Unveiling the Multifaceted Roles of Root Exudates: Chemical Interactions, Allelopathy, and Agricultural Applications
Abstract
1. Introduction
1.1. Background of Root Exudates
1.2. Significance of Allelopathic Activity
2. Classes of Metabolites in Root Exudates
2.1. Organic Compounds
2.1.1. Phenolic Compounds
2.1.2. Organic Acids
2.1.3. Terpenoids
2.1.4. Alkaloids
2.1.5. Benzoxazinoids
3. Allelopathic Mechanisms
3.1. Chemical Signaling
3.2. Effects on Plant Growth
- Inhibition of germination;
- Inhibition of root development and growth;
- Inhibition of/uncontrolled increase in cell division, with consequences on seedling development;
- Morphological alterations of the root system and shoots.
| Tested Exudates | Species Tested with Exudate | Methodology | Phytotoxic Effects Observed | References |
|---|---|---|---|---|
| Ageratum conyzoides Centella asiatica Commelina benghalensis Cynodon dactylon Heliotropium indicum Leucas aspera Marsilea quadrifolia Mikania micrantha Phyllanthus niruri Physalis heterophylla Polygonum hydropiper Rotala indica Sida acuta Solanum nigrum Spilanthes acmella | Triticum aestivum | In vitro germination inhibition test in Petri dishes with filter paper. Germination and growth inhibition tests in pots with garden soil. | The most inhibitory exudate for T. aestivum germination was from C. benghalensis (22%). A total of 10.41% of inhibition in the shoot growth by M. micrantha exudate. Presence of byproducts resulting from oxidative stress. | [217] |
| Colocasia esculenta Cyperus rotundus Ludwigia hyssopifolia Marselia quadrifolia Colocasia esculenta | Vigna radiata Vigna unguiculata | In vitro germination inhibition test in Petri dishes with filter paper. | Inhibition of germination up to 30% for V. radiata and up to 50% for V. unguiculata. Reduction in root growth dependent on the species’ sensitivity to different exudates. | [216] |
| Triticum aestivum (cv. Adesso, Element, Maurizio, and NS 40S) | Lolium rigidum Portulaca oleracea | In vitro tests with equal-compartment-agar method employing pregerminated seeds. | T. aestivum cv. Maurizio caused, in both treated species, an inhibition in germination. Reduction in total weight, shoot length, and root length (56%, 55%, and 94%, respectivley) on L. rigidum, and reduction in total weight and root length (84% and 86%, respectivley) in P. oleracea. | [218] |
| Ageratum conyzoides | Lactuca sativa | Germination and growth inhibition test in pots with infested and not-infested forest soil. | Inhibition of germination of 6.67% in a dose-dependent manner. Significant mitotic inhibition in the root cells. | [219] |
| Bidens pilosa | Pteris multifida | Test in in vitro conditions using applications of undecane and palmitic acid, main compounds in B. Pilosa root exudate. | Down-regulation of alpha−linolenic acid, starch, and sucrose metabolism by the undecane. Reduction in flavonoid biosynthesis, arginine biosynthesis, pentose, and glucuronate interconversions, and the proteins related to spliceosome pathway production by the palmitic acid. | [220] |
| Bidens pilosa | Lactuca sativa, Phaseolus vulgaris, Zea mays, and Sorghum bicolor | Root exudates recirculating system. | Inhibition of germination and growth ranging from 30 to 50%, monocots are more sensitive than dicots. | [221] |
| Oryza sativa | Echinochloa crus-galli | Co-cultivation with rice seedlings in a bioassay medium. | Inhibition of roots and stem growth. | [222] |
| Triticum turgidum durum cv. Khapli | Lolium rigidum | In vitro growth inhibition tests in beakers with agar medium. | Inhibition of root and shoot growth with a maximum effect after 6–8 days of treatment. | [223] |
| Triticum aestivum cv. Pishgam | Amaranthus retroflexus | Growth tests in pots with sterilized soil and peat moss. Intercropping with ratios (wheat:amaranth) 100:0, 75:25, 50:50, and 25:75. | As wheat increased (particularly at 75:25), there was a reduction in the fresh weight of the roots and chlorophyll content. Additionally, there was a decrease in shoot protein content (−42%) but an increase in roots (+285%). Induction of oxidative stress. | [92] |
| Amaranthus retroflexus | Triticum aestivum cv. Pishgam | Growth tests in pots with sterilized soil and peat moss. Intercropping with ratios (amaranth:wheat) 0:100, 25:75, 50:50, and 75:25. | As amaranth increased (particularly at 75:25), there was a decrease in the fresh weight of the roots, but an increase in protein content (74%) at the same. Induction of oxidative stress. | [92] |
| Hordeum vulgare | Bromus diandrus Lolium rigidum Hordeum vulgare | Growth and germination tests in in vitro conditions with filter paper. “Seed-to-seed” protocol. | Greater inhibitory effect on weeds, affecting their root and coleoptile growth. In B. diandrus, inhibition ranged from 65 to 74% for radicle growth and 42% for coleoptile growth (25 barley seeds). In L. rigidum, inhibition ranged from 55 to 65% for radicle growth and 18% for coleoptile growth (25 barley seeds). | [224] |
| Heracleum mantegazzianum Heracleum sphondylium Dactylis glomerata Plantago lanceolata | Centaurea jacea Dactylis glomerata Plantago lanceolata | Tests in pots with sterilized garden soil and sand (ratio 2:3). Soil microbiota treatments (added vs. sterilized) and activated carbon treatments (20 mL of powder vs. without powder). | D. glomerata exudates suppressed C. jacea biomass in soil without carbon, while P. lanceolata exudates also suppressed biomass, but within the same species. The addition of activated carbon in the soil reversed the negative effects of all exudates on the tested plants. | [225] |
| Solanum rostratum | Triticum aestivum Brassica campestris | Growth and germination in vitro tests in Petri dishes with agar medium (exudate concentrations: 0.1 g fw/mL, 0.2 g fw/mL, and 0.4 g fw/mL). | The germination rate decreased in both wheat (by approximately 18.5%) and cabbage (by approximately 23%) with increasing concentrations of exudates. The wheat shoot growth decreased starting from the concentration of 0.1 g fw/mL. Conversely, the cabbage shoot growth increased, although not significantly. | [226] |
| Oryza sativa | Oryza sativa Cyperus difformis Echinochloa crus-galli Eclipta prostrata Leptochloa chinensis | Tests with window rhizoboxes and root segregation methods. | Rice interfered with weeds by altering root placement patterns and root interactions, except for wild rice. | [210] |
| Sorghum bicolor | Triticum aestivum Triticum durum Hordeum spontaneum Avena fatua Phalaris minor | Growth and chlorophyll content tests in a greenhouse with a modified stair step tool. | Phalaris minor was the most sensitive plant to sorghum root exudates. Compared to other species, its dry weight, and its length (41.68 cm, 294.87 mg) were strongly inhibited, along with its chlorophyll content. | [227] |
| Sorghum bicolor Solidago canadensis | Bromus sterilis Veronica persica Youngia japonica Rumex acetosa | Germination and growth tests in pots using coco peat and sand (1:1) in a glasshouse. | The invasive species exhibited variable growth responses, while the native species showed greater sensitivity to the exudates across all evaluated parameters in both shoot and root growth. | [228] |
| Tithonia diversifolia | Amaranthus dubius Solanum melongena | Germination, growth, and chlorophyll tests were conducted in in vitro conditions (Petri dishes with filter paper) and subsequently in pots filled with humus soil. | Reduction in germination rates was observed for both species (from 91% to 72.5% in A. dubius, and from 53.3% to 12.5% in S. melongena). Shoot length and leaf area of A. dubius were significantly inhibited. | [229] |
| Ageratina adenophora | Osbeckia stellata Elsholtzia blanda | Growth tests in a greenhouse were conducted using pots filled with soil from uninvaded areas and root leachate (10 g root/100 mL distilled water). | Adenophora root leachate reduced the shoot length of E. blanda and the chlorophyll content in both tested weeds. | [230] |
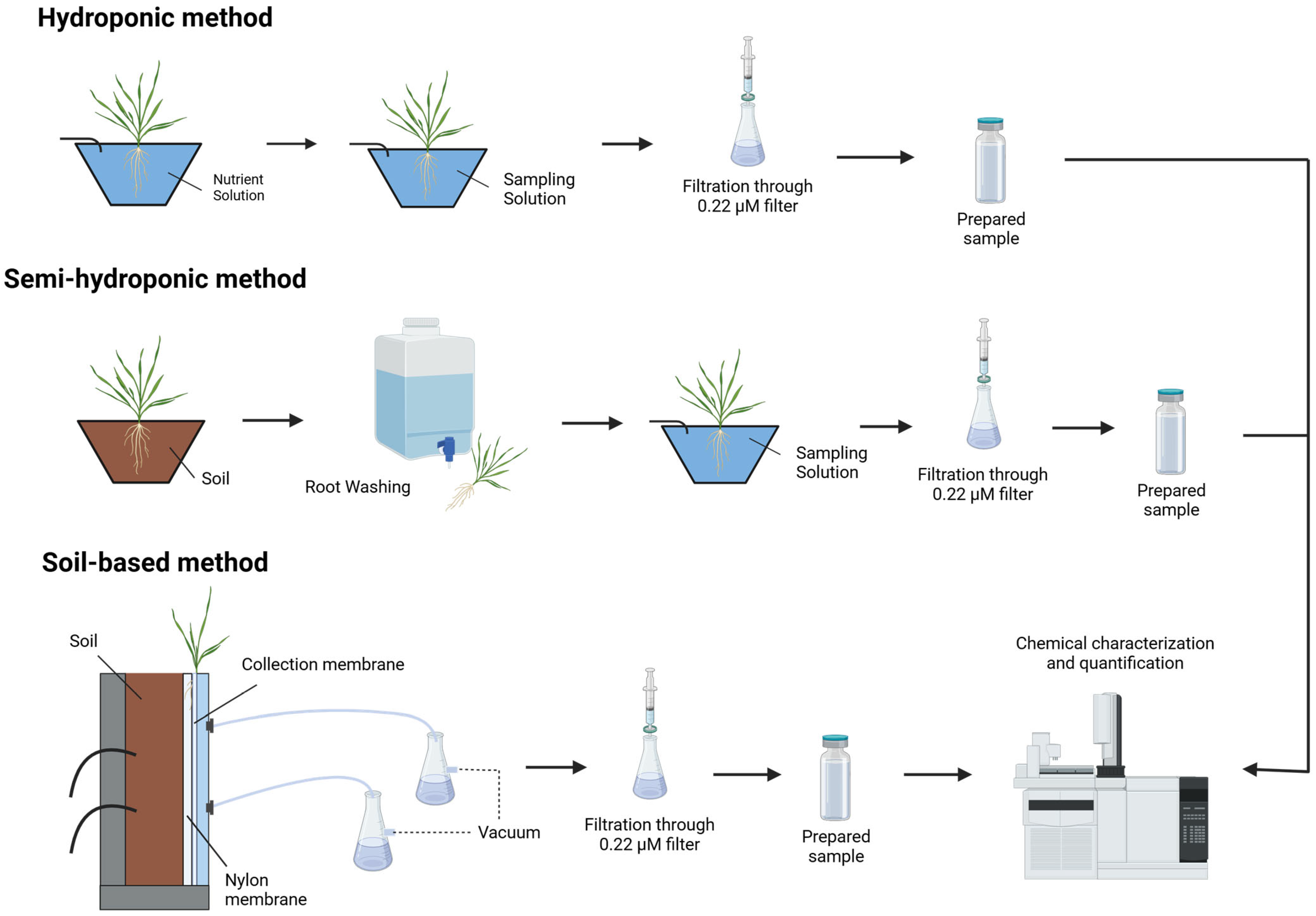
4. Collection Methods for Root Exudates
