Abstract
While forage grasses in southern China exhibit yield and nutritional advantages, the impact of nutrient solutions on alfalfa (Medicago sativa BC4) growth and elemental accumulation remains understudied. We conducted a pot-based controlled substrate cultivation trial using a nitrogen-poor substrate to compare four treatments: MS, Hoagland, B5 nutrient solutions, and RO water (control). From the V1 to R1 stages, the plant height was monitored continuously, with leaf dimensions and soluble proteins (Bradford method) measured at R1. ICP-MS quantified macro elements (Na+, K+, and Mg2+) and microelements (Cu2+, Fe2+, Mn2+, and Zn2+). The growth rates followed the order: MS > Hoagland > RO water > B5. Both the MS and Hoagland solutions significantly increased the leaf length at the R1 stage (p < 0.001 vs control), with Hoagland showing the greatest leaf expansion. The soluble protein content decreased significantly in all groups (p < 0.05) except MS-treated plants. An elemental analysis revealed treatment-specific accumulation patterns, most notably 1.17-fold higher Fe and 1.48-fold higher Mn in the MS group versus control (p < 0.001). Magnesium levels showed no significant differences among treatments. These results demonstrate the MS nutrient solution’s superior efficacy in enhancing the alfalfa growth parameters (height and leaf size) while maintaining the soluble protein content and promoting Fe/Mn accumulation. The findings provide empirical evidence for optimizing alfalfa cultivation in a nitrogen-deficient soil-based mix substrate through nutrient solution selection.
1. Introduction
Alfalfa (Medicago sativa L.), a perennial herbaceous plant of the Leguminosae (or Fabaceae) family, is a widely cultivated forage in Eurasia and around the world, and is regarded as one of the most important pastures for supporting livestock production [1]. Moreover, alfalfa grown in the southern region of China (the south of the Qinling–Huaihe Line) has the advantage of a higher yield and nutrients compared to other regions of China. However, after converting natural grassy hills and slopes to artificial grassland and farms in southern China, the soils contained significantly low amounts of total carbon, organic carbon, and total nitrogen [2]. Correspondingly, soil fertility and management practices should be considered in the production of alfalfa in southern China [3]. It is commonly believed that the nutrient solution affects a variety of components related to alfalfa quality, such as the mineral, nitrate, and chlorophyll content [4]. Meanwhile, alfalfa has a well-developed root system [5], and it is well-known that the root system is an important organ for plants to absorb, transform, and store nutrients, and its growth and development are affected by a combination of its own genetic factors and the external environment. In addition, root-drenching application is a method of pouring the nutrient solution directly into the soil around the roots of seedlings. Nutrient solution application represents a cornerstone of controlled soil fertility management, enabling the precise modulation of macro/micronutrient ratios while mitigating soil heterogeneity [6]. This hydroponic-derived approach has been adapted for soil systems to study genotype × nutrition interactions, particularly in legume-breeding programs targeting stress resilience [7]. Thus, plant root growth can be promoted by this method [8,9] or regulated [10]; and fertilizer can be saved, which ultimately improves the efficiency of fertilizer utilization. Likewise, it is also used in the field of pesticides for the effective control of subterranean pests and diseases [11].
The characteristic of the alfalfa seedling population physiology with element accumulation has, until recently, been rare. For research on the effect of the nutritional regulation on alfalfa population characteristics and growth, we focus on the alfalfa backcross germplasm in order to recover elite genotypes and create children that are genetically identical or closer to parents [12]. For this purpose, alfalfa backcrossed third-generation (BC3) material [13], which was cultivated in the wet-summer climate of the Jianghuai region, was utilized as the test material in this study and the experiment was carried out in an artificial light incubator. This material was obtained by combining BC2 material [14], which has the characteristics of being “multifoliate” and having a high aboveground biomass, as the maternal parent, with ‘Huaiyin Alfalfa’, a landrace in the Jianghuai area, which has the characteristics of having a “high plant height” and “good resistance”, as the paternal parent. The BC4 population was obtained by backcrossing with ‘Huaiyin Alfalfa’ as the donor and BC3 material as the recurrent parent for another one year. It should also be noted that repeated backcrossing (BC) allows for the insertion of desirable traits in a crop from one variety to another while maintaining the integrity of the recipient variety [15,16]. In theory, as the number of backcrosses increases, the recurrent parent maintains the trait of being multifoliate while the donor’s higher plant height trait gradually experiences an introgression, and, by employing this method, the progeny can be made to maintain the trait of multiple leaves while obtaining a taller plant height with a higher resistance trait, like heat or humidity [17,18]. As populations are established, we may carry on with our discussions about genotype frequencies or polymorphisms, allele frequencies, and the population-level abundance of gene pools sensitive to fertilizer nutrition.
The efficacy of nutrient assimilation in alfalfa is intrinsically linked to genotype–environment interactions. Emerging research reveals that the precise modulation of the red–blue–green LED spectra and nitrogen concentrations (15–20 mmol L−1) in plant factories elevates alfalfa productivity by 38% through the coordinated activation of nitrogen metabolic enzymes and photomorphogenic pathways, while mitigating light-stress markers like H2O2 [19]. Environmental parameters critically modulate these responses. Legumes generally require more phosphorus than grasses, and can acquire it less easily due to thicker roots and shorter root hairs. In comparison to non-fertilized control levels, a meta-analysis result showed that fertilization enhanced the alfalfa quality by raising the crude protein by 7.7%, and decreasing the acid detergent fiber by 2.9%, and the neutral detergent fiber by 1.8%. It also raised the alfalfa yield by 19.2% [20]. Specifically, another meta-analysis of 105 papers published between 2003 and 2023 looked at how environmental conditions and fertilizer affected alfalfa quality and output [21].
On the one hand, different nutrient solutions provide different nutrient elements to the plant, and, in addition to the essential elements represented by NH4+ (supports nitrogen), other elements, particularly trace elements, are also required for the morphology and quality of legumes such as alfalfa [22]. Zinc (Zn) is an essential trace element in plants and a transition metal required for the synthesis and operation of many enzymes, auxin, and chlorophyll [23]. Iron deficiency chlorosis (IDC), meanwhile, causes soybean leaves to shrivel, stunting plant growth and frequently resulting in significant output losses [24].
On the other hand, from the perspective of alfalfa pasture products, mineral nutrients are critical for the adequate growth, productivity, and health of all food-producing animals. Among the minerals, the seven macro minerals essential to the diet of farm animals are calcium (Ca), phosphorus (P), magnesium (Mg), sulfur (S), sodium (Na), chlorine (Cl), and potassium (K); of these, the insufficient absorption of Mg in ruminants results in a magnesium deficiency, which is manifested by the clinical signs of tetany (grass tetany) or paresis (milk fever) [25]. Micronutrients, such as zinc (Zn), are also crucial for milk production and cow health [26], while it is a critical component of innate and adaptive immune responses [27,28,29]. Manganese (Mn) deficiency caused a number of detrimental effects, such as impaired growth, poor bone formation and skeletal defects, reduced fertility and birth defects, abnormal glucose tolerance, and an altered lipid and carbohydrate metabolism in both animals and humans [30]. Beyond ruminant nutrition, the elemental profile of alfalfa directly modulates its own stress resilience. Jaghdani et al. discovered that chloroplast photosynthesis is Mg-dependent, with a minimum threshold of 1.5 mg g−1 DM for optimal yield and dry matter production [31]. Moreover, Mg-IAA interaction enhanced quantum yields and electron transfer rates while reducing O2− accumulation in aluminum-stressed seedlings [32]. Such elemental dysregulation cascades into stunted root architectures—a modification in the root architecture is necessary for acquiring Zn during particular circumstances of Zn deficiency. Zn deficiency impaired primary root elongation but enhanced lateral root proliferation (increased density and length) in Arabidopsis [33]. The F-bZIP-transcription-factor-regulated Zn deficiency adaptation mechanism in Arabidopsis thaliana is also evolutionarily conserved in the legume model Medicago truncatula [34].
Consequently, the scheme of conducting the nutrient solution to the roots of alfalfa seedlings by a root-drenching operation to impact alfalfa growth is quite practicable. In this study, by applying nutrient solutions of different compositions, we compared the alfalfa plant height, leaf length and width, fresh-to-dry ratio, leaf protein accumulation, and nutrient element accumulation before and after the root-drenching operation, and, then, by systematically comparing the differences between the results obtained, we can determine the nutrient solution compositions that are the most suitable for alfalfa growth.
2. Materials and Methods
2.1. Materials
The test material was chosen from the 3rd generation of backcrossed alfalfa numbered B33 (BC3: B33), which has notable multifoliate characteristics and a reasonably large foliage mass. Through artificial pollination, 765 grains of seed material were collected from the BC4 alfalfa, which can be used for the test, using the BC3 material as the maternal parent and the ‘Huaiyin Alfalfa’ as the paternal parent. Full, golden-colored seeds were selected as test materials and stored at 4 °C ± 1 °C and 60% RH ± 3% RH for 60 d after-ripening treatment to eliminate seed dormancy [35,36]. Before the test began, the germination rate was determined using the method outlined in the Chinese national standard GB/T 2930.4-2017 Rules of seed testing for forage, turfgrass and other herbaceous plant—The germination test. Standards Press of China: Beijing, China, 2017, and the seedling survival rate (SS) is defined by Equation (1) (Table S2), based on the morphological traits that indicate when the seedling enters the V2 stage [37]. As a consequence, robust seedlings were selected as suitable seed material for formal trial:
where SS refers to seedling survival rate (%), while G refers to number of germinated seed, and SM refers to seedling mortality.
2.2. Methods
2.2.1. Culture Substrate and Water
The growth medium comprised a soil-based mix substrate (peat:perlite:sterilized field soil = 1:1:1 v/v/v), which was made by combining sandy loam soil extracted from Yangzhou University’s Yang–Tzu–Chin Grass Science Teaching and Practice Base (121°23′9″ E, 28°22′19″ N) with 0–6 mm Pindstrup® Orange Gold substrate (Ryomgaard, Denmark) and 3–7 mm perlite (Sandantian, Xinyang, China). Then, the compounded culture soil prepared in 1:1 ratio was determined for bulk weight, pH [38,39,40,41], and ammonium nitrogen [42]. All potting soil received identical batches of microwave-sterile, nitrogen-poor substrate (initial pH 7.23 ± 0.195; ammonium-nitrogen 8.64 mg kg−1), with full replacement between treatments to eliminate carryover effects (Tables S1 and S2).
