Abstract
Nutrient seed priming is a promising technique for enhancing nutrient uptake and improving crop growth, especially under water stress conditions. This study investigated the effects of various priming treatments on water stress tolerance and the uptake of essential nutrients, including nitrogen (N), phosphorus (P), potassium (K), and zinc, in Capsicum annuum L. plants grown under varying moisture conditions (30% field capacity (FC), 50% FC, and 80% FC). Seed priming was conducted using two nutrient solutions: potassium nitrate (KnO3) and zinc oxide (ZnO) with best-performing concentrations, i.e., ZnO 20 mg/L and KnO3 10 g/L and the best priming duration of 12 h obtained from a previous preliminary glasshouse experiment. The study examined the effectiveness of different priming solutions, zinc oxide, potassium nitrate (KnO3), and water (H2O), at various field capacities (30%, 50%, and 80%). The results demonstrated that nutrient priming significantly influenced nutrient uptake, with KnO3 and H2O priming showing the most pronounced effects on N, P, and K uptake. ZnO-primed seedlings absorbed 54.63% more nitrogen compared to the control and 25.7% more phosphorus. Zn uptake was significantly influenced by the interaction between priming treatment and moisture content, while ZnO priming generally resulted in 25.6% lower Zn uptake compared to the control and other treatments. The highest Zn concentrations of 32 mg/kg were observed in control plants grown under very low and optimum moisture conditions (30% and 80% field capacity). The results imply that the ideal absorption of Zn is influenced by both priming and moisture factors. Overall, this study highlights that nutrient seed priming, especially with KnO3 and water, effectively enhances nitrogen, phosphorous, and potassium uptake in Capsicum annuum plants. Optimizing priming treatments, especially in conjunction with appropriate moisture management (50–80% FC), is crucial for maximizing nutrient acquisition and plant growth and development. The complex interaction between ZnO priming and moisture content highlights the species-specific nature of priming responses, particularly for Zn absorption.
1. Introduction
Chili peppers (Capsicum annum L.) are regarded as high-value crops for farmers and play an important role in strengthening the economy of South Africa [1]. The high demand for chilies has the potential to boost income for South African small-scale farmers. The entire chili produce is usually sold out due to a shortage in production in the country [1]. According to [2], despite this huge demand, several economic and environmental production factors hinder production, resulting in the inability of South African small-scale farmers to keep up with the growing demand for produce. During germination, chili seeds are mostly vulnerable to injury and environmental stress, such as drought, salinity, and diseases, leading to crop deterioration and low yields. In drought-prone countries like South Africa, chili production and quality can be negatively impacted by insufficient water and excessive heat, leading to stunted growth and lower overall yields. Water stress affects crops’ ability to absorb nutrients and interferes with biochemical processes such as photosynthesis, resulting in reduced inorganic phosphate reserves, increased starch production, and increased abscisic acid accumulation [3].
Previous researchers have proven that prolonged drought can result in stock shortages of chili crops due to limited access to water for irrigation [4,5]. Studies have also indicated that water stress, induced by drought conditions, can significantly reduce root and shoot development, resulting in decreased yield and poor crop quality [6]. Water deficit has been associated with an increase in the pungency of chili peppers, affecting their taste and market value [7]. Moreover, insufficient water due to drought stress compromises the physiological and biochemical responses of chili crops, affecting their antioxidant content [8]. These negative impacts of water scarcity on chili crop yield highlight the importance of creating innovative water management procedures and crop resilience strategies. This can be achieved by adopting sustainable agricultural practices that conserve water resources, such as nutrient seed priming.
Nutrient seed priming has been found to be a sustainable strategy for enhancing crop performance and resilience during drought [9,10]. This technique involves pre-treating seeds with nutrient solutions to activate metabolic processes associated with germination, leading to faster and uniform emergence. Seedling vigor and the plant’s ability to withstand periods of drought are also improved through seed priming [11]. Nutrient seed priming has the added advantage of supplying the seed with nutrients that aid in its early development. Recent studies have demonstrated that nutrient priming can enhance a plant’s ability to be resilient to water stress [12,13,14]; it has been proven to significantly improve germination, seedling growth, and physiological responses [14,15]. According to [14], priming resulted in increased growth and betacyanin content under water-deficit conditions in Beetroots (Beta vulgaris L.).
Morden agricultural practices and concepts necessitate the enhancement of crop productivity and nutritional value while minimizing environmental consequences. Nutrient seed priming is a developing concept that has been proven to enhance crop production by supplying the required nutrients to crop seed prior to sowing. Previous research shows that seed priming can increase nutrient uptake and improve chlorophyll levels, ultimately increasing photosynthesis [16]. In the crops studied, nutrient seed priming positively impacted mineral nutrition and improved yield and the nutritional quality of the crop [17]. This method also improves seedling development and nutrient uptake in plants exposed to stress conditions. For example, priming with ZnO promotes salt stress tolerance in maize [18]. Moreover, micronutrient seed priming positively influences mineral absorption, biomass accumulation, and water utilization efficiency in crops [19]. Furthermore, it is associated with improved seedling vigor, root growth, and nutrient uptake, resulting in better plant competency. Studies have shown that seed nano-priming can lead to improved plant growth, increased productivity, and enhanced nutritional quality of food [20].
Several studies have been conducted on seed priming of chilies with various agents, and there have been varying results. Priming chili seeds with salicylic acid produces higher germination rates due to the modulation of ethylene levels and increasing total soluble sugar contents and superoxide dismutase (SOD) activity [21]. Additionally, the use of manganese nanoparticles in seed priming has been demonstrated to control salinity-modulated molecular responses in Capsicum annuum [22]. The focus of this study is on N, P, K, and Zn, which are all crucial for plant growth and development. Nitrogen, phosphorus, and potassium are important macronutrients that are involved in energy metabolism, structural development, and stress tolerance. Additionally, zinc plays a significant role in enzyme activation and cellular function. It is essential to understand how nutrient seed priming affects the uptake and accumulation of these nutrients in chili peppers, as this will help to optimize agricultural practices and ensure sustainable production under a variety of environmental conditions.
While nutrient seed priming has been studied extensively across various crops and conditions, its specific role in enhancing water stress tolerance on chilies remains largely unexplored within the South African agricultural and climatic context. Given the increasing challenges posed by water scarcity and the vulnerability of high-value crops like chilies to drought stress, there is a need for sustainable solutions, especially for resource-poor farmers. As indicated earlier, existing studies have demonstrated the efficacy of seed priming in improving drought resilience in crops such as wheat and maize. However, the physiological and agronomic responses of chilies to nutrient priming in terms of macronutrients and zinc uptake have not been adequately addressed. Furthermore, the interaction between nutrient uptake and resilience to drought under South African soil and environmental conditions is poorly understood. By addressing these gaps, this study aims to provide valuable insights for improving the productivity and resilience of chilies, which have become a vital crop for local consumption and economic growth. Most importantly, these findings will not only advance the scientific understanding of seed priming under stress conditions but also contribute to sustainable farming practices and food security in water-scarce regions.
2. Materials and Methods
The experiment was conducted as a pot trial under glasshouse conditions at the Agricultural Research Council—Vegetable, Industrial, and Medicinal Plants (ARC-VIMP) in Roodeplaat, Pretoria (Figure 1). In November 2023, minimum/maximum ambient temperatures were maintained at an average of 13/25 °C, with maximum temperatures controlled using thermostatically activated fans in the glasshouse, and NPK and Zn were analyzed at the Agricultural Research Council—Vegetable, Industrial and Medicinal Plants (ARC-VIMP) in Rustenburg, Northwest.
Figure 1.
ARC-VIMP map.
2.1. Plant Material
Chili seeds used in this study were obtained from Starke Ayres (Pty), Pepper Hot-STAR 6603 F1 hybrid. The seed mix is characterized by a germination period of 16–18 days and reaches maturity in 75–80 days. The ideal sowing conditions for this is full sun exposure during the spring and summer months. The seeds were stored in a cool, dry place prior to the experiments.
2.2. Soil Material
The soil used for the trial was obtained from the ARC-VIMP in Roodeplaat. Soil samples were collected from the 0 to 20 cm depth after removing surface litter using an auger and were homogenized thoroughly to make a composite sample. It was naturally air-dried and sieved (2 mm). Subsamples were then transported to the laboratory in sampling bags for analysis. The following physio-chemical properties were analyzed: soil pH, texture, exchangeable bases (Ca, Mg, K, and Na), soil organic carbon (SOC), electrical conductivity (EC), cation exchangeable capacity (CEC), and phosphorus (P). Soil pH was measured in water at a soil water ratio of 1:2.5 using a pH meter (model PH25, Crison Instruments, Johannesburg, South Africa) after shaking the suspensions for 30 min and equilibrating for 30 min [23]. EC was determined from the same suspension, similar to pH, using an EC meter (model PH25, Crison Instruments, Johannesburg, South Africa). Particle size distribution was measured using the pipette method after oxidizing SOM with hydrogen peroxide [23] Soil organic carbon was determined using the Walkley–Black chromic acid wet oxidation method [24]. Exchangeable bases (Ca, Mg, Na, and K) were determined by treating the samples with 1 M ammonium acetate buffered at pH 7.0, while CEC was calculated from the cations. Phosphorus was determined following the Bray 1 method [23,24].
2.3. Priming Treatments
Seed priming was conducted using two nutrient solutions: potassium nitrate (KnO3) and zinc oxide (ZnO) using the best-performing concentrations from the preliminary experiment, i.e., ZnO 20 mg/L and KnO3 10 mg/L. The seeds were immersed in the respective concentrations of the nutrient solutions for 12 h at room temperature.