5. Species with Noteworthy Allelopathic Root Exudates
5.1. Crops
5.1.1. Wheat
| Chemical Class | Compound | Allelopathic Effect | Production Site | References |
|---|---|---|---|---|
| Phenolic acids | p-Hydroxybenzoic acid | Oxidative stress, membrane depolarization, hydraulic conductivity reduction, affection of respiration and transpiration, and inhibition of germination and plant growth. | Root, shoot, leaf, seed, and straw. | [81,247,248,249,250] |
| Vanillic acid | ||||
| Cis-coumaric acid | ||||
| Syringic acid | ||||
| Trans-coumaric acid | ||||
| Trans-ferulic acid | ||||
| Hydroxamic acids | DIBOA | Physiological, biochemical, and oxidative stress, affection of photosynthesis and respiration, damage in membrane transport, germination, and root and shoot growth. | Root, shoot, and leaf. | [218,223,242,248] |
| DIMBOA |
5.1.2. Rice
| Chemical Class | Compound | Allelopathic Effect | Production Site | References |
|---|---|---|---|---|
| Phenolic acids | Salicylic acid | Affection of photosynthesis and metabolism. Inhibition of germination, roots growth, and plant growth. | Roots and leaves. | [262,263,264] |
| Ferulic acid | ||||
| p-Hydroxybenzoic acid | ||||
| Vanillic acid | ||||
| p-Coumaric acid | ||||
| 2,4-Dimethoxybenzoic acid | ||||
| Flavonoids | Tricin | Inhibitory activity on weeds and pathogens. | Roots | [91,263,265] |
| Momilactones | Momilactone A | Inhibition of germination and seedling growth. | Roots, leaves, husks, and seeds. | [73,260,266] |
| Momilactone B |
5.2. Weeds
5.2.1. Bidens pilosa L.
5.2.2. Ageratum conyzoides L.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VOCs | Volatile Organic Compounds |
| ABA | Abscisic Acid |
| TPS23 | Terpene Synthase 23 |
| BXZs | Benzoxazinoids |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| QTLs | Quantitative Trait Loci |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| MA | Momilactone A |
| MB | Momilactone B |
| BOA | Benzoxazolin-2-one |
| MBOA | 6-Methoxy-2-Benzoxazolinone |
| DIBOA | 2,4-Dihydroxy-1,4-Benzoxazin-3-one |
| DIMBOA | 2,4-Dihydroxy-7-Methoxy-1,4-Benzoxazin-3-one |
References
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271. [Google Scholar] [CrossRef]
- Dreyer, I.; Gomez-Porras, J.L.; Riaño-Pachón, D.M.; Hedrich, R.; Geiger, D. Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front. Plant Sci. 2012, 3, 263. [Google Scholar]
- Koo, B.J.; Adriano, D.C.; Bolan, N.S.; Barton, C.D. Root Exudates and Microoorganisms. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 421–428. [Google Scholar]
- Zwetsloot, M.J.; Kessler, A.; Bauerle, T.L. Phenolic root exudate and tissue compounds vary widely among temperate forest tree species and have contrasting effects on soil microbial respiration. New Phytol. 2018, 218, 530–541. [Google Scholar]
- Peters, N.K.; Frost, J.W.; Long, S.R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 1986, 233, 977–980. [Google Scholar]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2012, 20, 5–15. [Google Scholar]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef]
- Wen, Z.; White, P.J.; Shen, J.; Lambers, H. Linking root exudation to belowground economic traits for resource acquisition. New Phytol. 2022, 233, 1620–1635. [Google Scholar] [CrossRef]
- De Andrade, S.A.L.; Borghi, A.A.; De Oliveira, V.H.; Gouveia, L.D.M.; Martins, A.P.I.; Mazzafera, P. Phosphorus shortage induces an increase in root exudation in fifteen eucalypts species. Agronomy 2022, 12, 2041. [Google Scholar] [CrossRef]
- Khorassani, R.; Hettwer, U.; Ratzinger, A.; Steingrobe, B.; Karlovsky, P.; Claassen, N. Citramalic acid and salicylic acid in sugar beet root exudates solubilize soil phosphorus. BMC Plant Biol. 2011, 11, 121. [Google Scholar]
- Hazrati, H.; Fomsgaard, I.S.; Kudsk, P. Targeted metabolomics unveil alteration in accumulation and root exudation of flavonoids as a response to interspecific competition. J. Plant Interact. 2021, 16, 53–63. [Google Scholar]
- Chaïb, S.; Pistevos, J.C.; Bertrand, C.; Bonnard, I. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Muzell Trezzi, M.; Vidal, R.A.; Balbinot Junior, A.A.; von Hertwig Bittencourt, H.; da Silva Souza Filho, A.P. Allelopathy: Driving mechanisms governing its activity in agriculture. J. Plant Interact. 2016, 11, 53–60. [Google Scholar]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: Principles and basic aspects for agroecosystem control. In Sustainable Agriculture Reviews 28: Ecology for Agriculture; Gaba, S., Smith, B., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2018; Volume 28, pp. 47–101. [Google Scholar]
- Wang, B.L.; Shen, J.B.; Zhang, W.H.; Zhang, F.S.; Neumann, G. Citrate exudation from white lupin induced by phosphorus deficiency differs from that induced by aluminum. New Phytol. 2007, 176, 581–589. [Google Scholar]
- Kong, C.H.; Zhang, S.Z.; Li, Y.H.; Xia, Z.C.; Yang, X.F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar]
- Wang, P.; Chai, Y.N.; Roston, R.; Dayan, F.E.; Schachtman, D.P. The Sorghum bicolor root exudate sorgoleone shapes bacterial communities and delays network formation. MSystems 2021, 6, e00749-20. [Google Scholar]
- Wojciechowski, T.; Kant, J. In How sorghum root traits can contribute to cereal yield increase. In Cereal Grains; IntechOpen: London, UK, 2021; Volume 2. [Google Scholar]
- Mohammadi, G.R. Alternative weed control methods: A review. In Larramendy Weed and Pest Control-Conventional and New Challenges; IntechOpen: London, UK, 2013; pp. 117–159. [Google Scholar]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Lotina-Hennsen, B.; King-Díaz, B.; Pereda-Miranda, R. Tricolorin A as a natural herbicide. Molecules 2013, 18, 778–788. [Google Scholar] [CrossRef]
- Uddin, M.R.; Park, K.W.; Kim, Y.K.; Park, S.U.; Pyon, J.Y. Enhancing sorgoleone levels in grain sorghum root exudates. J. Chem. Ecol. 2010, 36, 914–922. [Google Scholar]
- Peres, M.T.L.P.; Lopes, J.R.R.; Silva, C.B.D.; Cândido, A.C.S.; Simionatto, E.; Cabral, M.R.P.; Oliveira, R.M.; Facco, J.T.; Cardoso, C.A.L.; Simas, P.H. Phytotoxic and antioxidant activity of seven native fruits of Brazil. Acta Bot. Bras. 2013, 27, 836–846. [Google Scholar]
- Tibugari, H.; Chiduza, C.; Mashingaidze, A.B.; Mabasa, S. Incorporated sorghum residues reduce emergence and seedling growth of some crops. Cien. Investig. Agrar. 2021, 48, 97–107. [Google Scholar]
- Hussain, M.I.; Reigosa, M.J. Evaluation of photosynthetic performance and carbon isotope discrimination in perennial ryegrass (Lolium perenne L.) under allelochemicals stress. Ecotoxicology 2017, 26, 613–624. [Google Scholar]
- Miller, S.B.; Heuberger, A.L.; Broeckling, C.D.; Jahn, C.E. Non-targeted metabolomics reveals sorghum rhizosphere-associated exudates are influenced by the belowground interaction of substrate and sorghum genotype. Int. J. Mol. Sci. 2019, 20, 431. [Google Scholar] [CrossRef]
- Takao, L.K.; Ribeiro, J.P.N.; Lima, M.I.S. Allelopathic effects of Ipomoea cairica (L.) Sweet on crop weeds. Acta Bot. Bras. 2011, 25, 858–864. [Google Scholar]
- Tesfamariam, T.; Yoshinaga, H.; Deshpande, S.P.; Srinivasa Rao, P.; Sahrawat, K.L.; Ando, Y.; Subbarao, G.V. Biological nitrification inhibition in sorghum: The role of sorgoleone production. Plant Soil 2014, 379, 325–335. [Google Scholar]
- Meazza, G.; Scheffler, B.E.; Tellez, M.R.; Rimando, A.M.; Romagni, J.G.; Duke, S.O.; Dayan, F.E. The inhibitory activity of natural products on plant p-Hydroxyphenylpyruvate dioxygenase. Phytochemistry 2002, 60, 281–288. [Google Scholar]
- Hejl, A.M.; Koster, K.L. The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J. Chem. Ecol. 2004, 30, 2181–2191. [Google Scholar] [CrossRef]
- Dayan, F.E.; Howell, J.L.; Weidenhamer, J.D. Dynamic root exudation of sorgoleone and its in planta mechanism of action. J. Exp. Bot. 2009, 60, 2107–2117. [Google Scholar]
- Dayan, F.E.; Rimando, A.M.; Pan, Z.; Baerson, S.R.; Gimsing, A.L.; Duke, S.O. Sorgoleone. Phytochemistry 2010, 71, 1032–1039. [Google Scholar] [CrossRef]