The water used for the experiments in this section was 0.02 μS cm−1 of UP water (Kezhi, Shanghai, China), and the glass electrode was a PB-10 fully automated pH/mV meter (Sartorius®, Goettingen, Germany).
2.2.2. Transplanting Protocol
Seedlings were propagated using standardized paper-based germination:
(1) Germination stage: Alfalfa seeds were rubbed through the seed coat with 150-mesh sandpaper (Suisun, Hongkong, China), then placed on qualitative filter paper (Cytiva Bio-technology, Hangzhou, China) in 9 cm Petri dishes moistened with 5 mL deionized water. Pre-germination of alfalfa seedling before V1 has been performed under 100 μmol m−2 s−1 straight T5 fluorescent tube light in the RXZ-430E artificial climate incubator (Zhejiang Jiangnan Pharmaceutical Machinery, Rui’an, China).
(2) Growth conditions: The dishes were maintained at 25 ± 2 °C under 16/8 h (light/dark) photoperiod (photon flux density: 150 μmol m−2 s−1) for 10 days until reaching the V1 developmental stage (emergence of first true leaf). The day/night temperature was 23–28 °C/18–20 °C, the relative humidity (RH) was 50–60%, and the CO2 concentration was ambient.
(3) Transplanting criteria: Seedlings (root length 2.5 ± 0.5 cm) were transplanted into 500 mL polyethylene pots containing soil-based mix substrate (bulk density 0.27 ± 0.122 g·cm−3; pH 7.23 ± 0.195).
(4) Acclimatization: Post-transplant irrigation was withheld for 72 h to minimize transplantation shock prior to initiating growth observations.
2.2.3. Methods of Preparing and Applying Nutrient Solutions
A randomized block group design was used to set up 3 classical plant culture nutrient solutions, as experimental groups, reverse osmosis water (RO water) as the substrate for nutrient solution preparation, and control group (CK), respectively, and the 3 solutions were prepared according to Table 1. Of these, Murashige–Skoog (MS) and Gamborg’s B5 (B5) both remove agar and sucrose from the original medium [43]. This is carried out to control for variables by removing the additional carbon source that sucrose provides, as well as to prevent disruption of substrate joints under test conditions because agar is below the melting point. Hoagland’s original formulation with Arnon is cited in Hoagland Nutrient Solution (HL) [6]. HL prioritizes balanced N-P-K with ammonium-enhanced micronutrient solubility, whereas MS emphasizes nitrate dominance and iron bioavailability for oxidative stress mitigation. Contrastingly, B5’s low-N formulation mirrors marginal substrate conditions, enabling identification of genotype-specific nutrient scavenging traits. Three biological replications are conducted with ten plants per treatment.

Table 1.
The compounds of 3 different nutrient solutions.
The water used for the experiments in this section was 0.07 μS cm−1 of RO water (Kezhi, Shanghai, China), and the supplier of chemical reagents was Macklin (Shanghai, China), except for calcium nitrate, which was Sinopharm Chemical Reagent (Shanghai, China).
2.2.4. Research on Agronomic Traits and Growth Period
Phenological staging (from V1 to R1) was conducted daily under controlled greenhouse conditions (25 °C/20 °C day/night; 65% RH) at the Institute of Grassland Science, Yangzhou University (121°23′9″ E, 28°22′19″ N). Soybean-based growth period parameters were used [44]. A metal straightedge (Shinwa Rules, Niigata, Japan) was used to measure the height of the plumb line from the soil surface to the tallest point of the branches, which is known as plant height. The plant height variable y was then calculated as a function of time t to create a one-way linear regression equation . The average rate of change in plant height was then objectively compared by using the size of the slope k in the equation to calculate the plant’s growth rate [44]. Leaf width was also described as the length of the leaf blade spread out in the horizontal plane at its widest point perpendicular to the line of extension of the petiole, while leaf length was defined as the distance from the tip of the leaf blade to the base of the leaf blade. Fresh/hay yield ratio (FHR) was defined as the ratio of the weight of fresh alfalfa stems and leaves (g) cut 5 cm upward along the soil surface to the dry weight of the stems and leaves (g) dried at 105 °C for 0.5 h and then oven-drying at 85 °C for 48 h to constant weight in a parched state [45].
2.2.5. Determination of Soluble Protein Accumulation in Leaves
The quantification of soluble leaf proteins was prioritized due to alfalfa’s distinctive biomass allocation patterns. As reported by Cao [46], alfalfa exhibits high foliar abundance, with leaves constituting over 50% of the plant’s fresh biomass and containing approximately 2.5 times more protein than stems. Complementing this finding, Wang et al. [47] demonstrated that alfalfa leaves possess significantly higher total digestible nutrients compared to stems. These well-characterized nutritional properties establish leaf protein concentration as scientifically convenient biomarkers for assessing the efficacy of nutrient solutions in optimizing forage quality parameters. The Coomassie G-250 approach was used for the determination [48]. Protein quantification employed Bradford’s method rather than Kjeldahl analysis to exclude non-protein nitrogen interference, given its established reliability in legume studies [49,50]. To make the Coomassie G-250 assay solution (CBBG), Coomassie Brilliant Blue G-250 was pre-dissolved in 95% (v/v) ethanol; then, 100 mL of 85% (v/v) phosphoric acid was added and diluted to 1 L with RO water. Leaf protein content was evaluated by grinding 0.2 g of alfalfa leaves with a mortar and pestle, extracting the water-soluble proteins with 5 mL of RO water as the grinding solution, and then transferring the slurry to a centrifuge tube. The residue and protein leachate were separated using a Centrifuge 5810R (Eppendorf®, Oldenburg, Germany) at 20 °C for 10 min with a relative centrifugal force of 1825× g. The protein leachate was recovered by centrifuging 1.5 mL of RO water in a mortar. Transfer the supernatant to a 10 mL volumetric flask. Following that, 2 mL of RO water was added to the centrifuge tube to resuspend the precipitated pellets, which were then centrifuged for another 10 min, and the supernatants from the two centrifugations were mixed. Finally, the solution in the volumetric flask was diluted to the appropriate scale to be tested. Then, 0.1 mL of each test solution was aspirated by pipette into a pre-numbered test tube, and then reacted with CBBG for 2 min on a static surface. Colorimetric measurement was performed at 595 nm. The standard curve was determined by using CBBG as a blank control and aqueous bovine serum albumin solution with 0–100 μg mL−1. The protein content PR (g g−1) was calculated using Equation (2):
where A is the standard curve which calculated protein content (μg); V1 is the total volume of the extracted solution after dilution (mL); V2 is the volume aspirated for the test (mL); and W is the fresh weight of the sample (g).
The water used for the experiments in this section was 0.07 μS cm−1 RO water (Kezhi, Shanghai, China) and the supplier of the chemical reagents was Macklin (Shanghai, China).
2.2.6. Determination of the Elemental Content of the Leaves of Alfalfa
Inductively coupled plasma mass spectrometry (ICP-MS) has excellent performance in ultra-trace multi-element analysis and isotope analysis [51]. Thus, ICP-MS is often utilized to assess the elemental content of plant seedlings [52,53]. ICP-MS can determine the concentration of various elements with extremely low limits of detection (LOD) and high sensitivity. The method also has a wide linear dynamic range for consistent detection of trace elements in individual samples; thus, we used microwave digestion and ICP-MS to determine the contents of seven major nutrient elements [54], K, Na, Mg, Mn, Fe, Zn, and Cu, in nutrient solutions affecting alfalfa growth. Specifically, the cut alfalfa blades were fully dried using a hot air dryer (DHG-9141A, Jinghong, Shanghai, China) set to 105 °C. The drying process was halted after the material’s weight remained consistent. The dried samples were diced and weighed to 0.1 g ± 0.0001 g using an ML 204 analytical balance (Mettler-Toledo, Columbus, OH, USA), and then placed in PTFE microwave concentration and evaporation vessels. To wet the samples, a tiny amount of distilled water (Watsons, Canton, Guangzhou, China) was added, followed by 5 mL 14.4 mol L−1 HNO3, which was allowed to stand for 1 h. Then, 1 mL 10 mol L−1 H2O2 was added, mixed, capped, and sealed, and the mixed test samples were loaded into the MARS 6. The mixed test items were put onto a MARS 6 microwave digestion apparatus (CEM, Matthews, NC, USA), and the digestion was carried out using the digestion program (Table 2). The digested sample was placed on the XOSM-24N graphite acid catcher (ATPIO, Nanjing, Jiangsu, China) for acid evaporating treatment, and, when the liquid was only the size of a soybean, the volume was precisely adjusted to 50 mL using distilled water. To test the solution sample, the adjusted volume of the sample was passed through a 0.22 μm filter membrane. The NexION® 1000 ICP-MS Mass Spectrometer (PerkinElmer, Shelton, CT, USA) was used to identify the measured values.

Table 2.
Program of microwave digestion.
2.2.7. Statistical Methodologies and Software
The experimental data were collected and analyzed in Microsoft Excel 2021 (Microsoft, Redmond, WA, USA) for basic descriptive analysis, which included calculating and ranking arithmetic means and totals. SPSS Statistics 26 (IBM, Armonk, NY, USA) was used to conduct one-sample t-tests, two independent-sample t-tests, one-way ANOVA analyses, and linear regression fitting. Origin 2022 (OriginLab, Northampton, MA, USA) ran color plots. The study used a significance level of α = 0.05, unless otherwise noted.