2.4. Experimental Design
A factorial experiment was designed to evaluate the combined effects of seed priming treatments and water stress levels on chili growth. The experiment followed a completely randomized design (CRD) with a 4 × 3 factorial treatment structure. There were two factors: seed priming and water stress levels. The seed priming treatments consisted of the best performing KnO3 and ZnO concentrations from the preliminary experiments, i.e., ZnO at 20 mg/L, KnO3 at 10 g/L, as well as 2 additional priming treatments, i.e., hydropriming, which served as the positive control (seeds soaked in water for 12 h) and unprimed seeds (negative control) (Supplementary Materials). The water stress levels were as follows: 30% field capacity (FC) (severe water stress), 50% field capacity (FC) (moderate water stress), and 80% field capacity (FC) (no water stress, which served as the control). Field moisture content was measured using the decagon moisture meter; water stress was imposed by withholding water or adding based on the results produced by the decagon moisture sensors. Each time water levels reached below the required moisture level, water was re-added until the calculated required moisture was reached [25,26,27]. This factorial arrangement aimed to assess the interaction between priming treatments and water stress levels. Each treatment combination was replicated three times.
2.5. Experimental Procedures
The experimental pots were prepared by adding prepared soil into each pot. All the pots, including the control pots, were treated with NPK added to achieve the recommended rate for chilies for the soil form used. Seed priming was performed by dissolving the required mass of ZnO and KnO3 powder in distilled water and was stirred with a magnetic stirrer. After the priming treatment, three seeds of each treatment were sown in 25 cm plastic pots at a depth of 3 cm. Each pot was irrigated using 500 mL of tap water every second day for two weeks until the seedlings were well established. After seedling establishment, drought stress was imposed, as described above. Decagon Moisture Sensors were used to monitor and maintain the desired soil moisture levels. Irrigation was adjusted accordingly to maintain 30%, 50%, and 80% FC, re-watering to the respective percentage FC, while unstressed seedlings were irrigated continuously (Table 1) [28]. Table 1 shows the experimental treatments used in this study.

Table 1.
Experimental treatments.
2.6. Data Collection
Data were collected from November 2023 to May 2024.
The following parameters were measured:
- i.
- Emergence percentage (EP) was calculated when cumulative emerged seeds with normal radicle and plumule were visible using the following equation:
- ii.
- Days to emergence (DE), chlorophyll content index (CCI), stem diameter (SD), seedling height (SH), seedling weight (fresh (FSW) and dry (DSW)), and final root length (RL) were measured. The stem diameter was measured with a digital Vernier caliper (Mac Afric, Johannesburg, South Africa), chlorophyll content was measured using a chlorophyll meter (MINOLTA SPAD-502, Minolta Camera Co., Osaka, Japan), and seedling height was measured using a ruler. On the final day of the experiment, seedlings were uprooted and washed off over a 53 μm sieve to remove all the soil from the roots. Root length was measured with a ruler, whilst FWS was measured with a weighing balance. Dry seedling weight mass was measured after drying the seedlings at 65 °C in a forced air oven until constant weight was achieved.
Data on plant NPK and Zn uptake were also collected. Plant samples were dried at 60 °C to a constant weight, milled, and stored in sampling bags for analysis. Nitrogen was determined using the Kjeldahl method, phosphorus (P) using the Vanado-molybdate method, and potassium (K) using flame photometry, following [29]. Phosphorus was measured at 420 nm using a phospho-molybdovanadate complex. Potassium content was quantified at 766.5 nm with a flame photometer, calibrated within the range of 0–6.0% K. Zinc content was determined using atomic absorption spectroscopy (AAS) at 213.9 nm after sample digestion with a mixture of nitric acid and hydrogen peroxide [30].
2.7. Data Analysis
Analysis of variance (ANOVA) was carried out as a factorial treatment structure to test the effects of seed priming and soaking drought effects on chilies growth and development [30] using JMP 14.0 version 17 Pro statistical software (SAS Institute, Inc., Cary, NC, USA, 2022).
3. Results
The initial properties of the soil used in this study are shown in Table 2. The soil had a slightly alkaline pH of 7.25 and was characterized by a sandy loam texture, comprising 64% sand, 28% clay, and 8% silt. The total carbon and nitrogen content were relatively low, at 0.47% and 0.038%, respectively. Available phosphorus was 11.95 mg/kg, while available potassium and zinc were measured at 140 mg/kg and 4.03 mg/kg, respectively. The concentrations of available and exchangeable calcium and magnesium were notably higher compared to sodium and potassium.

Table 2.
The initial soil properties of the soil used.
3.1. The Effects of Nutrient Seed Priming and Water Stress on Germination Indices
Analysis of variance indicated that there were no significant interaction effects for all parameters except for dry seedling weight (DSW) (Table 3). There were significant nutrient (p < 0.05) effects on all parameters except for root length (RL) and CCI. Moisture content significantly (p < 0.05) affected all parameters except RL and SVI (Table 3).

Table 3.
ANOVA table for the effect of nutrient seed priming and drought stress on the number of leaves, stem height (SH), fresh seedling weight (FSW), dry seedling weight (DSW), stem diameter (SD), chlorophyll content index (CCI), and seedling vigor index (SVI).
3.2. The Effects of Nutrient Seed Priming and Water Stress on the Number of Leaves
The number of leaves was significantly affected by both nutrient seed priming and drought conditions (p < 0.05). Seeds primed with KnO3 had the highest number of leaves, amounting to approximately nine leaves per seedling, whilst the control produced six leaves, which was the lowest ((A) in Table 4). However, there were no differences in the number of leaves between seedlings primed with KnO3 and ZnO, but both were significantly higher than those primed with H2O and the unprimed control ((A) in Table 4). Similarly, drought stress had significant effects on the number of leaves (p < 0.05), with 80% FC resulting in the highest number of leaves, averaging nine leaves per seedling ((B) in Table 4). The number of leaves at 80% FC and 50% FC were not significantly different from each other but were both significantly higher than the number of leaves at 30% FC. ((B) in Table 4)

Table 4.
(A) Effect of nutrient seed priming on the number of leaves, stem height, fresh seedling weight, and stem diameter. (B) Effect of drought stress on the number of leaves, stem height, fresh seedling weight, and stem diameter. Different letters indicate significant differences at p < 0.05.
3.3. The Effects of Nutrient Seed Priming and Water Stress on Shoot Height
Shoot height was significantly influenced by nutrient seed priming. Both ZnO and KnO3 significantly increased shoot height compared to hydro-primed and un-primed seeds (negative control), but there was no significant difference between ZnO and KnO3. H2O primed. However, seeds primed with H2O were significantly taller than the control ((A) in Table 4). Seedlings primed with ZnO produced the tallest shoots, averaging about 10.5 cm, while the shortest shoots (8.1 cm) were observed in the unprimed control ((A) in Table 4). Water stress also had a significant effect on shoot height. The longest shoots (117 cm) were observed with 80% FC, which was significantly taller than 50% FC and 30% FC. Shoots under the 30% FC treatments, which represented severe drought stress, were the shortest shoots, averaging 7.1 cm ((B) in Table 4).
3.4. The Effects of Nutrient Seed Priming and Drought Stress on Fresh Seedling Weight
Fresh seedling weight was significantly influenced by nutrient seed priming. KnO3-primed seedlings produced the highest fresh biomass, averaging 2.4 g, while the unprimed control had the lowest fresh weight, averaging 1.4 g. However, there were no significant differences between KnO3 and ZnO treatments. Both KnO3 and ZnO were significantly different from H2O-primed and unprimed controls, whereas hydro-primed and unprimed were not significantly different ((A) in Table 4). Drought stress also had a significant impact on fresh seedling weight. Seedlings under 80% FC resulted in the highest fresh biomass (2.7 g), which was significantly greater than seedlings at 50% FC. Similarly, seedlings grown at at 50% FC had significantly higher weight than those exposed to 30% FC, which averaged 1.1 g ((B) in Table 4) p < 0.05.
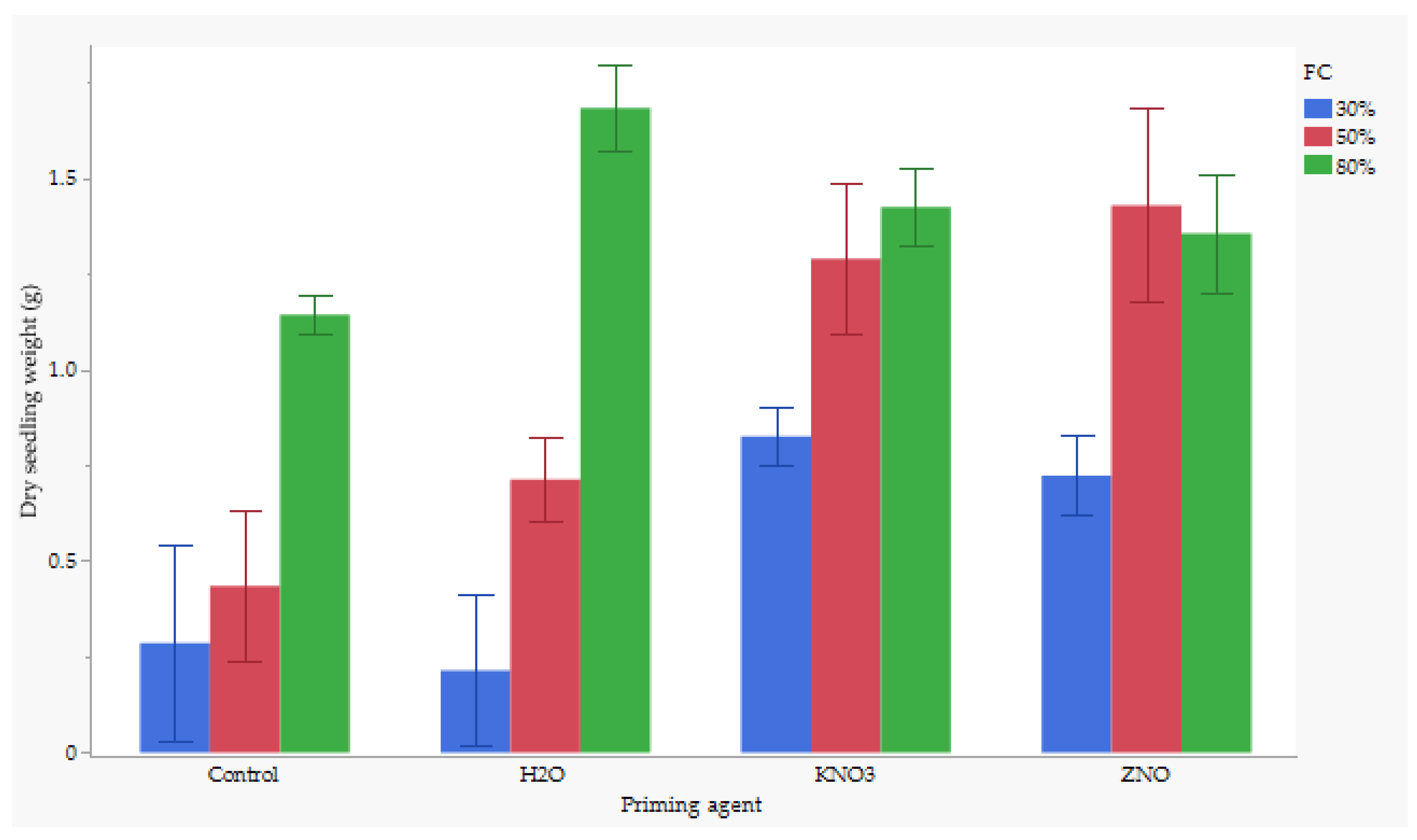
3.5. The Interaction of Nutrient and Water Stress Effects on Dry Seedling Weight
There were significant interaction effects between nutrient seed priming and water stress on dry seedling weight. Seeds primed with H2O primed seeds and maintained at 80% FC produced the highest dry seedling biomass, averaging around 1.7 g, while the lowest dry seed biomass weight was observed for H2O at 30% FC, averaging 0.2 g (Figure 2). However, the combination of H2O and 80% FC was not significantly different from ZnO at 50% FC, KnO3 at 80% FC, ZnO at 80% FC, or KnO3 at 50%. Additionally, KnO3 at 50% FC showed no significant difference compared to the control at 80% FC or KnO3 at 30% FC. However, KnO3 at 50% FC was significantly different from ZnO at 30% FC and H2O at 50% FC.
