Monitoring the Progression of Downy Mildew on Vineyards Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area

2.2. Remote-Sensing Data
2.2.1. Data Acquisition
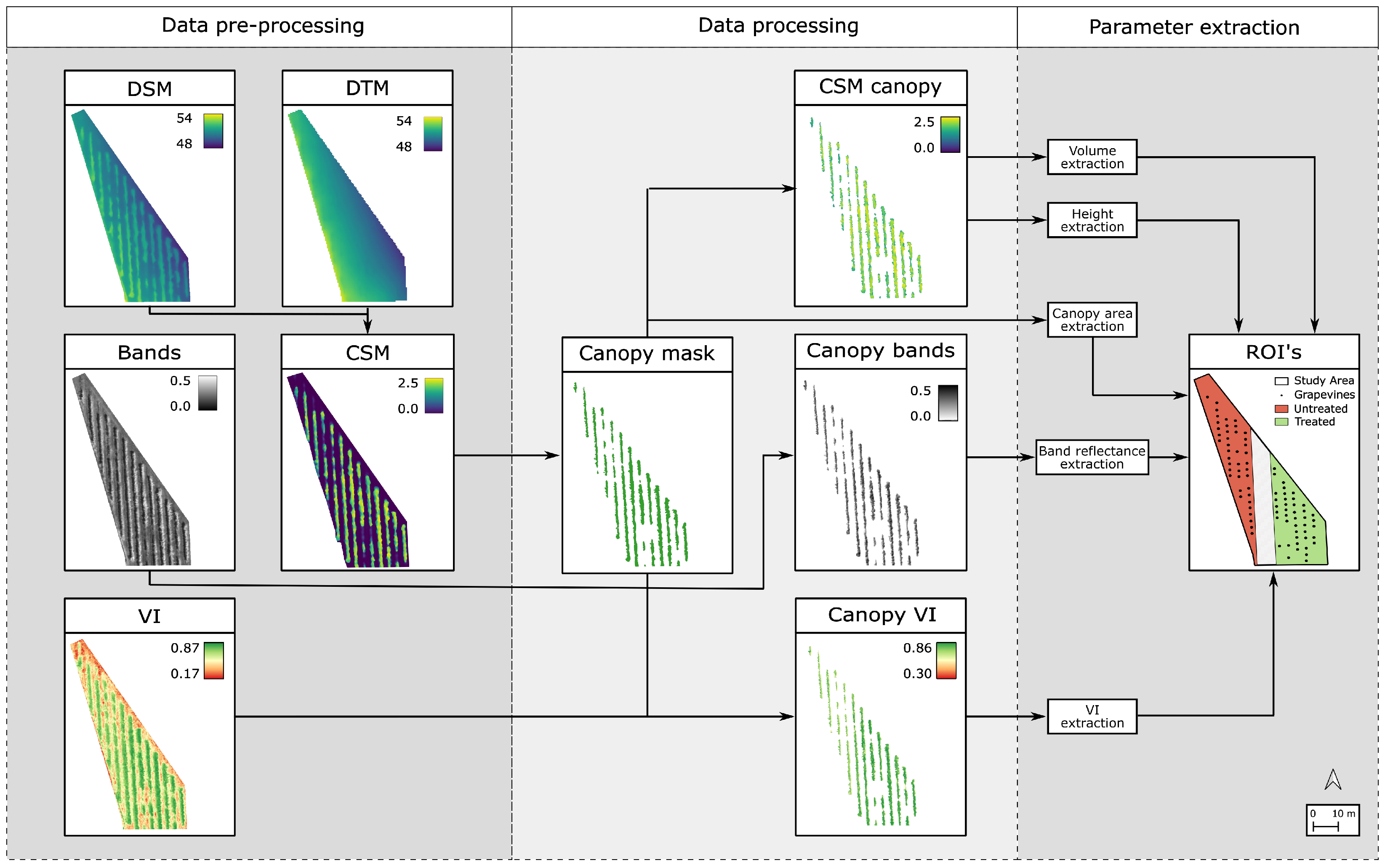
2.2.2. Data Processing
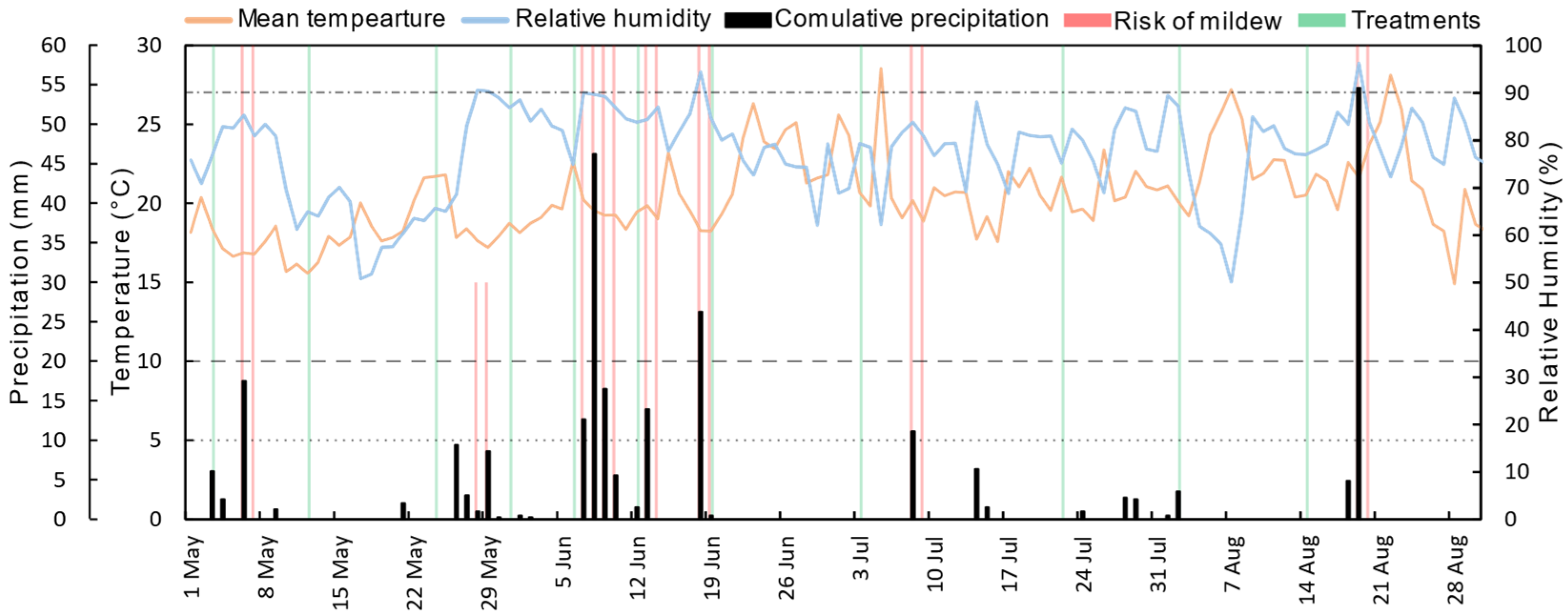
2.3. Climate Data
2.4. Phytosanitary Treatments
2.5. Statistical and Data Analysis
3. Results
3.1. Downy Mildew Forecasting from Climate Data and Treatments
3.2. Data Characterization
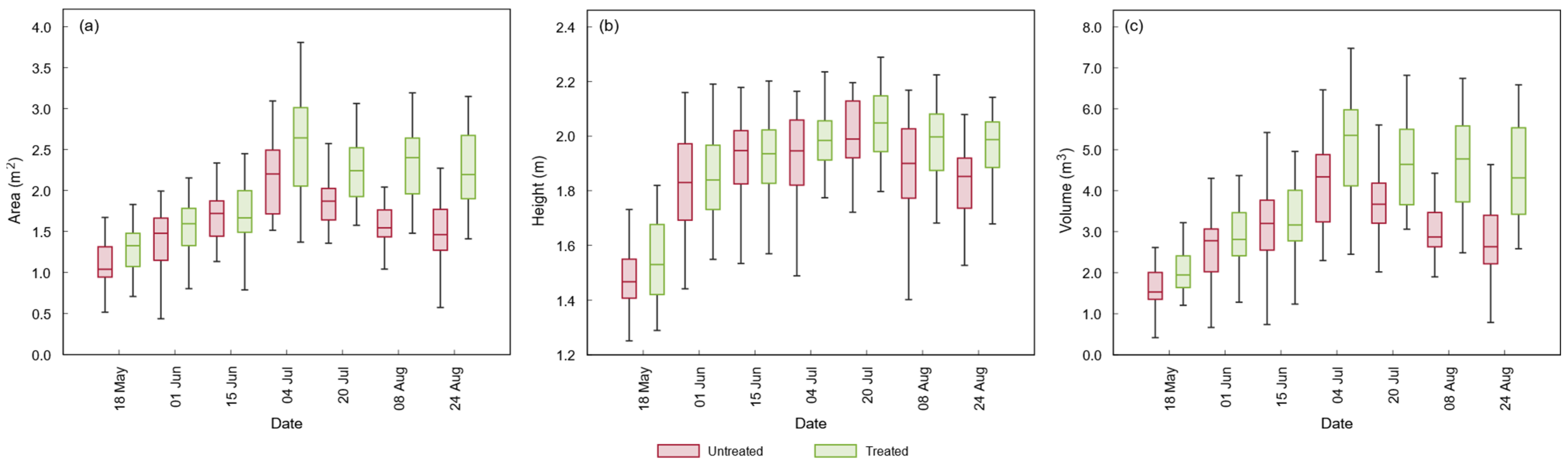
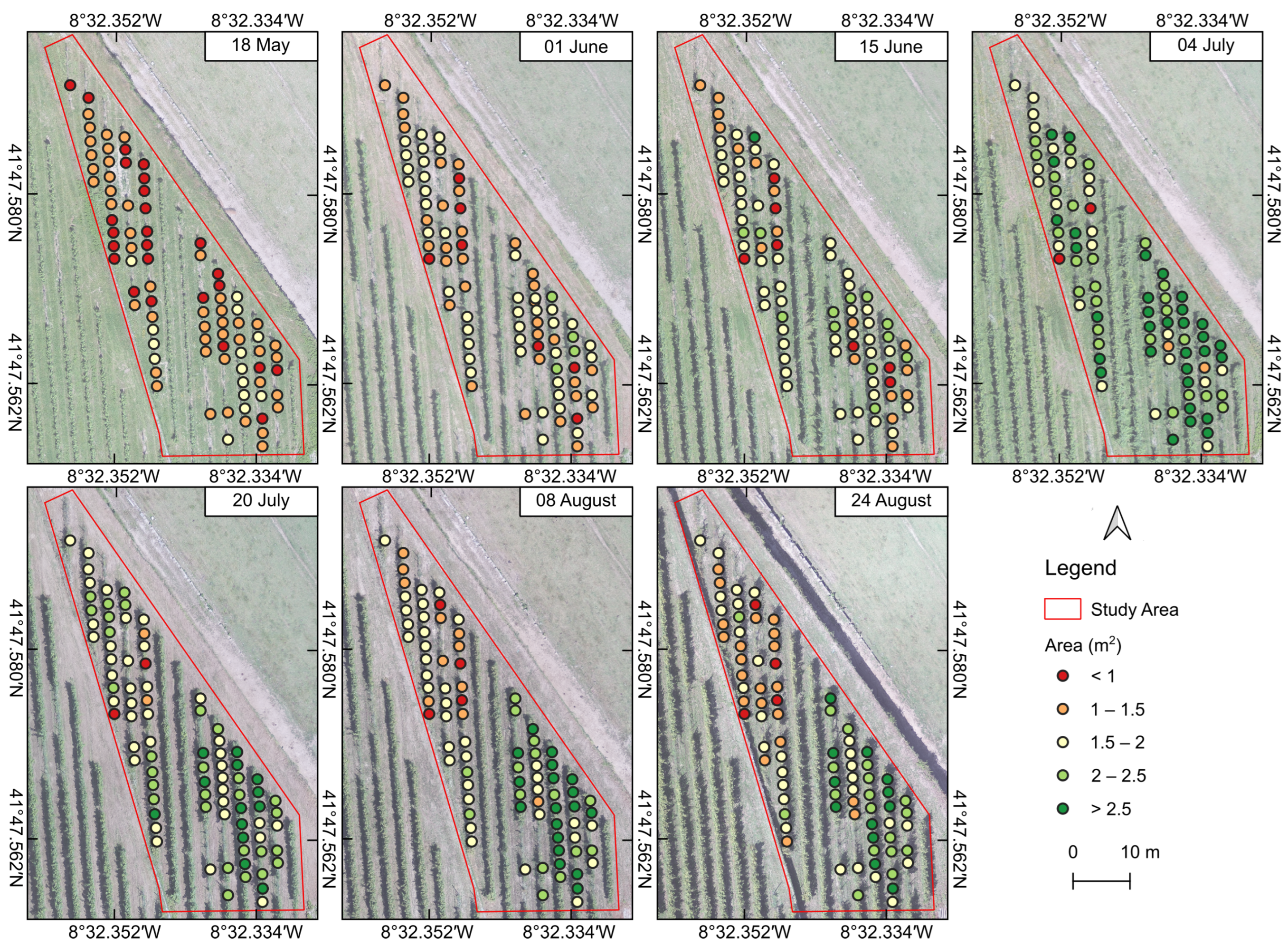
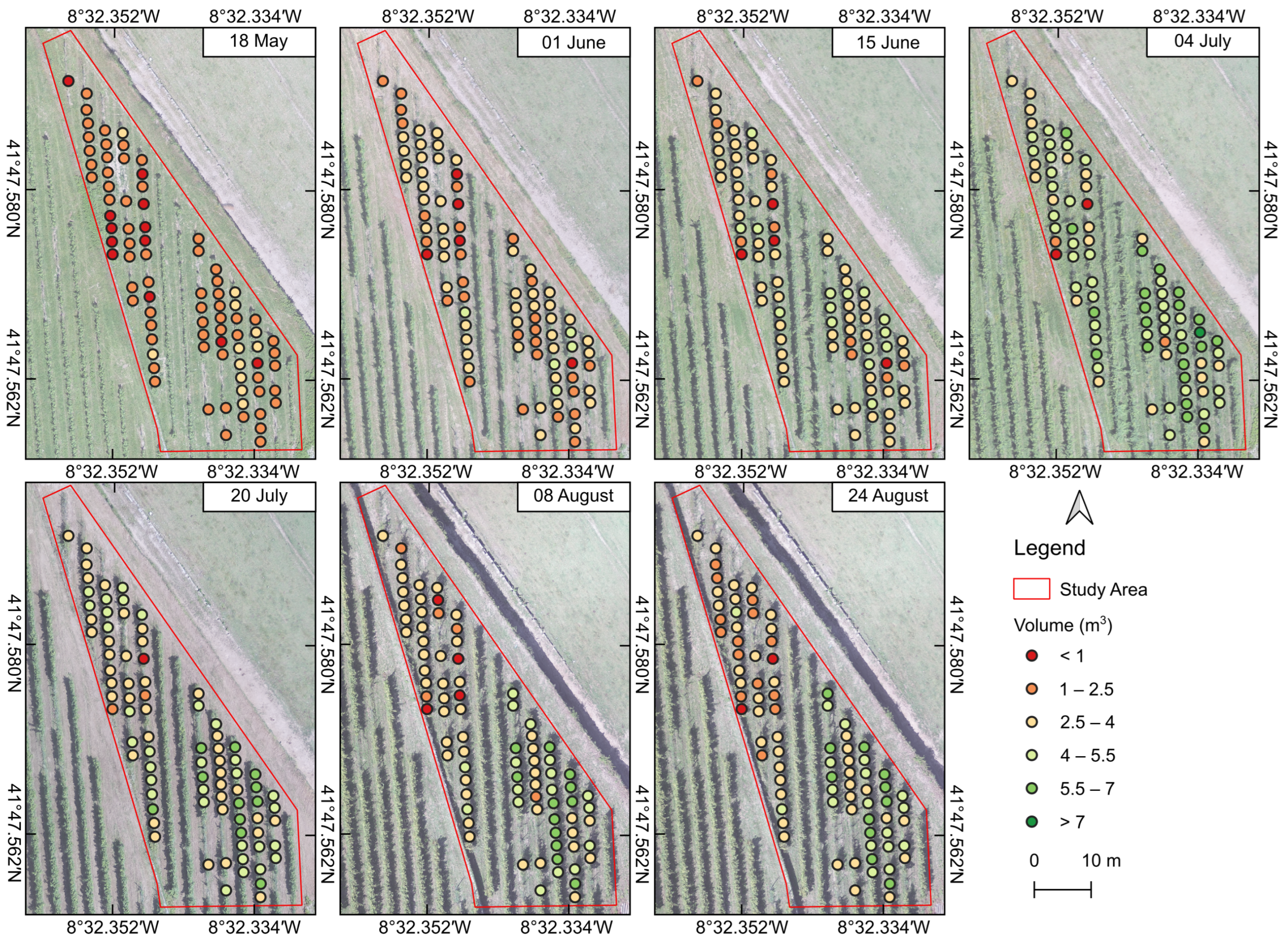
3.2.1. Morphometric Variables
3.2.2. Spectral Reflectance
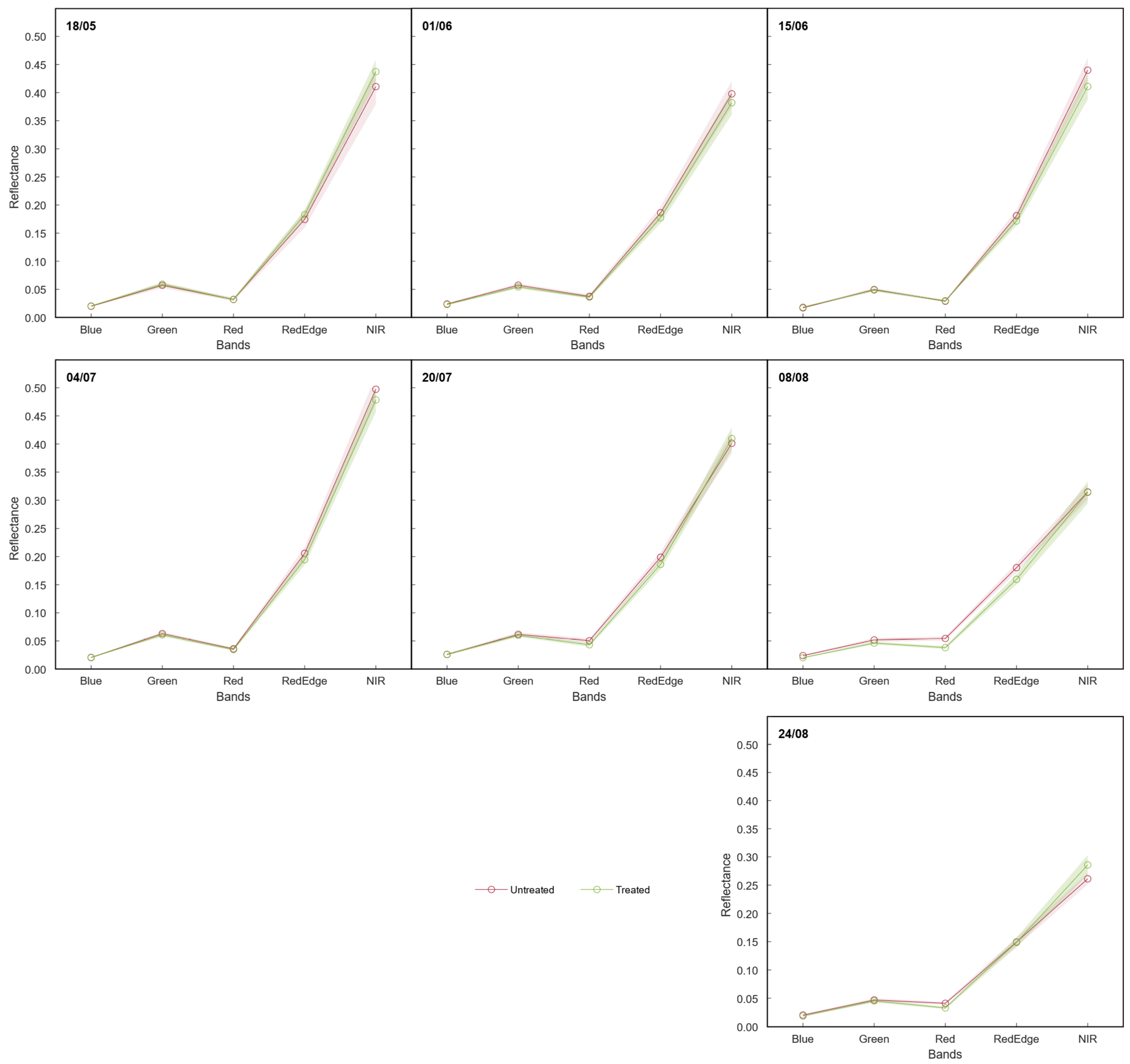
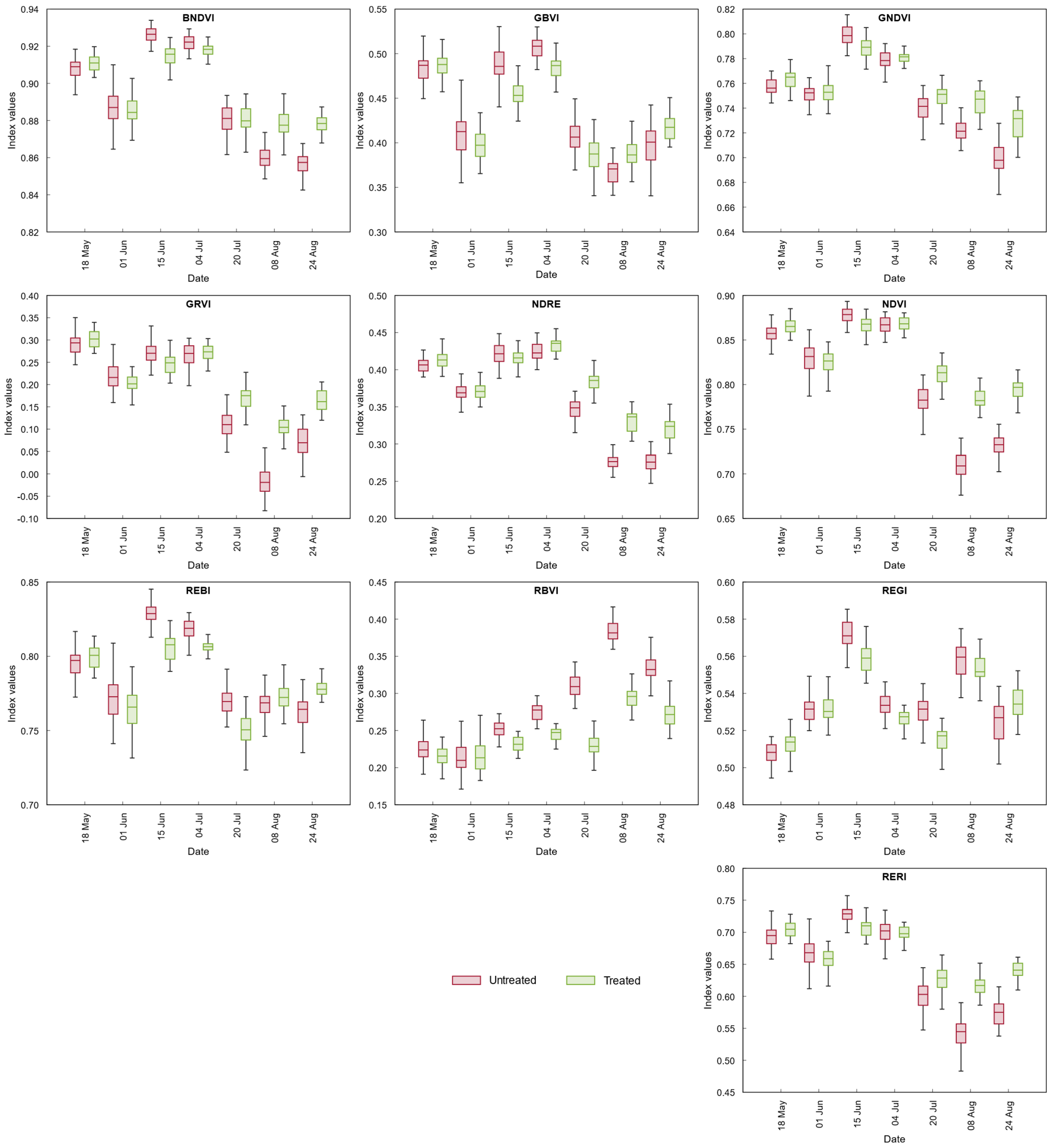
3.2.3. Vegetation Indices
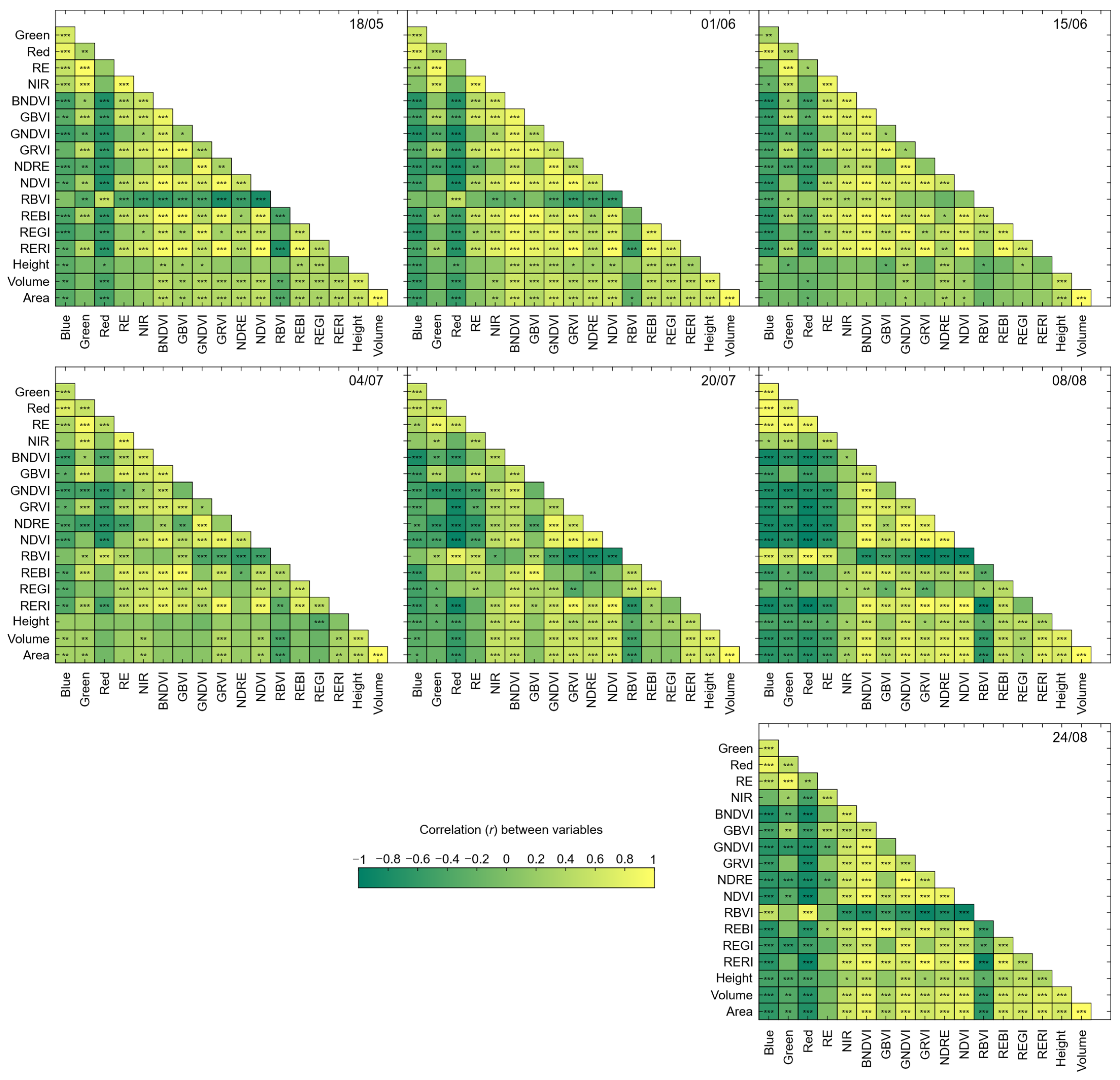
3.3. Statistical Analysis of UAV-Based Parameters

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Ferro, M.V.; Catania, P. Technologies and Innovative Methods for Precision Viticulture: A Comprehensive Review. Horticulturae 2023, 9, 399. [Google Scholar] [CrossRef]
- Tardaguila, J.; Stoll, M.; Gutiérrez, S.; Proffitt, T.; Diago, M.P. Smart Applications and Digital Technologies in Viticulture: A Review. Smart Agric. Technol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Santesteban, L.G. Precision Viticulture and Advanced Analytics. A Short Review. Food Chem. 2019, 279, 58–62. [Google Scholar] [CrossRef]
- Portela, F.; Sousa, J.J.; Araújo-Paredes, C.; Peres, E.; Morais, R.; Pádua, L. A Systematic Review on the Advancements in Remote Sensing and Proximity Tools for Grapevine Disease Detection. Sensors 2024, 24, 8172. [Google Scholar] [CrossRef]
- Matese, A.; Filippo Di Gennaro, S. Technology in Precision Viticulture: A State of the Art Review. Int. J. Wine Res. 2015, 7, 69–81. [Google Scholar] [CrossRef]
- Pádua, L.; Adão, T.; Hruška, J.; Sousa, J.J.; Peres, E.; Morais, R.; Sousa, A. Very High Resolution Aerial Data to Support Multi-Temporal Precision Agriculture Information Management. Procedia Comput. Sci. 2017, 121, 407–414. [Google Scholar] [CrossRef]
- Brook, A.; De Micco, V.; Battipaglia, G.; Erbaggio, A.; Ludeno, G.; Catapano, I.; Bonfante, A. A Smart Multiple Spatial and Temporal Resolution System to Support Precision Agriculture from Satellite Images: Proof of Concept on Aglianico Vineyard. Remote Sens. Environ. 2020, 240, 111679. [Google Scholar] [CrossRef]
- Cohen, B.; Edan, Y.; Levi, A.; Alchanatis, V. Early Detection of Grapevine (Vitis Vinifera) Downy Mildew (Peronospora) and Diurnal Variations Using Thermal Imaging. Sensors 2022, 22, 3585. [Google Scholar] [CrossRef]
- Zia-Khan, S.; Kleb, M.; Merkt, N.; Schock, S.; Müller, J. Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara Viticola) in Grapevine. Agriculture 2022, 12, 617. [Google Scholar] [CrossRef]
- Araújo-Paredes, C.; Portela, F.; Mendes, S.; Valín, M.I. Using Aerial Thermal Imagery to Evaluate Water Status in Vitis Vinifera Cv. Loureiro. Sensors 2022, 22, 8056. [Google Scholar] [CrossRef] [PubMed]
- Calderón, R.; Montes-Borrego, M.; Landa, B.B.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Detection of Downy Mildew of Opium Poppy Using High-Resolution Multi-Spectral and Thermal Imagery Acquired with an Unmanned Aerial Vehicle. Precis. Agric. 2014, 15, 639–661. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Santoni, M.; Faralli, M.; Bertamini, M.; Bruzzone, L. A Multispectral Acquisition System for Potential Detection of Flavescence Dorée. In Proceedings of the 2022 30th Telecommunications Forum (TELFOR), Belgrade, Serbia, 15–16 November 2022; IEEE: New York, NY, USA, 2022; pp. 1–4. [Google Scholar]