- Wu, J.; Long, J.; Lin, X.; Chang, Z.; Baerson, S.R.; Ding, C.; Zeng, R. Momilactone B Inhibits Arabidopsis Growth and Development via Disruption of ABA and Auxin Signaling. bioRxiv 2020. [Google Scholar] [CrossRef]
- Almeida Barbosa, L.C.; Alves Pereira, U.; Alvares Maltha, C.R.; Ricardo Teixeira, R.; Moreira Valente, V.M.; Oliveira Ferreira, J.R.; Costa-Lotufo, L.V.; Odorico Moraes, M.; Pessoa, C. Synthesis and Biological Evaluation of 2, 5-Bis (alkylamino)-1, 4-benzoquinones. Molecules 2010, 15, 5629–5643. [Google Scholar] [CrossRef] [PubMed]
- Mareya, C.R.; Tugizimana, F.; Steenkamp, P.; Piater, L.; Dubery, I.A. Lipopolysaccharides trigger synthesis of the allelochemical sorgoleone in cell cultures of Sorghum bicolor. Plant Signal. Behav. 2020, 15, 1796340. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, P.R.F.; Nepomuceno, M.P.; Oliveira, T.S.; Costa, C.; Alves, P.L.C. A Interspecific competition between sweet sorghum and weeds. Planta Daninha 2019, 37, e019209325. [Google Scholar] [CrossRef]
- Uddin, M.R.; Park, S.U.; Dayan, F.E.; Pyon, J.Y. Herbicidal activity of formulated sorgoleone, a natural product of sorghum root exudate. Pest Manag. Sci. 2014, 70, 252–257. [Google Scholar] [CrossRef]
- Zucareli, V.; Coelho, E.; Fernandes, W.; Peres, E.; Stracieri, J. Potencial Alelopático da Parte Aérea de Sorghum bicolor em Diferentes Fases Fenológicas. Planta Daninha 2019, 37, e019184017. [Google Scholar] [CrossRef]
- Uddin, M.R.; Won, O.J.; Pyon, J.Y. Herbicidal effects and crop selectivity of sorgoleone, a sorghum root exudate under greenhouse and field conditions. Korean J. Weed Sci. 2010, 30, 412–420. [Google Scholar] [CrossRef]
- Uddin, M.R.; Thwe, A.A.; Kim, Y.B.; Park, W.T.; Chae, S.C.; Park, S.U. Effects of jasmonates on sorgoleone accumulation and expression of genes for sorgoleone biosynthesis in sorghum roots. J. Chem. Ecol. 2013, 39, 712–722. [Google Scholar] [CrossRef]
- Denadai, M.S.; De Mello, L.M.; Chioderoli, C.A.; Gazola, R.D.N. Desiccation time of the spring sorghum as a predecessor crop for summer soybean and autumn bean in a no-tillage system. Eng. Agríc. 2016, 36, 94–101. [Google Scholar] [CrossRef]
- Biramahire, B.; Appiah, K.S.; Tojo, S.; Fujii, Y.; Chosa, T. Influence of Mowing and Trampling on the Allelopathy and Weed Suppression Potential of Digitaria ciliaris and Cyperus microiria. Sustainability 2022, 14, 16665. [Google Scholar] [CrossRef]
- Pan, Z.; Baerson, S.R.; Wang, M.; Bajsa-Hirschel, J.; Rimando, A.M.; Wang, X.; Duke, S.O. A cytochrome P450 CYP 71 enzyme expressed in Sorghum bicolor root hair cells participates in the biosynthesis of the benzoquinone allelochemical sorgoleone. New Phytol. 2018, 218, 616–629. [Google Scholar] [PubMed]
- Li, J.; Lin, S.; Zhang, Q.; Zhang, Q.; Hu, W.; He, H. Fine-root traits of allelopathic rice at the seedling stage and their relationship with allelopathic potential. PeerJ 2009, 7, e7006. [Google Scholar]
- Oda, Y.; Elmore, J.R.; Nelson, W.C.; Wilson, A.; Farris, Y.; Shrestha, R.; Egbert, R.G. Sorgoleone degradation by sorghum-associated bacteria; an opportunity for enforcing plant growth promotion. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ghatak, A.; Schindler, F.; Bachmann, G.; Engelmeier, D.; Bajaj, P.; Brenner, M.; Fragner, L.; Varshney, R.K.; Subbarao, G.V.; Chaturvedi, P.; et al. Root exudation of contrasting drought-stressed pearl millet genotypes conveys varying biological nitrification inhibition (BNI) activity. Biol. Fertil. Soils 2022, 58, 291–306. [Google Scholar]
- Moore, B.D.; Johnson, S.N. Get tough, get toxic, or get a bodyguard: Identifying candidate traits conferring belowground resistance to herbivores in grasses. Front. Plant Sci. 2017, 7, 1925. [Google Scholar]
- La Hovary, C.; Danehower, D.A.; Ma, G.; Reberg-Horton, C.; Williamson, J.D.; Baerson, S.R.; Burton, J.D. Phytotoxicity and benzoxazinone concentration in field grown cereal rye (Secale cereale L.). Int. J. Agron. 2016, 2016, 6463826. [Google Scholar] [CrossRef]
- Maharjan, B.; Vitha, S.; Okumoto, S. Developmental regulation and physical interaction among enzymes involved in sorgoleone biosynthesis. Plant J. 2023, 115, 820–832. [Google Scholar] [CrossRef]
- Soltane, S.; Benmeddour, T. In Silico Approaches for the Identification of Bioherbicidal Allelochemicals from Plant Species. In Proceedings of the 3rd International Conference on Innovative Academic Studies (ICIAS 2023), Konya, Turkey, 26–28 September 2023. [Google Scholar]
- Pan, Z.; Bajsa-Hirschel, J.; Vaughn, J.N.; Rimando, A.M.; Baerson, S.R.; Duke, S.O. In vivo assembly of the sorgoleone biosynthetic pathway and its impact on agroinfiltrated leaves of Nicotiana benthamiana. New Phytol. 2021, 230, 683–697. [Google Scholar] [CrossRef]
- Janke, C.K.; Wendling, L.A.; Fujinuma, R. Biological nitrification inhibition by root exudates of native species, Hibiscus splendens and Solanum echinatum. PeerJ 2018, 6, e4960. [Google Scholar] [CrossRef]
- Shehzad, T.; Okuno, K. Genetic analysis of QTLs controlling allelopathic characteristics in sorghum. PLoS ONE 2020, 15, e0235896. [Google Scholar]
- Kumar, R.; Parmar, B.S.; Walia, S.; Saha, S. Nitrification inhibitors: Classes and its use in nitrification management. In Nutrient Use Efficiency: From Basics to Advances; Amitava, R., Harikesh, B.S., Avijit, S., Eds.; Springer Publishing Company, Incorporated: New Delhi, India, 2015; pp. 103–122. [Google Scholar]
- Funnell-Harris, D.L.; Pedersen, J.F.; Sattler, S.E. Soil and root populations of fluorescent Pseudomonas spp. associated with seedlings and field-grown plants are affected by sorghum genotype. Plant Soil 2010, 335, 439–455. [Google Scholar]
- Honda, M.; Borthakur, D. Mimosine concentration in giant leucaena (Leucaena leucocephala subsp. glabrata) fluctuates with age and plant part: Mimosine concentration in giant leucaena. Trop. Grassl.-Forrajes Trop. 2024, 12, 11–23. [Google Scholar]
- Ikegami, F.; Mizuno, M.; Kihara, M.; Murakoshi, I. Enzymatic synthesis of the thyrotoxic amino acid mimosine by cysteine synthase. Phytochemistry 1990, 29, 3461–3465. [Google Scholar]
- Chruscinska, E.; Garribba, E.; Micera, G.; Panzanelli, A. L-Mimosine, an amino acid with maltol-type binding properties toward copper (II), oxovanadium (IV) and other metal ions. J. Inorg. Biochem. 1999, 75, 225–232. [Google Scholar]
- Corbi, P.P.; Massabni, A.C.; Costa-Neto, C.M. Synthesis and characterization of a new platinum (II) complex with L-mimosine. J. Coord. Chem. 2005, 58, 1477–1483. [Google Scholar]
- Honda, M.D.H.; Borthakur, D. Mimosine facilitates metallic cation uptake by plants through formation of mimosine–cation complexes. Plant Mol. Biol. 2020, 102, 431–445. [Google Scholar]
- Lalande, M. A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp. Cell Res. 1990, 186, 332–339. [Google Scholar] [PubMed]
- Gupta, H.K.; Atreja, P.P. Influence of ferric chloride treated Leucaena leucocephala on metabolism of mimosine and 3-hydroxy 4 (1H)-pyridone in growing rabbits. Anim. Feed Sci. Technol. 1998, 74, 45–55. [Google Scholar]
- Fallon, A.M. Effects of mimosine on Wolbachia in mosquito cells: Cell cycle suppression reduces bacterial abundance. In Vitro Cell. Dev. Biol.-Anim. 2015, 51, 958–963. [Google Scholar]
- Chou, C.H.; Kuo, Y.L. Allelopathic research of subtropical vegetation in Taiwan: III. Allelopathic exclusion of understory by Leucaena leucocephala (Lam.) de Wit. J. Chem. Ecol. 1986, 12, 1431–1448. [Google Scholar]
- Sahoo, U.K.; Upadhyaya, K.; Meitei, C.B. Allelopathic effects of Leucaena leucocephala and Tectona grandis on germination and growth of maize. Allelopath. J. 2007, 20, 135–144. [Google Scholar]
- Pires, N.D.M.; Prates, H.T.; Pereira Filho, I.A.; Oliveira, R.S.D., Jr.; Faria, T.C.L.D. Allelopathic activity of leucaena on weed species. Sci. Agric. 2001, 58, 61–65. [Google Scholar]
- Boaprem, P. Allelopathic effects of leucaena leaves extract (Leucaena leucocephala (Lam.) de Wit) on the growth of rice (Oryza sativa L.), wrinkle duck-beak (Ischaemum rugosum Salisb), and mung bean (Vigna radiata (L.) R. Wilczek). J. Sci. Technol. 2019, 41, 619–623. [Google Scholar]
