Progress and Opportunities of In Planta and Topical RNAi for the Biotechnological Control of Agricultural Pests
Abstract
1. Introduction
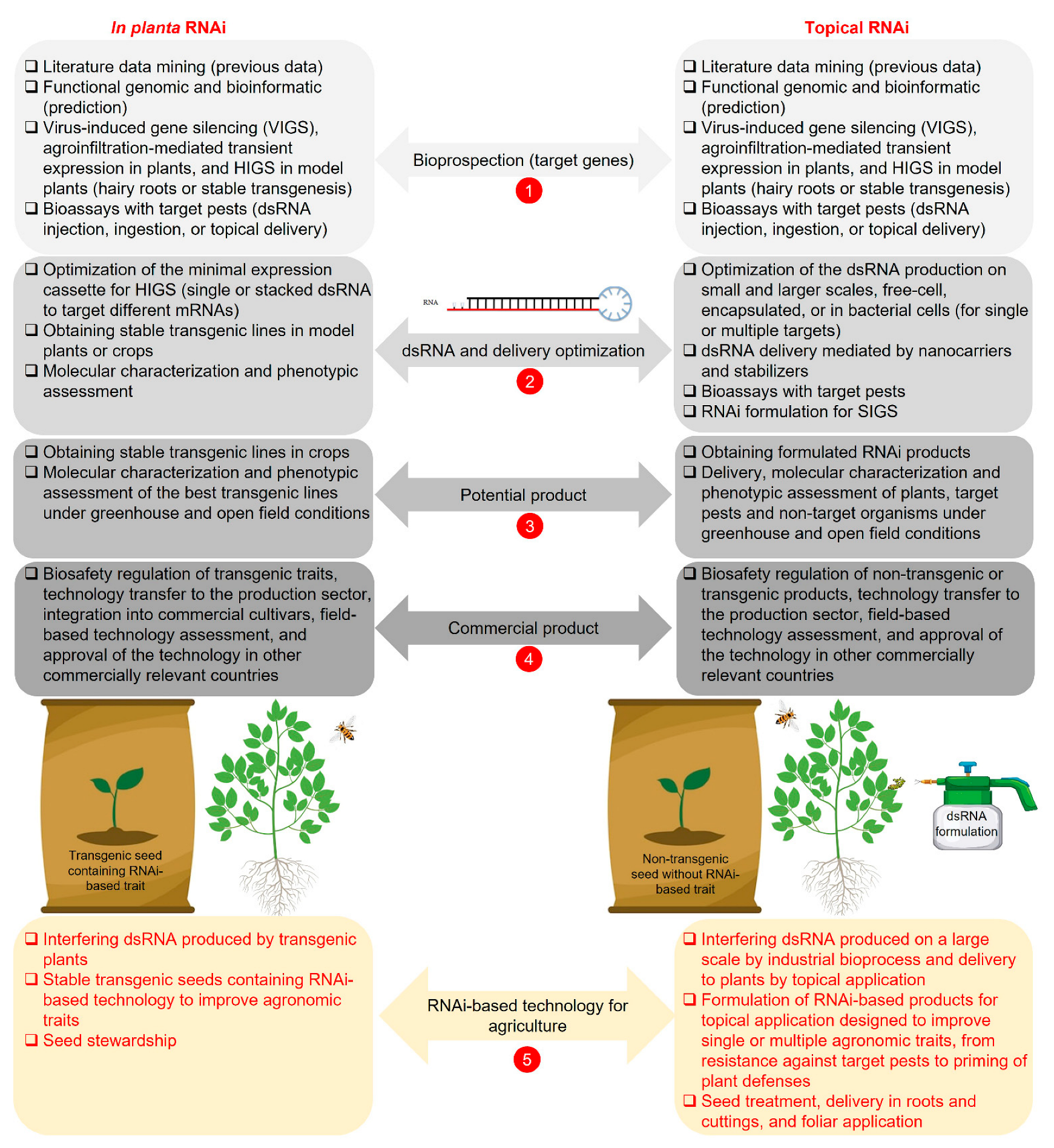
2. In Planta RNAi
2.1. Establishment of In Planta RNAi
2.2. In Planta RNAi for Insect Pest Control
2.3. In Planta RNAi to Control Plant-Parasitic Nematodes
2.4. In Planta RNAi to Control Other Important Plant Pathogens
2.5. Alternative Methods for In Planta RNAi
3. Topical RNAi
3.1. Establishment of Topical RNAi
3.2. Large-Scale dsRNA Production
3.3. Nanocarriers for Topical Delivery of dsRNA
3.4. Topical RNAi to Control Insect Pests
3.5. Topical RNAi to Control Major Plant Pathogens
4. Intellectual Property of RNAi-Based Technologies
4.1. Patent Search Methodology
4.2. Patent Scenario
5. Biosafety and Risk Assessment of In Planta and Topical RNAi-Based Technologies
5.1. Risk Assessment for Human Health
5.2. Environmental Risk Assessment
5.3. Possible Off-Target Effects of RNAi-Based Products Versus Decision Making
5.4. Current Regulatory Status of In Planta and Topical RNAi in Latin America
5.5. Resistance Against Exogenous dsRNA and RNAi
6. Major Contributions of RNAi to More Sustainable Agriculture
7. Concluding Remarks and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ali, N.; Datta, S.K.; Datta, K. RNA interference in designing transgenic crops. GM Crops 2010, 1, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rössner, C.; Lotz, D.; Becker, A. VIGS goes viral: How VIGS transforms our understanding of plant science. Annu. Rev. Plant Biol. 2022, 73, 703–728. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, K.; Faria, J.C.; Nogueira, E.O.; Mendes, E.A.; Aragão, F.J. RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol. Plant-Microbe Interact. 2007, 20, 717–726. [Google Scholar] [CrossRef]
- Aregger, M.; Borah, B.K.; Seguin, J.; Rajeswaran, R.; Gubaeva, E.G.; Zvereva, A.S.; Windels, D.; Vazquez, F.; Blevins, T.; Farinelli, L.; et al. Primary and secondary siRNAs in geminivirus-induced gene silencing. PLoS Pathog. 2012, 8, e1002941. [Google Scholar] [CrossRef]
- Yang, B.; Sugio, A.; White, F.F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA 2006, 103, 10503–10508. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Vasquez, D.D.N.; Macedo, L.L.P.; Lourenço-Tessutti, I.T.; Valença, D.C.; Oliveira-Neto, O.B.; Paes-de-Melo, B.; Rodrigues-Silva, P.L.; Firmino, A.A.P.; Basso, M.F.; et al. Stabilized double-stranded RNA strategy improves cotton resistance to CBW (Anthonomus grandis). Int. J. Mol. Sci. 2022, 23, 13713. [Google Scholar] [CrossRef]
- Basso, M.F.; Lourenço-Tessutti, I.T.; Mendes, R.A.G.; Pinto, C.E.M.; Bournaud, C.; Gillet, F.-X.; Togawa, R.C.; de Macedo, L.L.P.; de Almeida Engler, J.; Grossi-de-Sa, M.F. MiDaf16-like and MiSkn1-like gene families are reliable targets to develop biotechnological tools for the control and management of Meloidogyne incognita. Sci. Rep. 2020, 10, 6991. [Google Scholar] [CrossRef]
- Mendes, R.A.G.; Basso, M.F.; Amora, D.X.; Silva, A.P.; Paes-de-Melo, B.; Coiti Togawa, R.; Saliba Albuquerque, E.V.; Lisei-de-Sa, M.E.; Lima Pepino Macedo, L.; Lourenço-Tessutti, I.T.; et al. In planta RNAi approach targeting three M. incognita effector genes disturbed the process of infection and reduced plant susceptibility. Exp. Parasitol. 2022, 238, 108246. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Y.; Zhao, J.H.; Gao, F.; Zhou, B.J.; Fang, Y.Y.; Guo, H.S. Host-induced gene silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae. Mol. Plant 2016, 9, 939–942. [Google Scholar] [CrossRef]
- Cheng, W.; Lin, M.; Chu, M.; Xiang, G.; Guo, J.; Jiang, Y.; Guan, D.; He, S. RNAi-based gene silencing of RXLR effectors protects plants against the oomycete pathogen Phytophthora capsici. Mol. Plant-Microbe Interact. 2022, 35, 440–449. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Okada, K.; Takebe, I.; Matsui, C. Delivery of Tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (Liposomes). Mol. Gen. Genet. 1981, 184, 161–165. [Google Scholar] [CrossRef]
- Grossi-de-Sa, M.F.; Basso, M.F. Ciências agrárias e as revoluções na produção de alimentos: Do passado ao futuro. In Segurança alimentar e nutricional: O papel da ciência brasileira no combate à fome; Hungria, M.H., Ed.; Academia Brasileira de Ciências: Rio de Janeiro, Brazil, 2024; pp. 48–55. [Google Scholar]
- Basso, M.; Neves, M.; Grossi-de-Sá, M.F. Agriculture evolution, sustainability and trends, focusing on Brazilian agribusiness: A review. Front. Sustain. Food Syst. 2024, 7, 1296337. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Torres, J.B.; Rolim, G.G.; Arruda, L.S.; dos Santos, M.P.; Leite, S.A.; Neves, R.C.d.S. Insecticides in Use and Risk of Control Failure of Boll Weevil (Coleoptera: Curculionidae) in the Brazilian Cerrado. Neotrop. Entomol. 2022, 51, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Távora, F.T.P.K.; de Assis Dos Santos Diniz, F.; de Moraes Rêgo-Machado, C.; Chagas Freitas, N.; Barbosa Monteiro Arraes, F.; Chumbinho de Andrade, E.; Furtado, L.L.; Osiro, K.O.; Lima de Sousa, N.; Cardoso, T.B.; et al. CRISPR/Cas- and topical RNAi-based technologies for crop management and improvement: Reviewing the risk assessment and challenges towards a more sustainable agriculture. Front. Bioeng. Biotechnol. 2022, 10, 913728. [Google Scholar] [CrossRef]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef]
- Rosado, A.; Eriksson, D. Biosafety legislation and the regulatory status of the products of precision breeding in the Latin America and the Caribbean region. Plants People Planet 2022, 4, 214–231. [Google Scholar] [CrossRef]
- Basso, M.F.; Ferreira, P.C.G.; Kobayashi, A.K.; Harmon, F.G.; Nepomuceno, A.L.; Molinari, H.B.C.; Grossi-de-Sa, M.F. MicroRNAs and new biotechnological tools for its modulation and improving stress tolerance in plants. Plant Biotechnol. J. 2019, 17, 1482–1500. [Google Scholar] [CrossRef]
- Basso, M.F.; Lourenço-Tessutti, I.T.; Busanello, C.; Pinto, C.E.M.; de Oliveira Freitas, E.; Ribeiro, T.P.; de Almeida Engler, J.; de Oliveira, A.C.; Morgante, C.V.; Alves-Ferreira, M.; et al. Insights obtained using different modules of the cotton uceA1.7 promoter. Planta 2020, 251, 56. [Google Scholar] [CrossRef]
- Fragoso, R.R.; Arraes, F.B.M.; Lourenço-Tessutti, I.T.; Miranda, V.J.; Basso, M.F.; Ferreira, A.V.J.; Viana, A.A.B.; Lins, C.B.J.; Lins, P.C.; Moura, S.M.; et al. Functional characterization of the pUceS8.3 promoter and its potential use for ectopic gene overexpression. Planta 2022, 256, 69. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.F.; Arraes, F.B.M.; Grossi-de-Sa, M.; Moreira, V.J.V.; Alves-Ferreira, M.; Grossi-de-Sa, M.F. Insights into genetic and molecular elements for transgenic crop development. Front. Plant Sci. 2020, 11, 509. [Google Scholar] [CrossRef]
- Lindbo, J.A.; Silva-Rosales, L.; Proebsting, W.M.; Dougherty, W.G. Induction of a highly specific antiviral state in transgenic plants: Implications for regulation of gene expression and virus resistance. Plant Cell 1993, 5, 1749–1759. [Google Scholar] [CrossRef]
- Baulcombe, D.C.; Chapman, S.; Santa Cruz, S. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 1995, 7, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Voinnet, O.; Chappell, L.; Baulcombe, D. Two classes of short interfering RNA in RNA silencing. EMBO J. 2002, 21, 4671–4679. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Tao, X.-Y.; Xue, X.-Y.; Wang, L.-J.; Chen, X.-Y. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res. 2011, 20, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Shen, W.; Gao, X.; Li, X.; Zhou, P.; Duan, J. High-throughput construction of intron-containing hairpin RNA vectors for RNAi in plants. PLoS ONE 2012, 7, e38186. [Google Scholar] [CrossRef]
- Hu, D.; Chen, Z.-Y.; Zhang, C.; Ganiger, M. Reduction of Phakopsora pachyrhizi infection on soybean through host- and spray-induced gene silencing. Mol. Plant Pathol. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Zhang, D.; Zhong, C.; Smith, N.A.; de Feyter, R.; Greaves, I.K.; Swain, S.M.; Zhang, R.; Wang, M.B. Nucleotide mismatches prevent intrinsic self-silencing of hpRNA transgenes to enhance RNAi stability in plants. Nat. Commun. 2022, 13, 3926. [Google Scholar] [CrossRef]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef]
- Butel, N.; Yu, A.; Le Masson, I.; Borges, F.; Elmayan, T.; Taochy, C.; Gursanscky, N.R.; Cao, J.; Bi, S.; Sawyer, A.; et al. Contrasting epigenetic control of transgenes and endogenous genes promotes post-transcriptional transgene silencing in Arabidopsis. Nat. Commun. 2021, 12, 2787. [Google Scholar] [CrossRef]
