Abstract
Miscanthus × giganteus (Greef and Deuter ex Hodkinson and Renvoize) is a perennial, rhizomatous grass that has gained significant attention as an industrial crop, particularly as a bioenergy feedstock. It is a natural interspecific hybrid with 57 chromosomes (2n = 3x = 57). Due to its sterility, M. × giganteus has limited genetic variability, making traditional breeding methods ineffective for its improvement. Consequently, alternative approaches are being explored to enhance its cultivation and utility. The study aimed to investigate the potential for M. × giganteus plant regeneration through ovary and flower bud cultures. Indirect in vitro regeneration of M. × giganteus plants was successfully achieved using flower bud cultures. Embryogenic-like callus was derived from explants originating from inflorescences that had undergone a four-day pretreating at 10 °C. The most effective medium for callus induction was a modified MS medium supplemented with 5 mg·dm−3 dicamba, 0.2 mg dm−3 6-benzylaminopurine, 30 g dm−3 sucrose, and solidified with 8 g dm−3 agar or agarose. The optimal conditions for callus induction were achieved by culturing in the dark. The regenerated plants exhibited the characteristic chromosome number of the species, confirming that the regenerants did not develop from embryo sac cells. In contrast, ovary culture failed to produce callus or regenerated plants, highlighting its ineffectiveness for M. × giganteus regeneration. These findings underscore the potential of flower bud culture as a successful in vitro regeneration method while demonstrating the limitations of ovary culture for this species.
1. Introduction
Miscanthus × giganteus (Greef and Deuter ex Hodkinson and Renvoize) is a perennial, rhizomatous grass that has gained significant attention as an industrial crop, especially as a bioenergy feedstock. Its rapid growth, minimal agricultural input requirements, and ability to thrive on marginal lands make it a promising candidate for sustainable biomass production [1]. The cultivation of M. × giganteus requires minimal fertilizer input, largely due to the plant’s efficient nutrient translocation mechanisms. During late summer and autumn, nutrients are relocated from its above-ground parts—stems and leaves—to the rhizomes. This process enables the plant to store essential nutrients in the rhizomes, supporting rapid regrowth in the subsequent growing season [2]. This nutrient recycling contributes to the low concentrations of nitrogen, potassium, sulfur, chloride, and ash in the harvested biomass, enhancing its suitability as a bioenergy feedstock. M. × giganteus is notable for its low moisture content at harvest [3]. This low moisture content improves combustion efficiency and reduces transportation costs, making this species a highly efficient and economically viable biomass resource [4]. The high yield of M. × giganteus is attributed to its C4 photosynthesis pathway, which includes a CO2-concentrating mechanism that enables high rates of net CO2 assimilation under chilling conditions and other unfavorable environmental conditions [5]. Due to efficient photosynthesis, M. × giganteus stands out for its carbon sequestration capabilities. Its extensive root system contributes to significant below-ground carbon storage, enhancing soil carbon levels over time [6]. M. × giganteus can play a significant role in bioenergy with carbon capture and storage, known by the acronym “BECCS”. BECCS is a climate change mitigation technology that links energy generation based on existing technologies with the geological storage of sequestered atmospheric carbon [7]. M. × giganteus can provide lignocellulose-rich biomass to replace fossil fuels in energy production, while also sequestering carbon in the soil [6]. The biomass can be processed thermochemically through direct combustion [8], gasification, pyrolysis, or liquefaction [9], or converted biochemically via fermentation to produce biogas or bioethanol [10,11]. Furthermore, M. × giganteus biomass is of interest to the paper industry as an alternative to woody materials [12].