- Ishak, M.S.; Ismail, B.S.; Yusoff, N. Allelopathic potential of Leucaena leucocephala (Lam.) de Wit on the germination and seedling growth of Ageratum conyzoides L.; Tridax procumbens L. and Emilia sonchifolia (L.) DC. Allelopath. J. 2016, 37, 109–122. [Google Scholar]
- Chen, S.; Zhou, B.; Lin, S.; Li, X.; Ye, X. Accumulation of cinnamic acid and vanillin in eggplant root exudates and the relationship with continuous cropping obstacle. Afr. J. Biotechnol. 2011, 10, 2659–2665. [Google Scholar]
- Kato-Noguchi, H.; Ino, T.; Ota, K. Secretion of momilactone A from rice roots to the rhizosphere. J. Plant Physiol. 2008, 165, 691–696. [Google Scholar] [CrossRef]
- Friedman, J.; Waller, G.R. Allelopathy and autotoxicity. Trends Biochem. Sci. 1985, 10, 47–50. [Google Scholar]
- Kong, C.H.; Chen, L.C.; Xu, X.H.; Wang, P.; Wang, S.L. Allelochemicals and activities in a replanted Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) tree ecosystem. J. Agric. Food Chem. 2008, 56, 11734–11739. [Google Scholar]
- Chen, L.C.; Wang, S.L.; Wang, P.; Kong, C.H. Autoinhibition and soil allelochemical (cyclic dipeptide) levels in replanted Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 2014, 374, 793–801. [Google Scholar]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; da Silva, L.F.S.; Corrêa, A.P.F.; Bach, E.; Brandelli, A. Total polyphenols, antioxidant, antimicrobial and allelopathic activities of spend coffee ground aqueous extract. Waste Biomass Valorization 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Friedman, J.; Waller, G.R. Caffeine hazards and their prevention in germinating seeds of coffee (Coffea arabica L.). J. Chem Ecol. 1983, 9, 1099–1106. [Google Scholar] [CrossRef]
- Chon, S.U.; Kim, J.D. Biological activity and quantification of suspected allelochemicals from alfalfa plant parts. J. Agron. Crop Sci. 2002, 188, 281–285. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Shi, S.-L.; Li, X.-L.; Li, C.-N.; Zhang, C.-M.; A, Y.; Kang, W.-J.; Yin, G.-L. Effects of autotoxicity on alfalfa (Medicago sativa): Seed germination, oxidative damage and lipid peroxidation of seedlings. Agronomy 2021, 11, 1027. [Google Scholar] [CrossRef]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Cultivar difference in the exudation of phenolic acids. J. Agric. Food Chem. 2001, 49, 3742–3745. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–532. [Google Scholar]
- Blum, U. Plant-Plant Allelopathic Interactions III, 1st ed.; Springer: Cham, Switzerland, 2019; pp. 1–7. [Google Scholar]
- Chaudhuri, A.; Ray, S. Allelopathic potential of tannic acid and its equivalent phenolics extracted from aerial parts of Ampelocissus latifolia (Roxb.) Planch. J. Agric. Vet. Sci. 2016, 9, 90–100. [Google Scholar]
- Narasimhan, K.; Basheer, C.; Bajic, V.B.; Swarup, S. Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol. 2003, 132, 146–153. [Google Scholar] [CrossRef]
- Cooper, J.E. Multiple responses of rhizobia to flavonoids during legume root infection. Adv. Bot. Res. 2004, 41, 1–62. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 263–284. [Google Scholar]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; Elhadi, M.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 253–271. [Google Scholar]
- Zhang, Q.; Zheng, X.Y.; Lin, S.X.; Gu, C.Z.; Li, L.; Li, J.Y.; Fang, C.X.; He, H.B. Transcriptome analysis reveals that barnyard grass exudates increase the allelopathic potential of allelopathic and non-allelopathic rice (Oryza sativa) accessions. Rice 2019, 12, 30. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Hydrolysis products of glucosinolates in Brassica napus tissues as inhibitors of seed germination. Plant Soil 1996, 181, 307–316. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Pratley, J.E.; An, M.; Luckett, D.J.; Lemerle, D. Metabolomics differentiation of canola genotypes: Toward an understanding of canola allelochemicals. Front. Plant Sci. 2015, 5, 765. [Google Scholar]
- Alizadeh, Z.; Motafakkerazad, R.; Salehi-Lisar, S.Y.; Zarrini, G. Evaluation of the allelopathic effect of wheat and redroot pigweed on growth indices and antioxidant system activity in intercropping. J. Plant Prot. Res. 2023, 63, 97–112. [Google Scholar]
- Li, Y.; Xu, L.; Letuma, P.; Lin, W. Metabolite profiling of rhizosphere soil of different allelopathic potential rice accessions. BMC Plant Biol. 2020, 20, 265. [Google Scholar]
- Ma, J.; Wang, W.; Yang, J.; Qin, S.; Yang, Y.; Sun, C.; Huang, J. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar]
- Ma, J.; Xie, Y.; Yang, Y.; Jing, C.; You, X.; Yang, J.; Sun, C.; Qin, S.; Chen, J.; Cao, K.; et al. AMF colonization affects allelopathic effects of Zea mays L. root exudates and community structure of rhizosphere bacteria. Front. Plant Sci. 2022, 13, 1050104. [Google Scholar]
- Shi, J.B.; Gong, X.Y.; Rahman, M.K.U.; Tian, Q.; Zhou, X.A.; Wu, F.Z. Effects of wheat root exudates on bacterial communities in the rhizosphere of watermelon. Plant Soil Environ. 2021, 67, 721–728. [Google Scholar]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar]
- Kato-Noguchi, H.; Ino, T.; Kujime, H. The relation between growth inhibition and secretion level of momilactone B from rice root. J. Plant Interact. 2010, 5, 87–90. [Google Scholar]
- Li, Z.; Fu, J.; Zhou, R.; Wang, D. Effects of phenolic acids from ginseng rhizosphere on soil fungi structure, richness and diversity in consecutive monoculturing of ginseng. Saudi J. Biol. Sci. 2018, 25, 1788–1794. [Google Scholar]
- Wu, H.; Wu, L.; Zhu, Q.; Wang, J.; Qin, X.; Xu, J.; Lin, W. The role of organic acids on microbial deterioration in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Sci. Rep. 2017, 7, 3497. [Google Scholar] [CrossRef]
- Khandual, S. Flavonoids as signaling molecules and regulators of root nodule development. Dyn. Soil Dyn. Plant 2007, 1, 83–94. [Google Scholar]
- Auguy, F.; Abdel-Lateif, K.; Doumas, P.; Badin, P.; Guerin, V.; Bogusz, D.; Hocher, V. Activation of the isoflavonoid pathway in actinorhizal symbioses. Funct. Plant Biol. 2011, 38, 690–696. [Google Scholar] [CrossRef]
- Schlaman, H.R.M.; Phillips, D.A.; Kondorosi, E. Genetic Organization and Transcriptional Regulation of Rhizobial Nodulation Genes. In The Rhizobiaceae; Spaink, H.P., Kondorosi, A., Hooykaas, P.J.J., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 361–386. [Google Scholar]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Csepregi, K.; Hideg, É. Phenolic compound diversity explored in the context of photo-oxidative stress protection. Phytochem. Anal. 2018, 29, 129–136. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Yang, C.M.; Chang, F.; Lin, S.J.; Chou, C.H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: II. Stimulation of consumption-orientation. Bot. Bull. Acad. Sin. 2004, 45, 119–125. [Google Scholar]
- Ye, S.F.; Zhou, Y.H.; Sun, Y.; Zou, L.Y.; Yu, J.Q. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 2006, 56, 255–262. [Google Scholar] [CrossRef]
- Tanase, C.; Bujor, O.C.; Popa, V.I. Phenolic natural compounds and their influence on physiological processes in plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z.A. Allelopathy in agro-ecosystems: A critical review of wheat allelopathy-concepts and implications. Chemoecology 2017, 27, 1–24. [Google Scholar]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar]
- Macías, F.A.; Galindo, J.C.; Molinillo, J.M. (Eds.) Allelopathy: Chemistry and Mode of Action of Allelochemicals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 217–232. [Google Scholar]
- Barkosky, R.R.; Einhellig, F.A.; Butler, J.L. Caffeic acid-induced changes in plant–water relationships and photosynthesis in leafy spurge Euphorbia esula. J. Chem. Ecol. 2000, 26, 2095–2109. [Google Scholar]
- Fu, Y.H.; Quan, W.X.; Li, C.C.; Qian, C.Y.; Tang, F.H.; Chen, X.J. Allelopathic effects of phenolic acids on seedling growth and photosynthesis in Rhododendron delavayi Franch. Photosynthetica 2019, 57, 377–387. [Google Scholar]
- Huang, P.M.; Wang, M.C.; Wang, M.K. Catalytic transformation of phenolic compounds in the soils. In Principles and Practices in Plant Ecology, 1st ed.; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 287–306. [Google Scholar]