- Gennaro, S.D.; Battiston, E.; Marco, S.D.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-Based Remote Sensing to Monitor Grapevine Leaf Stripe Disease within a Vineyard Affected by Esca Complex. Phytopathol. Mediterr. 2016, 55, 262–275. [Google Scholar] [CrossRef]
- la Fuente, C.P.-D.; Valdés-Gómez, H.; Roudet, J.; Verdugo-Vásquez, N.; Mirabal, Y.; Laurie, V.F.; Goutouly, J.P.; Opazo, C.A.; Fermaud, M. Vigor Thresholded NDVI Is a Key Early Risk Indicator of Botrytis Bunch Rot in Vineyards. OENO One 2020, 54, 279–297. [Google Scholar] [CrossRef]
- Sousa, J.J.; Toscano, P.; Matese, A.; Di Gennaro, S.F.; Berton, A.; Gatti, M.; Poni, S.; Pádua, L.; Hruška, J.; Morais, R.; et al. UAV-Based Hyperspectral Monitoring Using Push-Broom and Snapshot Sensors: A Multisite Assessment for Precision Viticulture Applications. Sensors 2022, 22, 6574. [Google Scholar] [CrossRef]
- Gallo, R.; Ristorto, G.; Daglio, G.; Massa, M.; Berta, G.; Lazzari, M.; Mazzetto, F. New Solutions for the Automatic Early Detection of Diseases in Vineyards through Ground Sensing Approaches Integrating Lidar and Optical Sensors. Chem. Eng. Trans. 2017, 58, 673–678. [Google Scholar] [CrossRef]
- Abdelghafour, F.; Keresztes, B.; Germain, C.; Da Costa, J.-P. In Field Detection of Downy Mildew Symptoms with Proximal Colour Imaging. Sensors 2020, 20, 4380. [Google Scholar] [CrossRef]
- Tsouros, D.C.; Bibi, S.; Sarigiannidis, P.G. A Review on UAV-Based Applications for Precision Agriculture. Information 2019, 10, 349. [Google Scholar] [CrossRef]
- Giovos, R.; Tassopoulos, D.; Kalivas, D.; Lougkos, N.; Priovolou, A. Remote Sensing Vegetation Indices in Viticulture: A Critical Review. Agriculture 2021, 11, 457. [Google Scholar] [CrossRef]
- Pádua, L.; Marques, P.; Hruška, J.; Adão, T.; Peres, E.; Morais, R.; Sousa, J.J. Multi-Temporal Vineyard Monitoring through UAV-Based RGB Imagery. Remote Sens. 2018, 10, 1907. [Google Scholar] [CrossRef]
- García-Fernández, M.; Sanz-Ablanedo, E.; Pereira-Obaya, D.; Rodríguez-Pérez, J.R. Vineyard Pruning Weight Prediction Using 3D Point Clouds Generated from UAV Imagery and Structure from Motion Photogrammetry. Agronomy 2021, 11, 2489. [Google Scholar] [CrossRef]
- Ferro, M.V.; Catania, P.; Miccichè, D.; Pisciotta, A.; Vallone, M.; Orlando, S. Assessment of Vineyard Vigour and Yield Spatio-Temporal Variability Based on UAV High Resolution Multispectral Images. Biosyst. Eng. 2023, 231, 36–56. [Google Scholar] [CrossRef]
- Albetis, J.; Duthoit, S.; Guttler, F.; Jacquin, A.; Goulard, M.; Poilvé, H.; Féret, J.-B.; Dedieu, G. Detection of Flavescence Dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery. Remote Sens. 2017, 9, 308. [Google Scholar] [CrossRef]
- Mucalo, A.; Matić, D.; Morić-Španić, A.; Čagalj, M. Satellite Solutions for Precision Viticulture: Enhancing Sustainability and Efficiency in Vineyard Management. Agronomy 2024, 14, 1862. [Google Scholar] [CrossRef]