- Joshi, I.; Kumar, A.; Kohli, D.; Bhattacharya, R.; Sirohi, A.; Chaudhury, A.; Jain, P.K. Gall-specific promoter, an alternative to the constitutive CaMV35S promoter, drives host-derived RNA interference targeting Mi-msp2 gene to confer effective nematode resistance. Front. Plant Sci. 2022, 13, 1007322. [Google Scholar] [CrossRef]
- Li, J.; Jiang, D.; Zhou, H.; Li, F.; Yang, J.; Hong, L.; Fu, X.; Li, Z.; Liu, Z.; Li, J.; et al. Expression of RNA-interference/antisense transgenes by the cognate promoters of target genes is a better gene-silencing strategy to study gene functions in rice. PLoS ONE 2011, 6, e17444. [Google Scholar] [CrossRef]
- Moura, S.M.; Freitas, E.O.; Ribeiro, T.P.; Paes-de-Melo, B.; Arraes, F.B.M.; Macedo, L.L.P.; Paixão, J.F.R.; Lourenço-Tessutti, I.T.; Artico, S.; da Cunha Valença, D.; et al. Discovery and functional characterization of novel cotton promoters with potential application to pest control. Plant Cell Rep. 2022, 41, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Wintermantel, W.M. Optimizing efficient RNAi-mediated control of hemipteran pests (psyllids, leafhoppers, whitefly): Modified pyrimidines in dsRNA triggers. Plants 2021, 10, 1782. [Google Scholar] [CrossRef] [PubMed]
- Fitch, M.M.M.; Manshardt, R.M.; Gonsalves, D.; Slightom, J.L.; Sanford, J.C. Virus resistant papaya plants derived from tissues bombarded with the coat protein gene of Papaya ringspot virus. Nat. Biotechnol. 1992, 10, 1466–1472. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Pitz, K.Y.; Manshardt, R.; Zee, F.; Fitch, M.; Gonsalves, D. Virus coat protein transgenic papaya provides practical control of Papaya ringspot virus in Hawaii. Plant Dis. 2002, 86, 101–105. [Google Scholar] [CrossRef]
- Aragão, F.J.; Nogueira, E.O.; Tinoco, M.L.; Faria, J.C. Molecular characterization of the first commercial transgenic common bean immune to the Bean golden mosaic virus. J. Biotechnol. 2013, 166, 42–50. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C.; et al. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0197059. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Pest control. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W.; et al. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef]
- Svoboda, P.; Di Cara, A. Hairpin RNA: A secondary structure of primary importance. Cell. Mol. Life Sci. 2006, 63, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kutter, C.; Svoboda, P. miRNA, siRNA, piRNA: Knowns of the unknown. RNA Biol. 2008, 5, 181–188. [Google Scholar] [CrossRef]
- Abbasi, R.; Heschuk, D.; Kim, B.; Whyard, S. A novel paperclip double-stranded RNA structure demonstrates clathrin-independent uptake in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2020, 127, 103492. [Google Scholar] [CrossRef]
- Guan, R.B.; Li, H.C.; Fan, Y.J.; Hu, S.R.; Christiaens, O.; Smagghe, G.; Miao, X.X. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 2018, 293, 6011–6021. [Google Scholar] [CrossRef]
- Garcia, R.A.; Lima Pepino Macedo, L.; Cabral do Nascimento, D.; Gillet, F.X.; Moreira-Pinto, C.E.; Faheem, M.; Moreschi Basso, A.M.; Mattar Silva, M.C.; Grossi-de-Sa, M.F. Nucleases as a barrier to gene silencing in the cotton boll weevil, Anthonomus grandis. PLoS ONE 2017, 12, e0189600. [Google Scholar] [CrossRef]
- Gillet, F.X.; Garcia, R.A.; Macedo, L.L.P.; Albuquerque, E.V.S.; Silva, M.C.M.; Grossi-de-Sa, M.F. Investigating engineered ribonucleoprotein particles to improve oral RNAi delivery in crop insect pests. Front. Physiol. 2017, 8, 256. [Google Scholar] [CrossRef]
- He, G. Engineering chloroplasts for insect pest control. Proc. Natl. Acad. Sci. USA 2022, 119, e2205125119. [Google Scholar] [CrossRef]
- Wu, M.; Dong, Y.; Zhang, Q.; Li, S.; Chang, L.; Loiacono, F.V.; Ruf, S.; Zhang, J.; Bock, R. Efficient control of western flower thrips by plastid-mediated RNA interference. Proc. Natl. Acad. Sci. USA 2022, 119, e2120081119. [Google Scholar] [CrossRef]
- Liao, J.; Rong, H.; You, L.; Xia, K.; Wang, M.; Han, P.; Li, C.; Zhang, J. Identification of leaf chloroplast-specific promoter to efficiently control of Colorado potato beetle with reduced dsRNA accumulation in potato tubers. Pest Manag. Sci. 2023, 79, 3326–3333. [Google Scholar] [CrossRef]
- Ren, B.; Cao, J.; He, Y.; Yang, S.; Zhang, J. Assessment on effects of transplastomic potato plants expressing Colorado potato beetle β-Actin double-stranded RNAs for three non-target pests. Pestic. Biochem. Physiol. 2021, 178, 104909. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, M.; Li, Y.; He, W.; Li, S.; Zhang, J. Complete protection from Henosepilachna vigintioctopunctata by expressing long double-stranded RNAs in potato plastids. J. Integr. Plant Biol. 2023, 65, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Singh, N.D.; Li, L.; Zhang, X.; Daniell, H. Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa zea larval development and pupation. Plant Biotechnol. J. 2015, 13, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, M.; Zhang, Q.; Fu, J.; Loiacono, F.V.; Yang, Y.; Wang, Z.; Li, S.; Chang, L.; Bock, R.; et al. Control of a sap-sucking insect pest by plastid-mediated RNA interference. Mol. Plant 2022, 15, 1176–1191. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Wang, Z.; Wu, M.; Fu, J.; Guo, J.; Chang, L.; Zhang, J. Inaccessibility to double-stranded RNAs in plastids restricts RNA interference in Bemisia tabaci (whitefly). Pest Manag. Sci. 2020, 76, 3168–3176. [Google Scholar] [CrossRef]
- Burke, W.G.; Kaplanoglu, E.; Kolotilin, I.; Menassa, R.; Donly, C. RNA interference in the tobacco hornworm, Manduca sexta, using plastid-encoded long double-stranded RNA. Front. Plant Sci. 2019, 10, 313. [Google Scholar] [CrossRef]
- Fu, J.; Xu, S.; Lu, H.; Li, F.; Li, S.; Chang, L.; Heckel, D.G.; Bock, R.; Zhang, J. Resistance to RNA interference by plant-derived double-stranded RNAs but not plant-derived short interfering RNAs in Helicoverpa armigera. Plant Cell Environ. 2022, 45, 1930–1941. [Google Scholar] [CrossRef]
- He, W.; Xu, W.; Xu, L.; Fu, K.; Guo, W.; Bock, R.; Zhang, J. Length-dependent accumulation of double-stranded RNAs in plastids affects RNA interference efficiency in the Colorado potato beetle. J. Exp. Bot. 2020, 71, 2670–2677. [Google Scholar] [CrossRef]
- Bally, J.; McIntyre, G.J.; Doran, R.L.; Lee, K.; Perez, A.; Jung, H.; Naim, F.; Larrinua, I.M.; Narva, K.E.; Waterhouse, P.M. In-plant protection against Helicoverpa armigera by production of long hpRNA in chloroplasts. Front. Plant Sci. 2016, 7, 1453. [Google Scholar] [CrossRef]
- Sheridan, C. First self-amplifying mRNA vaccine approved. Nat. Biotechnol. 2024, 42, 4. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Luo, J.; Liang, S.; Li, J.; Xu, Z.; Li, L.; Zhu, B.; Li, Z.; Lei, C.; Lindsey, K.; Chen, L.; et al. A transgenic strategy for controlling plant bugs (Adelphocoris suturalis) through expression of double-stranded RNA homologous to fatty acyl-coenzyme A reductase in cotton. New Phytol. 2017, 215, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sparks, C.; Jones, H.; Riley, M.; Francis, F.; Du, W.; Xia, L. Silencing an essential gene involved in infestation and digestion in grain aphid through plant-mediated RNA interference generates aphid-resistant wheat plants. Plant Biotechnol. J. 2019, 17, 852–854. [Google Scholar] [CrossRef]
- Mao, C.; Zhu, X.; Wang, P.; Sun, Y.; Huang, R.; Zhao, M.; Hull, J.J.; Lin, Y.; Zhou, F.; Chen, H.; et al. Transgenic double-stranded RNA rice, a potential strategy for controlling striped stem borer (Chilo suppressalis). Pest Manag. Sci. 2022, 78, 785–792. [Google Scholar] [CrossRef]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef]
- Li, H.; Bowling, A.J.; Gandra, P.; Rangasamy, M.; Pence, H.E.; McEwan, R.E.; Khajuria, C.; Siegfried, B.D.; Narva, K.E. Systemic RNAi in western corn rootworm, Diabrotica virgifera virgifera, does not involve transitive pathways. Insect Sci. 2018, 25, 45–56. [Google Scholar] [CrossRef]
- Firmino, A.A.P.; Pinheiro, D.H.; Moreira-Pinto, C.E.; Antonino, J.D.; Macedo, L.L.P.; Martins-de-Sa, D.; Arraes, F.B.M.; Coelho, R.R.; Fonseca, F.C.A.; Silva, M.C.M.; et al. RNAi-mediated suppression of laccase2 impairs cuticle tanning and molting in the cotton boll weevil (Anthonomus grandis). Front. Physiol. 2020, 11, 591569. [Google Scholar] [CrossRef]
- Vasquez, D.D.N.; Pinheiro, D.H.; Teixeira, L.A.; Moreira-Pinto, C.E.; Macedo, L.L.P.; Salles-Filho, A.L.O.; Silva, M.C.M.; Lourenço-Tessutti, I.T.; Morgante, C.V.; Silva, L.P.; et al. Simultaneous silencing of juvenile hormone metabolism genes through RNAi interrupts metamorphosis in the cotton boll weevil. Front. Mol. Biosci. 2023, 10, 1073721. [Google Scholar] [CrossRef]