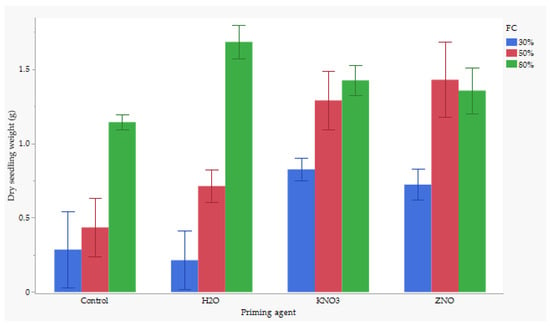
Figure 2.
Effect of nutrient × water content on dry shoot weight. Significant difference at p < 0.05.
3.6. The Effects of Nutrient Seed Priming and Drought Stress on Stem Diameter
Nutrient seed priming significantly affected stem diameter. Seedlings primed with ZnO produced the thickest stems (2.5 mm), while the control produced the thinnest stems (1.8 mm) (Table 4(A)). However, ZnO was not significantly different from KnO3. Both KnO3 and ZnO were significantly different from H2O and the control, while H2O and the control were not significantly different. (Table 4(A)). Stem diameter was significantly affected by drought. The largest stem diameter (2.6 mm) was observed at 80% field capacity (FC), which was significantly different from 50% FC and 30% FC. Stem diameter decreased as drought stress increased, with 30% FC producing the smallest stems, averaging 1.7 mm (Table 4(B)). Stems diameter at 80% FC was 21.91% thicker than 50% FC, 55.23% is thicker than 30% FC, and 50% FC is 27.32% thicker than 30% FC.
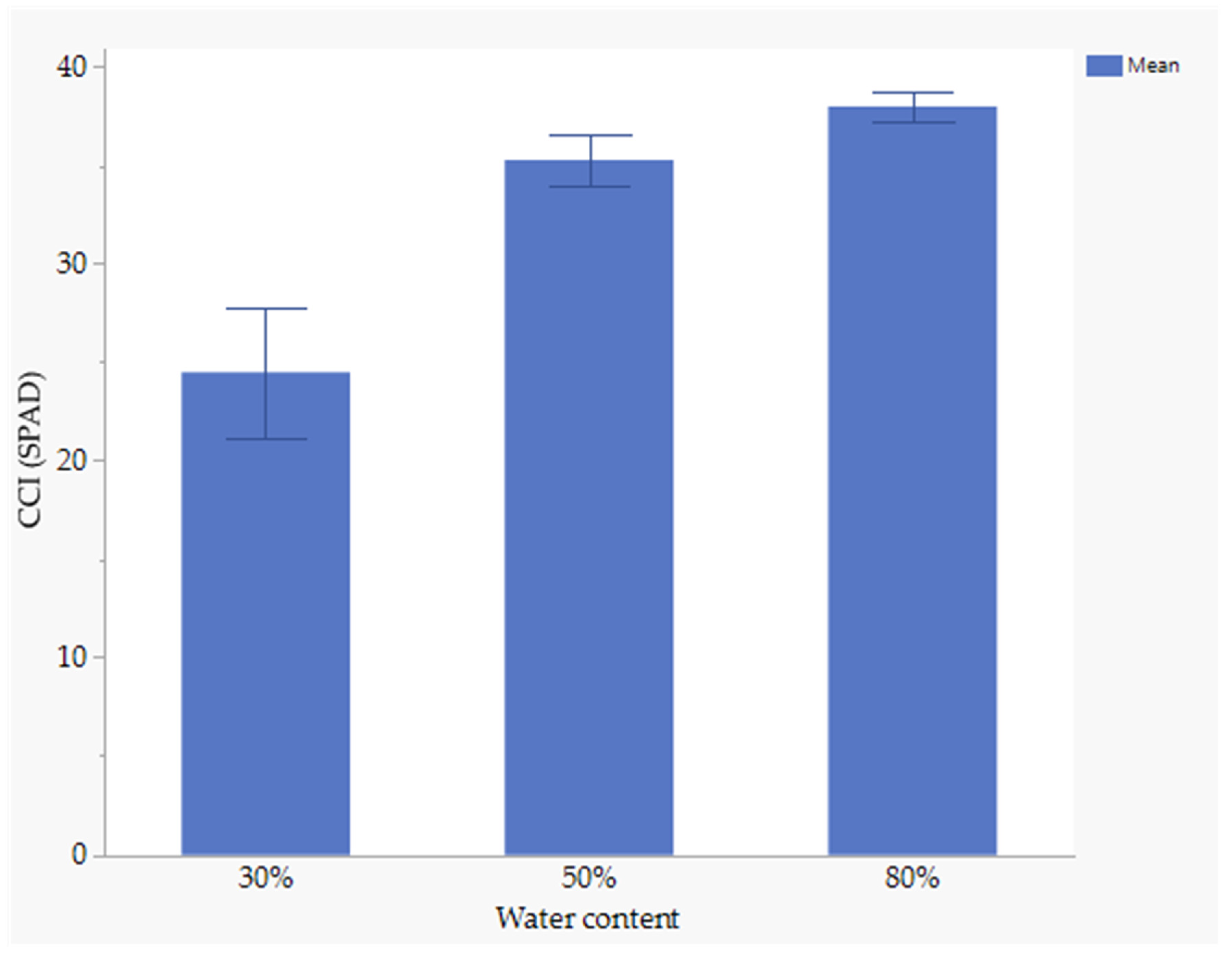
3.7. The Effect of Nutrient Seed Priming and Water Stress on Chlorophyll Content Index
Nutrient seed priming did not significantly affect the chlorophyll content index (CCI). However, water stress significantly influenced CCI.At 80% field capacity (FC), the chlorophyll content index was significantly higher (37.97.6 SPAD) compared to 30% FC. The lowest chlorophyll content index was observed at 30% FC (24.4 SPAD) (Figure 3).
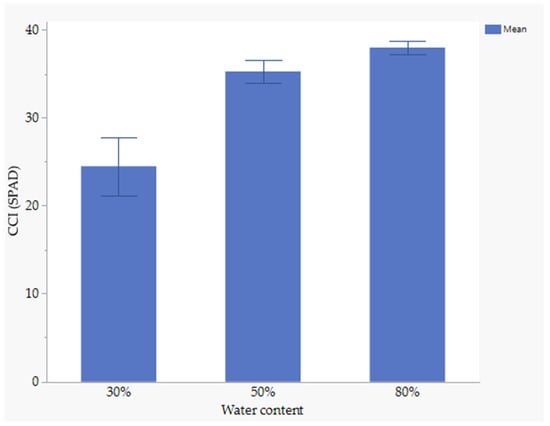
Figure 3.
Effect of water stress on CCI.