- Aneece, I.; Thenkabail, P.S. New Generation Hyperspectral Sensors DESIS and PRISMA Provide Improved Agricultural Crop Classifications. Photogramm. Eng. Remote Sens. 2022, 88, 715–729. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Liu, B.; Wu, W.; Ren, Y.; Ruan, C.; Geng, Y. Wheat Yellow Rust Detection Using UAV-Based Hyperspectral Technology. Remote Sens. 2021, 13, 123. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Tsiouni, M.; Lagoudas, C.; Loridas, A.; Baliktsis, S.; Vozikis, C. Unmanned Aerial Systems for Early Detection of Downy Mildew in Tobacco Fields: Enhancing Financial Outcomes through Precision Monitoring. In Proceedings of the Tenth International Conference on Remote Sensing and Geoinformation of the Environment (RSCy2024), 13 September 2024; Volume 13212, pp. 398–401. [Google Scholar]
- Garcia-Ruiz, F.; Sankaran, S.; Maja, J.M.; Lee, W.S.; Rasmussen, J.; Ehsani, R. Comparison of Two Aerial Imaging Platforms for Identification of Huanglongbing-Infected Citrus Trees. Comput. Electron. Agric. 2013, 91, 106–115. [Google Scholar] [CrossRef]
- Ye, H.; Huang, W.; Huang, S.; Cui, B.; Dong, Y.; Guo, A.; Ren, Y.; Jin, Y. Recognition of Banana Fusarium Wilt Based on UAV Remote Sensing. Remote Sens. 2020, 12, 938. [Google Scholar] [CrossRef]
- Castrignanò, A.; Belmonte, A.; Antelmi, I.; Quarto, R.; Quarto, F.; Shaddad, S.; Sion, V.; Muolo, M.R.; Ranieri, N.A.; Gadaleta, G.; et al. Semi-Automatic Method for Early Detection of Xylella Fastidiosa in Olive Trees Using UAV Multispectral Imagery and Geostatistical-Discriminant Analysis. Remote Sens. 2021, 13, 14. [Google Scholar] [CrossRef]
- Chandel, A.K.; Khot, L.R.; Sallato, B.C. Towards Rapid Detection and Mapping of Powdery Mildew in Apple Orchards. In Proceedings of the 2020 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento, Italy, 4–6 November 2020; pp. 288–292. [Google Scholar]
- Pádua, L.; Marques, P.; Martins, L.; Sousa, A.; Peres, E.; Sousa, J.J. Monitoring of Chestnut Trees Using Machine Learning Techniques Applied to UAV-Based Multispectral Data. Remote Sens. 2020, 12, 3032. [Google Scholar] [CrossRef]
- Kuswidiyanto, L.W.; Wang, P.; Noh, H.-H.; Jung, H.-Y.; Jung, D.-H.; Han, X. Airborne Hyperspectral Imaging for Early Diagnosis of Kimchi Cabbage Downy Mildew Using 3D-ResNet and Leaf Segmentation. Comput. Electron. Agric. 2023, 214, 108312. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.P.; Di Cicco, M.; Nardi, S.; Nardi, D. Mapping Infected Crops Through UAV Inspection: The Sunflower Downy Mildew Parasite Case. In Proceedings of the Advances and Trends in Artificial Intelligence. From Theory to Practice, Graz, Austria, 9–11 July 2019; Wotawa, F., Friedrich, G., Pill, I., Koitz-Hristov, R., Ali, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 495–503. [Google Scholar]
- Abdulridha, J.; Ampatzidis, Y.; Qureshi, J.; Roberts, P. Identification and Classification of Downy Mildew Severity Stages in Watermelon Utilizing Aerial and Ground Remote Sensing and Machine Learning. Front. Plant Sci. 2022, 13, 791018. [Google Scholar] [CrossRef] [PubMed]
- Power, A.; Truong, V.K.; Chapman, J.; Cozzolino, D. From the Laboratory to The Vineyard—Evolution of The Measurement of Grape Composition Using NIR Spectroscopy towards High-Throughput Analysis. High-Throughput 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Kerkech, M.; Hafiane, A.; Canals, R.; Ros, F. Vine Disease Detection by Deep Learning Method Combined with 3D Depth Information. In Proceedings of the Image and Signal Processing, Marrakesh, Morocco, 4–6 June 2020; El Moataz, A., Mammass, D., Mansouri, A., Nouboud, F., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 82–90. [Google Scholar]