- Moreira-Pinto, C.E.; Coelho, R.R.; Leite, A.G.B.; Silveira, D.A.; de Souza, D.A.; Lopes, R.B.; Macedo, L.L.P.; Silva, M.C.M.; Ribeiro, T.P.; Morgante, C.V.; et al. Increasing Anthonomus grandis susceptibility to Metarhizium anisopliae through RNAi-induced AgraRelish knockdown: A perspective to combine biocontrol and biotechnology. Pest Manag. Sci. 2021, 77, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, G.; Asokan, R.; Prasannakumar, N.R.; Kariyanna, B.; Karthi, S.; Alwahibi, M.S.; Elshikh, M.S.; Abdel-Megeed, A.; Ghaith, A.; Senthil-Nathan, S.; et al. RNA interference suppression of v-ATPase B and juvenile hormone binding protein genes through topically applied dsRNA on tomato leaves: Developing biopesticides to control the South American pinworm, Tuta absoluta (Lepidoptera: Gelechiidae). Front Physiol. 2021, 12, 742871. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Ma, W.; Wang, X.; Gao, M.; Dai, Y.; Wei, X.; Zhang, L.; Peng, Y.; Chen, S.; Ding, L.; et al. Next-generation transgenic cotton: Pyramiding RNAi and Bt counters insect resistance. Plant Biotechnol. J. 2017, 15, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-Z.; Lum, A.; Schepers, E.; Liu, L.; Weston, R.T.; McGinness, B.S.; Heckert, M.J.; Xie, W.; Kassa, A.; Bruck, D.; et al. Novel insecticidal proteins from ferns resemble insecticidal proteins from Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 2023, 120, e2306177120. [Google Scholar] [CrossRef]
- Mehlhorn, S.; Hunnekuhl, V.S.; Geibel, S.; Nauen, R.; Bucher, G. Establishing RNAi for basic research and pest control and identification of the most efficient target genes for pest control: A brief guide. Front. Zool. 2021, 18, 60. [Google Scholar] [CrossRef]
- Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Goodfellow, S.; Zhang, D.; Wang, M.B.; Zhang, R. Bacterium-mediated RNA interference: Potential application in plant protection. Plants 2019, 8, 572. [Google Scholar] [CrossRef]
- Chaudhary, S.; Dutta, T.K.; Tyagi, N.; Shivakumara, T.N.; Papolu, P.K.; Chobhe, K.A.; Rao, U. Host-induced silencing of Mi-msp-1 confers resistance to root-knot nematode Meloidogyne incognita in eggplant. Transgenic Res. 2019, 28, 327–340. [Google Scholar] [CrossRef]
- Moreira, V.J.V.; Pinheiro, D.H.; Lourenço-Tessutti, I.T.; Basso, M.F.; Lisei-de-Sa, M.E.; Silva, M.C.M.; Danchin, E.G.J.; Guimarães, P.M.; Grynberg, P.; Brasileiro, A.C.M.; et al. In planta RNAi targeting Meloidogyne incognita Minc16803 gene perturbs nematode parasitism and reduces plant susceptibility. J. Pest Sci. 2023, 97, 411–427. [Google Scholar] [CrossRef]
- Moreira, V.J.V.; Lourenço-Tessutti, I.T.; Basso, M.F.; Lisei-de-Sa, M.E.; Morgante, C.V.; Paes-de-Melo, B.; Arraes, F.B.M.; Martins-de-Sa, D.; Silva, M.C.M.; de Almeida Engler, J.; et al. Minc03328 effector gene downregulation severely affects Meloidogyne incognita parasitism in transgenic Arabidopsis thaliana. Planta 2022, 255, 44. [Google Scholar] [CrossRef]
- Mejias, J.; Chen, Y.; Bazin, J.; Truong, N.M.; Mulet, K.; Noureddine, Y.; Jaubert-Possamai, S.; Ranty-Roby, S.; Soulé, S.; Abad, P.; et al. Silencing the conserved small nuclear ribonucleoprotein SmD1 target gene alters susceptibility to root-knot nematodes in plants. Plant Physiol. 2022, 189, 1741–1756. [Google Scholar] [CrossRef]
- Lisei-de-Sá, M.E.; Rodrigues-Silva, P.L.; Morgante, C.V.; de Melo, B.P.; Lourenço-Tessutti, I.T.; Arraes, F.B.M.; Sousa, J.P.A.; Galbieri, R.; Amorim, R.M.S.; de Lins, C.B.J.; et al. Pyramiding dsRNAs increases phytonematode tolerance in cotton plants. Planta 2021, 254, 121. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, T.; Miroshnichenko, D.; Kirov, I.; Pushin, A.; Dolgov, S. Effect of Grafting on Viral Resistance of Non-transgenic Plum Scion Combined With Transgenic PPV-Resistant Rootstock. Front. Plant Sci. 2021, 12, 621954. [Google Scholar] [CrossRef]
- Rodrigues-Silva, P.; Moura, S.; Dantas, L.; Domiciano, G.; Silva, M.; Campos, M.; Grossi-de-Sa, M. RNAi and Bt approaches to insect-pest control: Analyses and perspectives on trends in global patent publications. Biotechnol. Res. Innov. 2023, 6, e2022204. [Google Scholar] [CrossRef]
- Voloudakis, A.E.; Kaldis, A.; Patil, B.L. RNA-based vaccination of plants for control of viruses. Annu. Rev. Virol. 2022, 9, 521–548. [Google Scholar] [CrossRef]
- Beyene, G.; Chauhan, R.D.; Ilyas, M.; Wagaba, H.; Fauquet, C.M.; Miano, D.; Alicai, T.; Taylor, N.J. A virus-derived stacked RNAi construct confers robust resistance to cassava brown streak disease. Front. Plant Sci. 2017, 7, 2052. [Google Scholar] [CrossRef]
- Tabein, S.; Jansen, M.; Noris, E.; Vaira, A.M.; Marian, D.; Behjatnia, S.A.A.; Accotto, G.P.; Miozzi, L. The induction of an effective dsRNA-mediated resistance against Tomato spotted wilt virus by exogenous application of double-stranded RNA largely depends on the selection of the viral RNA target region. Front. Plant Sci. 2020, 11, 533338. [Google Scholar] [CrossRef]
- Walker, P.L.; Ziegler, D.J.; Giesbrecht, S.; McLoughlin, A.; Wan, J.; Khan, D.; Hoi, V.; Whyard, S.; Belmonte, M.F. Control of white mold (Sclerotinia sclerotiorum) through plant-mediated RNA interference. Sci. Rep. 2023, 13, 6477. [Google Scholar] [CrossRef]
- Jin, B.J.; Chun, H.J.; Choi, C.W.; Lee, S.H.; Cho, H.M.; Park, M.S.; Baek, D.; Park, S.-Y.; Lee, Y.-H.; Kim, M.C. Host-induced gene silencing is a promising biological tool to characterize the pathogenicity of Magnaporthe oryzae and control fungal disease in rice. Plant Cell Environ. 2023, 47, 319–336. [Google Scholar] [CrossRef]
- Pérez, C.E.B.; Cabral, G.B.; Aragão, F.J.L. Host-induced gene silencing for engineering resistance to Fusarium in soybean. Plant Pathol. 2021, 70, 417–425. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Rusk, N. Prokaryotic RNAi. Nat. Methods 2012, 9, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef]
- Caccia, S.; Astarita, F.; Barra, E.; Di Lelio, I.; Varricchio, P.; Pennacchio, F. Enhancement of Bacillus thuringiensis toxicity by feeding Spodoptera littoralis larvae with bacteria expressing immune suppressive dsRNA. J. Pest Sci. 2020, 93, 303–314. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Chen, J.Z.; Li, M.W.; Zha, B.H.; Huang, P.R.; Chu, X.M.; Chen, J.; Yang, G. The combination of Bacillus thuringiensis and its engineered strain expressing dsRNA increases the toxicity against Plutella xylostella. Int. J. Mol. Sci. 2021, 23, 444. [Google Scholar] [CrossRef]
- Kolliopoulou, A.; Taning, C.N.T.; Smagghe, G.; Swevers, L. Viral Delivery of dsRNA for Control of Insect Agricultural Pests and Vectors of Human Disease: Prospects and Challenges. Front. Physiol. 2017, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Li, Y.; Zeng, J.; Wang, G.; Deng, C.; Guo, W. Host-induced gene silencing of a regulator of G protein signalling gene (VdRGS1) confers resistance to Verticillium wilt in cotton. Plant Biotechnol. J. 2018, 16, 1629–1643. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhao, C.; Jiang, X.; Gao, D. Responses of non-structural carbohydrates and biomass in plant to heavy metal treatment. Sci. Total Environ. 2024, 909, 168559. [Google Scholar] [CrossRef]
- Paciorek, T.; Chiapelli, B.J.; Wang, J.Y.; Paciorek, M.; Yang, H.; Sant, A.; Val, D.L.; Boddu, J.; Liu, K.; Gu, C.; et al. Targeted suppression of gibberellin biosynthetic genes ZmGA20ox3 and ZmGA20ox5 produces a short stature maize ideotype. Plant Biotechnol. J. 2022, 20, 1140–1153. [Google Scholar] [CrossRef]
- Basso, M.F.; Duarte, K.E.; Santiago, T.R.; de Souza, W.R.; Garcia, B.O.; da Cunha, B.D.B.; Kobayashi, A.K.; Molinari, H.B.C. Efficient genome editing and gene knockout in Setaria viridis with CRISPR/Cas9 directed gene editing by the non-homologous end-joining pathway. Plant Biotechnol. 2021, 38, 227–238. [Google Scholar] [CrossRef]
- Tuncel, A.; Pan, C.; Sprink, T.; Wilhelm, R.; Barrangou, R.; Li, L.; Shih, P.M.; Varshney, R.K.; Tripathi, L.; Van Eck, J.; et al. Genome-edited foods. Nat. Rev. Bioeng. 2023, 1, 799–816. [Google Scholar] [CrossRef]
- Siddiquee, R.; Pong, C.H.; Hall, R.M.; Ataide, S.F. A programmable seekRNA guides target selection by IS1111 and IS110 type insertion sequences. Nat. Commun. 2024, 15, 5235. [Google Scholar] [CrossRef]
- Gao, H.; Gadlage, M.J.; Lafitte, H.R.; Lenderts, B.; Yang, M.; Schroder, M.; Farrell, J.; Snopek, K.; Peterson, D.; Feigenbutz, L.; et al. Superior field performance of waxy corn engineered using CRISPR-Cas9. Nat. Biotechnol. 2020, 38, 579–581. [Google Scholar] [CrossRef]
- Shelake, R.M.; Kadam, U.S.; Kumar, R.; Pramanik, D.; Singh, A.K.; Kim, J.Y. Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: Targets, tools, challenges, and perspectives. Plant Commun. 2022, 3, 100417. [Google Scholar] [CrossRef] [PubMed]
- Usovsky, M.; Gamage, V.A.; Meinhardt, C.G.; Dietz, N.; Triller, M.; Basnet, P.; Gillman, J.D.; Bilyeu, K.D.; Song, Q.; Dhital, B.; et al. Loss-of-function of an α-SNAP gene confers resistance to soybean cyst nematode. Nat. Commun. 2023, 14, 7629. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef]