3. Results
3.1. Nutrient-Solution-Treated Alfalfa Had Earlier Growth Period, but B5 Inhibited Its Dry Matter Accumulation
3.1.1. Studies on the Growth Period of Alfalfa
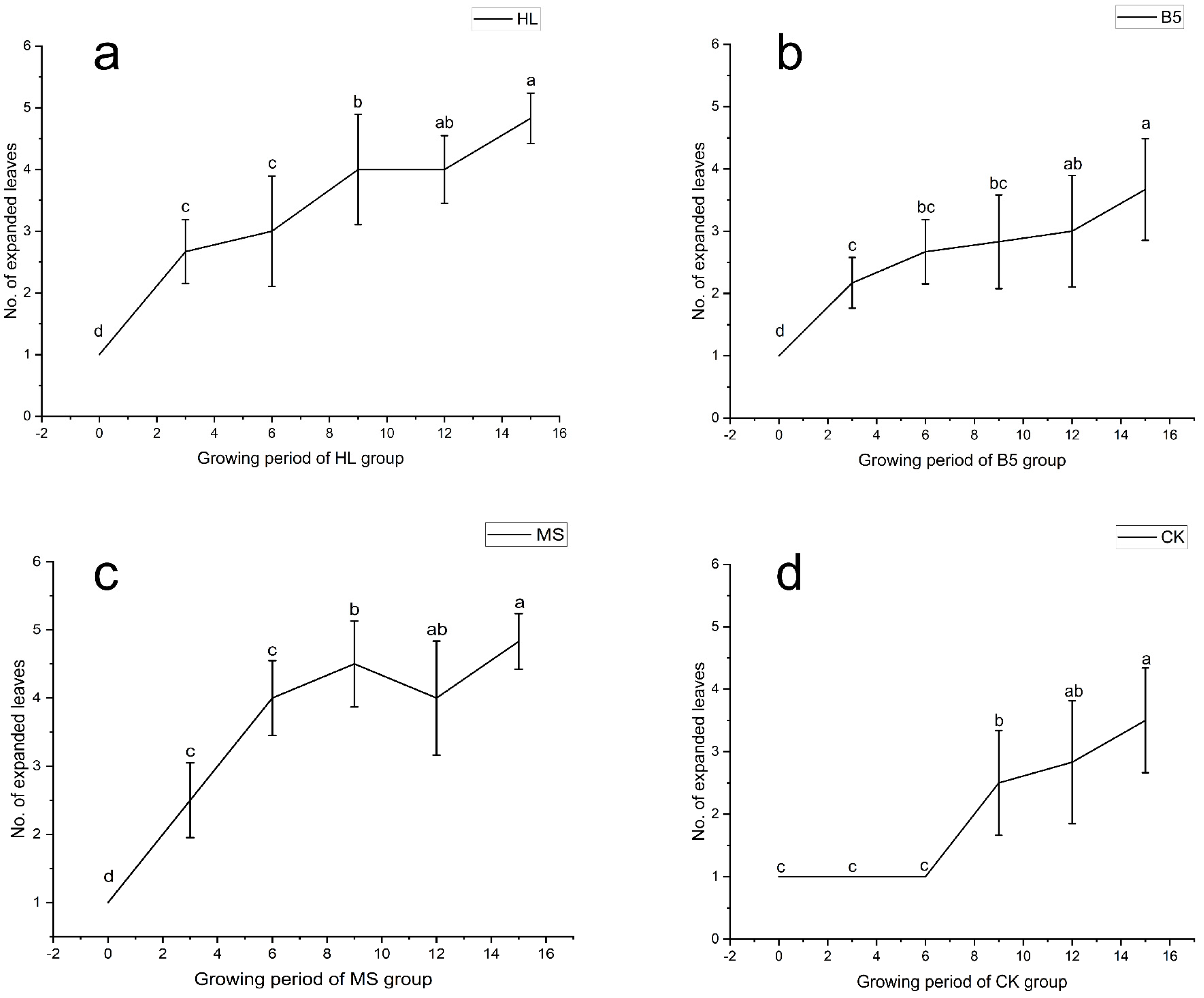
The results of the 16-day alfalfa growth period showed that all three nutrient solutions were effective in promoting the growth of alfalfa true leaves, resulting in a “phase shift” of the growth period, with alfalfa roots drenched in the nutrient solution growing 3 days earlier than the RO water control group. The effects of the MS (Figure 1c) and HL (Figure 1a) nutritional solutions were more obvious. Following the calculation of Duncan’s new multiple range method, the comparison obtained the average value of the day-to-day change in the true leaf number (as leaf age). The rate of leaf age of both MS and HL was similar, and both were relatively fast; however, the change in leaf age of the B5 nutrient solution treatment was smoother (Figure 1b), and it only began to grow more quickly than RO water (Figure 1d).
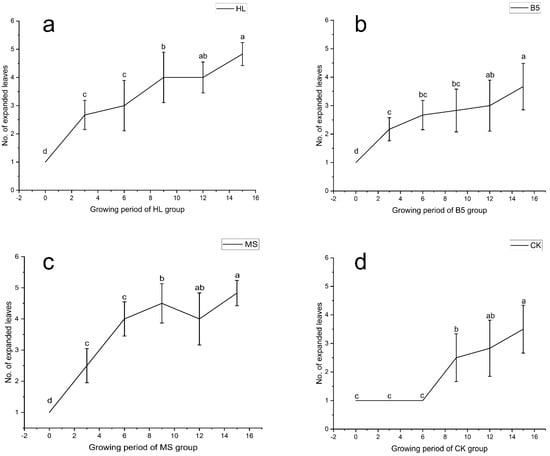
Figure 1.
Alfalfa number of fully expanded leaves (unitless count metric) during vegetative growth stage (V1–R1). (a) Hoagland nutrient solution treatment (HL); (b) B5 nutrient solution treatment (B5); (c) Murashige & Skoog (MS) nutrient solution treatment; (d) Reverse osmosis (RO) water control group (CK). Different lowercase letters denote statistically significant differences among treatments (p < 0.05, one-way ANOVA with Duncan’s Multiple Range). Error bars represent standard deviation (n = 5 biological replicates).
3.1.2. Determination of Alfalfa FHR
The dry matter fraction of alfalfa was determined by measuring fresh and oven-dried weights at R1 stage (beginning flowering) after applying different nutrient solutions via the soil drench to the soil-based substrate (see Section 2.2.1; Table 3). B5-treated plants exhibited significantly higher FHR (36.42%) compared to the MS (−10.25%), Hoagland (−12.55%), and RO water groups (13.6%). This elevated FHR reflects a reduced tissue dry matter content rather than superior biomass production. This finding implies that the B5 root-drenching treatment produced less dry matter accumulation capacity than the other treatments.

Table 3.
The FHR of groups and their anomalies.
3.2. Differences in the Effects of Different Nutrient Solutions on the Growth of Alfalfa Were Observed
3.2.1. Analysis of Alfalfa Plant Height Variation
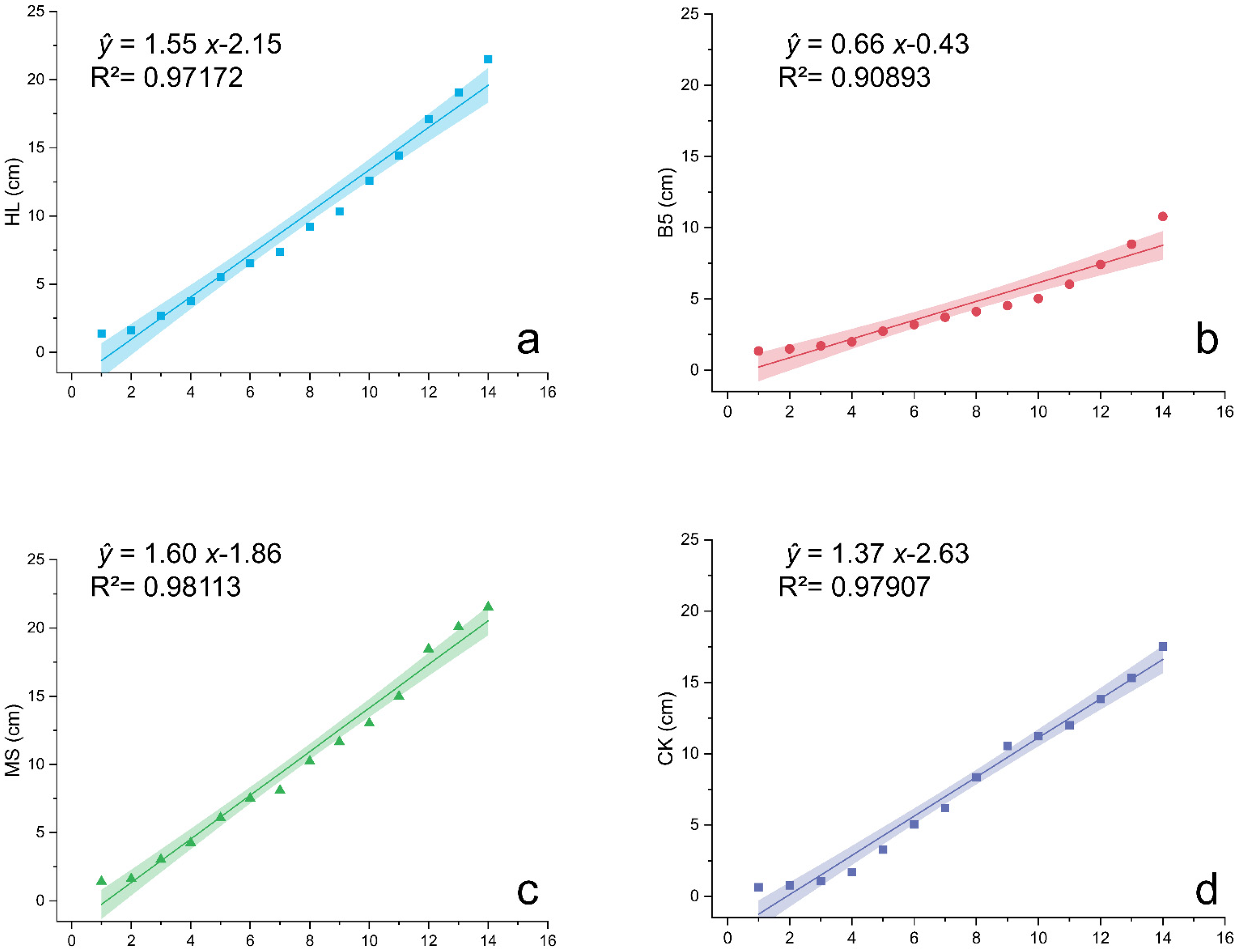
In terms of the plant height of alfalfa seedlings, both the HL (Figure 2a) and MS (Figure 2c) nutrient solutions were favorable; however, B5 (Figure 2b) had no positive significant influence on the plant height and hampered alfalfa growth. B5 had a considerably lower plant height than CK at the 14th measurement (Figure 2d) (ANOVA, p = 0.021 < 0.05). Furthermore, a study of growth rates using slope k of the linear fitting curve as a quantitative metric revealed that HL had the greatest influence on the increasing alfalfa seedling growth rates. The results specifically present features as follows: MS (k = 1.59967 ± 0.06405) > HL (k = 1.55366 ± 0.07652) > CK (k = 1.37396 ± 0.05799) > B5 (k = 0.65729 ± 0.06006).