M. × giganteus is a natural interspecific hybrid resulting from crosses between diploid M. sinensis and allotetraploid M. sacchariflorus [8]. This hybridization has led to an allotriploid species with 57 chromosomes (2n = 3x = 57), given the basic chromosome number of 19. Due to its triploid nature, M. × giganteus is sterile and does not produce viable seeds, which poses challenges for breeding methods aimed at improving its agricultural and economic traits. It produces panicles, but does not set seeds [13,14]. Grasses of the genus Miscanthus naturally inhabit tropical and subtropical regions. In 1935, Danish botanist and gardener Aksel Olsen introduced M. × giganteus to Europe from Yokohama (prefecture Kanagawa; Honshu; Japan) [2]. Subsequent introductions to England occurred around 1980, also directly from Japan [10]. Additional interspecies hybrids have been identified in their natural habitats in Japan in the Honshu regions of Hyogo and Gifu Prefectures, and in the Kyushu regions of Kumamoto and Miyazaki Prefectures [9,15]. Plants of M. × giganteus obtained from natural habitats often exhibit suboptimal agricultural and economic characteristics. The limited genetic variability of this sterile species renders traditional breeding methods ineffective for its improvement. Consequently, alternative approaches, such as in vitro regeneration, are being explored to enhance the cultivation and utility features of this grass [16,17].
M. × giganteus is propagated through rhizome division or in vitro micropropagation [2]. Indirect in vitro plant regeneration is common, with plants regenerated from embryogenic callus derived from immature inflorescences. Micropropagation via somatic embryogenesis in the callus is also possible using cultures of shoot apices, leaves, shoots, and root sections from in vitro-cultured or greenhouse-grown plants [2,18,19,20,21]. Direct in vitro plant regeneration can occur through bud development from axillary nodes and apical meristems [2,22].
Our research was part of a project aimed at expanding the genetic variability of M. × giganteus and restoring its fertility. We investigated the potential of in vitro cultures to produce androgenic and gynogenic M. × giganteus plants, as well as the feasibility of whole genome duplication. The presented results are part of an experiment focused on obtaining regenerants through gynogenesis. In 1979, San Noem [23] introduced the term gynogenesis into plant embryology, describing the development of haploid embryos regenerating plants from unfertilized embryo sac cells in in vitro cultures. The first haploid callus derived from unfertilized female gametophytes was obtained in in vitro cultures of Ginkgo biloba [24]. Subsequently, haploid callus was also obtained in cultures of maize ovaries and eggplant ovules [25]. The first haploid plants obtained by gynogenesis were regenerated in cultures of barley ovaries [26].
In previous studies, we attempted M. × giganteus plant regeneration in anther and isolated microspore cultures by inducing androgenesis [27]. Androgenesis is the process of developing haploid embryos from an immature male gametophyte. Our findings demonstrate that while inducing in vitro androgenesis in M. × giganteus is feasible, the process is hindered by several factors. The inflorescences produce microspores in limited quantities, and these microspores exhibit short viability periods. Consequently, the efficiency of androgenesis is exceedingly low, and there is a lack of regeneration in the androgenic structures, rendering this technique impractical. Cytological analysis suggests that the recalcitrance to androgenesis is due to the hybrid origin of M. × giganteus, which results from interploidy crosses leading to irregular meiosis in microsporocytes. During the induction of androgenic structure development, anthers and isolated microspores were co-cultured with immature ovaries obtained from the same inflorescence [27]. It was observed that the ovaries became enlarged, with some indications of callus formation (unpublished observation). Obtaining androgenic plants in species with an odd number of chromosomes may seem challenging. However, successful attempts have been made utilizing pentaploid hybrids of Lolium multiflorum and Festuca arundinacea (2n = 5x = 35). Through another culture, regenerants with chromosome numbers ranging from 14 to 42 have been obtained [28,29].
We also attempted to restore M. × giganteus fertility by doubling its genome [13]. Scientific articles have reported the successful production of hexaploid M. × giganteus plants [30,31,32,33], but there is no further information on their development and reproduction. Our research demonstrates that while it is feasible to produce hexaploid M. × giganteus plants through chromosome doubling techniques, these plants often do not survive to the flowering stage [13]. Whole-genome duplication leads to an increased gene dosage, resulting in the production of additional gene products. This overexpression can cause genomic instability, epigenetic remodeling, and abnormalities in both mitotic and meiotic processes [34,35,36].
Our study aimed to investigate the potential for M. × giganteus plant regeneration through ovary and flower bud cultures. We examined various factors that could influence regeneration efficiency and assessed the ploidy and chromosome numbers of the obtained regenerates, considering the possibility of plant regeneration from embryo sac cells.