- Mai, J.; Liao, L.; Ling, R.; Guo, X.; Lin, J.; Mo, B.; Chen, W.; Yu, Y. Study on RNAi-based herbicide for Mikania micrantha. Synth. and Syst. Biotechnol. 2021, 6, 437–445. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental fate and dissipation of applied dsRNA in soil, aquatic systems, and plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Bennett, M.; Deikman, J.; Hendrix, B.; Iandolino, A. Barriers to efficient foliar uptake of dsRNA and molecular barriers to dsRNA activity in plant cells. Front. Plant Sci. 2020, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a foliar spray: Efficiency and challenges to field applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- Schutter, K.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. Boosting dsRNA Delivery in Plant and Insect Cells with Peptide- and Polymer-Based Carriers: Case-Based Current Status and Future Perspectives; CABI: Oxfordshire, UK, 2021; pp. 102–116. [Google Scholar]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Rank, A.P.; Koch, A. Lab-to-field transition of RNA spray applications—How far are we? Front. Plant Sci. 2021, 12, 755203. [Google Scholar] [CrossRef] [PubMed]
- Jalaluddin, N.S.M.; Othman, R.Y.; Harikrishna, J.A. Global trends in research and commercialization of exogenous and endogenous RNAi technologies for crops. Crit. Rev. Biotechnol. 2019, 39, 67–78. [Google Scholar] [CrossRef]
- Hough, J.; Howard, J.D.; Brown, S.; Portwood, D.E.; Kilby, P.M.; Dickman, M.J. Strategies for the production of dsRNA biocontrols as alternatives to chemical pesticides. Front. Bioeng. Biotechnol. 2022, 10, 980592. [Google Scholar] [CrossRef]
- Necira, K.; Makki, M.; Sanz-García, E.; Canto, T.; Djilani-Khouadja, F.; Tenllado, F. Topical application of Escherichia coli-encapsulated dsRNA induces resistance in Nicotiana benthamiana to potato viruses and involves RDR6 and combined activities of DCL2 and DCL4. Plants 2021, 10, 644. [Google Scholar] [CrossRef]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef]
- Aalto, A.P.; Sarin, L.P.; van Dijk, A.A.; Saarma, M.; Poranen, M.M.; Arumäe, U.; Bamford, D.H. Large-scale production of dsRNA and siRNA pools for RNA interference utilizing bacteriophage phi6 RNA-dependent RNA polymerase. RNA 2007, 13, 422–429. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Deighan, P.; Kiner, E.; McReynolds, L.; Lieberman, J. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat. Biotechnol. 2013, 31, 350–356. [Google Scholar] [CrossRef]
- Ghoshal, K.; Theilmann, J.; Reade, R.; Maghodia, A.; Rochon, D. Encapsidation of Host RNAs by Cucumber Necrosis Virus Coat Protein during both Agroinfiltration and Infection. J. Virol. 2015, 89, 10748–10761. [Google Scholar] [CrossRef]
- Wuthisathid, K.; Chaijarasphong, T.; Chotwiwatthanakun, C.; Somrit, M.; Sritunyalucksana, K.; Itsathitphaisarn, O. Co-expression of double-stranded RNA and viral capsid protein in the novel engineered Escherichia coli DualX-B15(DE3) strain. BMC Microbiol. 2021, 21, 88. [Google Scholar] [CrossRef]
- Singh, I.K.; Singh, S.; Mogilicherla, K.; Shukla, J.N.; Palli, S.R. Comparative analysis of double-stranded RNA degradation and processing in insects. Sci. Rep. 2017, 7, 17059. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Q.; Mao, Y.; Wu, M.; Wang, Z.; Chang, L.; Zhang, J. Control of two insect pests by expression of a mismatch corrected double-stranded RNA in plants. Plant Biotechnol. J. 2024, 22, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Hanamasagar, Y.; Ramesha, N.M.; Mahapatra, S.; Panigrahi, C.K.; Vidhya, C.S.; Agnihotri, N.; Satapathy, S.N. Advancing RNAi for Sustainable Insect Management: Targeted Solutions for Eco-friendly Pest Control. J. Exp. Agric. Int. 2024, 46, 740–775. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Jalaluddin, N.S.M.; Asem, M.; Harikrishna, J.A.; Ahmad Fuaad, A.A.H. Recent progress on nanocarriers for topical-mediated RNAi strategies for crop protection—A review. Molecules 2023, 28, 2700. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Bi, S.; Tang, Y.; Zhang, G.; Yan, S.; Wan, F.; Lü, Z.; Liu, W. A promising approach to an environmentally friendly pest management solution: Nanocarrier-delivered dsRNA towards controlling the destructive invasive pest Tuta absoluta. Environ. Sci. Nano 2023, 10, 1003–1015. [Google Scholar] [CrossRef]
- Arjunan, N.; Thiruvengadam, V.; Sushil, S.N. Nanoparticle-mediated dsRNA delivery for precision insect pest control: A comprehensive review. Mol. Biol. Rep. 2024, 51, 355. [Google Scholar] [CrossRef]
- Afonin, K.A.; Viard, M.; Kagiampakis, I.; Case, C.L.; Dobrovolskaia, M.A.; Hofmann, J.; Vrzak, A.; Kireeva, M.; Kasprzak, W.K.; KewalRamani, V.N.; et al. Triggering of RNA interference with RNA-RNA, RNA-DNA, and DNA-RNA nanoparticles. ACS Nano 2015, 9, 251–259. [Google Scholar] [CrossRef]
- Zhou, H.; Wan, F.; Jian, Y.; Guo, F.; Zhang, M.; Shi, S.; Yang, L.; Li, S.; Liu, Y.; Ding, W. Chitosan/dsRNA polyplex nanoparticles advance environmental RNA interference efficiency through activating clathrin-dependent endocytosis. Int. J. Biol. Macromol. 2023, 253, 127021. [Google Scholar] [CrossRef]
- Almeida, A.A.; Santos, R.M.M.; Alves Rosa, M.A.; Pulcinelli, S.H.; John, V.M.; Santilli, C.V. MgAl-Layered Double Hydroxide Nanoparticles as Smart Nanofillers To Control the Rheological Properties and the Residual Porosity of Cement-Based Materials. ACS Appl. Nano Mater. 2022, 5, 7896–7907. [Google Scholar] [CrossRef]
- Kolge, H.; Kadam, K.; Galande, S.; Lanjekar, V.; Ghormade, V. New frontiers in pest control: Chitosan nanoparticles-shielded dsRNA as an effective topical RNAi spray for gram podborer biocontrol. ACS Appl. Bio Mater. 2021, 4, 5145–5157. [Google Scholar] [CrossRef]
- Islam, M.T.; Davis, Z.; Chen, L.; Englaender, J.; Zomorodi, S.; Frank, J.; Bartlett, K.; Somers, E.; Carballo, S.M.; Kester, M.; et al. Minicell-based fungal RNAi delivery for sustainable crop protection. Microb. Biotechnol. 2021, 14, 1847–1856. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.J.; Jin, H. Message in a bubble: Shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant Biol. 2021, 72, 497–524. [Google Scholar] [CrossRef]
- Amiri, A.; Bagherifar, R.; Ansari Dezfouli, E.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J. Transl. Med. 2022, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, W.; Li, M.; Zheng, A. Exosome-based carrier for RNA delivery: Progress and challenges. Pharmaceutics 2023, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Halilovic, L.; Shi, T.; Chen, A.; He, B.; Wu, H.; Jin, H. Extracellular vesicles: Cross-organismal RNA trafficking in plants, microbes, and mammalian cells. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 262–282. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Cai, Q. Plant exosome-like nanovesicles and their role in the innovative delivery of RNA therapeutics. Biomedicines 2023, 11, 1806. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Demirer, G.S.; Zhang, H.; Ye, T.; Goh, N.S.; Aditham, A.J.; Cunningham, F.J.; Fan, C.; Landry, M.P. DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. USA 2019, 116, 7543–7548. [Google Scholar] [CrossRef] [PubMed]
- Torney, F.; Trewyn, B.G.; Lin, V.S.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Buchman, Y.K.; Lellouche, E.; Zigdon, S.; Bechor, M.; Michaeli, S.; Lellouche, J.-P. Silica Nanoparticles and Polyethyleneimine (PEI)-Mediated Functionalization: A New Method of PEI Covalent Attachment for siRNA Delivery Applications. Bioconjugate Chem. 2013, 24, 2076–2087. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-nanocluster-mediated delivery of siRNA to intact plant cells for efficient gene knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- McGraw, E.; Roberts, J.D.; Kunte, N.; Westerfield, M.; Streety, X.; Held, D.; Avila, L.A. Insight into cellular uptake and transcytosis of peptide nanoparticles in Spodoptera frugiperda cells and isolated midgut. ACS Omega 2022, 7, 10933–10943. [Google Scholar] [CrossRef]
- Kolge, H.; Kadam, K.; Ghormade, V. Chitosan nanocarriers mediated dsRNA delivery in gene silencing for Helicoverpa armigera biocontrol. Pestic. Biochem. Physiol. 2023, 189, 105292. [Google Scholar] [CrossRef]
- Sandal, S.; Singh, S.; Bansal, G.; Kaur, R.; Mogilicherla, K.; Pandher, S.; Roy, A.; Kaur, G.; Rathore, P.; Kalia, A. Nanoparticle-shielded dsRNA delivery for enhancing RNAi efficiency in cotton spotted bollworm Earias vittella (Lepidoptera: Nolidae). Int. J. Mol. Sci. 2023, 24, 9161. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Lee, C.; Chon, J.; Jeong, D.; Yue, Y.; Sung, K.; Park, Y.; Jeong, K.C. Comprehensive in vitro and in vivo risk assessments of chitosan microparticles using human epithelial cells and Caenorhabditis elegans. J. Hazard. Mater. 2018, 341, 248–256. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan nanoparticle toxicity: A comprehensive literature review of in vivo and in vitro assessments for medical applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Hu, Q.; Li, J.; Chao, Z.; Cai, C.; Yin, M.; Du, X.; Shen, J. A Star Polycation Acts as a Drug Nanocarrier to Improve the Toxicity and Persistence of Botanical Pesticides. ACS Sustain. Chem. Eng. 2019, 7, 17406–17413. [Google Scholar] [CrossRef]