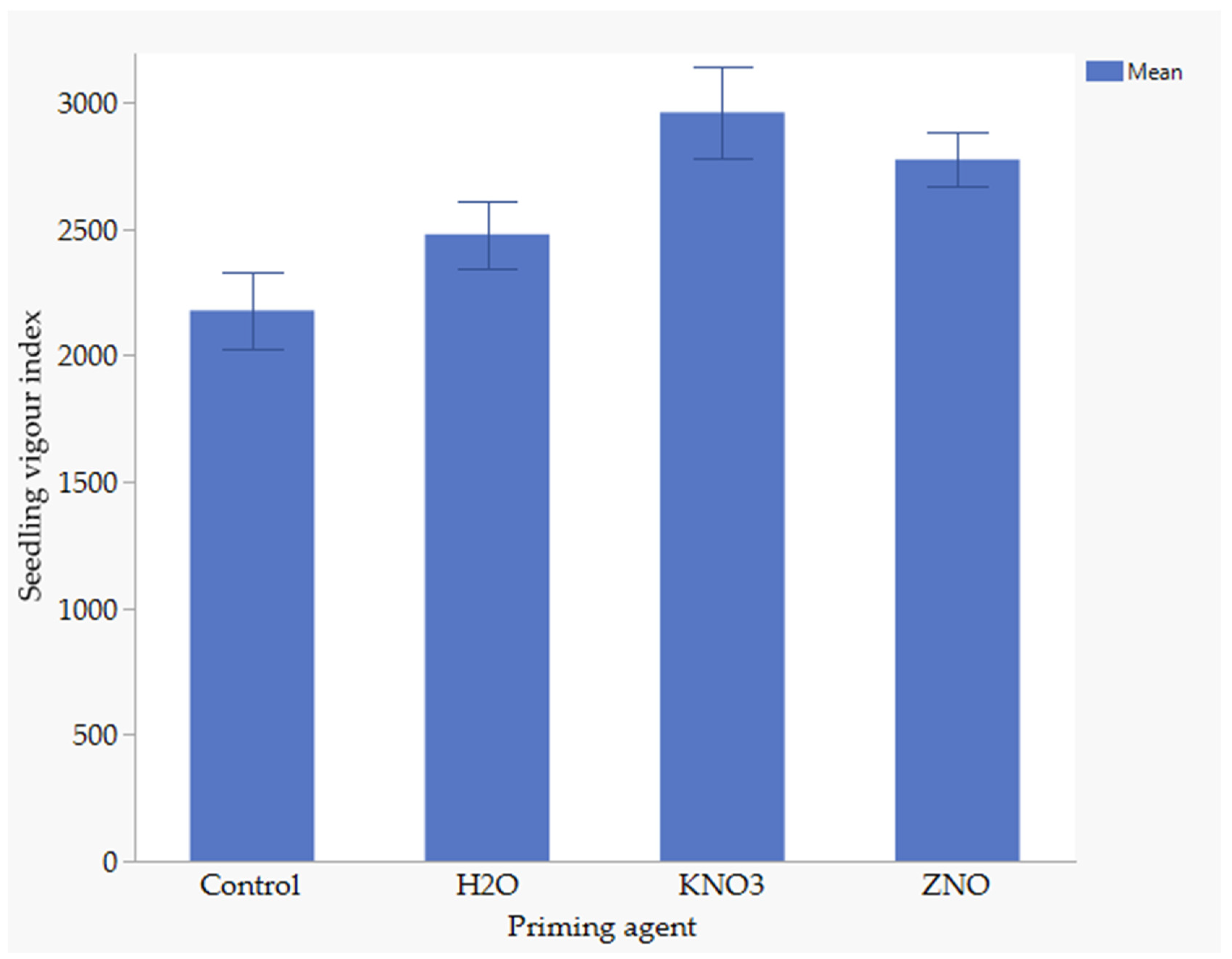
3.8. The Effect of Nutrient Seed Priming and Water Stress on Seedling Vigour Index
Water stress had no effects on seedling vigor index, whereas nutrient seed priming significantly had significant effects. The seedling vigor index ranged from 2177 to 2962, with the treatments ranked as follows from highest to lowest: KnO3, ZnO, H2O, and the control (Figure 4). There were no significant differences between KnO3 and ZnO or between H2O and ZnO on the seedling vigor index. Similarly, there was no significant difference between the unprimed control and hydroprimed seeds. However, significant differences were observed between KnO3 and the control as well as between KnO3 and H2O. KnO3 resulted in 16% higher seedling vigor compared to H2O and 26.5% higher seedling vigor than the control. Additionally, priming with H2O resulted in a 12.9% higher seedling vigor index than the control.
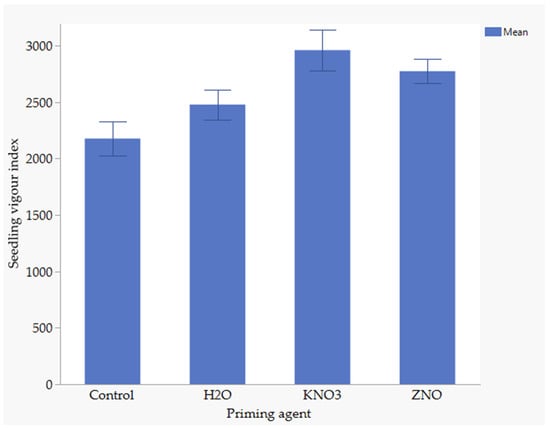
Figure 4.
Effect of nutrient on seedling vigour index, Error bars indicate significant difference at p < 0.05.
3.9. The Effect of Nutrient Priming and Water Stress on NPK Uptake
Nutrient priming had significant effects (p < 0.05) on the absorption of nitrogen (N), phosphorus (P), potassium (K), and zinc (Zn) (Table 5). However, neither moisture stress nor the interaction of nutrient priming and moisture stress had significant effects on N, P, or K uptake. The nutrient priming × moisture stress interaction significantly affected only Zn uptake (p < 0.05).