2. Materials and Methods
2.1. Experimental Design
Ovary culture, flower bud culture, and reducing explant darkening were conducted as independent experiments. In ovary culture and flower bud culture experiments, inflorescences from two different M. × giganteus clones, serving as the explant source for tissue culture, underwent a pretreatment involving incubation at one of eleven stress temperatures, or no incubation. Ovaries or flower buds were then isolated from the inflorescences and transferred to a callus induction medium. In ovary cultures, six different media were tested, while in flower bud cultures, 16 media were evaluated. These media varied in their composition of auxins, cytokinins, polyamines, sugars, and gelling agents. Additionally, in flower bud cultures, the effect of light was studied on selected media. In the experiment on plant regeneration from flower buds, callus formation occurred on only one type of medium. As a result, only modifications of this medium were developed, and the corresponding culture conditions were studied. In the reducing explant darkening experiment, ovaries and flower buds were isolated from untreated inflorescences of a single M. × giganteus clone and placed on a callus induction medium supplemented with one of four anti-browning agents.
To streamline tissue culture establishment, an incomplete factorial design was implemented, reducing working time, optimizing the use of limited explant sources within greenhouse box space, and focusing on culture conditions commonly used for other plant species by other research teams.
2.2. Plant Material and Growth Conditions
The material comprised two clones of M. × giganteus. The MG1 clone was obtained from the Institute of Plant Breeding and Acclimatization in Radzików (Poland), while the MG2 clone was received from the Horticultural Farm in Zabierzów (Poland). Amplified fragment length polymorphism (AFLP) analysis showed genetic variation between clones [16]. Unfortunately, the exact origin of the clones is unknown. Initially, the plants were grown in the experimental field (50o08′39″ N, 19o85′37″ E) belonging to the University of Agriculture in Kraków (Poland). In October 2015, after the growing season, rhizomes were transferred to a glasshouse. The plants were grown in 15 dm3 containers filled with commercial soil (pH 6.0), at 24 °C and 65% air humidity. Daylight was supplemented with light at 300 µmol m−2 s−1 from sodium lamps (AGRO Philips, Amsterdam, The Netherlands), for a 16 h photoperiod. The plants were fertilized once a month with a multi-component fertilizer, Azofoska (GRUPA INCO S.A., Warsaw, Poland).
2.3. Inflorescence Pretreatment
The inflorescences were harvested in March 2016, when bi- and trinuclear pollen grains were present in their middle part. When the panicle apex emerged from the leaf sheath, a flower bud was collected from the central part of the inflorescence. Anthers were isolated from the flower bud and squashed on a microscope slide in a 1% acetocarmine staining solution. The pollen grains were then observed under a Nikon Eclipse E600 microscope (Nikon Instruments Inc., Tokyo, Japan). Harvested inflorescences were wrapped in foil bags, placed in Hoagland’s medium [37], and pretreated with either cold or heat shock. Cold shock was induced by storing the panicles at 4 °C or 10 °C for 4, 7, 10, 14, or 21 days. Heat shock was applied by exposing the panicles to 33 °C for 2 days. Additionally, inflorescences that were cut directly from plants without pretreatment were used as explant donors. The panicles were sterilized in 70% ethanol.
2.4. Ovary Culture
Ovaries were extracted from the flowers using a binocular microscope and placed in 30 × 15 mm Petri dishes containing induction medium (Table 1 and Table 2). Two types of modified Murashige and Skoog (MS) media [38] were used: medium A (M A), as described by Sibi et al. [39], and Bv1 medium, as used by Wremerth-Weich and Levall [40] (Table 1). The basal composition of M A contains the same macro and micro elements as the MS medium, but with a modified vitamin and amino acid composition. Other organic ingredients, including 2.0 mg dm−3 2,4-dichlorophenoxyacetic acid (2,4-D), 0.5 mg dm−3 kinetin, 60 g dm−3 maltose, and 7 g dm−3 agar, as proposed by Sibi et al. [39], were used. The composition of the Bv1 medium differed from the MS medium in terms of plant growth regulators, and included 0.05 g dm−3 2,4-D and 0.3 g dm−3 6-benzylaminopurine (BA), as well as sucrose (80 g dm−3) and agarose (5.8 g dm−3). The effect of polyamine supplementation was also tested by adding putrescine (2.0 mM) or spermidine (0.1 mM) to the induction media (Table 2). The cultures were maintained on each medium for three weeks and then subcultured on a fresh medium of the same type for another three weeks. In total, three transplantations were performed. The cultures were incubated in the dark at 26 ± 0.5 °C. For each combination of medium and inflorescence pretreatment, 10 Petri dishes were prepared, with each dish containing 20 ovaries.