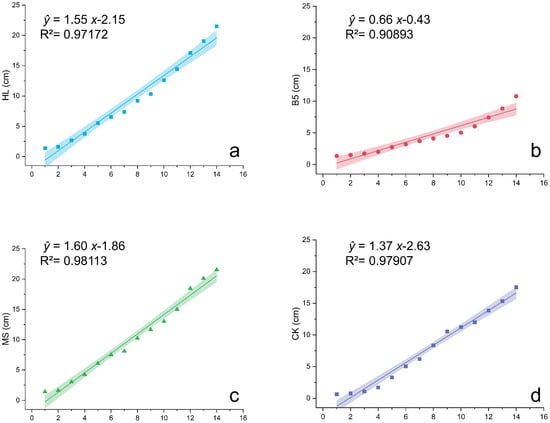
Figure 2.
Linear regression of alfalfa plant height under different nutrient treatments. (a) Hoagland nutrient solution treatment (HL); (b) B5 nutrient solution treatment (B5); (c) Murashige & Skoog (MS) medium treatment; (d) Reverse osmosis (RO) water control group (CK). Growth curves fitted via least squares method (solid lines) with 95% confidence intervals (shaded). Regression model: Plant height = Intercept + k × Days. Coefficient comparisons reveal highest growth rates (k) in HL (Hoagland’s solution) and MS (Murashige & Skoog medium), lowest in B5 (Gamborg’s B5). CK: RO water control. All regressions significant (R2 > 0.90, p < 0.001).
3.2.2. Analysis of Differences in Leaf Length and Width in Alfalfa
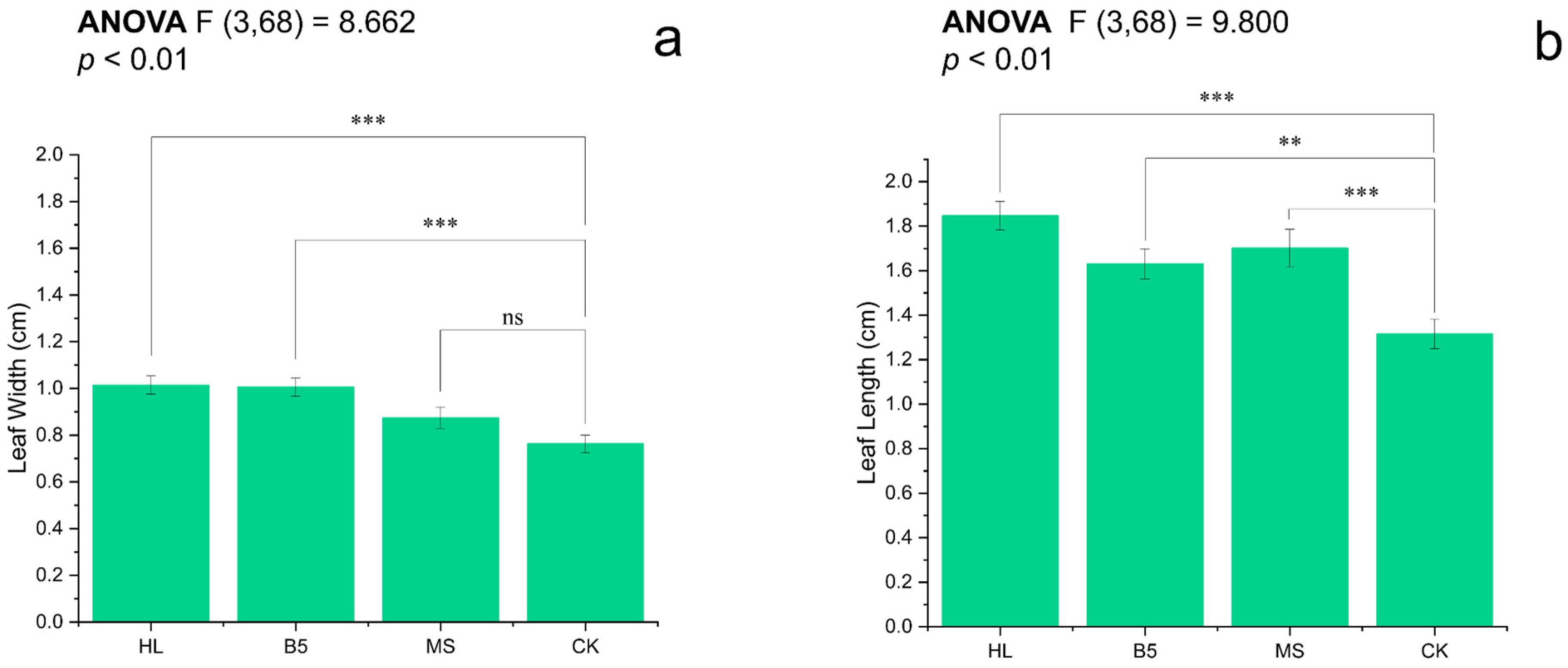
The leaf length and leaf width, important indicators of alfalfa seedling growth, differed. When the leaf length of alfalfa at R1 stage was compared to the means, HL > MS > B5 > CK (Figure 3b); and, when the leaf width of alfalfa at R1 stage was compared to the means, HL > B5 > MS > CK (Figure 3a). The leaf widths of alfalfa seedlings watered with HL and B5 were both considerably greater than those of CK (p < 0.001), whereas alfalfa seedlings watered with MS were not significantly different from CK (p = 0.058 > 0.05). The leaf length of alfalfa seedlings after root-drenching in all three nutrient solutions differed statistically considerably from that of alfalfa seedlings in CK (Figure 3a,b). Additionally, the differences in the leaf length of alfalfa seedlings after being root-drenched in HL and MS media were all significantly greater than those of alfalfa seedlings irrigated with CK (p < 0.001).
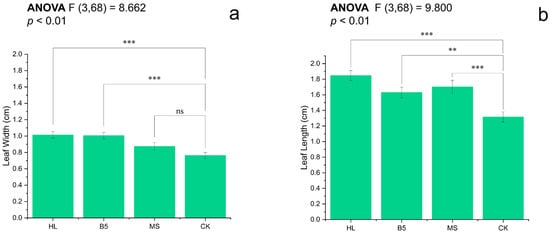
Figure 3.
Leaf dimensions of R1-stage alfalfa under nutrient treatments: (a) width; and (b) length. Treatments: HL (Hoagland’s), B5 (Gamborg’s), MS (Murashige-Skoog), and CK (RO water control). Bars show mean ± SEM. Different letters indicate significant differences (Fisher’s LSD, α = 0.05): *** p ≤ 0.001; ** p ≤ 0.01; ns = non-significant. All treatments exceeded CK in both parameters (p < 0.01), except MS leaf width (ns).
3.2.3. Analysis of Leaf Protein Differences in Alfalfa
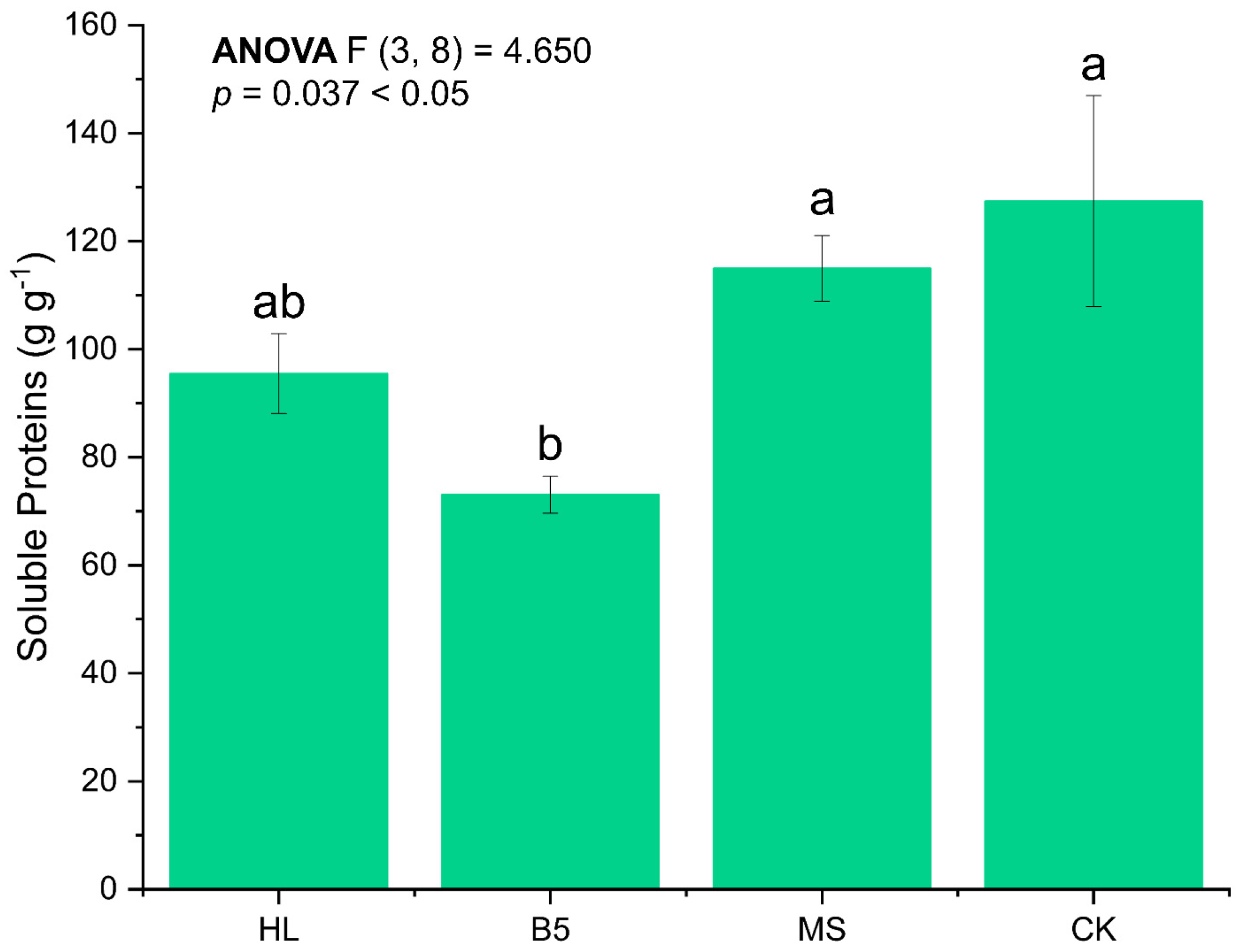
Meanwhile, when we compared the soluble protein accumulation in the leaves of alfalfa seedlings at the R1 stage after watering with different nutritional solutions, we discovered that, similarly to the plant height results, B5 had an inhibiting influence on seedling growth. Specifically, the soluble protein in the B5 group was significantly lower than that in the other groups; the differences in soluble protein accumulation in the other groups at the R1 stage were smaller (ANOVA, p = 0.037 < 0.05), and the post hoc test between CK and MS culture media revealed no statistically significant difference between the two (LSD, p = 0.074 > 0.05) (Figure 4).