- Molitor, D.; Baus, O.; Didry, Y.; Junk, J.; Hoffmann, L.; Beyer, M. BotRisk: Simulating the Annual Bunch Rot Risk on Grapevines (Vitis Vinifera L. Cv. Riesling) Based on Meteorological Data. Int. J. Biometeorol. 2020, 64, 1571–1582. [Google Scholar] [CrossRef]
- Campos, J.; García-Ruíz, F.; Gil, E. Assessment of Vineyard Canopy Characteristics from Vigour Maps Obtained Using UAV and Satellite Imagery. Sensors 2021, 21, 2363. [Google Scholar] [CrossRef]
- Franke, J.; Menz, G. Multi-Temporal Wheat Disease Detection by Multi-Spectral Remote Sensing. Precis. Agric. 2007, 8, 161–172. [Google Scholar] [CrossRef]
- Marucci, A.; Colantoni, A.; Zambon, I.; Egidi, G. Precision Farming in Hilly Areas: The Use of Network RTK in GNSS Technology. Agriculture 2017, 7, 60. [Google Scholar] [CrossRef]
- Kontogiannis, S.; Konstantinidou, M.; Tsioukas, V.; Pikridas, C. A Cloud-Based Deep Learning Framework for Downy Mildew Detection in Viticulture Using Real-Time Image Acquisition from Embedded Devices and Drones. Information 2024, 15, 178. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral Image Analysis Techniques for the Detection and Classification of the Early Onset of Plant Disease and Stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- Roşcăneanu, R.; Streche, R.; Osiac, F.; Bălăceanu, C.; Suciu, G.; Drăgulinescu, A.M.; Marcu, I. Detection of Vineyard Diseases Using the Internet of Things Technology and Machine Learning Algorithms. Aerul si Apa. Compon. ale Mediu. 2022, 128–139. [Google Scholar] [CrossRef]
- Pádua, L.; Marques, P.; Adão, T.; Guimarães, N.; Sousa, A.; Peres, E.; Sousa, J.J. Vineyard Variability Analysis through UAV-Based Vigour Maps to Assess Climate Change Impacts. Agronomy 2019, 9, 581. [Google Scholar] [CrossRef]
- Zherdev, A.V.; Vinogradova, S.V.; Byzova, N.A.; Porotikova, E.V.; Kamionskaya, A.M.; Dzantiev, B.B. Methods for the Diagnosis of Grapevine Viral Infections: A Review. Agriculture 2018, 8, 195. [Google Scholar] [CrossRef]
- Guo, Q.; Honesty, S.; Xu, M.L.; Zhang, Y.; Schoelz, J.; Qiu, W. Genetic Diversity and Tissue and Host Specificity of Grapevine Vein Clearing Virus. Phytopathology 2014, 104, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Sharma, S.G. Identification of Plant Diseases and Distinct Approaches for Their Management. Bull. Natl. Res. Cent. 2021, 45, 169. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, N.; Singh, S. A Review of Imaging Techniques for Plant Disease Detection. Artif. Intell. Agric. 2020, 4, 229–242. [Google Scholar] [CrossRef]
- Sassu, A.; Gambella, F.; Ghiani, L.; Mercenaro, L.; Caria, M.; Pazzona, A.L. Advances in Unmanned Aerial System Remote Sensing for Precision Viticulture. Sensors 2021, 21, 956. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Climate Factors Driving Wine Production in the Portuguese Minho Region. Agric. For. Meteorol. 2014, 185, 26–36. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis vinifera L. ssp. vinifera)—Codes and Descriptions According to the Extended BBCH Scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Gouveia, C.; Santos, R.B.; Paiva-Silva, C.; Buchholz, G.; Malhó, R.; Figueiredo, A. The Pathogenicity of Plasmopara Viticola: A Review of Evolutionary Dynamics, Infection Strategies and Effector Molecules. BMC Plant Biol. 2024, 24, 327. [Google Scholar] [CrossRef]
- Albetis, J.; Jacquin, A.; Goulard, M.; Poilvé, H.; Rousseau, J.; Clenet, H.; Dedieu, G.; Duthoit, S. On the Potentiality of UAV Multispectral Imagery to Detect Flavescence Dorée and Grapevine Trunk Diseases. Remote Sens. 2018, 11, 23. [Google Scholar] [CrossRef]