- Schmidt, S.K.; Ley, R.E. Microbial competition and soil structure limit the expression of allelochemicals in nature. In Principles and Practices in Plant Ecology: Allelochemical Interactions, 1st ed.; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 339–351. [Google Scholar]
- Jilani, G.; Akram, A.; Ali, R.M.; Hafeez, F.Y.; Shamsi, I.H.; Chaudhry, A.N.; Chaudhry, A.G. Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere microflora through organic and biofertilizers. Ann. Microbiol. 2007, 57, 177–184. [Google Scholar]
- Inderjit; Bhowmik, P.C. Sorption of benzoic acid onto soil colloids and its implications for allelopathy studies. Biol. Fertil. Soils 2004, 40, 345–348. [Google Scholar]
- Tharayil, N.; Bhowmik, P.C.; Xing, B. Preferential sorption of phenolic phytotoxins to soil: Implications for altering the availability of allelochemicals. J. Agric. Food Chem. 2008, 54, 3033–3040. [Google Scholar]
- Li, X.J.; Xia, Z.C.; Kong, C.H.; Xu, X.H. Mobility and microbial activity of allelochemicals in soil. J. Agric. Food Chem. 2013, 61, 5072–5079. [Google Scholar] [CrossRef]
- Macias-Benitez, S.; Garcia-Martinez, A.M.; Caballero Jimenez, P.; Gonzalez, J.M.; Tejada Moral, M.; Parrado Rubio, J. Rhizospheric organic acids as biostimulants: Monitoring feedbacks on soil microorganisms and biochemical properties. Front. Plant Sci. 2020, 11, 633. [Google Scholar]
- Aoki, M.; Fujii, K.; Kitayama, K. Environmental control of root exudation of low-molecular weight organic acids in tropical rainforests. Ecosystems 2012, 15, 1194–1203. [Google Scholar]
- Shahbaz, A.M.; Oki, Y.; Adachi, T.; Murata, Y.; Khan, M.H.R. Phosphorus starvation induced root-mediated pH changes in solublization and acquisition of sparingly soluble P sources and organic acids exudation by Brassica cultivars. Soil Sci. Plant Nutr. 2006, 52, 623–633. [Google Scholar]
- Yang, Y.; Yang, Z.; Yu, S.; Chen, H. Organic acids exuded from roots increase the available potassium content in the rhizosphere soil: A rhizobag experiment in Nicotiana tabacum. HortScience 2019, 54, 23–27. [Google Scholar]
- Wiesenbauer, J.; Gorka, S.; Jenab, K.; Schuster, R.; Kumar, N.; Rottensteiner, C.; Konig, A.; Kraemer, S.; Inselsbacher, E.; Kaiser, C. Preferential use of organic acids over sugars by soil microbes in simulated root exudation. Soil Biol. Biochem. 2025, 203, 109738. [Google Scholar]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plat Biol. 2001, 28, 897–906. [Google Scholar]
- Saber, N.; Abdel-Moneim, A.; Barakat, S. Role of organic acids in sunflower tolerance to heavy metals. Biol. Plant. 1999, 42, 65–73. [Google Scholar]
- Ghosh, S.; Narula, K.; Sinha, A.; Ghosh, R.; Jawa, P.; Chakraborty, N.; Chakraborty, S. Proteometabolomic analysis of transgenic tomato overexpressing oxalate decarboxylase uncovers novel proteins potentially involved in defense mechanism against Sclerotinia. J. Proteom. 2016, 143, 242–253. [Google Scholar]
- Freitas, L.B.; Boaventura, M.A.D.; Santos, W.L.; Stehmann, J.R.; Junior, D.D.; Lopes, M.T.; Magalhães, T.F.F.; Silva, D.; de Resende, M.A. Allelopathic, cytotoxic and antifungic activities of new dihydrophenanthrenes and other constituents of leaves and roots extracts of Banisteriopsis anisandra (Malpighiaceae). Phytochem. Lett. 2015, 12, 9–16. [Google Scholar]
- Syed, S.; Ahmed, Z.I.; Al-Haq, M.I.; Mohammad, A.; Fujii, Y. The possible role of organic acids as allelochemicals in Tamarindus indica L. leaves. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 511–517. [Google Scholar]
- Alsaadawi, I.S.; Dayan, F.E. Potentials and prospects of sorghum allelopathy in agroecosystems. Allelopath. J. 2009, 24, 255–270. [Google Scholar]
- Cheema, Z.A.; Khaliq, A.; Abbas, M.; Farooq, M. Allelopathic potential of sorghum (Sorghum bicolor L. Moench) cultivars for weed management. Allelopath. J. 2007, 20, 167. [Google Scholar]
- Kakar, K.; Xuan, T.D.; Khanh, T.D. Allelopathic potential of sweet sorghum root exudates and identification of the relevant allelochemicals. Agrochemicals 2023, 2, 96–105. [Google Scholar] [CrossRef]
- Rimando, A.M.; Dayan, F.E.; Czarnota, M.A.; Weston, L.A.; Duke, S.O. A new photosystem II electron transfer inhibitor from Sorghum bicolor. J. Nat. Prod. 1998, 61, 927–930. [Google Scholar] [PubMed]
- Seal, A.N.; Pratley, J.E.; Haig, T.; An, M. Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J. Chem. Ecol. 2004, 30, 1647–1662. [Google Scholar]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1999, 50, 47–65. [Google Scholar]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy: Fundamentals, Applications and Strategies; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H. Therapeutic and Biomedical Potentialities of Terpenoids-A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; Volume 148, pp. 63–106. [Google Scholar]
- Böttger, A.; Vothknecht, U.; Bolle, C.; Wolf, A. Terpenes and Terpenoids. In Lessons on Caffeine, Cannabis & Co.; Learning Materials in Biosciences; Springer: Cham, Switzerland, 2018; pp. 153–170. [Google Scholar]
- Berenbaum, M.R.; Zangerl, A.R. Facing the future of plant-insect interaction research: Le retour à la “raison d’être”. Plant Physiol. 2008, 146, 804–811. [Google Scholar]
- Srikanth, P.; Maxton, A.; Masih, S.A.; Sofo, A.; Khan, N.A. Isoprene: An Antioxidant to Guard Plants against Stress. Int. J. Plant Biol. 2024, 15, 161–174. [Google Scholar] [CrossRef]
- Lehning, A.; Zimmer, W.; Zimmer, I.; Schnitzler, J.P. Modeling of annual variations of oak (Quercus robur L.) isoprene synthase activity to predict isoprene emission rates. Geophys. Res. Atmos. 2001, 106, 3157–3166. [Google Scholar]
- Monson, R.K.; Winkler, B.; Rosenstiel, T.N.; Block, K.; Merl-Pham, J.; Strauss, S.H.; Ault, K.; Maxfield, J.; Moore, D.J.P.; Trahan, N.A.; et al. High productivity in hybrid-poplar plantations without isoprene emission to the atmosphere. Proc. Natl. Acad. Sci. USA 2020, 117, 1596–1605. [Google Scholar]
- Sun, Z.; Hüve, K.; Vislap, V.; Niinemets, Ü. Elevated [CO2] magnifies isoprene emissions under heat and improves thermal resistance in hybrid aspen. J. Exp. Bot. 2013, 64, 5509–5523. [Google Scholar]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene acts as a signaling molecule in gene networks important for stress responses and plant growth. Plant Physiol. 2019, 180, 124–152. [Google Scholar] [PubMed]
- Harvey, C.M.; Sharkey, T.D. Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell Environ. 2016, 39, 1251–1263. [Google Scholar]
- Xu, J.; Trainotti, L.; Li, M.; Varotto, C. Overexpression of Isoprene Synthase Affects ABA- and Drought-Related Gene Expression and Enhances Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 4276. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Várkonyi, Z.; Szabó, M.; Maslenkova, L.; Nogues, I.; Kovács, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 2011, 157, 905–916. [Google Scholar] [PubMed]
- Behnke, K.; Kaiser, A.; Zimmer, I.; Brüggemann, N.; Janz, D.; Polle, A.; Hampp, R.; Hänsch, R.; Popko, J.; Schmitt-Kopplin, P.; et al. RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: A transcriptomic and metabolomic analysis. Plant Mol. Biol. 2010, 74, 61–75. [Google Scholar]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar]
- Jasim, I.R.; Alwattar, M.T.; Yaqub, H.M. Terpenoids as Natural Allelopathic Compounds in Plants. Rafidain J. Sci. 2021, 30, 106–116. [Google Scholar]
- Zhang, K.; Jiang, Y.; Zhao, H.; Köllner, T.G.; Chen, S.; Chen, F.; Chen, F. Diverse terpenoids and their associated antifungal properties from roots of different cultivars of Chrysanthemum morifolium Ramat. Molecules 2020, 25, 2083. [Google Scholar] [CrossRef]
- Huang, A.C.; Osbourn, A. Plant terpenes that mediate below-ground interactions: Prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 2019, 75, 2368–2377. [Google Scholar]
- Hiltpold, I. Manipulation of Tritrophic Interactions: A Key for Belowground Biological Control? Doctoral Dissertation, University of Neuchâtel, Neuchâtel, Switzerland, 2008. [Google Scholar]