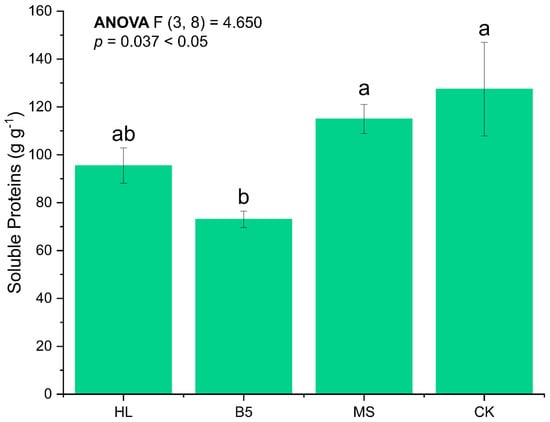
Figure 4.
Soluble protein content of leaves of alfalfa after root-drenching with nutrient solution. HL refers to Hoagland’s solution; B5 refers to Gamborg’s B5; MS refers to MS medium; and CK refers to control group which is the RO water group. Error bar refers to standard error of mean. Duncan’s method was chosen for the algorithm of multiple comparisons here. The Latin letters “a, b” indicate the significance level from high to low. The significance level of ANOVA analysis was designated as 0.05.
3.3. Different Nutritional Solution Showed Varying Effects on Alfalfa Element Accumulation over the R1 Stage
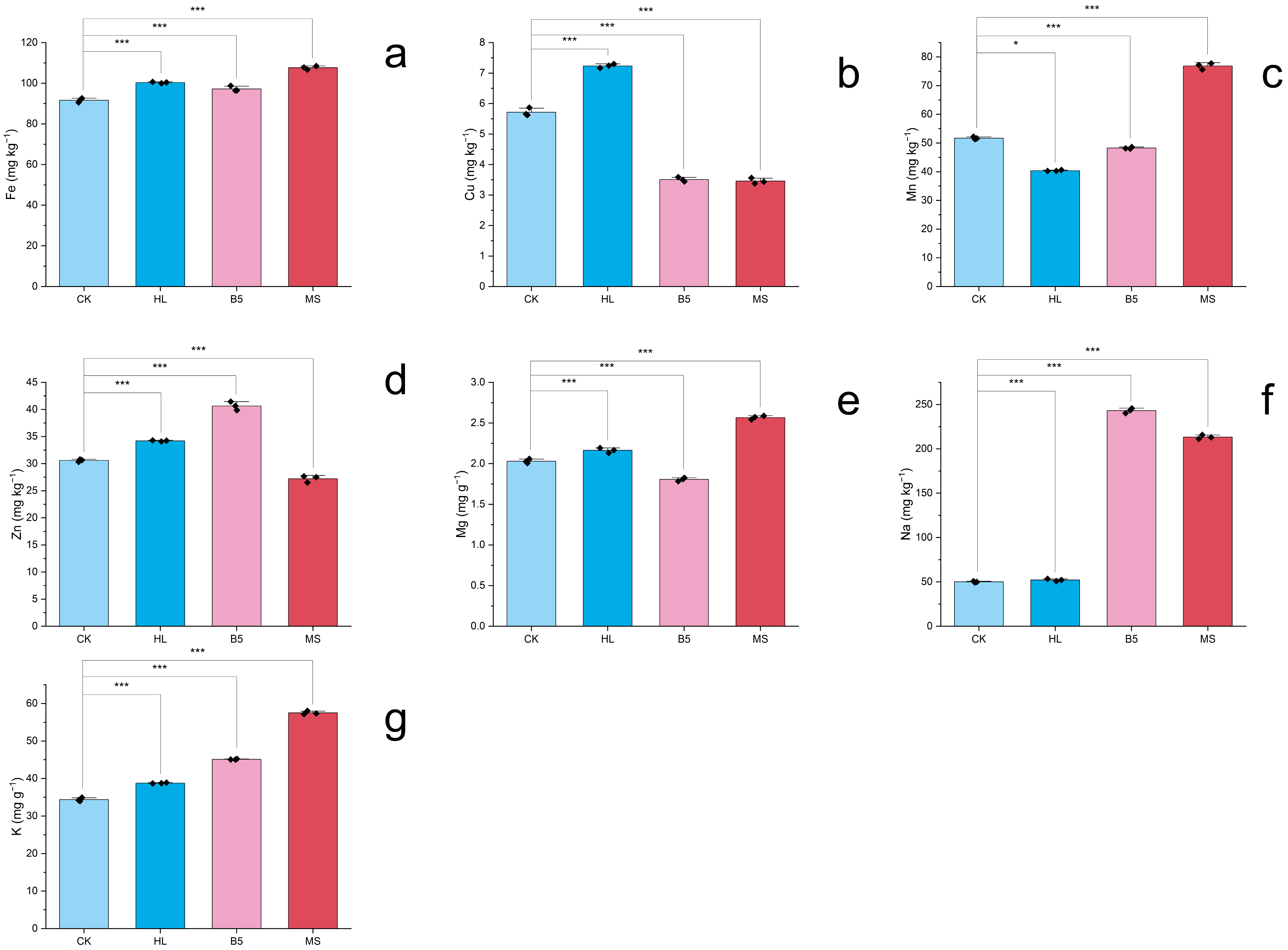
The one-way ANOVA revealed highly significant differences in elemental accumulation patterns among nutrient treatments (p < 0.001, η2 > 0.90), with LSD post hoc tests delineating distinct solution-specific profiles. Notably, the MS solution induced the most pronounced micronutrient enrichment, particularly elevating leaf Mn to 76.86 mg kg−1 (1.5-fold higher than control) while simultaneously boosting Fe (1.17×), Mg (1.26×), Na (4.26×), and K (1.67×) concentrations. In contrast, the HL solution demonstrated broader efficacy, achieving peak Cu accumulation at 7.24 mg kg−1 (1.3× control) alongside superior K enrichment (1.13× control) and comparable Fe/Mg/Na levels to MS. Meanwhile, the B5 treatment showed selective efficiency, primarily enhancing Na (4.68×) and K (45.11 mg g−1, 1.31× control), though its micronutrient performance lagged behind other treatments with only 1.06–1.33× increases in Fe/Zn. Crucially, while all solutions improved macronutrient acquisition versus control, their micronutrient specialization diverged markedly: MS excelled in Mn bioavailability, HL dominated Cu/Zn dynamics, and B5 exhibited a balanced but moderate multi-element promotion. These differential accumulation patterns, visualized in Figure 5a–g and tabulated in Table 4 and Table S3, underscore the nutrient solution composition as a critical determinant of alfalfa’s elemental profile.
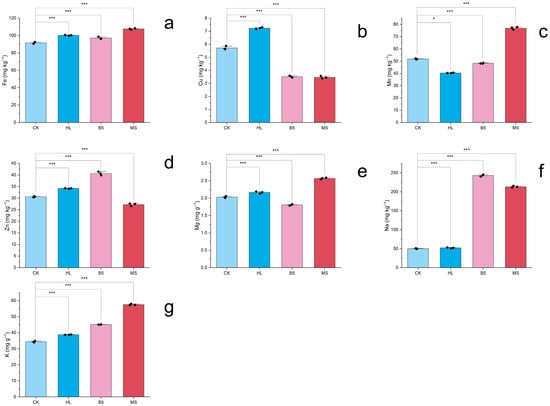
Figure 5.
Elemental profiles of alfalfa under nutrient treatments (50 d). ICP-MS quantified seven macro/microelements (Na+, K+, Mg2+, Cu2+, Fe2+, Mn2+, and Zn2+). (a) Iron (Fe); (b) Copper (Cu); (c) Manganese (Mn); (d) Zinc (Zn); (e) Magnesium (Mg); (f) Sodium (Na); (g) Potassium (K). Black diamond markers (♦) represent mean values of triplicate technical replicates per biological sample, while column heights indicate grand means pooled across three biological replicates (n = 9 total observations). Element concentrations expressed as mg kg−1 or mg g−1 dry weight (DW). Error bars: SEM (n = 3). Significant differences (Fisher’s LSD post hoc test, α = 0.05) marked: *** p < 0.001; * p < 0.05. Treatment codes: HL = Hoagland’s solution; MS = Murashige & Skoog; B5 = Gamborg’s B5; CK = RO water control.