- Avila, L.A.; Chandrasekar, R.; Wilkinson, K.E.; Balthazor, J.; Heerman, M.; Bechard, J.; Brown, S.; Park, Y.; Dhar, S.; Reeck, G.R.; et al. Delivery of lethal dsRNAs in insect diets by branched amphiphilic peptide capsules. J. Control. Release 2018, 273, 139–146. [Google Scholar] [CrossRef]
- Santos, V.S.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Wessel, E.M.; Tomich, J.M.; Todd, R.B. Biodegradable Drug-Delivery Peptide Nanocapsules. ACS Omega 2019, 4, 20059–20063. [Google Scholar] [CrossRef]
- Dhandapani, R.K.; Gurusamy, D.; Palli, S.R. Development of catechin, poly-l-lysine, and double-stranded RNA nanoparticles. ACS Appl. Bio Mater. 2021, 4, 4310–4318. [Google Scholar] [CrossRef]
- Khan, F.; Kim, M.; Kim, Y. Greenhouse test of spraying dsRNA to control the western flower thrips, Frankliniella occidentalis, infesting hot peppers. BMC Biotechnol. 2023, 23, 10. [Google Scholar] [CrossRef]
- Carroll, E.; Kunte, N.; McGraw, E.; Gautam, S.; Range, R.; Noveron-Nunez, J.A.; Held, D.W.; Avila, L.A. Gene silencing in adult Popillia japonica through feeding of double-stranded RNA (dsRNA) complexed with branched amphiphilic peptide capsules (BAPCs). Front. Insect Sci. 2023, 3, 1151789. [Google Scholar] [CrossRef]
- Su, C.; Liu, S.; Sun, M.; Yu, Q.; Li, C.; Graham, R.I.; Wang, X.; Wang, X.; Xu, P.; Ren, G. Delivery of methoprene-tolerant dsRNA to improve RNAi efficiency by modified liposomes for pest control. ACS Appl. Mater. Interfaces 2023, 15, 13576–13588. [Google Scholar] [CrossRef]
- Evangelista, A.R.; Amoroso, C.G.; Nitride, C.; Andolfo, G. Seed storage allergens tackled via next-generation research assistant. Front. Food Sci. Technol. 2024, 4, 1372770. [Google Scholar] [CrossRef]
- Strachan, J.B.; Dyett, B.P.; Nasa, Z.; Valery, C.; Conn, C.E. Toxicity and cellular uptake of lipid nanoparticles of different structure and composition. J. Colloid Interface Sci. 2020, 576, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef]
- Jain, R.G.; Fletcher, S.J.; Manzie, N.; Robinson, K.E.; Li, P.; Lu, E.; Brosnan, C.A.; Xu, Z.P.; Mitter, N. Foliar application of clay-delivered RNA interference for whitefly control. Nat. Plants 2022, 8, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, X.; Yin, M.; Shen, J.; Yan, S. Recent advances in nanoparticle-mediated co-delivery system: A promising strategy in medical and agricultural field. Int. J. Mol. Sci. 2023, 24, 5121. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef]
- Lyu, Z.; Xiong, M.; Mao, J.; Li, W.; Jiang, G.; Zhang, W. A dsRNA delivery system based on the rosin-modified polyethylene glycol and chitosan induces gene silencing and mortality in Nilaparvata lugens. Pest Manag. Sci. 2023, 79, 1518–1527. [Google Scholar] [CrossRef]
- De Vega, D.; Holden, N.; Hedley, P.E.; Morris, J.; Luna, E.; Newton, A. Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ. 2021, 44, 290–303. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Wang, J.; Ijaz, M.; Shahid, M.; Islam, M.S.; Azizullah; Manzoor, I.; Li, D.; Song, F. Nano-enabled crop resilience against pathogens: Potential, mechanisms and strategies. Crop Health 2023, 1, 15. [Google Scholar] [CrossRef]
- Wang, M.; Dean, R.A. Host induced gene silencing of Magnaporthe oryzae by targeting pathogenicity and development genes to control rice blast disease. Front. Plant Sci. 2022, 13, 959641. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, T.; Poupin, M.J.; Vega, A.; Urrutia, C.; Ruz, G.A.; González, B. Gene networks underlying the early regulation of Paraburkholderia phytofirmans PsJN induced systemic resistance in Arabidopsis. PLoS ONE 2019, 14, e0221358. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, J.; Wang, Q.; O’Hare, D.; Wan, Y. Layered Double Hydroxide Nanotransporter for Molecule Delivery to Intact Plant Cells. Sci. Rep. 2016, 6, 26738. [Google Scholar] [CrossRef]
- Petrônio, M.S.; Barros-Alexandrino, T.T.; Lima, A.M.F.; Assis, O.B.G.; Inoue-Nagata, A.K.; Nakasu, E.Y.T.; Tiera, M.J.; Pilon, L. Physicochemical and toxicity investigation of chitosan-based dsRNA nanocarrier formation. Biointerface Res. Appl. Chem. 2022, 12, 5266–5279. [Google Scholar]
- Lv, H.; Li, X.; Li, J.; Yu, C.; Zeng, Q.; Ning, G.; Wan, H.; Li, J.; Ma, K.; He, S. Overcoming resistance in insect pest with a nanoparticle-mediated dsRNA and insecticide co-delivery system. Chem. Eng. J. 2023, 475, 146239. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; Cheng, X.; Zhang, H.; Zheng, W. Nanomaterial-encapsulated dsRNA of ecdysone-induced early gene E75, a potential RNAi-based SIT strategy for pest control against Bactrocera dorsalis. Int. J. Biol. Macromol. 2024, 263, 130607. [Google Scholar] [CrossRef]
- Ivashuta, S.; Zhang, Y.; Wiggins, B.E.; Ramaseshadri, P.; Segers, G.C.; Johnson, S.; Meyer, S.E.; Kerstetter, R.A.; McNulty, B.C.; Bolognesi, R.; et al. Environmental RNAi in herbivorous insects. RNA 2015, 21, 840–850. [Google Scholar] [CrossRef]
- Okuda, T.; Niidome, T.; Aoyagi, H. Cytosolic soluble proteins induce DNA release from DNA—Gene carrier complexes. J. Control. Release 2004, 98, 325–332. [Google Scholar] [CrossRef]
- Bertschinger, M.; Backliwal, G.; Schertenleib, A.; Jordan, M.; Hacker, D.L.; Wurm, F.M. Disassembly of polyethylenimine-DNA particles in vitro: Implications for polyethylenimine-mediated DNA delivery. J. Control. Release 2006, 116, 96–104. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Bakhshian Nik, A.; Zare, H.; Razavi, S.; Mohammadi, H.; Torab Ahmadi, P.; Yazdani, N.; Bayandori, M.; Rabiee, N.; Izadi Mobarakeh, J. Smart drug delivery: Capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020, 299, 110115. [Google Scholar] [CrossRef]
- Chu, D.; Xu, S.; Li, X.; Li, H.; Miao, X.; An, S.; Guan, R. Combination of diflubenzuron and RNAi technology to improve the control effect of Helicoverpa armigera. Entomol. Gen. 2024, 44, 223–232. [Google Scholar] [CrossRef]
- Hou, R.; Li, C.; Tan, Y.; Wang, Y.; Huang, S.; Zhao, C.; Zhang, Z. Eco-friendly O-carboxymethyl chitosan base chlorfenapyr nanopesticide for effective pest control and reduced toxicity to honey bees. Int. J. Biol. Macromol. 2023, 224, 972–983. [Google Scholar] [CrossRef]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2020, 2, 12. [Google Scholar] [CrossRef]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest suppression. Southwest. Entomol. 2012, 37, 85–87. [Google Scholar]
- Baum, J.A.; Roberts, J.K. Chapter Five—Progress Towards RNAi-Mediated Insect Pest Management. In Advances in Insect Physiology; Dhadialla, T.S., Gill, S.S., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 47, pp. 249–295. [Google Scholar]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double strand RNA delivery system for plant-sap-feeding insects. PLoS ONE 2017, 12, e0171861. [Google Scholar] [CrossRef]
- Andrade, E.; Hunter, W. RNA interference—Natural gene-based technology for highly specific pest control (HiSPeC). In RNA Interference; Ibrokhim, Y.A., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 19. [Google Scholar]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.-W.; et al. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in colorado potato beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef]
- Christiaens, O.; Tardajos, M.G.; Martinez Reyna, Z.L.; Dash, M.; Dubruel, P.; Smagghe, G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Gurusamy, D.; Mogilicherla, K.; Palli, S.R. Chitosan nanoparticles help double-stranded RNA escape from endosomes and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21677. [Google Scholar] [CrossRef]
- Pei, Y.; Hao, H.; Zuo, Y.; Xue, Y.; Aioub, A.A.A.; Hu, Z. Functional validation of CYP304A1 associated with haedoxan A detoxification in Aedes albopictus by RNAi and transgenic drosophila. Pest Manag. Sci. 2023, 79, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shen, J. Application of nanoparticle-mediated RNAi for efficient gene silencing and pest control on soybean aphids. Methods Mol. Biol. 2022, 2360, 307–315. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhong, X.; Tian, J.; Segers, A.; Xia, L.; Francis, F. RNA-interference-mediated aphid control in crop plants: A review. Agriculture 2022, 12, 2108. [Google Scholar] [CrossRef]
- Dhandapani, R.K.; Gurusamy, D.; Palli, S.R. Protamine-lipid-dsRNA nanoparticles improve RNAi efficiency in the Fall Armyworm, Spodoptera frugiperda. J. Agric. Food Chem. 2022, 70, 6634–6643. [Google Scholar] [CrossRef]