- Yang, C.; Everitt, J.H.; Bradford, J.M.; Murden, D. Airborne Hyperspectral Imagery and Yield Monitor Data for Mapping Cotton Yield Variability. Precis. Agric. 2004, 5, 445–461. [Google Scholar] [CrossRef]
- KAWASHIMA, S.; NAKATANI, M. An Algorithm for Estimating Chlorophyll Content in Leaves Using a Video Camera. Ann. Bot. 1998, 81, 49–54. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial Color Infrared Photography for Determining Early In-Season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident Detection of Crop Water Stress, Nitrogen Status and Canopy Density Using Ground-Based Multispectral Data. In Proceedings of the 5th International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000; pp. 1–15. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA: Washington, DC, USA, 1974.
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel Algorithms for Remote Estimation of Vegetation Fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Matese, A. Evaluation of Novel Precision Viticulture Tool for Canopy Biomass Estimation and Missing Plant Detection Based on 2.5D and 3D Approaches Using RGB Images Acquired by UAV Platform. Plant Methods 2020, 16, 91. [Google Scholar] [CrossRef]
- Stolarski, O.; Fraga, H.; Sousa, J.J.; Pádua, L. Synergistic Use of Sentinel-2 and UAV Multispectral Data to Improve and Optimize Viticulture Management. Drones 2022, 6, 366. [Google Scholar] [CrossRef]
- Pádua, L.; Adão, T.; Sousa, A.; Peres, E.; Sousa, J.J. Individual Grapevine Analysis in a Multi-Temporal Context Using UAV-Based Multi-Sensor Imagery. Remote Sens. 2020, 12, 139. [Google Scholar] [CrossRef]
- Hnatiuc, M.; Ghita, S.; Alpetri, D.; Ranca, A.; Artem, V.; Dina, I.; Cosma, M.; Abed Mohammed, M. Intelligent Grapevine Disease Detection Using IoT Sensor Network. Bioengineering 2023, 10, 1021. [Google Scholar] [CrossRef]
- Mezei, I.; Lukić, M.; Berbakov, L.; Pavković, B.; Radovanović, B. Grapevine Downy Mildew Warning System Based on NB-IoT and Energy Harvesting Technology. Electronics 2022, 11, 356. [Google Scholar] [CrossRef]
- Chen, M.; Brun, F.; Raynal, M.; Makowski, D. Forecasting Severe Grape Downy Mildew Attacks Using Machine Learning. PLoS ONE 2020, 15, e0230254. [Google Scholar] [CrossRef]
- Kleb, M.; Merkt, N.; Zörb, C. New Aspects of In Situ Measurements for Downy Mildew Forecasting. Plants 2022, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Caffi, T.; Rossi, V.; Bugiani, R. Evaluation of a Warning System for Controlling Primary Infections of Grapevine Downy Mildew. Plant Dis. 2010, 94, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.V.; Ellis, M.A.; Lalancette, N.; Hughes, G.; Wilson, L.L. Evaluation of a Disease Warning System for Downy Mildew of Grapes. Plant Dis. 2000, 84, 549–554. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, A.; Serra, S.; Ligios, V.; Satta, D.; Nieddu, G. Assessing the Effects of Vineyard Soil Management on Downy and Powdery Mildew Development. Horticulturae 2021, 7, 209. [Google Scholar] [CrossRef]
- Fontaine, F.; Pinto, C.; Vallet, J.; Clément, C.; Gomes, A.C.; Spagnolo, A. The Effects of Grapevine Trunk Diseases (GTDs) on Vine Physiology. Eur. J. Plant. Pathol. 2016, 144, 707–721. [Google Scholar] [CrossRef]
- Lacotte, V.; Peignier, S.; Raynal, M.; Demeaux, I.; Delmotte, F.; da Silva, P. Spatial–Spectral Analysis of Hyperspectral Images Reveals Early Detection of Downy Mildew on Grapevine Leaves. Int. J. Mol. Sci. 2022, 23, 10012. [Google Scholar] [CrossRef]