Table 4.
The one-way ANOVA of 7 elements’ accumulation result of alfalfa after 50 d nutrient solution irrigation *.
4. Discussion
The outcomes of this study have shed light on the characteristics in which several plant nutritional solutions influenced the growth and nutrient accumulation of alfalfa. Although previous research looked at the impacts of nutrient solutions on growth, the results of seedling nitrogen fertilizer requirements varied, and there was no explicit discussion of specific element accumulation. While this study focused on plant-level nutrient responses, the absence of longitudinal soil chemistry data precludes the direct assessment of substrate–nutrient interactions. We recognize this as a methodological constraint and commit to incorporating sequential soil sampling in future iterations of this research program to fully resolve the nutrient flux dynamics. We know that, when the ammonium nitrogen concentration stays constant, soil NH3 volatilization increases tenfold for every unit increase in pH [55]. Thus, the use of a compounded soil-based mix substrate in this study ensured the accuracy of the experiments.
In this study, it was discovered that the MS treatment increased the stem elongation rate by 16.79% versus controls (based on k-value) under the effect of different nutrient solution rooting treatments; HL was the second fastest; and B5, which is considered promising in the field of plant microspore cultivation [56], performed mediocrely or even showed growth inhibition. This may lead to the conclusion that, when using mix substrates for alfalfa cultivating, MS or HL, with relatively high inorganic salt concentrations, particularly NO3−, K+, and NH4+, are preferable to B5, with a relatively low NH4+ content. In addition to the macronutrient elements represented by N, the amount of irrigation water used may have an impact on alfalfa yields. Despite the fact that alfalfa is more drought-tolerant than most legume forages [57], water deprivation can still have an impact on its growth, dry matter accumulation, and quality. Several studies have found that water deficits hinder N fixation in legumes, resulting in nitrogen deficiency [58,59,60]. However, the findings may be limited due to the lack of experiments on irrigation water limitations. However, we remain persuaded that it will be one of the criteria to consider in future investigations.
FHR is currently employed as one of the markers of fodder quality [61], and it is frequently connected with nitrogen accumulation [62], which is an important sign of crop production due to the dry matter accumulation [63]. On the one hand, it has been stated that the nitrogen fertilization of perennial alfalfa is ineffective and may even be harmful, and that N, P, and K have no effect on the FHR during harvest [64]. On the other hand, it has been stated that alfalfa rhizobacteria fix only 50–60% of alfalfa’s nitrogen requirements [65], necessitating external nitrogen delivery. While nodulation quantification was not a predefined study objective, the observed MS-induced nodule proliferation (Figure S1, Table S4) tentatively suggests the nutritional regulation of rhizobial symbiosis efficiency—a phenomenon warranting a dedicated investigation with proper negative controls and isotopic tracing. Notably, our findings revealed the lowest FHR following HL treatment, while B5 had the greatest FHR. As previously pointed out, B5 included less ammonium, whereas HL’s nitrogen sources, ammonium sulfate and diammonium hydrogen phosphate, provided significantly more nitrogen, with 210 mg kg−1 N and 31 mg kg−1 P. Previous research has shown that an insufficient amount of nitrogen will prevent alfalfa from growing properly [66]. As a result, it is plausible to infer that exogenous nitrogen fertilizer is required to maintain or promote growth when cultivating alfalfa in soil.
It is generally accepted that proteins serve as the primary executors of gene functions in order to maintain the effective and orderly conduct of numerous complicated biochemical processes in organisms, hence ensuring organisms’ normal life activities. Many of the proteins obtained from alfalfa tissues using RO water milling in the present study are enzymes engaged in various metabolic processes. The majority of enzymatic proteins in plants are water-soluble [67], including, for example, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) [68], which is likewise water-soluble and catalyzes the initial step of carbon fixation, and whose abundance reflects the intensity of plant metabolism. Additionally, as a plant whose principal function is the utilization of foliage, alfalfa leaves exhibit less variance in nutrient content than alfalfa stems [69]. As a result, determining the protein content is useful for examining the plant’s health during growth. Our findings indicated that MS showed comparable protein levels to CK (Fisher’s LSD, p = 0.447), while other treatments exhibited 9.77–42.64% reductions (ANOVA, p < 0.05). The physiological mechanisms regulating the leaf protein content are not yet fully understood. Nitrogen deficiency is widely recognized to reduce amino acid synthesis and subsequent protein production, leading to diminished plant growth. This limitation simultaneously promotes the accumulation of non-nitrogen metabolites by increasing the photoassimilate availability for secondary metabolic processes, including ascorbic acid synthesis and organic acid production [70]. Studies on Gramineae species indicate that nitrogen regulates root growth through multiple biological mechanisms, particularly affecting cell development, phenylpropanoid biosynthesis, and protein synthesis pathways [71]. Our findings demonstrate similar patterns to rice cultivation, with root growth being enhanced under low nitrogen conditions but inhibited under high nitrogen availability. This differential response appears mediated by nitrogen-induced variations in cellular processes, specifically through altered rates of cell division and expansion. The physiological impact of nitrogen extends to photosynthetic efficiency, as nitrogen availability significantly influences both leaf structural development and internal nitrogen allocation patterns [72]. A similar resource reallocation exists in soybean [Glycine max (L.) Merr.] [73]. These combined effects explain the observed variations in alfalfa morphology and biochemistry. Specifically, the above-ground plant height and protein accumulation in alfalfa demonstrate direct correlations with nitrogen availability from both soil sources and supplemental nutrient solutions. The integration of these nitrogen-mediated processes underscores the complex relationship between nitrogen nutrition and plant developmental outcomes.
While protein dynamics reflect physiological status, their nutritional value also merits an exploration in sustainable agriculture. Plant-based proteins have received a lot of attention for their health benefits and are seen as alternatives to animal proteins for the development of sustainable food systems [74], and alfalfa-leaf-protein-related food development and utilization could be a very promising direction if future improvements in leaf protein extraction and purification strategies are made [75].
Finally, MS substantially boosts the accumulation of three essential elements in alfalfa leaves: Fe, Mn, and Na. While the MS medium was initially developed for tobacco cell culture, its successful adaptation in alfalfa tissue culture systems suggests cross-species applicability [76], potentially attributable to its balanced micronutrient profile including 10.4 mg kg−1 Fe and 7.1 mg kg−1 Mn—concentrations aligning with reported alfalfa micronutrient demands [77,78]. Our ICP-MS analysis confirmed a 1.17-fold higher leaf Fe and 1.49-fold higher Mn accumulation in MS-treated plants versus controls (p < 0.001), suggesting the efficient translocation of these micronutrients from the substrate to foliage. We know that Fe is required for the normal functioning of enzymes involved in the stress response and development as cofactors of enzyme activity in animals, and that iron deficiency is a nutritional deficiency with serious effects on the immune response and overall animal health; however, Mn is required for the normal functioning of enzymes involved in the stress response and development [79]. More importantly, Fe and Mn are “complementary”, which means that Fe influences Mn metabolism and absorption, low Fe produces abnormal Mn accumulation, and an excess of or deficiency in Mn affects the animal’s Fe level [80]. As a result, it is reasonable to believe that applying an MS nutritional solution has a good influence on trace element accumulation in the edible sections of alfalfa. The results for HL and B5 were similar, but not improved, on aspects associated with lactation in dairy cattle.