Table 1.
Basal media composition used for callus induction in flower bud and ovary cultures, as well as for plant regeneration of Miscanthus × giganteus.

Table 2.
The composition of basal media supplemented with plant growth regulators and other organic compounds used for callus induction in flower bud and ovary cultures, as well as for plant regeneration of Miscanthus × giganteus.
2.5. Flower Bud Culture
Flower buds were placed in 60 × 15 mm Petri dishes containing the following induction media (Table 1 and Table 2): modified MS medium or medium A (M A), as described by Martínez [41]. The basal composition of MS medium was supplemented with 0.2 mg dm−3 BA, 5 mg dm−3 2,4-D, 30 g dm−3 sucrose, and was solidified with 8 g dm−3 agarose. This medium was modified to test different auxin compositions by using either 2 mg dm−3 2,4-D or 2 mg dm−3, or 5 mg dm−3 dicamba. The effect of polyamines was also tested by adding putrescine (2.0 mM) or spermidine (0.1 mM) to the basic MS medium (Table 2). Furthermore, the effect of sugar concentration was examined by increasing the sucrose concentration to 50 g dm−3. The influence of gelling agents was investigated by solidifying the medium with 8 g dm−3 agar instead of agarose. The basal composition of M A was supplemented with 2.0 mM putrescine or 0.1 mM spermidine, 100 g dm−3 sucrose, and was solidified with 7.5 g dm−3 agar, as proposed by Martínez [41] (Table 2). The cultures were maintained on each medium for three weeks and then subcultured on a fresh medium of the same type for another three weeks. In total, three transplantations were performed. The cultures were incubated in the dark at 26 ± 0.5 °C. For the basic MS medium, the effect of light was also studied. A portion of the flower buds was cultured in complete darkness, while others were exposed to dim light (100 μmol m−2 s−1 PPFD) with a 16 h photoperiod. For each combination of media, inflorescence pretreatment, and light conditions, 10 Petri dishes were prepared, with each dish containing 40 flower buds.
2.6. Reducing Explant Darkening
To reduce explant darkening, the induction media were supplemented with honey (30 g dm−3), L-proline (2.88 g dm−3), L-cysteine (50 mg dm−3), or reduced glutathione (GSH) (30 mg·dm−3). Commercial multifloral honey was used as a sugar substitute at the same concentration. In ovary culture, the effect of these compounds was tested using medium O1 (Table 2.). In flower bud culture, they were added to the F1 medium (Table 2). Explants were taken from the MS2 clone, without inflorescences pretreatment.
2.7. Plant Regeneration
Plant regeneration was carried out as described previously [21]. After 12 weeks of culture, calli were transferred to the regeneration medium (RM), consisting of MS medium supplemented with 0.2 mg dm−3 BA, 0.05 mg dm−3 kinetin, 30 g dm−3 sucrose, and solidified with 8 g dm−3 agar (Table 2). The cultures were maintained at 20 ± 0.5 °C under a 16 h photoperiod with a light intensity of 300 μmol m−2 s−1 PPFD. The regenerants were transferred to small pots (Ø 7 cm) containing sterile perlite soaked with Hoagland’s medium [37] and grown under phytotronic conditions (20 ± 0.5 °C, 16 h photoperiod with a light intensity of 300 μmol m−2 s−1 PPFD) for acclimatization. The regenerants were covered with transparent lids. Subsequently, the plants were re-potted into larger pots (Ø 13 cm) filled with a soil–peat–sand mixture (2:2:1 v/v/v) at pH 6.0 and grown in a greenhouse at 24 ± 2 °C. Daylight was supplemented with light from sodium lamps (AGRO Philips) at 300 μmol m−2 s−1 PPFD, under a 16 h photoperiod. For estimating plant regeneration capacity, the number of experimental replicates corresponded to the number of Petri dishes containing the same medium, on which the explants were placed and cultured under the same conditions.