- Hernández, I.; Gutiérrez, S.; Ceballos, S.; Iñíguez, R.; Barrio, I.; Tardaguila, J. Artificial Intelligence and Novel Sensing Technologies for Assessing Downy Mildew in Grapevine. Horticulturae 2021, 7, 103. [Google Scholar] [CrossRef]
- Kerkech, M.; Hafiane, A.; Canals, R. Deep Leaning Approach with Colorimetric Spaces and Vegetation Indices for Vine Diseases Detection in UAV Images. Comput. Electron. Agric. 2018, 155, 237–243. [Google Scholar] [CrossRef]
- Grishina, A.; Lysov, M.; Ageyeva, M.; Diakova, V.; Sherstneva, O.; Brilkina, A.; Vodeneev, V. Bacterial and Viral-Induced Changes in the Reflectance Spectra of Nicotiana Benthamiana Plants. Horticulturae 2024, 10, 1363. [Google Scholar] [CrossRef]
- Bendel, N.; Kicherer, A.; Backhaus, A.; Klück, H.-C.; Seiffert, U.; Fischer, M.; Voegele, R.T.; Töpfer, R. Evaluating the Suitability of Hyper- and Multispectral Imaging to Detect Foliar Symptoms of the Grapevine Trunk Disease Esca in Vineyards. Plant Methods 2020, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Simic Milas, A.; Romanko, M.; Reil, P.; Abeysinghe, T.; Marambe, A. The Importance of Leaf Area Index in Mapping Chlorophyll Content of Corn under Different Agricultural Treatments Using UAV Images. Int. J. Remote Sens. 2018, 39, 5415–5431. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ostendorf, B.; Pagay, V. Detecting Grapevine Virus Infections in Red and White Winegrape Canopies Using Proximal Hyperspectral Sensing. Sensors 2023, 23, 2851. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Pádua, L.; Sousa, J.J.; Fernandes-Silva, A. Assessing the Water Status and Leaf Pigment Content of Olive Trees: Evaluating the Potential and Feasibility of Unmanned Aerial Vehicle Multispectral and Thermal Data for Estimation Purposes. Remote Sens. 2023, 15, 4777. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Pereira-Obaya, D.; Rodríguez-Pérez, J.R. Field-Deployed Spectroscopy from 350 to 2500 Nm: A Promising Technique for Early Identification of Powdery Mildew Disease (Erysiphe Necator) in Vineyards. Agronomy 2024, 14, 634. [Google Scholar] [CrossRef]
- Zottele, F.; Crocetta, P.; Baiocchi, V. How Important Is UAVs RTK Accuracy for the Identification of Certain Vine Diseases? In Proceedings of the 2022 IEEE Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Perugia, Italy, 3 November 2022; IEEE: New York, NY, USA, 2022; pp. 239–243. [Google Scholar]
- Calamita, F.; Imran, H.A.; Vescovo, L.; Mekhalfi, M.L.; La Porta, N. Early Identification of Root Rot Disease by Using Hyperspectral Reflectance: The Case of Pathosystem Grapevine/Armillaria. Remote Sens. 2021, 13, 2436. [Google Scholar] [CrossRef]
- Liu, Y.; Su, J.; Zheng, Z.; Liu, D.; Song, Y.; Fang, Y.; Yang, P.; Su, B. GLDCNet: A Novel Convolutional Neural Network for Grapevine Leafroll Disease Recognition Using UAV-Based Imagery. Comput. Electron. Agric. 2024, 218, 108668. [Google Scholar] [CrossRef]
- Marques, P.; Carvalho, R.; Fernandes-Silva, A. Preliminary Assessment of the Relationship between Pigments in Olive Leaves and Vegetation Indices. Proc. Latv. Acad. Sciences. Sect. B. Nat. Exact Appl. Sci. 2022, 76, 517–525. [Google Scholar] [CrossRef]
- Solano-Alvarez, N.; Valencia-Hernández, J.A.; Vergara-Pineda, S.; Millán-Almaraz, J.R.; Torres-Pacheco, I.; Guevara-González, R.G. Comparative Analysis of the NDVI and NGBVI as Indicators of the Protective Effect of Beneficial Bacteria in Conditions of Biotic Stress. Plants 2022, 11, 932. [Google Scholar] [CrossRef]