5. Conclusions
The MS nutrient solution demonstrated superior efficacy in alfalfa BC population development, significantly enhancing key growth parameters versus controls: 16.8% faster stem elongation (p < 0.001), 3.35% higher dry matter, and advanced phenology (4–5 vs. 3-leaf stage). Element-specific accumulation was most pronounced for Fe (1.17-fold) and Mn (1.48-fold) (p < 0.001), suggesting an optimized micronutrient uptake under MS treatment. These results provide actionable insights for breeding programs—prioritizing MS formulation during seedling establishment can concurrently improve growth vigor and mineral acquisition traits. However, substrate-specific responses observed in this pot trial necessitate field validation across soil types. Future studies should integrate transcriptomic analyses to decipher the nutrient-solution-induced regulation of ion transporter genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15040902/s1, Table S1: Basic soil physical and chemical characteristics; Table S2: The pH of soil substrate; Table S3: Descriptive statistics on the accumulation of 7 elements in alfalfa at R1 after root-drenching treatment by 3 nutrient solutions; Table S4: Exploratory nodulation analysis under nutrient treatments; Figure S1: Nodulation of alfalfa rhizomes (scale 2 cm). Reference [81] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.L.; data curation, J.L., Y.Z., H.C. and C.W.; formal analysis, J.L.; funding acquisition, Z.W.; investigation, J.L., Y.Z., H.C. and C.W.; methodology, J.L.; project administration, Z.W.; resources, J.L., Z.W., H.C., K.C. and X.Z.; software, J.L. and H.C.; supervision, Z.W.; validation, J.L.; visualization, J.L.; writing—original draft, J.L.; writing—review and editing, Z.W., K.C. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Biological Breeding—National Science and Technology Major Project, grant number 2023ZD04060; Jiangsu Key R&D Program, “Key Technology Research on Breeding and Production Application of ‘Huaiyang No. 4’ Alfalfa Cultivar”, grant number BE2023383; Shanghai Agriculture Applied Technology Development Program, China, grant number T20200102; National Extension Program for Scientific and Technological Achievements in Forestry and Grassland, State Forestry and Grassland Administration, China, grant number 2023133122; and Central Finance Forestry Science and Technology Extension Demonstration Fund Project, grant number Jiangsu [2023] TG12.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
Haowen Cheng, College of Animal Science and Technology, Yangzhou University, provided support and assistance with the research materials, for which we are very thankful.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dhakal, M.; West, C.P.; Villalobos, C.; Sarturi, J.O.; Deb, S.K. Trade-off between Nutritive Value Improvement and Crop Water Use for an Alfalfa–Grass System. Crop Sci. 2020, 60, 1711–1723. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuang, Y.; Yan, R.; Qin, Q.; Jin, J.; Yang, P.; Liu, Y.; Xiong, J.; Xin, X. Characteristics of soil fertility under different long-term land-use patterns in south China: A case study in Huoshaoping Township, Changyang County, Hubei Province. J. Plant Nutr. Fertil. 2023, 29, 188–200. (In Chinese) [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and Quality Properties of Alfalfa (Medicago sativa L.) and Their Influencing Factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Giro, A.; Ferrante, A. Postharvest Physiology of Corchorus Olitorius Baby Leaf Growing with Different Nutrient Solutions. J. Hortic. Sci. Biotechnol. 2018, 93, 400–408. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.; Li, J.; Wei, S.; Liang, P.; Liu, X.; Nan, L. Influences of drought stress on physiological characteristics and anatomical structure of alfalfa roots of different root-types. Grassl. Turf 2023, 43, 132–137. (In Chinese) [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; University of California, College of Agriculture: Berkeley, CA, USA, 1938; Volume 347. [Google Scholar]
- Roosta, H.R.; Sharifi Azad, H.; Mirdehghan, S.H. Comparison of the growth, fruit quality and physiological characteristics of cucumber fertigated by three different nutrient solutions in soil culture and soilless culture systems. Sci. Rep. 2025, 15, 203. [Google Scholar] [CrossRef]
- Percival, G.C.; Fraser, G.A. Use of Sugars to Improve Root Growth and Increase Transplant Success of Birch (Betula Pendula Roth.). Arboric. Urban For. (AUF) 2005, 31, 66–77. [Google Scholar] [CrossRef]
- Xin, X.; Nepal, J.; Wright, A.L.; Yang, X.; He, Z. Carbon Nanoparticles Improve Corn (Zea mays L.) Growth and Soil Quality: Comparison of Foliar Spray and Soil Drench Application. J. Clean. Prod. 2022, 363, 132630. [Google Scholar] [CrossRef]
- Kemp, H.T.; Fuller, R.G.; Davidson, R.S. Inhibition of Plant Growth by Root-Drench Applications of Kinetin. Science 1957, 126, 1182. [Google Scholar] [CrossRef]
- Srivastava, P.; George, S.; Marois, J.J.; Wright, D.L.; Walker, D.R. Saccharin-Induced Systemic Acquired Resistance against Rust (Phakopsora pachyrhizi) Infection in Soybean: Effects on Growth and Development. Crop Prot. 2011, 30, 726–732. [Google Scholar] [CrossRef]
- Vogel, K.E. Backcross Breeding. Methods Mol. Biol. 2009, 526, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Olom, O.I.M.; Wei, Z. Effect of 3-Indole Acetic Acid and Gibberellic Acid on Growth and Yield of Alfalfa BC3 Hybrid. Appl. Ecol. Environ. Res. 2023, 21, 4931–4942. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Olom, O.I. Improvement Effect of Agronomic Traits in the BC2 Population of Multifoliate Alfalfa. Acta Agrestia Sin. 2021, 29, 488–494. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.Y.; Kim, D.Y.; Jeong, H.J.; Um, I.S.; Rho, I.R. Effects of Backcrossing on Quality of Strawberry Fruit. Hortic. Environ. Biotechnol. 2018, 59, 225–230. [Google Scholar] [CrossRef]
- Vilà, C.; Seddon, J.; Ellegren, H. Genes of Domestic Mammals Augmented by Backcrossing with Wild Ancestors. Trends Genet. 2005, 21, 214–218. [Google Scholar] [CrossRef]
- Falke, K.C.; Sušić, Z.; Wilde, P.; Wortmann, H.; Möhring, J.; Piepho, H.-P.; Geiger, H.H.; Miedaner, T. Testcross Performance of Rye Introgression Lines Developed by Marker-Assisted Backcrossing Using an Iranian Accession as Donor. Theor. Appl. Genet. 2009, 118, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.H.; Shikari, A.B.; Vaishnavi, R.; Najeeb, S.; Padder, B.A.; Bhat, Z.A.; Parray, G.A.; Bhat, M.A.; Kumar, R.; Singh, N.K. Marker-Assisted Introgression of Three Dominant Blast Resistance Genes into an Aromatic Rice Cultivar Mushk Budji. Sci. Rep. 2018, 8, 4091. [Google Scholar] [CrossRef]
- Chen, Y. Regulation Mechanisms on Alfalfa Yield, Quality Andnitrogen Metabolism by Optimizing LED Red, Blue and Green Light Combinations and Light Modes. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2024. (In Chinese). [Google Scholar]
- Wan, W.; Li, Y.; Li, H. Yield and quality of alfalfa (Medicago sativa L.) in response to fertilizer application in China: A meta-analysis. Front. Plant Sci. 2022, 23, 1051725. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z. Fertilisation and environmental factors affect the yield and quality of alfalfa in China. Plant Soil Environ. 2024, 70, 276–286. [Google Scholar] [CrossRef]
- Song, K.; Gao, J.; Li, S.; Sun, Y.; Sun, H.; An, B.; Hu, T.; He, X. Experimental and Theoretical Study of the Effects of Rare Earth Elements on Growth and Chlorophyll of Alfalfa (Medicago sativa L.) Seedling. Front. Plant Sci. 2021, 12, 731838. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Chopra, P.; Chhillar, H.; Ahanger, M.A.; Hussain, S.J.; Maheshwari, C. Regulatory Hubs and Strategies for Improving Heavy Metal Tolerance in Plants: Chemical Messengers, Omics and Genetic Engineering. Plant Physiol. Biochem. 2021, 164, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Merry, R.; Dobbels, A.A.; Sadok, W.; Naeve, S.; Stupar, R.M.; Lorenz, A.J. Iron Deficiency in Soybean. Crop Sci. 2022, 62, 36–52. [Google Scholar] [CrossRef]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. The Contribution of Dietary Magnesium in Farm Animals and Human Nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Oconitrillo, M.; Wickramasinghe, J.; Omale, S.; Beitz, D.; Appuhamy, R. Effects of Elevating Zinc Supplementation on the Health and Production Parameters of High-Producing Dairy Cows. Animals 2024, 14, 395. [Google Scholar] [CrossRef]
- Fraker, P.J.; Haas, S.M.; Luecke, R.W. Effect of Zinc Deficiency on the Immune Response of the Young Adult A/J Mouse. J. Nutr. 1977, 107, 1889–1895. [Google Scholar] [CrossRef]
- Mills, C.F.; Quarterman, J.; Williams, R.B.; Dalgarno, A.C.; Panić, B. The Effects of Zinc Deficiency on Pancreatic Carboxypeptidase Activity and Protein Digestion and Absorption in the Rat. Biochem. J. 1967, 102, 712–718. [Google Scholar] [CrossRef]
- Maret, W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional Aspects of Manganese Homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Jaghdani, S.J.; Jahns, P.; Tränkner, M. Mg deficiency induces photo-oxidative stress primarily by limiting CO2 assimilation and not by limiting photosynthetic light utilization. Plant Sci. 2021, 302, 110751. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Zhou, P.; An, Y. Auxin Is Involved in Magnesium-Mediated Photoprotection in Photosystems of Alfalfa Seedlings Under Aluminum Stress. Front. Plant Sci. 2020, 11, 746. [Google Scholar] [CrossRef]
- Jain, A.; Sinilal, B.; Dhandapani, G.; Meagher, R.B.; Sahi, S.V. Effects of deficiency and excess of zinc on morphophysiological traits and spatiotemporal regulation of zinc-responsive genes reveal incidence of cross talk between micro-and macronutrients. Environ. Sci. Technol. 2013, 47, 5327–5335. [Google Scholar] [CrossRef]
- Liao, F.; Lilay, G.H.; Castro, P.H.; Azevedo, H.; Assunção, A.G.L. Regulation of the Zinc Deficiency Response in the Legume Model Medicago truncatula. Front. Plant Sci. 2022, 13, 916168. [Google Scholar] [CrossRef]
- Carrera, E.; Holman, T.; Medhurst, A.; Dietrich, D.; Footitt, S.; Theodoulou, F.L.; Holdsworth, M.J. Seed After-Ripening Is a Discrete Developmental Pathway Associated with Specific Gene Networks in Arabidopsis. Plant J. 2008, 53, 214–224. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, Y.; Chen, X.; Zhu, P.; Jiang, S.; Chen, S.; Xie, T.; Luo, S.; Yang, Z.; Zhang, H.; et al. Preliminary Identification of the Changes of Physiological Characteristics and Transcripts in Rice After-Ripened Seeds. Seed Biol. 2023, 2, 5. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Kalra, Y.P. Determination of pH of Soils by Different Methods: Collaborative Study. J. AOAC Int. 1995, 78, 310–324. [Google Scholar] [CrossRef]