- Capra, E.; Colombi, C.; De Poli, P.; Nocito, F.F.; Cocucci, M.; Vecchietti, A.; Marocco, A.; Stile, M.R.; Rossini, L. Protein profiling and tps23 induction in different maize lines in response to methyl jasmonate treatment and Diabrotica virgifera infestation. J. Plant Physiol. 2015, 175, 68–77. [Google Scholar]
- de Oliveira, M.S.; Silva, S.; Da Costa, W.A. (Eds.) Essential Oils: Bioactive Compounds, New Perspectives and Applications; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Weiner, J. Plant allelochemical interference or soil chemical ecology? Perspect. Plant Ecol. Evol. Syst. 2001, 4, 3–12. [Google Scholar]
- De Oliveira, M.S.; Souza Filho, A.P.D.S. (Eds.) Terpenoids: Recent Advances in Extraction, Biochemistry and Biotechnology; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 181–191. [Google Scholar]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [PubMed]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Sanches, S.A., Nabavi, F.S., Saeedi, M., Mohammad, N.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–15. [Google Scholar]
- Adibah, K.Z.M.; Azzreena, M.A. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2019, 6, 21–29. [Google Scholar]
- Srivasatava, P. Use of alkaloids in plant protection. In Plant Protection: From Chemicals to Biologicals; Walter de Gruyter GmbH & Co KG, Ed.; De Gruyter: Berlin, Germany, 2022; pp. 337–352. [Google Scholar]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar]
- Berenyi, S.; Csutoras, C.; Sipos, A. Recent developments in the chemistry of thebaine and its transformation products as pharmacological targets. Curr. Med. Chem. 2009, 16, 3215–3242. [Google Scholar]
- Mohsin, H.F.; Wahab, I.A.; Nasir, N.I.M.; Zulkefl, N.H.; Nasir, N.I.S.M. The chemical investigation of Papaver seeds. Int. J. Adv. Sci. Eng. Inform. Technol. 2012, 2, 38–41. [Google Scholar]
- Sahu, P.K.; Pradhan, S.P.; Kumar, P.S. Isolation, elucidation, and structure–activity relationships of phytoalkaloids from Solanaceae. Stud. Nat. Prod. Chem. 2022, 72, 371–389. [Google Scholar]
- Ibragic, S.; Sofić, E. Chemical composition of various Ephedra species. Bosn. J. Basic Med. Sci. 2015, 15, 21. [Google Scholar]
- Mitchell, G.; Lafrance, M.; Boulanger, S.; Séguin, D.L.; Guay, I.; Gattuso, M.; Marsault, É.; Bouarab, K.; Malouin, F. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J. Antimicrob. Chemother. 2012, 67, 559–568. [Google Scholar]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013, 81, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Cretton, S.; Dorsaz, S.; Azzollini, A.; Favre-Godal, Q.; Marcourt, L.; Ebrahimi, S.N.; Voinesco, F.; Michellod, E.; Sanglard, D.; Gindro, K.; et al. Antifungal quinoline alkaloids from Waltheria indica. J. Nat. Prod. 2016, 79, 300–307. [Google Scholar] [CrossRef]
- Ogunsusi, M.; Akinlalu, A.O.; Komolafe, I.J.; Oyedapo, O.O. Allelopathic effects of alkaloid fraction of Crotalaria retusa Linn on growth and some biochemical parameters of bean seedlings (Phaseolus vulgaris). Int. J. Plant Physiol. Biochem. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, Y.; Shi, K.; Liu, Y.; Hu, Y.; Shao, H. Phytotoxic activity of alkaloids in the desert plant Sophora alopecuroides. Toxins 2021, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Niculaes, C.; Abramov, A.; Hannemann, L.; Frey, M. Plant protection by benzoxazinoids—Recent insights into biosynthesis and function. Agronomy 2018, 8, 143. [Google Scholar] [CrossRef]
- de Bruijn, W.J.; Gruppen, H.; Vincken, J.P. Structure and biosynthesis of benzoxazinoids: Plant defence metabolites with potential as antimicrobial scaffolds. Phytochemistry 2018, 155, 233–243. [Google Scholar] [CrossRef]
- Hazrati, H.; Fomsgaard, I.S.; Kudsk, P. Root-exuded benzoxazinoids: Uptake and translocation in neighboring plants. J. Agric. Food Chem. 2020, 68, 10609–10617. [Google Scholar] [CrossRef]
- Wu, W.H.; Chen, T.Y.; Lu, R.W.; Chen, S.T.; Chang, C.C. Benzoxazinoids from Scoparia dulcis (sweet broomweed) with antiproliferative activity against the DU-145 human prostate cancer cell line. Phytochemistry 2012, 83, 110–115. [Google Scholar] [CrossRef]
- Makowska, B.; Bakera, B.; Rakoczy-Trojanowska, M. The genetic background of benzoxazinoid biosynthesis in cereals. Acta Physiol. Plant. 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Frey, M.; Chomet, P.; Glawischnig, E.; Stettner, C.; Grün, S.; Winklmair, A.; Eisenreich, W.; Bacher, A.; Meeley, R.B.; Briggs, S.P.; et al. Analysis of a chemical plant defense mechanism in grasses. Science 1997, 277, 696–699. [Google Scholar] [CrossRef]
- Melanson, D.; Chilton, M.D.; Masters-Moore, D.; Chilton, W.S. A deletion in an indole synthase gene is responsible for the DIMBOA-deficient phenotype of bxbx maize. Proc. Natl. Acad. Sci. USA 1997, 94, 13345–13350. [Google Scholar] [CrossRef]
- Von Rad, U.; Hüttl, R.; Lottspeich, F.; Gierl, A.; Frey, M. Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize. Plant J. 2001, 28, 633–642. [Google Scholar] [PubMed]
- Stahl, E. New insights into the transcriptional regulation of benzoxazinoid biosynthesis in wheat. J. Exp. Bot. 2022, 73, 5358–5360. [Google Scholar]
- Krogh, S.S.; Mensz, S.J.; Nielsen, S.T.; Mortensen, A.G.; Christophersen, C.; Fomsgaard, I.S. Fate of benzoxazinone allelochemicals in soil after incorporation of wheat and rye sprouts. J. Agric. Food Chem. 2006, 54, 1064–1074. [Google Scholar] [PubMed]
- Hussain, M.I.; Vieites-Álvarez, Y.; Otero, P.; Prieto, M.A.; Simal-Gandara, J.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Weed pressure determines the chemical profile of wheat (Triticum aestivum L.) and its allelochemicals potential. Pest Manag. Sci. 2022, 78, 1605–1619. [Google Scholar]
- Rice, C.P.; Otte, B.A.; Kramer, M.; Schomberg, H.H.; Mirsky, S.B.; Tully, K.L. Benzoxazinoids in roots and shoots of cereal rye (Secale cereale) and their fates in soil after cover crop termination. Chemoecology 2022, 32, 117–128. [Google Scholar] [CrossRef]
- Hietala, P.K.; Virtanen, A.I.; Norén, B.; Levitin, N.E.; Westin, G. Precursors of benzoxazolinone in rye plants. II. Precursor I, the glucoside. Acta Chem. Scand. 1960, 14, 502. [Google Scholar]
- Kato-Noguchi, H.; Macías, F.A.; Molinillo, J.M. Structure–activity relationship of benzoxazinones and related compounds with respect to the growth inhibition and α-amylase activity in cress seedlings. J. Plant Phyiol. 2010, 167, 1221–1225. [Google Scholar]
- Hussain, M.I.; Araniti, F.; Schulz, M.; Baerson, S.; Vieites-Álvarez, Y.; Rempelos, L.; Bilsborrow, P.; Chinchilla, N.; Macìas, F.A.; Weston, L.A.; et al. Benzoxazinoids in wheat allelopathy–From discovery to application for sustainable weed management. Environ. Exp. Bot. 2022, 202, 104997. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2 H-1, 4-benzoxazin-3 (4 H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar]
- Maag, D.; Köhler, A.; Robert, C.A.; Frey, M.; Wolfender, J.L.; Turlings, T.C.; Glauser, G.; Erb, M. Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize. Plant J. 2016, 88, 976–991. [Google Scholar]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kaur, S.; Kohli, R.K. 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol. Biochem. 2006, 44, 819–827. [Google Scholar] [PubMed]
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Sicker, D.; Baluška, F.; Sablofski, T.; Scherer, H.W.; Ritter, F.M. Benzoxazolinone detoxification and degradation—A molecule’s journey. In Herbicides-Properties, Synthesis and Control of Weeds; Hasaneen, M.N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 17–42. [Google Scholar]
- Sánchez-Moreiras, A.M.; de la Peña, T.C.; Reigosa, M.J. The natural compound benzoxazolin-2 (3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry 2008, 69, 2172–2179. [Google Scholar]
- Mikić, S.; Ahmad, S. Benzoxazinoids-protective secondary metabolites in cereals: The role and application. Field Veg. Crops Res. 2018, 55, 49–57. [Google Scholar]
