14.87 ± 0.77 * | 25.31 ± 1.51 * | 13.35 ± 0.07 * | 75.71 ± 0.46 * | 64.44 ± 1.51 * |
| P31 | 62.83 ± 0.04 | 43.17 ± 0.44 | 49.14 ± 0.19 | 31.94 ± 0.09 | 83.37 ± 0.12 | 82.56 ± 0.21 | |
| 49 days | P1 | 33.52 ± 1.51 * | 21.57 ± 2.00 * | 26.97 ± 1.08 * | 17.88 ± 1.47 * | 76.73 ± 0.96 * | 70.75 ± 2.67 * |
| P31 | 64.27 ± 0.34 | 43.30 ± 2.16 | 49.91 ± 0.75 | 32.25 ± 2.28 | 86.09 ± 0.27 | 82.67 ± 1.09 | |
| Pathway | Compounds | Upregulated | Downregulated | |
|---|---|---|---|---|
| P1_Leaf_7d-P31_Leaf_7d | 37 | 26 | 15 | 11 |
| P1_Leaf_14d-P31_Leaf_14d | 37 | 26 | 17 | 9 |
| P1_Leaf_21d-P31_Leaf_21d | 37 | 26 | 19 | 7 |
| P1_Root_7d-P31_Root_7d | 37 | 26 | 18 | 8 |
| P1_Root_14d-P31_Root_14d | 37 | 26 | 14 | 12 |
| P1_Root_21d-P31_Root_21d | 37 | 26 | 13 | 13 |
| P1_Nodule_7d-P31_Nodule_7d | 37 | 26 | 18 | 8 |
| P1_Nodule_14d-P31_Nodule_14d | 37 | 26 | 19 | 7 |
| P1_Nodule_21d-P31_Nodule_21d | 37 | 26 | 12 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Liu, X. Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans. Agronomy 2025, 15, 836. https://doi.org/10.3390/agronomy15040836
Yao Y, Liu X. Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans. Agronomy. 2025; 15(4):836. https://doi.org/10.3390/agronomy15040836
Chicago/Turabian StyleYao, Yubo, and Xinlei Liu. 2025. "Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans" Agronomy 15, no. 4: 836. https://doi.org/10.3390/agronomy15040836
APA StyleYao, Y., & Liu, X. (2025). Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans. Agronomy, 15(4), 836. https://doi.org/10.3390/agronomy15040836