- Niu, L.; Yan, H.; Sun, Y.; Zhang, D.; Ma, W.; Lin, Y. Nanoparticle facilitated stacked-dsRNA improves suppression of the Lepidoperan pest Chilo suppresallis. Pesticide Biochem. Physiol. 2022, 187, 105183. [Google Scholar] [CrossRef]
- Sharma, Y.; Padha, S.; Dhar, A.; Baweja, V.; Singh, I.K. RNAi-Based Biopesticides: A Review of Recent Studies in Lepidopteran Insects. Proc. Zool. Soc. 2023, 76, 373–381. [Google Scholar] [CrossRef]
- Geng, K.; Zhang, Y.; Zhao, X.; Zhang, W.; Guo, X.; He, L.; Liu, K.; Yang, H.; Hong, H.; Peng, J.; et al. Fluorescent nanoparticle-RNAi-mediated silencing of sterol carrier protein-2 gene expression suppresses the growth, development, and reproduction of Helicoverpa armigera. Nanomaterials 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Long, G.-J.; Liu, X.-Z.; Guo, H.; Zhang, M.-Q.; Gong, L.-L.; Ma, Y.-F.; Dewer, Y.; Mo, W.-J.; Ding, L.-W.; Wang, Q.; et al. Oral-based nanoparticle-wrapped dsRNA delivery system: A promising approach for controlling an urban pest, Blattella germanica. J. Pest Sci. 2023, 97, 739–775. [Google Scholar] [CrossRef]
- Jain, M.; Bansal, J.; Rajkumar, M.S.; Garg, R. An integrated transcriptome mapping the regulatory network of coding and long non-coding RNAs provides a genomics resource in chickpea. Commun. Biol. 2022, 5, 1106. [Google Scholar] [CrossRef]
- Pallis, S.; Alyokhin, A.; Manley, B.; Rodrigues, T.; Barnes, E.; Narva, K. Effects of low doses of a novel dsRNA-based biopesticide (Calantha) on the Colorado potato beetle. J. Econ. Entomol. 2023, 116, 456–461. [Google Scholar] [CrossRef]
- Christiaens, O.; Smagghe, G. The challenge of RNAi-mediated control of hemipterans. Curr. Opin. Insect Sci. 2014, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Ottati, S.; Bucci, L.; Fusco, A.; Abbà, S.; Bosco, D.; Marzachì, C.; Galetto, L. Lab-scale method for plant-mediated delivery of dsRNAs to phloem-feeding leafhoppers. J. Pest Sci. 2024, 97, 455–467. [Google Scholar] [CrossRef]
- Li, H.; Guan, R.; Guo, H.; Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 2015, 38, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.C.; Wise, A.G.; Rakotondravelo, M.; Vandervoort, C.; Seeve, C.; Fabbri, B. Trunk injection delivery of dsRNA for RNAi-based pest control in apple trees. Pest Manag. Sci. 2022, 78, 3528–3533. [Google Scholar] [CrossRef]
- Harrison, J.F. Insect acid-base physiology. Annu. Rev. Entomol. 2001, 46, 221–250. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive nanoparticles for drug delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, A.; Gill, S.S.; Gupta, O.P.; Dahuja, A.; Jain, P.K.; Sirohi, A. RNA interference: A novel source of resistance to combat plant parasitic nematodes. Front. Plant Sci. 2017, 8, 834. [Google Scholar] [CrossRef]
- Iqbal, S.; Fosu-Nyarko, J.; Jones, M.G.K. Attempt to silence genes of the RNAi pathways of the root-knot nematode, Meloidogyne incognita results in diverse responses including increase and no change in expression of some genes. Front. Plant Sci. 2020, 11, 328. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Delgado-Martín, J.; Ruiz, L.; Janssen, D.; Velasco, L. Exogenous application of dsRNA for the control of viruses in cucurbits. Front. Plant Sci. 2022, 13, 895953. [Google Scholar] [CrossRef] [PubMed]
- Konakalla, N.C.; Kaldis, A.; Berbati, M.; Masarapu, H.; Voloudakis, A.E. Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 2016, 244, 961–969. [Google Scholar] [CrossRef]
- Rego-Machado, C.M.; Nakasu, E.Y.T.; Silva, J.M.F.; Lucinda, N.; Nagata, T.; Inoue-Nagata, A.K. siRNA biogenesis and advances in topically applied dsRNA for controlling virus infections in tomato plants. Sci. Rep. 2020, 10, 22277. [Google Scholar] [CrossRef]
- Kalyandurg, P.B.; Sundararajan, P.; Dubey, M.; Ghadamgahi, F.; Zahid, M.A.; Whisson, S.C.; Vetukuri, R.R. Spray-induced gene silencing as a potential tool to control potato late blight disease. Phytopathol. Res. 2021, 111, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Degnan, R.M.; McTaggart, A.R.; Shuey, L.S.; Pame, L.J.S.; Smith, G.R.; Gardiner, D.M.; Nock, V.; Soffe, R.; Sale, S.; Garrill, A.; et al. Exogenous double-stranded RNA inhibits the infection physiology of rust fungi to reduce symptoms in planta. Mol. Plant Pathol. 2023, 24, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Song, X.S.; Gu, K.X.; Duan, X.X.; Xiao, X.M.; Hou, Y.P.; Duan, Y.B.; Wang, J.X.; Yu, N.; Zhou, M.G. Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mol. Plant Pathol. 2018, 19, 2543–2560. [Google Scholar] [CrossRef]
- Ackah, M.; Jin, X.; Zhang, Q.; Amoako, F.K.; Wang, L.; Attaribo, T.; Zhao, M.; Yuan, F.; Herman, R.A.; Qiu, C.; et al. Long noncoding RNA transcriptome analysis reveals novel lncRNAs in Morus alba ‘Yu-711’ response to drought stress. Plant Genome 2024, 17, e20273. [Google Scholar] [CrossRef]
- Mosa, M.A.; Youssef, K. Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClay™ prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef]
- Trippe, A.J. Guidelines for Preparing Patent Landscape Reports; WIPO: Geneva, Switzerland, 2015. [Google Scholar]
- Banerjee, G. Assessing visibility of research organization: A fuzzy analytic network process approach. J. Sci. Ind. Res. 2014, 73, 283–289. [Google Scholar]
- Park, S.; Lee, S.-J.; Jun, S. A network analysis model for selecting sustainable technology. Sustainability 2015, 7, 13126–13141. [Google Scholar] [CrossRef]
- Kay, L.; Youtie, J.; Shapira, P. Signs of things to come? What patent submissions by small and medium-sized enterprises say about corporate strategies in emerging technologies. Technol. Forecast. Soc. Change 2014, 85, 17–25. [Google Scholar] [CrossRef]
- Das, P.R.; Sherif, S.M. Application of exogenous dsRNAs-induced RNAi in agriculture: Challenges and triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Soto, A.; Chacón-Cerdas, R. RNAi crop protection advances. Int. J. Mol. Sci. 2021, 22, 12148. [Google Scholar] [CrossRef]
- Government of Brazil. Lei nº 9.279, de 14 de maio de 1996. Regula Direitos e Obrigações Relativos à Propriedade Industrial; Diário Oficial da União: Brasília, DF, Brazil, 1996; Volume 134, p. 8353.
- US Patent Law. 35 USC Sec. 103. United States Code, Supplement 5, Title 35—Conditions for Patentability; Non-Obvious Subject Matter. 2001.
- US Patent Law. 35 USC Sec. 102. United States Code, Supplement 5, Title 35—Patents, Part II—Patentability of Inventions and Grant of Patents, Chapter 10—Patentability of Inventions. 2001.
- Federal Register, Environmental Protection Agency (EPA). 2001. Available online: https://www.govinfo.gov/content/pkg/FR-2001-01-05/pdf/01-84.pdf (accessed on 15 January 2025).
- Justia US Supreme Court. Certiorari to the United States Court of Appeals for the Federal Circuit. Assoc. for Molecular Pathology v. Myriad Genetics, Inc., 569 U.S. 576. Available online: https://supreme.justia.com/cases/federal/us/569/576/ (accessed on 15 January 2025).
- Bramlett, M.; Plaetinck, G.; Maienfisch, P. RNA-based biocontrols-A new paradigm in crop protection. Engineering 2020, 6, 522–527. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Petrick, J.S. Safety considerations for humans and other vertebrates regarding agricultural uses of externally applied RNA molecules. Front. Plant Sci. 2020, 11, 407. [Google Scholar] [CrossRef]
- Liu, S.; Jaouannet, M.; Dempsey, D.A.; Imani, J.; Coustau, C.; Kogel, K.H. RNA-based technologies for insect control in plant production. Biotechnol. Adv. 2020, 39, 107463. [Google Scholar] [CrossRef]
- Albright, V.C., 3rd; Wong, C.R.; Hellmich, R.L.; Coats, J.R. Dissipation of double-stranded RNA in aquatic microcosms. Environ. Toxicol. Chem. 2017, 36, 1249–1253. [Google Scholar] [CrossRef]
- Taning, C.N.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.G.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef]
- Christiaens, O.; Sweet, J.; Dzhambazova, T.; Urru, I.; Smagghe, G.; Kostov, K.; Arpaia, S. Implementation of RNAi-based arthropod pest control: Environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 2022, 95, 1–15. [Google Scholar] [CrossRef]
- Mendelsohn, M.L.; Gathmann, A.; Kardassi, D.; Sachana, M.; Hopwood, E.M.; Dietz-Pfeilstetter, A.; Michelsen-Correa, S.; Fletcher, S.J.; Székács, A. Summary of discussions from the 2019 OECD conference on RNAi based pesticides. Front. Plant Sci. 2020, 11, 740. [Google Scholar] [CrossRef]
- OECD. Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied dsRNA-based Pesticides. ENV/JM/MONO 2020 Paris 2020, 26. Available online: https://www.oecd.org/en/publications/considerations-for-the-environmental-risk-assessment-of-the-application-of-sprayed-or-externally-applied-ds-rna-based-pesticides_576d9ebb-en.html (accessed on 15 January 2025).