2.8. Ploidy and Chromosome Number Determination
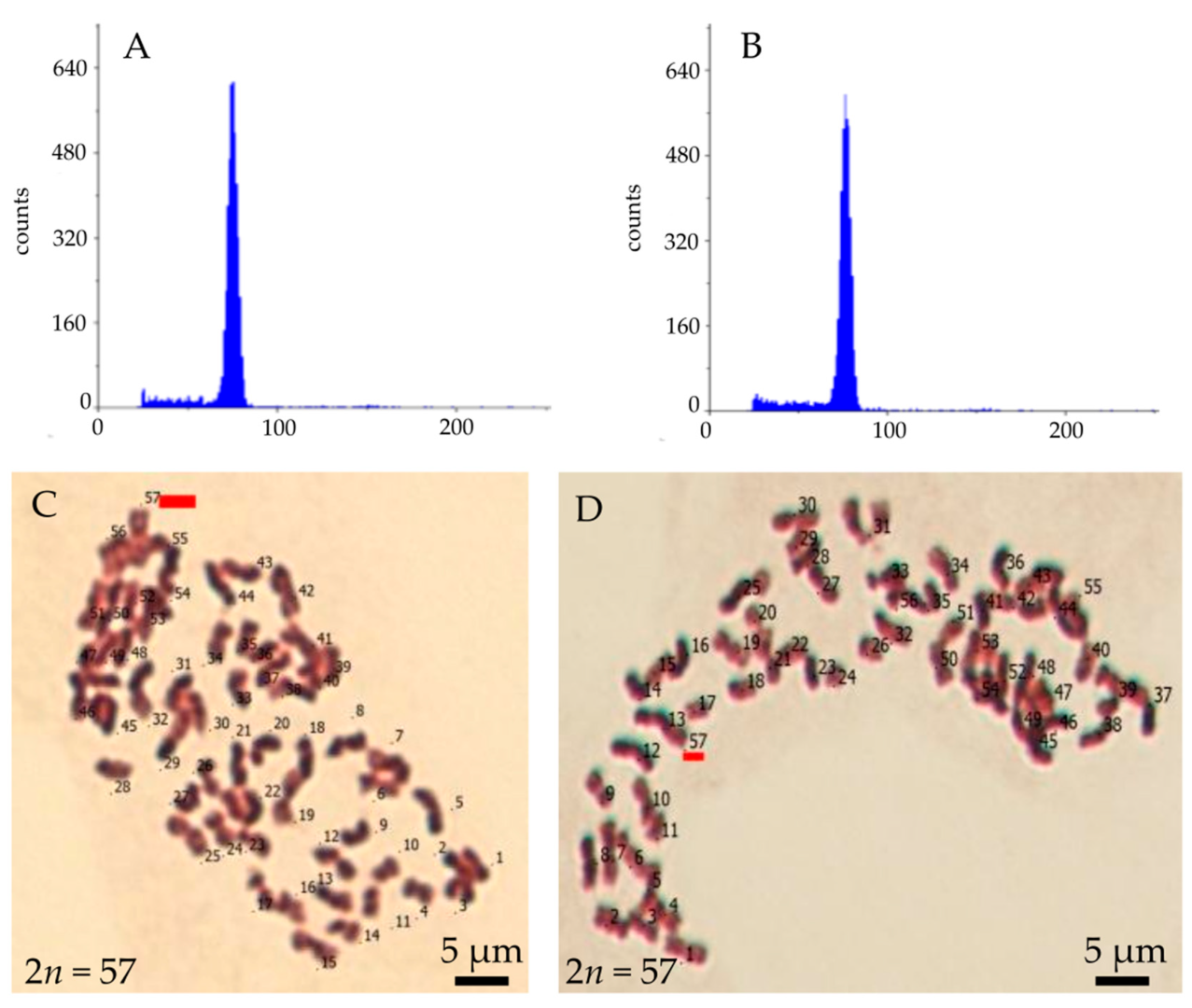
Flow cytometry analysis was performed on leaf fragments, as described by Kopeć and Płażek [13] in the Cytogenetics Laboratory of White Beet Breeding in Kutno (Poland). The ploidy composition of the regenerants was estimated by comparing the peaks in the nuclear DNA histogram with those of the explant donor plants.