Table 5.
ANOVA effects of nutrient seed priming and drought stress on NPK and Zn uptake.
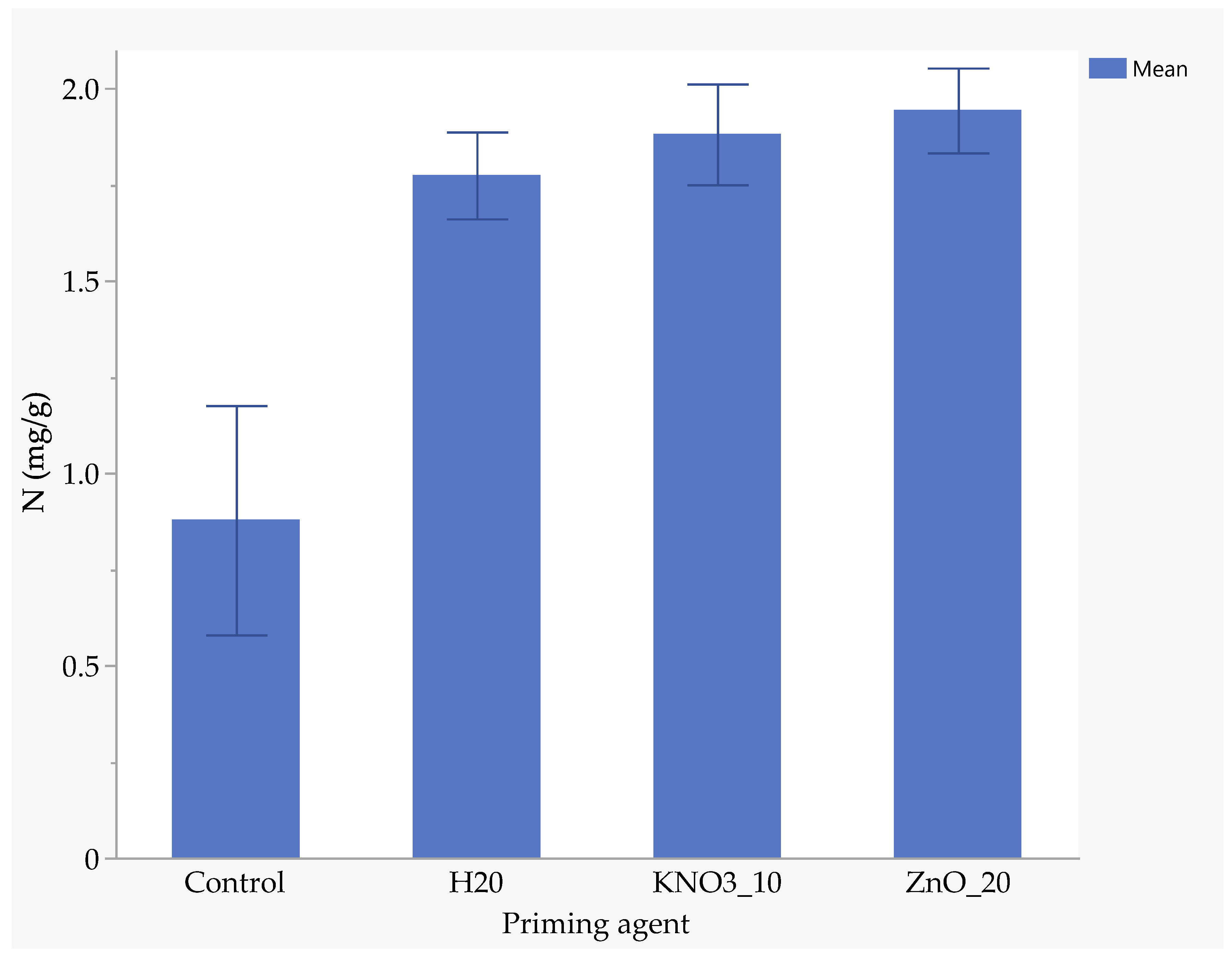
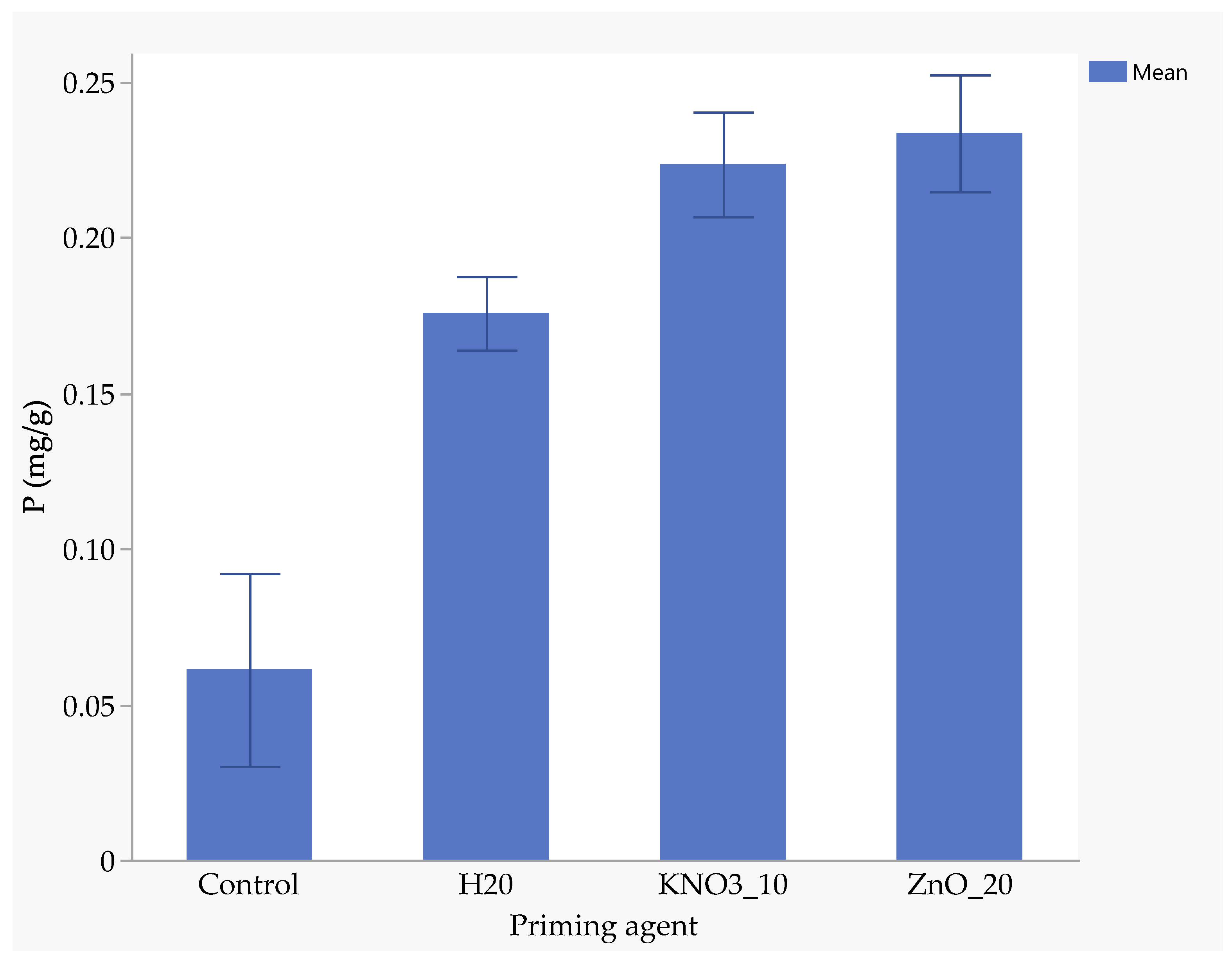
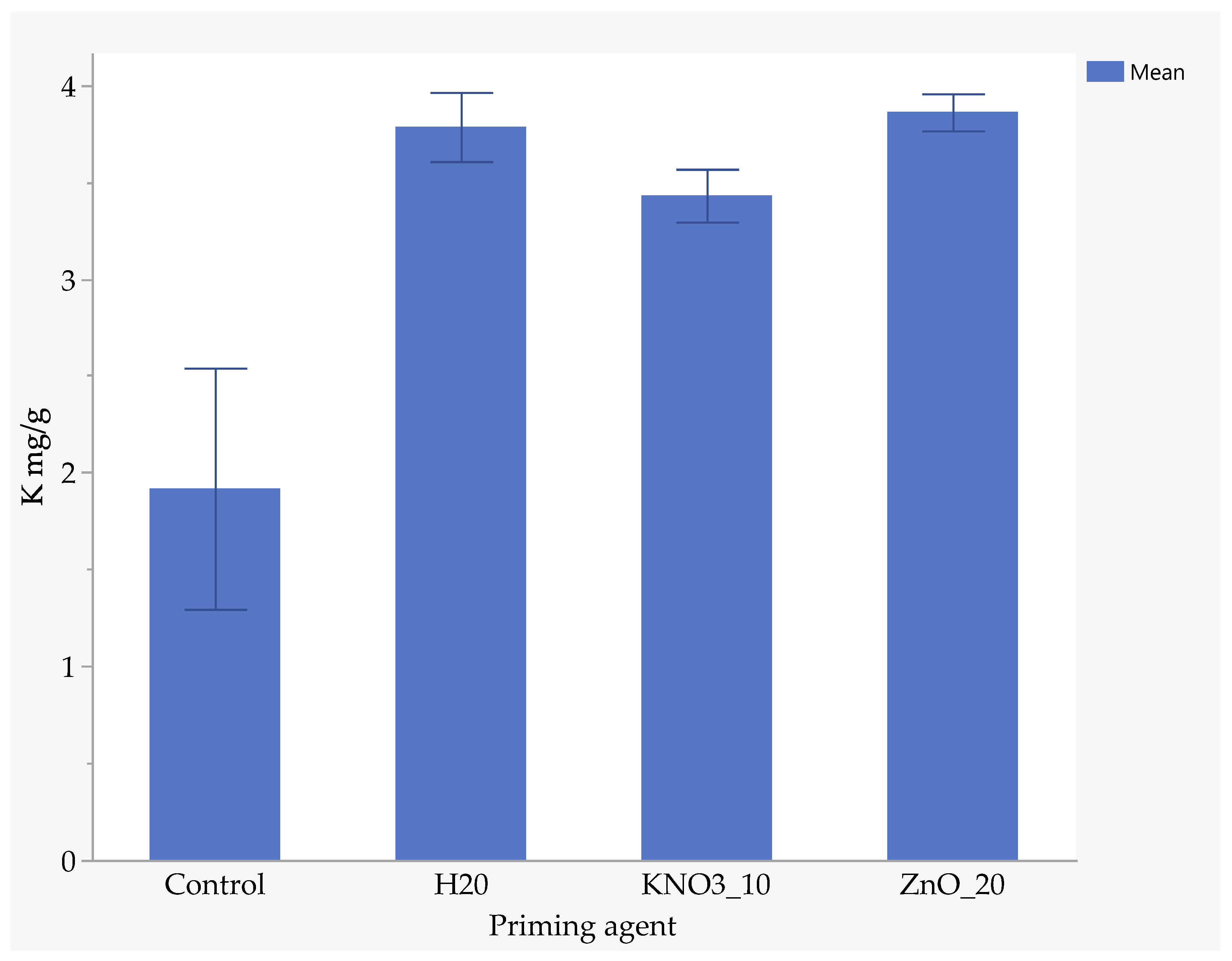
Seeds primed with ZnO had the highest nitrogen uptake (1.94 mg/kg), followed by those primed with KnO3 (1.88 mg/kg) and hydro-primed seeds (1.77 mg/kg). Un-primed seeds absorbed significantly less nitrogen (0.88 mg/kg), highlighting the benefits of nutrient priming (Figure 5). ZnO-primed seeds absorbed 25.7% more phosphorus than hydro-primed seeds and 117% more than the un-primed seeds (Figure 6). The uptake of phosphorus by KnO3-primed seeds was comparable to that of ZnO and hydro-primed seeds, but it was significantly (p < 0.05) higher than the un-primed seeds (Figure 6). The absorption of potassium was not significantly different among ZnO (3.83 mg/kg), hydro-primed (3.78 mg/kg), and KnO3 (3.43 mg/kg) primed seeds. However, all these treatments were significantly higher than un-primed seeds (1.92 mg/kg) (Figure 7).
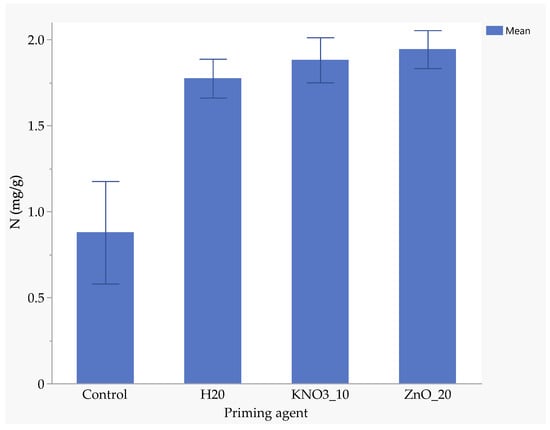
Figure 5.
Effect of priming agent on the absorption of nitrogen.
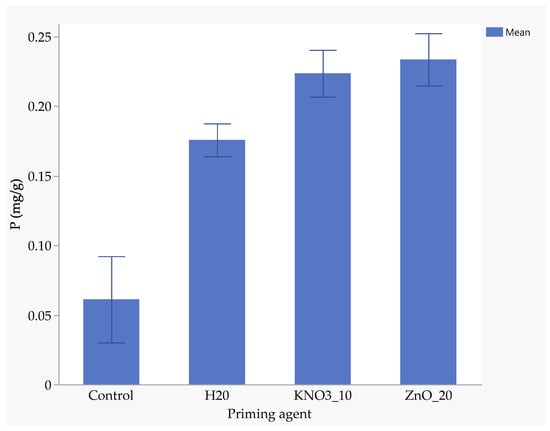
Figure 6.
Effect of priming agent on phosphorus absorption.
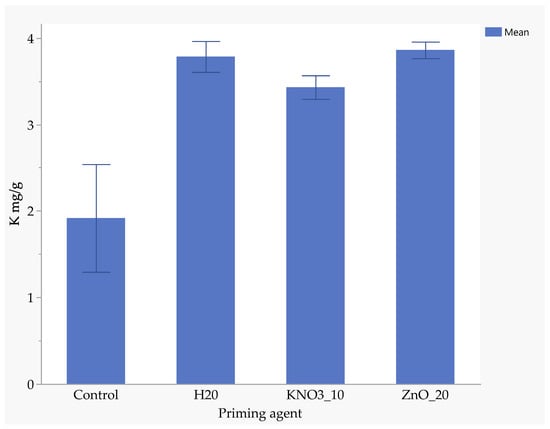
Figure 7.
Effect of priming agent on potassium absorption.