- Sherman, J.H.; Munyikwa, T.; Chan, S.Y.; Petrick, J.S.; Witwer, K.W.; Choudhuri, S. RNAi technologies in agricultural biotechnology: The Toxicology Forum 40th Annual Summer Meeting. Regul. Toxicol. Pharmacol. 2015, 73, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Dzhambazova, T.; Kostov, K.; Arpaia, S.; Joga, M.R.; Urru, I.; Sweet, J.; Smagghe, G. Literature review of baseline information on RNAi to support the environmental risk assessment of RNAi-based GM plants. EFSA Support. Publ. 2018, 15, 1424E. [Google Scholar] [CrossRef]
- Petrick, J.S.; Brower-Toland, B.; Jackson, A.L.; Kier, L.D. Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regul. Toxicol. Pharmacol. 2013, 66, 167–176. [Google Scholar] [CrossRef]
- Raybould, A.; Burns, A. Problem formulation for off-target effects of externally applied double-stranded RNA-based products for pest control. Front. Plant Sci. 2020, 11, 424. [Google Scholar] [CrossRef]
- Mamta, B.; Rajam, M.V. RNAi technology: A new platform for crop pest control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, N.L.; Smagghe, G.; Taning, C.N.T.; Oliveira, E.E.; Christiaens, O. Risk assessment of RNAi-based pesticides to non-target organisms: Evaluating the effects of sequence similarity in the parasitoid wasp Telenomus podisi. Sci. Total Environ. 2022, 832, 154746. [Google Scholar] [CrossRef]
- Taning, C.N.T.; Gui, S.; De Schutter, K.; Jahani, M.; Castellanos, N.L.; Christiaens, O.; Smagghe, G. A sequence complementarity-based approach for evaluating off-target transcript knockdown in Bombus terrestris, following ingestion of pest-specific dsRNA. J. Pest Sci. 2021, 94, 487–503. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Chen, X.; Koo, J.; Gurusamy, D.; Mogilicherla, K.; Reddy Palli, S. Caenorhabditis elegans systemic RNA interference defective protein 1 enhances RNAi efficiency in a lepidopteran insect, the fall armyworm, in a tissue-specific manner. RNA Biol. 2021, 18, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Mingels, L.; Vogel, E.; Wang, L.; Christiaens, O.; Cappelle, K.; Wynant, N.; Gansemans, Y.; Van Nieuwerburgh, F.; Smagghe, G.; et al. Generation of virus- and dsRNA-derived siRNAs with species-dependent length in insects. Viruses 2019, 11, 738. [Google Scholar] [CrossRef]
- Haller, S.; Widmer, F.; Siegfried, B.D.; Zhuo, X.; Romeis, J. Responses of two ladybird beetle species (Coleoptera: Coccinellidae) to dietary RNAi. Pest Manag. Sci. 2019, 75, 2652–2662. [Google Scholar] [CrossRef]
- Niu, J.; Smagghe, G.; De Coninck, D.I.; Van Nieuwerburgh, F.; Deforce, D.; Meeus, I. In vivo study of Dicer-2-mediated immune response of the small interfering RNA pathway upon systemic infections of virulent and avirulent viruses in Bombus terrestris. Insect Biochem. Mol. Biol. 2016, 70, 127–137. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi Efficiency, Systemic Properties, and Novel Delivery Methods for Pest Insect Control: What We Know So Far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Romeis, J.; Widmer, F. Assessing the risks of topically applied dsRNA-based products to non-target arthropods. Front. Plant Sci. 2020, 11, 679. [Google Scholar] [CrossRef]
- Vesprini, F.; Maggi, A.I.; López Olaciregui, M.; Módena, N.A. Transportability of conclusions from confined field trials: A case study using the virus resistant transgenic bean developed in Brazil. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Bachman, P.M.; Anderson, J.A.; Burns, A.; Chakravarthy, S.; Goodwin, L.; Privalle, L.; Song, S.; Storer, N. Data transportability for studies performed to support an environmental risk assessment for genetically modified (GM) crops. J. Regul. Sci. 2021, 9, 38–44. [Google Scholar]
- Tachikawa, M.; Matsuo, M. Divergence and convergence in international regulatory policies regarding genome-edited food: How to find a middle ground. Front. Plant Sci. 2023, 14, 1105426. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Tang, S.; Tao, M. Development of resistance to RNAi in mammalian cells. Ann. N. Y. Acad. Sci. 2005, 1058, 105–118. [Google Scholar] [CrossRef]
- Dadami, E.; Dalakouras, A.; Zwiebel, M.; Krczal, G.; Wassenegger, M. An endogene-resembling transgene is resistant to DNA methylation and systemic silencing. RNA Biol. 2014, 11, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Laurie, J.D.; Ali, S.; Linning, R.; Mannhaupt, G.; Wong, P.; Güldener, U.; Münsterkötter, M.; Moore, R.; Kahmann, R.; Bakkeren, G.; et al. Genome comparison of barley and maize smut fungi reveals targeted loss of RNA silencing components and species-specific presence of transposable elements. Plant Cell 2012, 24, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Pollari, M.; De, S.; Wang, A.; Mäkinen, K. The potyviral silencing suppressor HCPro recruits and employs host ARGONAUTE1 in pro-viral functions. PLoS Pathog. 2020, 16, e1008965. [Google Scholar] [CrossRef]
- Mishra, G.P.; Aski, M.S.; Bosamia, T.; Chaurasia, S.; Mishra, D.C.; Bhati, J.; Kumar, A.; Javeria, S.; Tripathi, K.; Kohli, M.; et al. Insights into the host-pathogen interaction pathways through RNA-seq analysis of Lens culinaris Medik. in response to Rhizoctonia bataticola infection. Genes 2021, 13, 90. [Google Scholar] [CrossRef]
- Mishra, S.; Dee, J.; Moar, W.; Dufner-Beattie, J.; Baum, J.; Dias, N.P.; Alyokhin, A.; Buzza, A.; Rondon, S.I.; Clough, M.; et al. Selection for high levels of resistance to double-stranded RNA (dsRNA) in Colorado potato beetle (Leptinotarsa decemlineata Say) using non-transgenic foliar delivery. Sci. Rep. 2021, 11, 6523. [Google Scholar] [CrossRef] [PubMed]
- Kyre, B.R.; Dupuis, J.R.; Zúñiga, G.; Rieske, L.K. Variation in RNA interference sensitivity in the southern pine beetle, Dendroctonus frontalis (Coleoptera: Curculionidae). Biol. J. Linn. Soc. 2024, 142, 147–154. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Sahoo, D.K.; Maiti, I.B.; Palli, S.R. Identification of target genes for RNAi-mediated control of the Twospotted Spider Mite. Sci. Rep. 2018, 8, 14687. [Google Scholar] [CrossRef]
- Mehlhorn, S.G.; Geibel, S.; Bucher, G.; Nauen, R. Profiling of RNAi sensitivity after foliar dsRNA exposure in different European populations of Colorado potato beetle reveals a robust response with minor variability. Pestic Biochem. Physiol. 2020, 166, 104569. [Google Scholar] [CrossRef]
- Neves, M.F.; Casagrande, B.P.; Cambaúva, V.; Teixeira, G.d.O.; Toledo, P.J.F. Agriculture 6.0: A New Proposal for the Future of Agribusiness. Revista de Gestão Social e Ambiental 2023, 17, e04004. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Asseng, S.; Boote, K.; Durand, J.L.; Ewert, F.; Martre, P.; MacCarthy, D.S. Climate change impacts on crop yields. Nat. Rev. Earth Environ. 2023, 4, 831–846. [Google Scholar] [CrossRef]
- Martha Júnior, G.B.; Lopes, M.A. Charting new sustainable agricultural innovation pathways in Brazil. Sci. Agric. 2023, 80. [Google Scholar] [CrossRef]
- Silva, F.T.d.; Baierle, I.C.; Correa, R.G.d.F.; Sellitto, M.A.; Peres, F.A.P.; Kipper, L.M. Open innovation in agribusiness: Barriers and challenges in the transition to Agriculture 4.0. Sustainability 2023, 15, 8562. [Google Scholar] [CrossRef]
- Munaweera, T.I.K.; Jayawardana, N.U.; Rajaratnam, R.; Dissanayake, N. Modern plant biotechnology as a strategy in addressing climate change and attaining food security. Agric. Food Secur. 2022, 11, 26. [Google Scholar] [CrossRef]
- Dalakouras, A.; Papadopoulou, K.K. Epigenetic modifications: An unexplored facet of exogenous RNA application in plants. Plants 2020, 9, 673. [Google Scholar] [CrossRef]



| Crop | Trade Name | Event Code (Name) | Developer | Target Gene | Modified Plant Trait | Regulatory Approvals | First Approval |
|---|---|---|---|---|---|---|---|
| Carica papaya | Rainbow, SunUp | CUH-CP551-8 (55-1) | Cornell University and the University of Hawaii | CP | Improved plant resistance to Papaya ringspot virus | Canada, Japan, and USA | 1996 |
| not available | CUH-CP631-7 (63-1) | Cornell University and the University of Hawaii | CP | USA | 1996 | ||
| Huanong No. 1 | Huanong No. 1 | South China Agricultural University | Rep | China | 2006 | ||
| not available | UFL-X17CP-6 (X17-2) | University of Florida | CP | USA | 2008 | ||
| Solanum lycopersicum | FLAVR SAVR™ | CGN-89564-2 (FLAVR SAVR™) | Monsanto | PGs | Delayed fruit softening | Mexico, Canada, and USA | 1992 |
| not available | SYN-ØØØØB-6 (B) | Zeneca Plant Science and Petoseed | PGs | Delayed fruit softening | Mexico and USA | 1994 | |
| not available | SYN-ØØØDA-9 (Da) | Zeneca Plant Science and Petoseed | PGs | Delayed fruit softening | Mexico and USA | 1995 | |