- Mejías, F.J.; Schwaiger, S.; Varela, R.M.; Molinillo, J.M.; Chinchilla, N.; Macías, F.A. Synthesis of Benzoxazinones Sulphur Analogs and Their Application as Bioherbicides: 1.4-Benzothiazinones and 1.4-Benzoxathianones for Weed Control. Agronomy 2023, 13, 1694. [Google Scholar] [CrossRef]
- Ji, S.; Wu, D.; Li, W.; Lv, G.; He, X. Mediating role of root-exuded secondary metabolites in intraspecific interactions with Haloxylon ammodendron. Plant Soil 2024, 1–20. [Google Scholar] [CrossRef]
- Rivoal, A.; Fernandez, C.; Greff, S.; Montes, N.; Vila, B. Lo stress da competizione diminuisce il potenziale allelopatico? Biochem. Syst. Ecol. 2011, 39, 401–407. [Google Scholar]
- Broz, A.K.; Broeckling, C.D.; De-la-Peña, C.; Lewis, M.R.; Greene, E.; Callaway, R.M.; Sumner, L.W.; Vivanco, J.M. Plant neighbor identity influences plant biochemistry and physiology related to defense. BMC Plant Biol. 2010, 10, 115. [Google Scholar] [CrossRef]
- Dudley, S.A.; Murphy, G.P.; File, A.L. Kin recognition and competition in plants. Funct. Ecol. 2013, 27, 898–906. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 9, 58. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Van Tol, R.W.; Van Der Sommen, A.T.; Boff, M.I.; Van Bezooijen, J.; Sabelis, M.W.; Smits, P.H. Plants protect their roots by alerting the enemies of grubs. Ecol. Lett. 2001, 4, 292–294. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, L.L.; Meiners, S.J.; Kong, C.H. Root placement patterns in allelopathic plant–plant interactions. New Phytol. 2023, 237, 563–575. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.A.; Prasad, P.R.C. Assessing the allelopathy and autotoxicity effects of Parthenium hysterophorus L.; Senna uniflora (Mill.) HS Irwin and Barneby and Hyptis suaveolens (L.) Poit. Russ. J. Biol. Invasions 2018, 9, 290–298. [Google Scholar] [CrossRef]
- Real, M.; Gámiz, B.; López-Cabeza, R.; Celis, R. Sorption, persistence, and leaching of the allelochemical umbelliferone in soils treated with nanoengineered sorbents. Sci. Rep. 2019, 9, 9764. [Google Scholar] [CrossRef]
- Jabran, K. (Ed.) Brassicaceae allelopathy for weed control. In Manipulation of Allelopathic Crops for Weed Control; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 21–27. [Google Scholar]
- Perniola, O.S.; Chorzempa, S.E.; Staltari, S.; Molina, M.D.C. Biofumigación con Brassica juncea: Efecto sobre la flora arvense. Rev. Fac. Agron. 2019, 118, 25–35. [Google Scholar] [CrossRef]
- Carvalho, M.S.S.; Andrade-Vieira, L.F.; dos Santos, F.E.; Correa, F.F.; das Graças Cardoso, M.; Vilela, L.R. Allelopathic potential and phytochemical screening of ethanolic extracts from five species of Amaranthus spp. in the plant model Lactuca sativa. Sci. Hortic. 2019, 245, 90–98. [Google Scholar] [CrossRef]
- Akter, P.; Sultana, B. Allelopathic effects, yields and qualitative phytochemical screening of root exudates of five weeds species. Malays. J. Sustain. Agric. 2019, 3, 44–48. [Google Scholar] [CrossRef]
- Akter, P.; Ahmed, A.A.; Promie, F.K.; Haque, M.E. Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L. Agronomy 2023, 13, 381. [Google Scholar] [CrossRef]
- Vieites-Álvarez, Y.; Otero, P.; Prieto, M.A.; Simal-Gandara, J.; Reigosa, M.J.; Sánchez-Moreiras, A.M.; Hussain, M.I. Testing the role of allelochemicals in different wheat cultivars to sustainably manage weeds. Pest Manag. Sci. 2023, 79, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Syngkli, R.B.; Lallianpuii, S.; Rai, P.K. Microcosm investigation on the allelochemic potential of Ageratum conyzoides on selected food crop. Ecol. Environ. Conserv. 2022, 28, S298–S304. [Google Scholar]
- Zhang, K.; Ebihara, A.; Tong, S.; White, J.C.; Shen, Y. Bidens pilosa root exudates modulate Pteris multifida gametophyte development: A proteomic investigation. Ind. Crops Prod. 2023, 205, 117499. [Google Scholar] [CrossRef]
- Stevens, G.A., Jr.; Tang, C.S. Inhibition of seedling growth of crop species by recirculating root exudates of Bidens pilosa L. J. Chem. Ecol. 1985, 11, 1411–1425. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791. [Google Scholar] [CrossRef]
- Huang, Z.; Haig, T.; Wu, H.; An, M.; Pratley, J. Correlation between phytotoxicity on annual ryegrass (Lolium rigidum) and production dynamics of allelochemicals within root exudates of an allelopathic wheat. J. Chem. Ecol. 2003, 29, 2263–2279. [Google Scholar] [PubMed]
- Bouhaouel, I.; Gfeller, A.; Fauconnier, M.L.; Rezgui, S.; Slim-Amara, H.; du Jardin, P. Allelopathic and autotoxicity effects of barley (Hordeum vulgare L. ssp. vulgare) root exudates. BioControl 2015, 60, 425–436. [Google Scholar] [CrossRef]
- Jandová, K.; Dostál, P.; Cajthaml, T.; Kameník, Z. Intraspecific variability in allelopathy of Heracleum mantegazzianum is linked to the metabolic profile of root exudates. Ann. Bot. 2015, 115, 821–831. [Google Scholar] [CrossRef]
- Shao, M.; Ma, Y.; Wang, Y.; Xu, S.; Miao, Q.; Zhai, Q.; Qu, B. A preliminary study on allelopathy and potential allelochemicals of root exudates from Solanum rostratum Dunal. Biotechnol. J. Int. 2022, 26, 31–39. [Google Scholar] [CrossRef]
- Naby, K.Y.; Ali, K.A. Allelopathic potential of Sorghum bicolor L. root exudates on growth and chlorophyll content of wheat and some grassy weeds. IOP Conf. Ser. Earth Environ. Sci. 2021, 761, 012085. [Google Scholar]
- Afzal, M.R.; Naz, M.; Ullah, R.; Du, D. Persistence of Root Exudates of Sorghum bicolor and Solidago canadensis: Impacts on Invasive and Native Species. Plants 2023, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Otusanya, O.O.; Sokan-Adeaga, A.A.; Ilori, O.J. Allelopathic Effect of the Root Exudates of Tithonia diversifolia on the germination, growth and Chlorophyll Accumulation of Amaranthus dubius L. and Solanum melongena L. Res. J. Bot. 2014, 9, 13. [Google Scholar]
- Darji, T.B.; Adhikari, B.; Pathak, S.; Neupane, S.; Thapa, L.B.; Bhatt, T.D.; Pant, R.R.; Pant, G.; Pal, K.B.; Bishwakarma, K. Phytotoxic effects of invasive Ageratina adenophora on two native subtropical shrubs in Nepal. Sci. Rep. 2021, 11, 13663. [Google Scholar]
- Gransee, A.; Wittenmayer, L. Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J. Plant Nutr. Soil Sci. 2000, 163, 381–385. [Google Scholar]
- Gottardi, S. Studio Fisiologico e Molecolare dei Meccanismi di Rilascio di Essudati Radicali e dell’Assorbimento di Fe e P. Ph.D. Thesis, University of Udine (IT), Udine, Italy, 2012. [Google Scholar]
- Goh, Y.S.; Hum, Y.C.; Lee, Y.L.; Lai, K.W.; Yap, W.S.; Tee, Y.K. A meta-analysis: Food production and vegetable crop yields of hydroponics. Sci. Hortic. 2023, 321, 112339. [Google Scholar]
- Sgherri, C.; Cecconami, S.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010, 123, 416–422. [Google Scholar]
- Mašková, T.; Klimeš, A. The effect of rhizoboxes on plant growth and root: Shoot biomass partitioning. Front. Plant Sci. 2020, 10, 1693. [Google Scholar]
- Yee, M.O.; Kim, P.; Li, Y.; Singh, A.K.; Northen, T.R.; Chakraborty, R. Specialized plant growth chamber designs to study complex rhizosphere interactions. Front. Microbiol. 2021, 12, 625752. [Google Scholar]
- Williams, A.; Langridge, H.; Straathof, A.L.; Fox, G.; Muhammadali, H.; Hollywood, K.A.; Xu, Y.; Goodacre, R.; de Vries, F.T. Comparing root exudate collection techniques: An improved hybrid method. Soil Biol. Biochem. 2021, 161, 108391. [Google Scholar]
- Döll, S.; Koller, H.; van Dam, N.M. A simple, cost-effective and optimized protocol for collecting root exudates from soil grown plants. Rhizosphere 2024, 30, 100899. [Google Scholar]
- Knee, E.M.; Gong, F.C.; Gao, M.; Teplitski, M.; Jones, A.R.; Foxworthy, A.; Mort, A.J.; Bauer, W.D. Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol. Plant-Microbe Interact. 2001, 14, 775–784. [Google Scholar] [PubMed]
- Delory, B.M.; Weidlich, E.W.; van Duijnen, R.; Pagès, L.; Temperton, V.M. Measuring plant root traits under controlled and field conditions: Step-by-step procedures. Methods Mol. Biol. 2018, 1761, 3–22. [Google Scholar]
- FAO Food Price Index Unchanged in September. Available online: https://www.fao.org/newsroom/detail/fao-food-price-index-unchanged-in-september/it (accessed on 22 April 2024).