3.10. The Effect of Nutrient Seed Priming and Water Stress on Zn Uptake
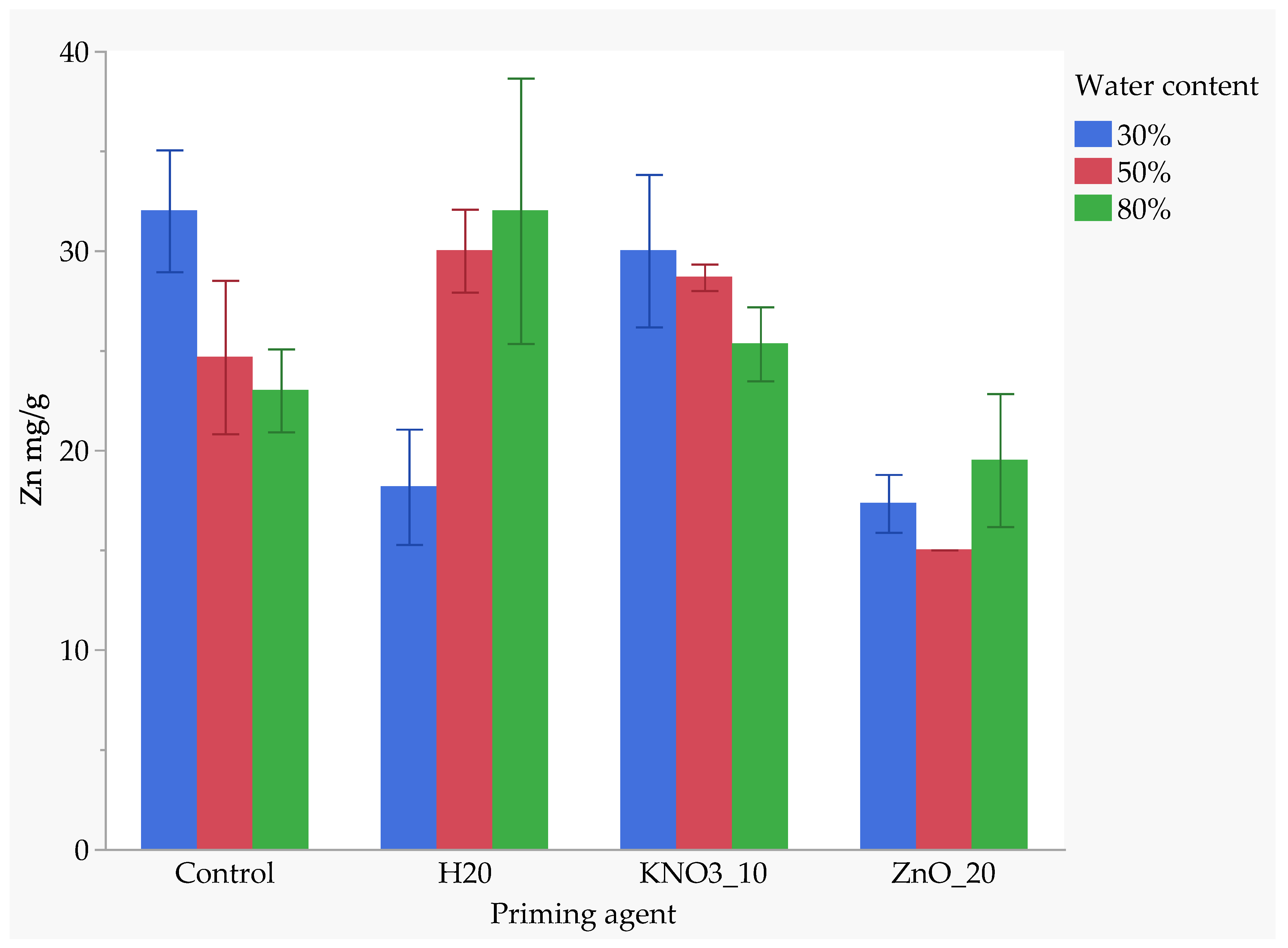
The interaction between nutrient seed priming and soil moisture levels significantly influenced zinc (Zn) uptake. The combination of the unprimed control treatment and 30% FC resulted in the highest Zn uptake, with a mean value of approximately 31 mg/kg, similar to the control at 30% FC, with no significant difference. KnO3-primed seeds maintained at 30% FC also demonstrated high Zn absorption similar to the control at 30% FC and H2O at 80% FC; H2O-primed and KnO3-primed seeds at 50% FC exhibited similarly high Zn uptake, with no significant differences among these treatments. However, Zn uptake decreased slightly in KnO3-primed seeds at 80% FC, which was significantly different from the highest uptake treatments. In the unprimed control treatment at 50% and 80% FC, Zn uptake further declined, showing significantly lower values than those in the control at 30% FC and H2O-primed seeds at 80% FC. Among all treatments, ZnO-primed seeds exhibited the lowest Zn uptake, particularly at 50% FC, where it was significantly lower than all other treatments. At 80% FC, ZnO priming resulted in a slight increase in Zn uptake compared to 50% FC but remained significantly lower than the highest-uptake treatments, indicating that Zn uptake is highly influenced by both the type of nutrient priming and the soil moisture content (Figure 8).
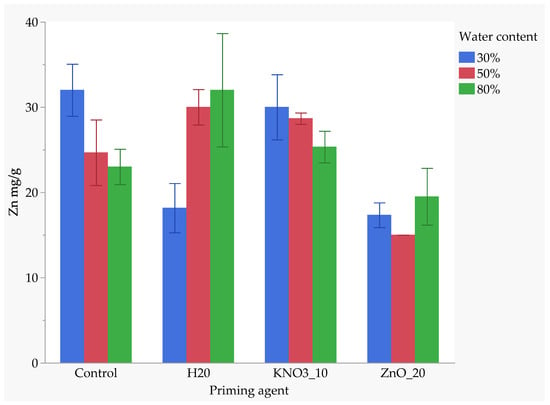
Figure 8.
Effect of priming agent and drought on zinc absorption.
4. Discussion
This study hypothesizes that ZnO and KnO3 priming enhances seedling growth by facilitating nutrient uptake and stress tolerance mechanisms. The positive effects of ZnO and KnO3 on the studied growth parameters show that nutrient seed priming can significantly improve early seedling growth by ensuring the availability of nutrients essential for plants to thrive under stressful conditions. The observed improvements align with the pre-mobilization hypothesis, which suggests that priming enhances metabolic readiness, leading to increased germination and seedling growth. This is because primed seeds have increased enzymatic activity and metabolic preconditioning, allowing for a more efficient transition from germination to seedling growth [20].
Nitrogen is essential for various physiological development processes such as photosynthesis, respiration, and protein synthesis [31]. This was evident in this study, as photosynthesis was significantly influenced by seed priming, as reflected by variations in the chlorophyll content index (Figure 3). According to the chlorophyll stability theory, potassium and zinc play vital roles in chlorophyll biosynthesis and stability. Potassium enhances electron transport efficiency, while zinc prevents chlorophyll degradation by stabilizing membrane structures. These findings are consistent with [32], who reported that seed priming with gibberellic acid (GA3) improved chili plant performance under water deficit conditions by enhancing nutrient uptake and stress tolerance.GA3 is essential for stress tolerance as it aids in seed germination, boosts cell elongation, and regulates hormonal balance, enabling plants to cope with the negative impacts of drought and nutrient shortages. GA3 also affects the expression of genes responsive to stress, highlighting its significance in plant physiological reactions to environmental challenges [33,34,35].
Zinc is essential in synthesizing growth hormones, maintaining membrane integrity, and protecting the plant against oxidative stress [36]. It also plays a key role in synthesizing growth hormones, maintaining membrane integrity, and protecting plants against oxidative stress. It influences several metabolic activities, including the activity of hydrogenase and carbonic anhydrase, the synthesis of cytochrome, the stabilization of ribosomal fractions, and auxin metabolism [36]. Similarly, potassium is essential for osmotic adjustment, stomatal regulation, and water and nutrients transportation within the plant. Our findings are consistent with previous research demonstrating the benefits of seed priming on crop performance under abiotic stress [13]. However, they contradict studies reporting increased nutrient content in drought-stressed plants [15], highlighting the context-dependent nature of plant responses influenced by crop species and priming treatments.
Adequate water content levels proved crucial for chili seedling growth, with 80% field capacity (FC) resulting in the best performance. This can be explained by the water potential gradient theory, which states that optimal moisture levels improve nutrient solubility and transport, facilitating root absorption. All studied parameters, except root length, improved significantly under higher moisture conditions (Table 3). These could be due to the importance of water in different physiological processes like cell expansion, nutrient uptake, and photosynthesis [35]. Root growth exhibited a moisture-depended response. Under water stress (30% and 50% FC), root systems elongated, likely reflecting resource allocation towards water acquisition. Conversely, at 80% FC, root elongation was reduced as plants prioritized shoots and leaf development due to readily available water. Optimum moisture level (80%FC) showed the best performance in terms of growth and development [36]. The results are consistent with previous studies on other crops that have demonstrated the positive effects of optimal moisture levels on seedling growth and vigor [37]. However, severe drought conditions (30% FC) significantly reduced growth, as evidenced by declines in the number of leaves, stem height, fresh seedling weight, and chlorophyll content index. Water deficit can result in stomatal closure, reduced transpiration, and reduced photosynthetic rates, hindering plant development and growth. These reductions can be attributed to stomatal closure, resulting in reduced transpiration and photosynthetic rates, thereby limiting plant development and biomass accumulation [38]. Additionally, water stress can cause nutrient imbalances, oxidative stress, and hormonal disturbances, which result in negative effects on plant growth. These findings are different from a study where hydro-priming was found to improve the performance of rice seedlings under water stress conditions, indicating the crop-specific nature of seed priming responses [39].