| not available | SYN-ØØØØF-1 (F) | Zeneca Plant Science and Petoseed | PGs | Delayed fruit softening | Mexico, Canada, and USA | 1995 | |
| not available | 1345-4 | DNA Plant Technology Corporation | ACC | Reduced synthesis of endogenous ethylene | Mexico, Canada, and USA | 1995 | |
| not available | Huafan No. 1 | Huazhong Agricultural University | ACO | Delayed ripening by suppressing the production of ethylene by silencing the ACO gene | China | 1997 | |
| not available | PK-TM8805R (8805R) | Beijing University | CP | Improved plant resistance to Cucumber mosaic virus | China | 1999 | |
| Cucurbita pepo | not available | SEM-ØZW2Ø-7 (ZW20) | Seminis Vegetable Seeds (Canada) and Monsanto (Asgrow) | CP | Improved plant resistance to Zucchini yellow mosaic virus, and Watermelon mosaic virus 2 | USA | 1994 |
| not available | SEM-ØCZW3-2 (CZW3) | Seminis Vegetable Seeds (Canada) and Monsanto (Asgrow) | CP | Improved plant resistance to Cucumber mosaic virus, Zucchini yellow mosaic virus, and Watermelon mosaic virus 2 | Canada and USA | 1996 | |
| Dianthus caryophyllus | not available | FLO-ØØØ66-8 (66) | Florigene Pty Ltd. | ACC | Reduced synthesis of ethylene | Australia and Norway | 1995 |
| Capsicum annuum | not available | PK-SP01 | Beijing University | CP | Improved plant resistance to Cucumber mosaic virus | China | 1998 |
| Solanum tuberosum | Hi-Lite NewLeaf™ Y potato | HLMT15-15 HLMT15-3 HLMT15-46 | Monsanto | CP | Improved plant resistance to Potato virus Y | USA | 1998 |
| Shepody NewLeaf™ Y potato | NMK-89935-9 (SEMT15-02) SEMT15-07 NMK-8993Ø-4 (SEMT15-15) | Monsanto | CP | Improved plant resistance to Potato virus Y | Australia, Canada, Japan, Mexico, New Zealand, Philippines, South Korea, and USA | 1998 | |
| New Leaf™ Y Russet Burbank potato | NMK-89653-6 (RBMT15-101) NMK-89684-1 (RBMT21-129) RBMT21-152 NMK-89896-6 (RBMT22-082) RBMT22-186 RBMT22-238 RBMT22-262 | Monsanto | ORF1 and ORF2 | Improved plant resistance to Potato leaf roll virus | Australia, Canada, Japan, Mexico, New Zealand, Philippines, South Korea, and USA | 1998 | |
| Amflora™ | BPS-25271-9 (EH92-527-1) | BASF | gbss | Reduced levels of amylose and increased levels of amylopectin in starch granules | European Union | 2010 | |
| Starch Potato | BPS-A1Ø2Ø-5 (AM04-1020) | BASF | gbss | USA | 2014 | ||
| Simplot Innate | SPS-ØØØZ6-5 (Gen2-Z6) | J.R. Simplot Co. | asn1, ppo5, PhL, and Vlnv | Improved black spot bruise tolerance, reduced levels of cold-induced sweetening, and reduced levels of acrylamide | Canada and USA | 2020 | |
| not available | SPS-ØØØW8-4 (W8) | J.R. Simplot Co. | asn1, ppo5, R1, PhL, and Vlnv | Australia, Canada, New Zealand, and USA | 2015 | ||
| Innate® Acclimate | SPS-ØØX17-5 (X17) | J.R. Simplot Co. | asn1, ppo5, R1, PhL, and Vlnv | Australia, Canada, New Zealand, Philippines, and USA | 2016 | ||
| Innate® Hibernate | SPS-ØØØY9-7 (Y9) | J.R. Simplot Co. | asn1, ppo5, R1, PhL, and Vlnv | Australia, Canada, New Zealand, Philippines, and USA | 2016 | ||
| Innate® Cultivate | SPS-ØØE12-8 (E12) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | Australia, Canada, Japan, Malaysia, Mexico, New Zealand, Philippines, Singapore, and USA | 2014 | ||
| not available | SPS-ØØE24-2 (E24) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | USA | 2014 | ||
| not available | SPS-ØØE56-7 (E56) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | Australia and New Zealand | 2017 | ||
| Innate® Generate Innate® Accelerate | SPS-ØØF10-7 (F10) SPS-ØØØJ3-4 (J3) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | Australia, Canada, Mexico, New Zealand, and USA | 2014 | ||
| not available | SPS-ØØF37-7 (F37) SPS-ØØG11-9 (G11) SPS-ØØH37-9 (H37) SPS-ØØH50-4 (H50) SPS-ØØJ78-7 (J78) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | USA | 2014 | ||
| not available | SPS-ØØJ55-2 (J55) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | Canada and USA | 2014 | ||
| Innate® Invigorate | SPS–ØØV11–6 (V11) | J.R. Simplot Co. | asn1, ppo5, PhL, and R1 | Australia, New Zealand, and USA | 2016 | ||
| not available | TIC-AR233-5 | Technoplant Argentina | CP | Improved plant resistance to Potato virus Y | Argentina | 2018 | |
| Nicotiana tabacum | not available | Vector 21-41 | Vector Tobacco Inc. | NtQPT1 | Reduced production of nicotinic acid | USA | 2002 |
| Medicago sativa | HarvXtra™ | MON-ØØ179-5 (KK179) | Monsanto and Forage Genetics International | CCOMT | Reduces content of guaiacyl (G) lignin | Australia, Canada, Japan, Mexico, New Zealand, Philippines, Singapore, South Korea, and USA | 2013 |
| Zea mays | not available | MON-87411-9 (MON87411) | Monsanto | DvSnf7 | Improved plant resistance against Diabrotica virgifera virgifera | Argentina, Australia, Brazil, Canada, Colombia, Japan, Mexico, New Zealand, Philippines, South Korea, Taiwan, and USA | 2014 |
| Malus domestica | Arctic™ “Golden Delicious” Apple | OKA-NBØØ1-8 (GD743) | Okanagan Specialty Fruits Incorporated | PPOs | Apples with a non-browning phenotype | Canada and USA | 2015 |
| Arctic™ | OKA-NBØØ2-9 (GS784) | Okanagan Specialty Fruits Incorporated | PPOs | Apples with a non-browning phenotype | Canada and USA | 2015 | |
| Arctic™ Fuji Apple | OKA-NBØØ3-1 (NF872) | Okanagan Specialty Fruits Incorporated | PPOs | Apples with a non-browning phenotype | Canada and USA | 2018 | |
| Ananas comosus | Rosé | FDP-ØØ114-5 (EF2-114) | Del Monte Fresh Produce | b-Lyc and e-Lyc | Increased lycopene accumulation | Canada and USA | 2016 |
| Gossypium hirsutum | not available | TAM-66274-5 (TAM66274) | Texas A&M AgriLife Research University | dCS | Reduced gossypol biosynthesis | USA | 2018 |
| Carthamus tinctorius | not available | GOR-73226-6 (Event 26) GOR-7324Ø-2 (Event 40) | Go Resources Pty Ltd. | fatB and fad2.2 | Modified oil/fatty acid | Australia | 2018 |
| Glycine max | not available | DD-Ø26ØØ5-3 (260-05) | DuPont (Pioneer Hi-Bred International Inc.) | gm-fad2-1 | Blocked conversion of oleic acid to linoleic acid | Australia, Canada, Japan, New Zealand, and USA | 1997 |
| Treus™, Plenish™ | DP-3Ø5423-1 (DP305423) | DuPont (Pioneer Hi-Bred International Inc.) | gm-fad2-1 | Blocked conversion of oleic acid to linoleic acid | Australia, Brazil, Canada, China, Colombia, European Union, Indonesia, Iran, Japan, Malaysia, Mexico, New Zealand, Philippines, Singapore, South Africa, South Korea, Taiwan, Turkey, and USA | 2008 | |
| Vistive Gold™ | MON-877Ø5-6 (MON87705) | Monsanto | fad2.1A and fatb1-A | Blocked conversion of oleic acid to linoleic acid and decreased transport of saturated fatty acids out of the plastid | Australia, Canada, China, Colombia, European Union, Indonesia, Japan, Mexico, New Zealand, Nigeria, Philippines, Singapore, South Korea, Taiwan, Turkey, Vietnam, and USA | 2011 | |
| Phaseolus vulgaris | BRS FC401 RMD | EMB-PVØ51-1 (EMBRAPA 5.1) | Embrapa | AC1 | Improved plant resistance to Bean golden mosaic virus | Brazil | 2011 |
| Prunus domestica | not available | ARS-PLMC5-6 (C-5) | USDA—Agricultural Research Service | CP | Improved plant resistance to Plum pox virus | USA | 2007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, M.F.; Vásquez, D.D.N.; Campos-Pinto, E.R.; Pinheiro, D.H.; Cruz, B.; Maktura, G.C.; Guidelli, G.V.; Marques-Souza, H.; Grossi-de-Sa, M.F. Progress and Opportunities of In Planta and Topical RNAi for the Biotechnological Control of Agricultural Pests. Agronomy 2025, 15, 859. https://doi.org/10.3390/agronomy15040859
Basso MF, Vásquez DDN, Campos-Pinto ER, Pinheiro DH, Cruz B, Maktura GC, Guidelli GV, Marques-Souza H, Grossi-de-Sa MF. Progress and Opportunities of In Planta and Topical RNAi for the Biotechnological Control of Agricultural Pests. Agronomy. 2025; 15(4):859. https://doi.org/10.3390/agronomy15040859
Chicago/Turabian StyleBasso, Marcos Fernando, Daniel David Noriega Vásquez, Eduardo Romano Campos-Pinto, Daniele Heloísa Pinheiro, Bread Cruz, Grazielle Celeste Maktura, Giovanna Vieira Guidelli, Henrique Marques-Souza, and Maria Fatima Grossi-de-Sa. 2025. "Progress and Opportunities of In Planta and Topical RNAi for the Biotechnological Control of Agricultural Pests" Agronomy 15, no. 4: 859. https://doi.org/10.3390/agronomy15040859
APA StyleBasso, M. F., Vásquez, D. D. N., Campos-Pinto, E. R., Pinheiro, D. H., Cruz, B., Maktura, G. C., Guidelli, G. V., Marques-Souza, H., & Grossi-de-Sa, M. F. (2025). Progress and Opportunities of In Planta and Topical RNAi for the Biotechnological Control of Agricultural Pests. Agronomy, 15(4), 859. https://doi.org/10.3390/agronomy15040859