- Radócz, L.; Szabó, A.; Tamás, A.; Illés, Á.; Bojtor, C.; Ragán, P.; Vad, A.; Széles, A.; Harsányi, E.; Radócz, L. Investigation of the Detectability of Corn Smut Fungus (Ustilago Maydis DC. Corda) Infection Based on UAV Multispectral Technology. Agronomy 2023, 13, 1499. [Google Scholar] [CrossRef]
- Kanaley, K.; Combs, D.B.; Paul, A.; Jiang, Y.; Bates, T.; Gold, K.M. Assessing the Capacity of High-Resolution Commercial Satellite Imagery for Grapevine Downy Mildew Detection and Surveillance in New York State. Phytopathology 2024, 114, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Daglio, G.; Cesaro, P.; Todeschini, V.; Lingua, G.; Lazzari, M.; Berta, G.; Massa, N. Potential Field Detection of Flavescence Dorée and Esca Diseases Using a Ground Sensing Optical System. Biosyst. Eng. 2022, 215, 203–214. [Google Scholar] [CrossRef]
- Ferreira, L.; Sousa, J.J.; Lourenço, J.M.; Peres, E.; Morais, R.; Pádua, L. Comparative Analysis of TLS and UAV Sensors for Estimation of Grapevine Geometric Parameters. Sensors 2024, 24, 5183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Sun, H.; An, L.; Zhao, R.; Liu, M.; Tang, W.; Li, M.; Yan, X.; Ma, Y.; et al. Exploring Multi-Features in UAV Based Optical and Thermal Infrared Images to Estimate Disease Severity of Wheat Powdery Mildew. Comput. Electron. Agric. 2024, 225, 109285. [Google Scholar] [CrossRef]
- Zeng, T.; Fang, J.; Yin, C.; Li, Y.; Fu, W.; Zhang, H.; Wang, J.; Zhang, X. Recognition of Rubber Tree Powdery Mildew Based on UAV Remote Sensing with Different Spatial Resolutions. Drones 2023, 7, 533. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ampatzidis, Y.; Roberts, P.; Kakarla, S.C. Detecting Powdery Mildew Disease in Squash at Different Stages Using UAV-Based Hyperspectral Imaging and Artificial Intelligence. Biosyst. Eng. 2020, 197, 135–148. [Google Scholar] [CrossRef]
- Zeng, T.; Wang, Y.; Yang, Y.; Liang, Q.; Fang, J.; Li, Y.; Zhang, H.; Fu, W.; Wang, J.; Zhang, X. Early Detection of Rubber Tree Powdery Mildew Using UAV-Based Hyperspectral Imagery and Deep Learning. Comput. Electron. Agric. 2024, 220, 108909. [Google Scholar] [CrossRef]
- Acosta, M.; Pena, J.; Sherafat, A.; Gonzalez, C.; Sherman, T.; Bhandari, S.; Raheja, A. Investigating the Potential of UAV-Based Hyperspectral Sensor in Detecting Powdery Mildew in Grapes. In Proceedings of the Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping IX, National Harbor, MD, USA, 7 June 2024; SPIE: Bellingham, WA, USA; Volume 13053, pp. 26–34. [Google Scholar]
- Portela, F.; Paredes, C.A.; Sousa, J.J.; Pádua, L. The Potential of Thermal Imaging to Assist Winemakers in the Detection of Downy Mildew in Vitis Vinifera Cv Loureiro. Procedia Comput. Sci. 2025, 256, 78–85. [Google Scholar] [CrossRef]
- Dutta, A.; Tyagi, R.; Chattopadhyay, A.; Chatterjee, D.; Sarkar, A.; Lall, B.; Sharma, S. Early Detection of Wilt in Cajanus Cajan Using Satellite Hyperspectral Images: Development and Validation of Disease-Specific Spectral Index with Integrated Methodology. Comput. Electron. Agric. 2024, 219, 108784. [Google Scholar] [CrossRef]



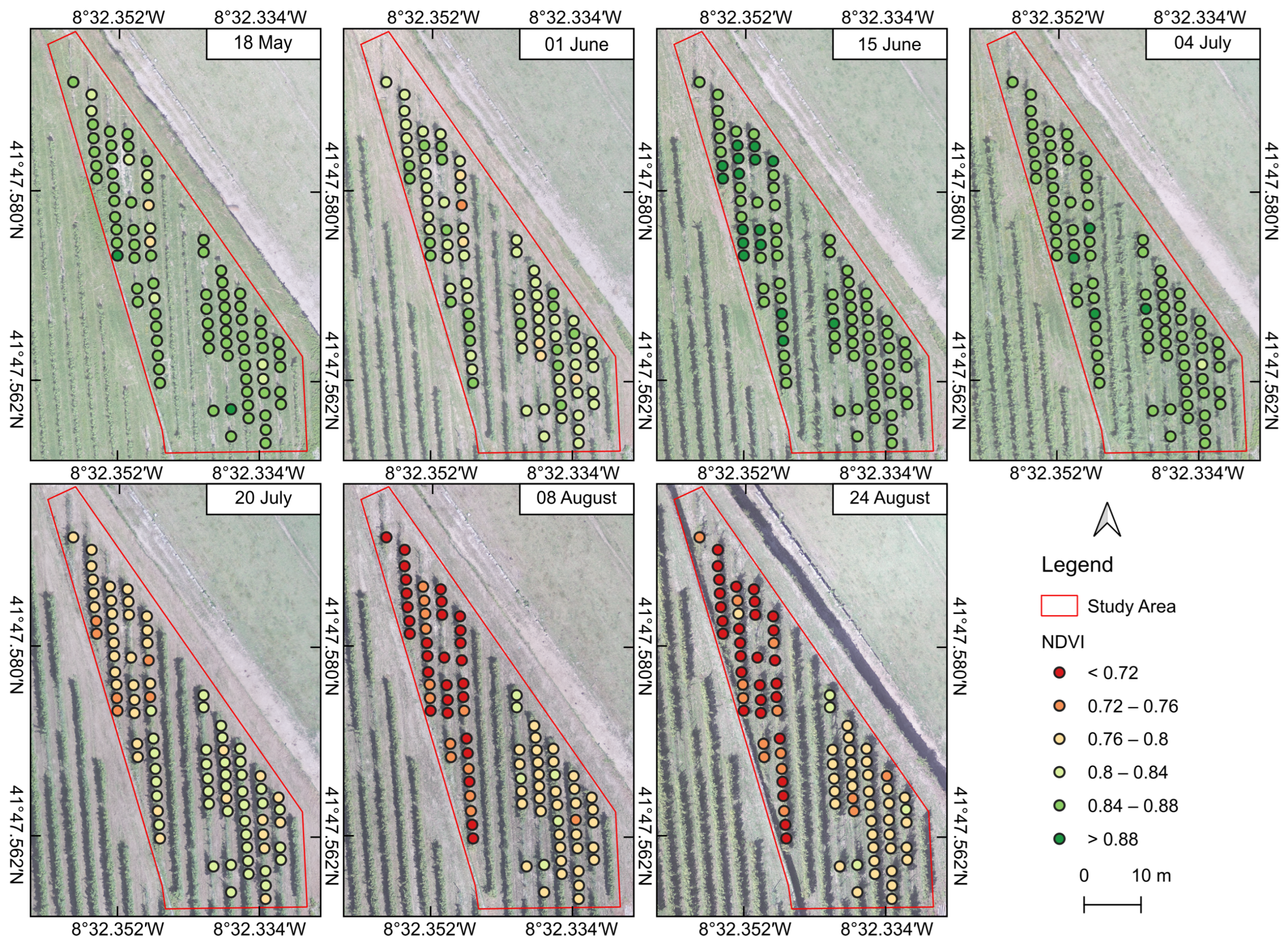
| Month | Acquisition Date | Difference (Days) | Growth Stage | BBCH Code |
|---|---|---|---|---|
| May | 18 May 2023 | — | 6 | 67 |
| June | 1 June 2023 | 14 | 7 | 71 |
| 15 June 2023 | 14 | 73 | ||
| July | 4 July 2023 | 19 | 79 | |