Drought conditions significantly reduced the number of leaves, stem height, fresh seedling weight, and chlorophyll content index, showing compromised plant growth and physiological functions under water scarcity. The significant decrease in chlorophyll content index under drought conditions suggests impaired photosynthetic efficiency, a critical factor influencing plant biomass accumulation and yield [40].
Moreover, the negative impact of drought on fresh seedling weight highlights the challenge of maintaining adequate water balance for metabolic activities essential for growth and development [41]. The lack of significant differences in the effects of water stress and nutrient priming on root length observed in this study could be due to the ability of the chili plant’s root system to maintain its growth and functionality under drought conditions. This can also be due to root plasticity, enabling plants subjected to water stress to allocate more resources towards root elongation, thereby improving access to water and nutrients in deeper soil layers. The maintenance of root growth is a crucial adaptive strategy for plants to access water and nutrients from deeper soil layers, thereby enhancing their tolerance to water deficits. However, nutrient-primed seedlings under water stress may have accumulated more proline and compatible solutes, enhancing osmotic adjustment and water-use efficiency [42]. Proline is an amino acid that builds up in plants due to environmental stress, contributing to maintaining balance within cells and signaling. Increased proline levels in plants under stress boost cell ability to repair itself and enhance plant resilience to environmental challenges [43]. Compatible solutes are small organic molecules that are highly soluble and are used by cyanobacteria to enhance their ability to withstand drought and salt stress [44]. These compounds maintain cell turgor, protect cellular structures, and regulate essential metabolic processes for survival and productivity during drought [45]. The increased activities of antioxidant enzymes, including superoxide dismutase, catalase, and peroxidase, in nutrient-primed seedlings under water stress indicate an enhanced capacity to scavenge reactive oxygen species and reduce oxidative damage. In addition to its direct effects on plant physiology and biochemistry, nutrient priming can also modulate root–microbiome interactions, which can contribute to improved plant performance under stress conditions. For example, inoculation of chili plants with beneficial rhizobacteria or mycorrhizal fungi can enhance their nutrient and water uptake as well as their tolerance to abiotic stresses [46]. Similar results have been reported by [47], which researched quinoa. Significant interactions between nutrient priming and drought were observed for dry seedling weight but not for other parameters like the number of leaves and stem diameter, indicating that the effects of nutrient seed priming on biomass accumulation were modulated under drought stress. No significant interactions were found for other parameters, such as the number of leaves and stem diameter. This interaction was due to the synergistic effects of priming nutrients and water stress on the physiological processes. Synergistic effects are the cooperative interaction of photosynthesis and efficient mineral nutrition in plants, which is heightened when there is a maximum difference between internal and external temperatures [48]; for this study, synergistic effects were observed.
The enhanced nutrient absorption in plants through nutrient priming can boost their ability to withstand water stress [49,50], showing that nutrients like potassium are essential for osmotic regulation, aiding plants in preserving cell turgor and water absorption during periods of water stress. Nutrient priming promotes the production of antioxidants, which helps by reducing oxidative damage brought on by water stress. Zinc acts as a shielding cell from harm. Treatment with potassium can improve this ability for osmotic adjustment. This is consistent with the findings of previous studies on the effects of nutrient priming on the growth and yield of various crops under water-limited conditions [51,52].
Nutrient seed priming significantly improved NPK and zinc uptake (Figure 5, Figure 6, Figure 7 and Figure 8). ZnO and KnO3 priming resulted in higher absorption of nitrogen, phosphorus, and potassium compared to unprimed seeds, reflecting enhanced nutrient mobilization and translocation [31], which is the release and movement of stored nutrients from one part of the plant into an actively growing region where they are required for metabolic processes. Specifically, NPK and zinc stored in seeds are remobilized to developing plant parts [48]. Zinc promotes enzymatic activity and hormonal production, supporting nutrient uptake [53]. Moisture levels influenced nutrient uptake, with higher field capacities (50% and 80%) promoting NPK and zinc absorption (Table 5). However, at lower moisture levels (30% FC), ZnO priming reduced zinc uptake, potentially due to disruptions in zinc absorption mechanisms. Zinc absorption depends on soil moisture since water acts as a medium for nutrient dissolution and transportation. At 30% field capacity (FC), there is less available water, reducing the solubility of ZnO and making it less accessible for root absorption. Zinc moves through diffusion, which is restricted under low field capacity. Under water stress, plants often increase the uptake of other stress-related nutrients such as potassium and calcium to maintain osmotic balance, creating competitive inhibition, where other nutrients outcompete zinc for uptake at root absorption sites. These results align with findings by [54] but differ from studies suggesting greater nutrient uptake under water-limited conditions with specific priming agents like zinc oxide nanoparticles [55]. This suggests that the availability and uptake of zinc may be sensitive to drought conditions, with excess water or drought potentially limiting its absorption [31]. The results of these experiments are consistent with previous research on the efficacy of nutrient priming for improving crop performance and nutrient status [31,53]. The use of nanoparticle-based priming agents, such as zinc oxide, results in greater nutrient uptake in various crop species [31,53,56]. The positive effects of nutrient priming on NPK and zinc absorption observed in this study align with previous research on the benefits of seed priming for crop productivity [35]. For example, a study on rice found that seed priming techniques like hydro-priming improve nutrient uptake and, ultimately, grain yield [35]. Drought plays a crucial role in nutrient accumulation. These results showed that a higher field capacity (50% and 80%) promotes N, P, K, and Zn uptake, except in treatments with high ZnO concentration. Water availability enables efficient nutrient transport and uptake. However, lower field capacities (30%) combined with ZnO priming exacerbate nutrient stress, leading to reduced Zn uptake.
Overall, the findings of these studies show that nutrient seed priming, particularly with zinc oxide, potassium nitrate, and water, can be a valuable strategy for improving the NPK and zinc content of capsicum annuum plants. The optimization of priming treatments and moisture conditions can be crucial for maximizing the benefits of this approach. Our findings support the species-specific priming hypothesis, which suggests that the response to seed priming is highly dependent on crop genotype and environmental conditions. The study was conducted under controlled conditions, which may not fully replicate field variability such as temperature and soil condition. Future perspectives can focus on field trials under different Agro-climatic conditions.
5. Conclusions
This study shows that nutrient seed priming significantly enhances early growth and chill seedling vigor under water stress conditions. This suggests that nutrient seed priming, particularly with zinc and potassium, can be an effective strategy to enhance the water stress tolerance of chili plants. Seed priming could potentially contribute to sustainable agriculture by reducing the dependency on irrigation water and mitigating the impact of climate variability on crop yield. Nutrient seed priming, particularly with ZnO, KnO3, and H₂O, presents a promising approach to enhancing NPK and zinc uptake in Capsicum annuum plants. However, the effects of ZnO priming on zinc absorption appear to be moisture-dependent. While ZnO improved overall nutrient availability, its effectiveness in enhancing zinc uptake was reduced under severe drought conditions (30% FC). Optimizing priming solutions and moisture conditions is crucial for maximizing nutrient acquisition and plant resilience, making nutrient priming a valuable tool for addressing challenges posed by climate variability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15040930/s1, Table S1: ANOVA table of the effect of nutrient concentration, duration and interaction on Germination Rate (GR), Germination Energy (GE), Mean Germination Time (MGT)and Germination Rate Index (GRI); Table S2: Effect of Nutrient concentration and duration on germination energy; Table S3: Some chemical characteristics of the soil used; Table S4: Effects on germination and seedling emergence indices; Table S5: ANOVA table presents the effect of nutrient seed priming duration, nutrient type and their interaction on the measured parameters.