- Miller, R.O.; Kissel, D.E. Comparison of Soil pH Methods on Soils of North America. Soil Sci. Soc. Am. J. 2010, 74, 310–316. [Google Scholar] [CrossRef]
- Libohova, Z.; Wills, S.; Odgers, N.P.; Ferguson, R.; Nesser, R.; Thompson, J.A.; West, L.T.; Hempel, J.W. Converting pH 1:1 H2O and 1:2CaCl2 to 1:5 H2O to Contribute to a Harmonized Global Soil Database. Geoderma 2014, 213, 544–550. [Google Scholar] [CrossRef]
- Merl, T.; Rasmussen, M.R.; Koch, L.R.; Søndergaard, J.V.; Bust, F.F.; Koren, K. Measuring Soil pH at in Situ like Conditions Using Optical pH Sensors (pH-Optodes). Soil Biol. Biochem. 2022, 175, 108862. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, H.; Yuan, M.; Xu, R.; Xue, L. Determination of Ammonium Nitrogen and Nitrate Nitrogen in Soil by Gas Phase Molecular Absorption Spectrometry. YKCS 2021, 40, 165–171. [Google Scholar] [CrossRef]
- Nartop, P. Chapter 9—Engineering of Biomass Accumulation and Secondary Metabolite Production in Plant Cell and Tissue Cultures. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 169–194. ISBN 978-0-12-812689-9. [Google Scholar]
- Hilty, J.; Muller, B.; Pantin, F.; Leuzinger, S. Plant Growth: The What, the How, and the Why. New Phytol. 2021, 232, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, D.; Li, H. The relationship between growth characteristics and the quality of alfalfa under sprinkler irrigation in the northwest arid area of China. Acta Paraculture Sin. 2018, 27, 54–65. (In Chinese) [Google Scholar] [CrossRef]
- Cao, Z. Cultivation and Utilization of High-Quality Alfalfa; China Agricultural Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Wang, Z.; Xu, X.; Yang, Y.; Yan, W.; Cai, G. Experimental study on the introduction of different alfalfa varieties. J. Northwest Agric. For. Univ. (Nat. Sci. Ed.) 2002, 3, 29–31. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Wei, Z.; Min, X.; Zhao, P.; Yang, L.; Liu, N. Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability. Agronomy 2023, 13, 787. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dent, T.; LeMinh, A.; Maleky, F. Comparison of Colorimetric Methods for Measuring the Solubility of Legume Proteins. Gels 2024, 10, 551. [Google Scholar] [CrossRef]
- Hassan, S.; Mazhar, W.; Farooq, S.; Ali, A.; Musharraf, S.G. Assessment of Heavy Metals in Calcium Carbide Treated Mangoes by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Food Addit. Contam. Part A 2019, 36, 1769–1776. [Google Scholar] [CrossRef]
- Gulmez, O.; Tiryaki, D.; Atici, O.; Baris, O. Boron-Resistant Alternaria alternata (OG14) Mitigates Boron Stress by Improving Physiological and Antioxidative Response in Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2023, 202, 107911. [Google Scholar] [CrossRef]
- Prerna, D.I.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Vasantharaja, R.; Chaturvedi, S.; Shkolnik, D. Influence of Nanoscale Micro-Nutrient α-Fe2O3 on Seed Germination, Seedling Growth, Translocation, Physiological Effects and Yield of Rice (Oryza sativa) and Maize (Zea mays). Plant Physiol. Biochem. 2021, 162, 564–580. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, J.; Shen, P.; Zhao, Y.; Li, Y.; Liu, Y.; Bi, Z.; Liu, Z. Determination of 33 kinds of metallic elements in food aquatic products and animal tissues by microwave digestion and ICP-MS method. J. Food Saf. Qual. 2015, 6, 953–961. [Google Scholar] [CrossRef]
- Martines, A.M.; Nogueira, M.A.; Santos, C.A.; Nakatani, A.S.; Andrade, C.A.; Coscione, A.R.; Cantarella, H.; Sousa, J.P.; Cardoso, E.J.B.N. Ammonia Volatilization in Soil Treated with Tannery Sludge. Bioresour. Technol. 2010, 101, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Moieni, A.; Sabet, M.S.; Mokhtassi-Bidgoli, A.; Mojarrad Nanas, S. Introducing Gamborg’s B5, a High-Potential Medium for Isolated Microspore Culture, and Presenting a New MS Medium-Based Protocol for Androgenic Plant Regeneration in Eggplant (Solanum melongena L.). Plant Cell Tiss. Organ. Cult. 2024, 156, 79. [Google Scholar] [CrossRef]
- Yu, M.; Tian, Y.; Gao, Q.; Xu, X.; Wen, S.; Fan, Z.; Li, X.; Gong, J.; Liu, Y. Nonlinear Response of Lucerne (Medicago sativa) Biomass and Biological Nitrogen Fixation to Different Irrigations and Sowing Modes. Appl. Soil Ecol. 2018, 125, 257–263. [Google Scholar] [CrossRef]
- Devries, J.D.; Bennett, J.M.; Albrecht, S.L.; Boote, K.J. Water Relations, Nitrogenase Activity and Root Development of Three Grain Legumes in Response to Soil Water Deficits. Field Crops Res. 1989, 21, 215–226. [Google Scholar] [CrossRef]
- Hungria, M.; Vargas, M.A.T. Environmental Factors Affecting N2 Fixation in Grain Legumes in the Tropics, with an Emphasis on Brazil. Field Crops Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- González, E.M.; Larrainzar, E.; Marino, D.; Wienkoop, S.; Gil-Quintana, E.; Arrese-Igor, C. Physiological Responses of N2-Fixing Legumes to Water Limitation. In Legume Nitrogen Fixation in a Changing Environment: Achievements and Challenges; Sulieman, S., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 5–33. ISBN 978-3-319-06212-9. [Google Scholar]
- Zhang, X.; Chen, C.; Zhang, J.; Zeng, Y.; Bao, M.; Zhang, S.; Shang, J.; Sha, X.; Wu, J.; Zhang, G.; et al. Analysis and Comprehensive Evaluation of Agronomic and Yield Traits of 55 Alfalfa Varieties. Acta Agrestia Sin. 2023, 31, 3453–3461. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Li, C.; Li, M.; Xiong, T.; Tang, Y. Dry Matter and Nitrogen Accumulation, Partitioning, and Translocation in Synthetic-Derived Wheat Cultivars under Nitrogen Deficiency at the Post-Jointing Stage. Field Crops Res. 2020, 248, 107720. [Google Scholar] [CrossRef]
- Meng, Q.; Yue, S.; Chen, X.; Cui, Z.; Ye, Y.; Ma, W.; Tong, Y.; Zhang, F. Understanding Dry Matter and Nitrogen Accumulation with Time-Course for High-Yielding Wheat Production in China. PLoS ONE 2013, 8, e68783. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Han, Q. The Effect of Different Fertilization on Stem/Leaf Ratio and FW/DW Ratio of Alfalfa. Acta Agric. Boreali-Occident. Sin. 2007, 16, 121–125. (In Chinese) [Google Scholar]
- de Oliveira, W.S.; Oliveira, P.P.A.; Corsi, M.; Duarte, F.R.S.; Tsai, S.M. Alfalfa yield and quality as function of nitrogen fertilization and symbiosis with Sinorhizobium meliloti. Sci. Agric. 2004, 61, 433–438. (In Spanish) [Google Scholar] [CrossRef]
- Liu, Z.; Yu, N.; Camberato, J.J.; Gao, J.; Liu, P.; Zhao, B.; Zhang, J. Crop Production Kept Stable and Sustainable with the Decrease of Nitrogen Rate in North China Plain: An Economic and Environmental Assessment over 8 Years. Sci. Rep. 2019, 9, 19335. [Google Scholar] [CrossRef]
- Rasheed, F.; Markgren, J.; Hedenqvist, M.; Johansson, E. Modeling to Understand Plant Protein Structure-Function Relationships—Implications for Seed Storage Proteins. Molecules 2020, 25, 873. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Selling, G.W.; Hatfield, R.; Digman, M. Extraction, Composition, and Functional Properties of Dried Alfalfa (Medicago sativa L.) Leaf Protein. J. Sci. Food Agric. 2017, 97, 882–888. [Google Scholar] [CrossRef]
- Motsinger, L.A.; Young, A.Y.; Feuz, R.; Larsen, R.; Brady, T.J.; Briggs, R.K.; Reichhardt, C.C.; Pratt, C.; Thornton, K.J. Replacing Alfalfa Hay with a Novel Alfalfa Leaf Pellet Product (ProLEAF MAX) and/or Alfalfa Stems (ProFiber Plus) in the Diet of Developing Dairy Heifers Alters Dry Matter Intake, but Does Not Negatively Impact Growth or Development. Transl. Anim. Sci. 2024, 8, txae038. [Google Scholar] [CrossRef] [PubMed]
- Maia, V.M.; Pegoraro, R.F.; Aspiazú, I.; Oliveira, F.S.; Nobre Danúbia, A.C. Chapter 50—Diagnosis and management of nutrient constraints in pineapple. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Oxford, UK, 2020; pp. 739–760. [Google Scholar]
- Xin, W.; Zhang, L.; Gao, J.; Zhang, W.; Yi, J.; Zhen, X.; Bi, C.; He, D.; Liu, S.; Zhao, X. Adaptation Mechanism of Roots to Low and High Nitrogen Revealed by Proteomic Analysis. Rice 2021, 14, 5. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. PPB 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Study on Soybean Root Systems Along with Its Relations with Upperground Characteristics. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2006. [Google Scholar]
- Anoop, A.A.; Pillai, P.K.S.; Nickerson, M.; Ragavan, K.V. Plant Leaf Proteins for Food Applications: Opportunities 624 and Challenges. Compr. Rev. Food Sci. Food Saf. 2023, 22, 473–501. [Google Scholar] [CrossRef]
- Heppner, S.; Livney, Y.D. Green Leaves as a Promising Source for Sustainable Food Protein: Seeking the Productivity-Functionality Balance. Trends Food Sci. Technol. 2023, 142, 104207. [Google Scholar] [CrossRef]
- Jiang, Q.; Fu, C.; Wang, Z.-Y. A Unified Agrobacterium-Mediated Transformation Protocol for Alfalfa (Medicago sativa L.) and Medicago truncatula. In Transgenic Plants: Methods and Protocols; Kumar, S., Barone, P., Smith, M., Eds.; Springer: New York, NY, USA, 2019; pp. 153–163. ISBN 978-1-4939-8778-8. [Google Scholar]
- Gherardi, M.J.; Rengel, Z. The Effect of Manganese Supply on Exudation of Carboxylates by Roots of Lucerne (Medicago sativa). Plant Soil 2004, 260, 271–282. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahmed, M.B.; Alotaibi, F.; Alotaibi, K.D.; Ziadi, N.; Lee, K.-W.; Kabir, A.H. Growth and Physiological Impairments in Fe-Starved Alfalfa Are Associated with the Downregulation of Fe and S Transporters along with Redox Imbalance. Chem. Biol. Technol. Agric. 2021, 8, 36. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace Metals and Animal Health: Interplay of the Gut Microbiota with Iron, Manganese, Zinc, and Copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Trakooljul, N.; Liu, H.-C.; Moeser, A.J.; Spears, J.W. Iron Transporters Are Differentially Regulated by Dietary Iron, and Modifications Are Associated with Changes in Manganese Metabolism in Young Pigs. J. Nutr. 2009, 139, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Li, Y. Study on the rapid determination technology of glucosinolate in rapeseed grain. Chin. J. Oil Crop Sci. 1998, 20, 66–69. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).