| 20 July 2023 | 16 | 79 | ||
| August | 8 August 2023 | 19 | 8 | 81 |
| 24 August 2023 | 16 | 83 |
| Vegetation Index | Equation | Reference |
|---|---|---|
| Blue Normalized Difference Vegetation Index | [56] | |
| Normalized Green–Blue Difference Index | [57] | |
| Green Normalized Difference Vegetation Index | [58] | |
| Green–Red Vegetation Index | [59] | |
| Normalized Difference Red-Edge | [60] | |
| Normalized Difference Vegetation Index | [61] | |
| Red-edge Green Index | [55] | |
| Red-edge Red Index | [55] | |
| Red-edge Blue Index | Adapted from REGI and RERI | |
| Red–Blue Vegetation Index | [62] |
| Application Date | Difference (Days) | Active Substance |
|---|---|---|
| 10 April 2023 | — | Fosetyl-Al |
| 25 April 2023 | 15 | Fosetyl-Al |
| 3 May 2023 | 8 | Metalaxyl |
| 12 May 2023 | 9 | Cymoxanil and folpet |
| 24 May 2023 | 12 | Folpet and iprovalicarb |
| 31 May 2023 | 7 | Cymoxanil and folpet |
| 6 June 2023 | 6 | Cymoxanil and folpet |
| 12 June 2023 | 6 | Metalaxyl-M and folpet |
| 19 June 2023 | 7 | Cymoxanil and folpet |
| 3 July 2023 | 14 | Copper oxychloride and iprovalicarb |
| 22 July 2023 | 19 | Cimoxanil and folpet |
| 2 August 2023 | 11 | Copper sulfate and calcium |
| 14 August 2023 | 12 | Copper sulfate and calcium |
| Period | 18 May | 1 June | 15 June | 4 July | 20 July | 8 August | 24 August |
|---|---|---|---|---|---|---|---|
| Blue | * | n.s. | *** | n.s. | n.s. | *** | *** |
| Green | n.s. | *** | n.s. | ** | n.s. | *** | n.s. |
| Red | n.s. | n.s. | * | ** | *** | *** | *** |
| Red Edge | *** | ** | *** | *** | *** | *** | n.s. |
| NIR | *** | ** | *** | *** | n.s. | n.s. | *** |
| BNDVI | * | n.s. | *** | *** | n.s. | *** | *** |
| GBVI | n.s. | ** | *** | *** | *** | *** | *** |
| GNDVI | *** | n.s. | *** | n.s. | ** | *** | *** |
| GRVI | n.s. | * | *** | n.s. | *** | *** | *** |
| NDRE | * | n.s. | n.s. | ** | *** | *** | *** |
| NDVI | * | n.s. | *** | n.s. | *** | *** | *** |
| RBVI | n.s. | n.s. | *** | *** | *** | *** | *** |
| REBI | n.s. | n.s. | *** | *** | *** | n.s. | *** |
| REGI | ** | n.s. | *** | *** | *** | * | ** |
| RERI | * | n.s. | *** | n.s. | *** | *** | *** |
| Height | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | *** |
| Volume | * | n.s. | n.s. | *** | *** | *** | *** |
| Area | ** | n.s. | n.s. | ** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portela, F.; Sousa, J.J.; Araújo-Paredes, C.; Peres, E.; Morais, R.; Pádua, L. Monitoring the Progression of Downy Mildew on Vineyards Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Data. Agronomy 2025, 15, 934. https://doi.org/10.3390/agronomy15040934
Portela F, Sousa JJ, Araújo-Paredes C, Peres E, Morais R, Pádua L. Monitoring the Progression of Downy Mildew on Vineyards Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Data. Agronomy. 2025; 15(4):934. https://doi.org/10.3390/agronomy15040934
Chicago/Turabian StylePortela, Fernando, Joaquim J. Sousa, Cláudio Araújo-Paredes, Emanuel Peres, Raul Morais, and Luís Pádua. 2025. "Monitoring the Progression of Downy Mildew on Vineyards Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Data" Agronomy 15, no. 4: 934. https://doi.org/10.3390/agronomy15040934
APA StylePortela, F., Sousa, J. J., Araújo-Paredes, C., Peres, E., Morais, R., & Pádua, L. (2025). Monitoring the Progression of Downy Mildew on Vineyards Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Data. Agronomy, 15(4), 934. https://doi.org/10.3390/agronomy15040934