Author Contributions
Conceptualization, M.M. and A.D.N.; methodology, M.M.; software, A.D.N.; validation, M.M. and A.D.N.; formal analysis, A.D.N.; investigation, M.M.; resources, A.D.N.; data curation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, A.D.N.; visualization, M.M and A.D.N.; supervision, A.D.N.; project administration, A.D.N.; funding acquisition, A.D.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that financial support was received for the research and authorship of this article. This study was financially supported by The Agricultural Research Council and The University of South Africa.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We extend our sincere gratitude to Stark Ayers (pty) for donating seeds. We would also like to Thank Tshepang F. Mabaso, Hlayisani Z. Mabasa, and Kaya Mrubata for their invaluable technical assistance. Our appreciation also goes to the Agricultural Research Council–Natural Resources and Engineering (ARC-NRE) for their support with all the analysis, which significantly contributed to this study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Anwar, A.; Yu, X.; Li, Y. Seed priming as a promising technique to improve growth, chlorophyll, photosynthesis and nutrient contents in cucumber seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 116–127. [Google Scholar] [CrossRef]
- Mpanza, F.N.; Mavengahama, S. Variable germination and high cost of commercial chilli seed: Implications for communal area cash crop chilli production in northern KwaZulu-Natal. Acta Hortic. 2018, 1204, 249–254. [Google Scholar]
- Mardiyati, S.; Natsir, M. Fluctuations and trends in the prices of red chilies and cayenne peppers in the traditional markets of Makassar City. IOP Conf. Ser. Earth Environ. Sci. 2024, 1302, 012124. [Google Scholar] [CrossRef]
- Jiao, X.; Yu, X.; Ding, J.; Du, Q.; Zhang, J.; Song, X.; Bai, P.; Li, J. Effects of rising VPD on the nutrient uptake, water status and photosynthetic system of tomato plants at different nitrogen applications under low temperature. Sci. Hortic. 2022, 304, 111335. [Google Scholar] [CrossRef]
- Silva, V.F.; Bezerra, C.V.C.; Nascimento, E.C.S.; Ferreira, T.N.F.; Lima, V.L.A.; Andrade, L.O. Production of chili pepper under organic fertilization and irrigation with treated wastewater. Rev. Bras. De Eng. Agric. E Ambient. 2019, 23, 84–89. [Google Scholar] [CrossRef]
- Sudiana, I.M.; Chandra, N.D.; Mangunwardoyo, W.; Kanti, A.; Napitupulu, T.P.; Idris Sumerta, I.N. Effects of funneliformis mosseae inoculation on chili pepper growth under repeated drought stress. J. Teknol. 2022, 84, 69–80. [Google Scholar] [CrossRef]
- Siaga, E.; Rini, D.S.; Widuri, L.I.; Sakagami, J.I.; Lakitan, B.; Yabuta, S. Growth and morpho-physiological assessments of Indonesian red chili cultivars on early vegetative stage under water stress conditions: A comparison of waterlogging and drought. Chil. J. Agric. Res. 2024, 84, 425–438. [Google Scholar] [CrossRef]
- Rathnayaka, R.M.S.M.B.; Kondo, F.; Prabandaka, S.S.; Nemoto, K.; Matsushima, K. Drought stress induced an increase in the pungency and expression of capsaicinoid biosynthesis genes in chili pepper (Capsicum annuum L.). Hortic. J. 2021, 90, 410–419. [Google Scholar] [CrossRef]
- Agyemang Duah, S.; Silva e Souza, C.; Nagy, Z.; Pék, Z.; Neményi, A.; Daood, H.G.; Vinogradov, S.; Helyes, L. Effect of water supply on physiological response and phytonutrient composition of chili peppers. Water 2021, 13, 1284. [Google Scholar] [CrossRef]
- Aswathi, K.P.R.; Kalaji, H.M.; Puthur, J.T. Seed priming of plants aiding in drought stress tolerance and faster recovery: A Review. Plant Growth Regul. 2020, 97, 235–253. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Habib, M.M.; Khan, M.S.; Ahmad, I.; Farooq, S.; Siddique, K.H.M. Influence of seed priming techniques on grain yield and economic returns of bread wheat planted at different spacings. Crop Pasture Sci. 2020, 71, 725–738. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Baath, G.S.; Shukla, M.K.; Bosland, P.W.; Steiner, R.L.; Walker, S.J. Irrigation water salinity influences at various growth stages of Capsicum annuum. Agric. Water Manag. 2017, 179, 246–253. [Google Scholar] [CrossRef]
- Patel, V.; Gandhi Krishi Vishwavidyalaya, I.; Sengar, I.S.; Singh, I.R.; Singh, I.A.; Rajesh Kumar, I.; Onkar Singh, I.; Sengar, S.; Singh, R.; Singh, A.; et al. Primary nutrient content and its uptake in little millet (Panicum sumatrense) as influenced by different nutrient management and seed priming. J. Pharmacogn. Phytochem. 2020, 9, 1228–1233. [Google Scholar]
- Sitompul, S.M.; Roviq, M.; Riedo, E. Growth and betacyanin content of beetroots (Beta vulgaris L.) under water deficit in a tropical condition. Agrivita 2019, 41, 491–503. [Google Scholar] [CrossRef]
- Muslimin; Taufik, M.; Thamrin, M.; Suddin, A.F. Financial analysis of red chili farming business with green cultivation technology in South Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2021, 911, 012079. [Google Scholar] [CrossRef]
- Yafizham; Herwibawa, B. The effects of sodium azide on seed germination and seedling growth of chili pepper (Capsicum annum L. cv. Landung). IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012052. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef]
- Muhammad, I.; Volker, R.; Günter, N. Accumulation and Distribution of Zn and Mn in Soybean Seeds after Nutrient Seed Priming and its Contribution to Plant Growth under Zn and Mn Deficient Conditions. J. Plant Nutr. 2017, 40, 695–708. [Google Scholar] [CrossRef]
- Adhikary, S.; Biswas, B.; Chakraborty, D.; Timsina, J.; Pal, S.; Chandra Tarafdar, J.; Banerjee, S.; Hossain, A.; Roy, S. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. 2022, 12, 7103. [Google Scholar] [CrossRef]
- Pereira AD, E.S.; Oliveira, H.C.; Fraceto, L.F.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. Through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual; TSBF-CIAT and SACRED Africa: Nairobi, Kenya, 2000. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis. Part 3-Chemical Methods; Sparks, D.L., Ed.; ASA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Phelan, D.C.; Harrison, M.T.; McLean, G.; Cox, H.; Pembleton, K.G.; Dean, G.J.; Parsons, D.; do Amaral Richter, M.E.; Pengilley, G.; Hinton, S.J.; et al. Advancing a farmer decision support tool for agronomic decisions on rainfed and irrigated wheat cropping in Tasmania. Agric. Syst. 2018, 167, 113–124. [Google Scholar] [CrossRef]
- Sui, R. Irrigation Scheduling Using Soil Moisture Sensors. J. Agric. Sci. 2017, 10, 1. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles promote nutrient utilization and plant growth in rice by upregulating nutrient uptake genes and stimulating growth hormone production. Sci. Total. Environ. 2021, 800, 149627. [Google Scholar] [CrossRef]
- Samota, M.K.; Sasi, M.; Awana, M.; Yadav, O.P.; Mithra, S.V.A.; Tyagi, A.; Kumar, S.; Singh, A. Elicitor-induced biochemical and molecular manifestations to improve drought tolerance in rice (Oryza sativa L.) through seed-priming. Front. Plant Sci. 2017, 8, 934. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef]
- Frungillo, L. Mapping the genomic basis of developmental and metabolic responses to nitrogen. Plant Cell 2022, 34, 4663–4664. [Google Scholar] [CrossRef]
- E’rahim, N.I.K.; Nordin, M.S.; Salleh, M.S. Effects of Seed Priming on Seed Germination and Early Seedling Growth of Chili (Capsicum annum L.) Under Water Deficit Condition. Trop. Agrobiodiversity 2021, 2, 37–41. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of Zinc in Plant Nutrition-A Review. Am. J. Exp. Agric. 2013, 50, 374–391. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Khan, M.B. Seed priming improves growth of nursery seedlings and yield of transplanted rice. Arch. Agron. Soil Sci. 2007, 53, 315–326. [Google Scholar] [CrossRef]
- Nakao, Y.; Asea, G.; Yoshino, M.; Kojima, N.; Hanada, H.; Miyamoto, K.; Yabuta, S.; Kamioka, R.; Sakagami, J.-I. Development of Hydropriming Techniques for Sowing Seeds of Upland Rice in Uganda. Am. J. Plant Sci. 2018, 9, 2170–2182. [Google Scholar] [CrossRef][Green Version]
- Hou, D.; Bi, J.; Ma, L.; Zhang, K.; Li, D.; Rehmani, M.I.A.; Tan, J.; Bi, Q.; Wei, Y.; Liu, G.; et al. Effects of Soil Moisture Content on Germination and Physiological Characteristics of Rice Seeds with Different Specific Gravity. Agronomy 2022, 12, 500. [Google Scholar] [CrossRef]
- Sethy, B.; Patra, C.; Das, S.; Mohanty, S.K. Response of Naturally Aged Paddy Seed to Halo, Hormonal and Hydropriming. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 593–599. [Google Scholar] [CrossRef]
- Hussain, M.; Hussain, M.; Farooq, M.; Farooq, M.; Lee, D.; Lee, D. Evaluating the role of seed priming in improving drought tolerance of pigmented and non-pigmented rice. J. Agron. Crop Sci. 2017, 203, 269–276. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Hussain, T.; Rasheed, A.; Alwahibi, M.S.; Elshikh, M.S.; Hussain, M.I.; Zulfiqar, F.; Mansoor, S.; Abbas, Z. Biomass Production and Predicted Ethanol Yield Are Linked with Optimum Photosynthesis in Phragmites karka under Salinity and Drought Conditions. Plants 2022, 11, 1657. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Escalante-Magaña, C.; Aguilar-Caamal, L.F.; Echevarría-Machado, I.; Medina-Lara, F.; Cach, L.S.; Martínez-Estévez, M. Contribution of glycine betaine and proline to water deficit tolerance in pepper plants. HortScience 2019, 54, 1044–1054. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Vyrides, I.; Stuckey, D.C. Compatible solute addition to biological systems treating waste/wastewater to counteract osmotic and other environmental stresses: A review. Crit. Rev. Biotechnol. 2017, 37, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Nivetha, N.; Kiruthika, A.; Asha, A.D.; Lavanya, A.K.; Vikram, K.V.; Manjunatha, B.S.; Paul, S. Osmotolerant Rhizobacteria Improve Seedling Vigour and Plant Growth of Mustard under Water Scarcity. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1928–1937. [Google Scholar] [CrossRef]
- Aslam, M.U.; Raza, M.A.S.; Saleem, M.F.; Waqas, M.; Iqbal, R.; Ahmad, S.; Haider, I. Improving Strategic Growth Stage-based Drought Tolerance in Quinoa by Rhizobacterial Inoculation. Commun. Soil Sci. Plant Anal. 2020, 51, 853–868. [Google Scholar] [CrossRef]
- Grishin, A.; Grishin, A.; Grishin, V.; Pavlova, E. Software Algorithm for the Implementation of a Synergistic Effect in the Production Processes of Plants. In International Conference on Digital Transformation: Informatics, Economics, and Education (DTIEE2023); SPIE: Bellingham, WA, USA, 2023. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Alghanem, S.M.S.; Alhaithloul, H.A.S.; Saleem, M.H.; Abeed, A.H.A. Combined exposure of PVC-microplastic and mercury chloride (HgCl2) in sorghum (Pennisetum glaucum L.) when its seeds are primed titanium dioxide nanoparticles (TiO2–NPs). Environ. Sci. Pollut. Res. 2024, 31, 7837–7852. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; De Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Anandaraj, K.; Natarajan, N. Effect of Nanoparticles for Seed Quality Enhancement in Onion [Allium cepa (Linn) cv. CO (On)] 5. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3714–3724. [Google Scholar] [CrossRef]
- Marngar, E.; Dawson, J. Effect of Biofertilizers, Levels of Nitrogen and Zinc on Growth and Yield of Hybrid Maize (Zea mays L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3614–3622. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Aref, F. Evaluation of application methods and rates of zinc and boron on nitrogen, phosphorus and potassium contents of maize leaf. J. Plant Nutr. 2012, 35, 1210–1224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).