- Qasem, J.R. Applied allelopathy in weed management: An update. In Allelopathy: Current Trends and Future Applications; Cheema, Z.A., Farooq, M., Wahid, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 251–297. [Google Scholar]
- Villagrasa, M.; Guillamón, M.; Labandeira, A.; Taberner, A.; Eljarrat, E.; Barceló, D. Benzoxazinoid allelochemicals in wheat: Distribution among foliage, roots, and seeds. J. Agric. Food Chem. 2006, 54, 1009–1015. [Google Scholar] [PubMed]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Distribution and exudation of allelochemicals in wheat Triticum aestivum. J. Chem. Ecol. 2000, 26, 2141–2154. [Google Scholar]
- Wu, H.; Pratley, J.; Ma, W.; Haig, T. Quantitative trait loci and molecular markers associated with wheat allelopathy. Theor. Appl. Genet. 2003, 107, 1477–1481. [Google Scholar]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Evaluation of seedling allelopathy in 453 wheat (Triticum aestivum) accessions against annual ryegrass (Lolium rigidum) by the equal-compartment-agar method. Aust. J. Agric. Res. 2000, 51, 937–944. [Google Scholar]
- Ma, Y. Allelopathic studies of common wheat (Triticum aestivum L.). Weed Biol. Manag. 2005, 5, 93–104. [Google Scholar]
- Rizvi, S.J.H.; Rizvi, V. (Eds.) Exploitation of allelochemicals in improving crop productivity. In Allelopathy; Springer: Dordrecht, The Netherlands, 1992; pp. 443–472. [Google Scholar]
- Bai, J.; Bai, X.; Yang, Y.; Tao, B. Extraction of allelochemicals from germinated wheat seeds and their inhibitory effects on cucumber. Agron. J. 2021, 113, 3124–3134. [Google Scholar]
- FAO Rice Price Update. Available online: https://www.fao.org/markets-and-trade/commodities/rice/fao-rice-price-update/en/ (accessed on 22 April 2024).
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 14, 3861–3864. [Google Scholar]
- Hohn, T.M. Cloning and expression of terpene synthase genes. In Comprehensive Natural Products Chemistry; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 201–215. [Google Scholar]
- Zhang, J.; Li, C.; Wu, C.; Xiong, L.; Chen, G.; Zhang, Q.; Wang, S. RMD: A rice mutant database for functional analysis of the rice genome. Nucleic Acids Res. 2006, 34 (Suppl. S1), D745–D748. [Google Scholar] [PubMed]
- Toyomasu, T.; Usui, M.; Sugawara, C.; Otomo, K.; Hirose, Y.; Miyao, A.; Hirochika, H.; Okada, K.; Shimizu, T.; Koga, J.; et al. Reverse-genetic approach to verify physiological roles of rice phytoalexins: Characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiol. Plant 2014, 150, 55–62. [Google Scholar] [PubMed]
- Chung, I.M.; Ham, T.H.; Cho, G.W.; Kwon, S.W.; Lee, Y.; Seo, J.; An, Y.-J.; Kim, S.-Y.; Kim, S.-H.; Lee, J. Study of quantitative trait loci (QTLs) associated with allelopathic trait in rice. Genes 2020, 11, 470. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Convergent or parallel molecular evolution of momilactone A and B: Potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 2011, 168, 1511–1516. [Google Scholar]
- Minh, T.N.; Xuan, T.D. Momilactone B and Potential in Biological Control of Weeds. In Plant Phenolics in Biotic Stress Management; Lone, R., Khan, S., Mohammed Al-Sadi, A., Eds.; Springer Nature: Singapore, 2024; pp. 367–388. [Google Scholar]
- Serra Serra, N.; Shanmuganathan, R.; Becker, C. Allelopathy in rice: A story of momilactones, kin recognition, and weed management. J. Exp Bot. 2021, 72, 4022–4037. [Google Scholar] [PubMed]
- Xu, M.; Galhano, R.; Wiemann, P.; Bueno, E.; Tiernan, M.; Wu, W.; Chung, I.-M.; Gershenzon, J.; Tudzynski, B.; Sesma, A.; et al. Genetic evidence for natural product-mediated plant–plant allelopathy in rice (Oryza sativa). New Phytol. 2012, 193, 570–575. [Google Scholar]
- Xu, Y.; Cheng, H.F.; Kong, C.H.; Meiners, S.J. Intra-specific kin recognition contributes to inter-specific allelopathy: A case study of allelopathic rice interference with paddy weeds. Plant Cell Environ. 2021, 44, 3709–3721. [Google Scholar]
- Anten, N.P.; Chen, B.J. Kin discrimination in allelopathy and consequences for agricultural weed control. Plant Cell Environ. 2021, 44, 3705. [Google Scholar]
- Hartley, R.D.; Whitehead, D.C. Phenolic Acids in Soils and their Influence on Plant Growth and Soil Microbial Processes. In Soil Organic Matter and Biological Activity; Vaughan, D., Malcolm, R.E., Eds.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1985; Volume 16, pp. 109–149. [Google Scholar]
- Ho, T.L.; Nguyen, V.L.; Phan, L.K.; Nguyen, C.T.; Nguyen, T.H.; Van, V.L.; Reid, S.J. Phytotoxicity in aqueous methanolic extracts of rice against junglerice and total activities of identified phytotoxic compounds. Ann. Appl. Biol. 2022, 180, 196–210. [Google Scholar]
- Kong, C.H.; Li, H.B.; Hu, F.; Xu, X.H.; Wang, P. Allelochemicals released by rice roots and residues in soil. Plant Soil 2006, 288, 47–56. [Google Scholar]
- Kato-Noguchi, H.; Ino, T. Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 2005, 162, 718–721. [Google Scholar] [PubMed]
- Kato-Noguchi, H.; Ota, K.; Kujime, H.; Ogawa, M. Effects of momilactone on the protein expression in Arabidopsis germination. Weed Biol. Manag. 2013, 13, 19–23. [Google Scholar]
- Invasive Species Compendium, Bidens pilosa. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.9148 (accessed on 18 March 2025).
- Wang, R.; Zhang, M.; Song, Y.; Hu, L.; Su, Y.; Zeng, R. Allelopathic effect of forage legumes on four common cropland weeds and rice. Ecol. Environ. 2010, 19, 2307. [Google Scholar]
- Chiang, Y.M.; Chuang, D.Y.; Wang, S.Y.; Kuo, Y.H.; Tsai, P.W.; Shyur, L.F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004, 95, 409–419. [Google Scholar] [PubMed]
- Hong, N.H.; Xuan, T.D.; Eiji, T.; Khanh, T.D. Paddy weed control by higher plants from Southeast Asia. Crop Prot. 2004, 23, 255–261. [Google Scholar]
- Del Fabbro, C.; Güsewell, S.; Prati, D. Allelopathic effects of three plant invaders on germination of native species: A field study. Biol. Invasions 2014, 16, 1035–1042. [Google Scholar]
- Khasabulli, B.D.; Musyimi, D.M.; George, O.; Gichuhi, M.N. Allelopathic effect of Bidens pilosa on seed germination and growth of Amaranthus dubius. J. Asian Sci. Res. 2018, 8, 103. [Google Scholar]
- Cheng, H.; Wang, S.; Wei, M.; Yu, Y.; Wang, C. Effect of leaf water extracts of four Asteraceae alien invasive plants on germination performance of Lactuca sativa L. under acid deposition. Plant Ecol. 2021, 222, 433–443. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants 2024, 13, 356. [Google Scholar] [CrossRef]
- Pereira, R.L.; Ibrahim, T.; Lucchetti, L.; da Silva, A.J.R.; de Moraes, V.L.G. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology 1999, 43, 31–37. [Google Scholar]
- Kamboj, A.; Saluja, A.K. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). Int. J. Pharm. Pharm. Sci. 2011, 3, 94–96. [Google Scholar]
- Widodo, G.P.; Sukandar, E.Y.; Sukrasno, S.; Adnyana, I.K. A coumarin from Ageratum leaves (Ageratum conyzoides L.). Int. J. Pharmacol. 2008, 4, 56–59. [Google Scholar]
- Kong, C.H.; Hu, F.; Xu, X.H.; Liang, W.; Zhang, C. Allelopathic plants. Ageratum conyzoides L. Allelopath. J. 2004, 14, 1–12. [Google Scholar]
- Javaid, N.; Shah, M.H.; Khan, I.H.; Javaid, A.; Waleed, S.M. Herbicidal activity of Ageratum conyzoides against parthenium. Pak. J. Weed Sci. Res. 2020, 26, 137–146. [Google Scholar]
- Kato-Noguchi, H. Assessment of the allelopathic potential of Ageratum conyzoides. Biol. Plant. 2001, 44, 309–311. [Google Scholar]
- Lalrindiki, J.A.; Zohmachhuana, A.; Lalnunmawia, F. Phytochemical Screening and Allelopathic Effects of Ageratum conyzoides L. Sci. Technol. J. 2020, 8, 29–33. [Google Scholar]
- Erida, G.; Saidi, N.; Hasanuddin, H.; Syafruddin, S. Herbicidal effects of ethyl acetate extracts of billygoat weed (Ageratum conyzoides L.) on spiny amaranth (Amaranthus spinosus L.) growth. Agronomy 2021, 11, 1991. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambelli, A.; Nocito, F.F.; Araniti, F. Unveiling the Multifaceted Roles of Root Exudates: Chemical Interactions, Allelopathy, and Agricultural Applications. Agronomy 2025, 15, 845. https://doi.org/10.3390/agronomy15040845
Zambelli A, Nocito FF, Araniti F. Unveiling the Multifaceted Roles of Root Exudates: Chemical Interactions, Allelopathy, and Agricultural Applications. Agronomy. 2025; 15(4):845. https://doi.org/10.3390/agronomy15040845
Chicago/Turabian StyleZambelli, Alice, Fabio Francesco Nocito, and Fabrizio Araniti. 2025. "Unveiling the Multifaceted Roles of Root Exudates: Chemical Interactions, Allelopathy, and Agricultural Applications" Agronomy 15, no. 4: 845. https://doi.org/10.3390/agronomy15040845
APA StyleZambelli, A., Nocito, F. F., & Araniti, F. (2025). Unveiling the Multifaceted Roles of Root Exudates: Chemical Interactions, Allelopathy, and Agricultural Applications. Agronomy, 15(4), 845. https://doi.org/10.3390/agronomy15040845