Chromosome numbers were counted from root meristem preparations according to [42], using acetic orcein staining. Microscope slides were examined under a Nikon Eclipse E800 or E600 microscope (Nikon Instruments Inc., Tokyo, Japan). The images were captured with a Nikon DS-2MBWc or DS-Ri1 digital camera (Nikon Instruments Inc., Tokyo, Japan) and processed using NIS Elements AR 4.00 software.
Flow cytometry analysis and chromosome number observations were performed for all obtained regenerants.
2.9. Statistical Analysis
Callus formation efficiency on inducing media was expressed as the callus induction rate (CIR)—the number of obtained calli per 100 explants. The efficiency of plant regeneration from calli on RM was expressed as the plantlet regeneration rate (PRR)—the number of plantlets obtained per 100 calli. The total regeneration efficiency (TRE) represented the number of plantlets obtained per 100 explants and served as a summary parameter for the conducted cultures. These parameters were selected based on the research by Ślusarkiewicz-Jarzina et al. [43].
The results of CIR, PRR, and TRE, reflecting the influence of auxin types, sucrose concentrations, gelling agents, and light conditions in cultures, were presented using Tukey’s box plots. The data were analyzed using the Lilliefors test (p > 0.05) for normality and the Brown–Forsythe test (p > 0.05) for the equality of group variances. The tests indicated non-normal data distribution and unequal group variances. Since the assumptions of analysis of variance (ANOVA) were not met, each experimental factors was analyzed separately using the nonparametric test, the Mann–Whitney U test (p > 0.05). Interactions between variables were not analyzed due to their low anticipated impact on the regeneration capacity of plants from ovaries or flower buds. However, investigating these interactions could be valuable for future studies aimed at enhancing plant regeneration efficiency. Statistical analysis and data visualization were performed using R 4.4.2 [44], along with the dplyr (v1.1.4) [45] and ggplot2 (v3.5.1) [46] packages.
3. Results and Discussion
3.1. Ovary Culture
In the experiment aimed at plant regeneration in ovary cultures, 29,600 ovaries were placed on the induction media (Figure 1). Regardless of the M. × giganteus clone used as the source of explants, as well as the pretreatment and induction media applied, plant regeneration from ovary tissues was not achieved. After one week, the ovaries began to darken (Figure 1), a common phenomenon in tissue cultures of the Miscanthus genus [2]. Explant darkening in tissue culture is primarily caused by enzymatic processes. Enzymatic browning occurs when explants secrete phenols, which subsequently undergo oxidation by enzymes, forming quinones. Additionally, quinones can interact with proteins or polymerize, producing dark-colored melanin compounds that disrupt tissue metabolism. The melanin compounds inhibit growth and ultimately lead to the darkening and death of the explants [47]. Many modifications to media have been attempted to prevent this phenomenon in Miscantus tissue cultures, often without success. During plant regeneration from immature inflorescences, α-aminooxyacetic acid (AOA), an inhibitor of phenylalanine ammonialyase (PAL)—the key enzyme of the phenylpropanoid pathway—was used, as well as polyvinylpyrrolidone (PVP). However, neither component had a positive effect on reducing explant darkening [48]. In ovary culture, supplements such as honey, L-cysteine, L-proline, and GSH were tested to prevent tissue darkening (Figure 1). Płażek and Dubert [21] successfully reduced the darkening of immature inflorescences fragments in culture by replacing sucrose with honey. Holme et al. [19] observed, that proline had a beneficial effect on reducing the explants browning in cultures of shoot apices, immature inflorescences, and immature leaves of in vitro-formed shoots. Gubišová et al. [49] also successfully used the addition of L-cysteine in the cultures of immature inflorescences and non-detached axillary buds. Glutathione, a major reducing agent in living cells, has not yet been used in tissue cultures of plants of M. × giganteus, though it has been applied in other plants. For instance, GSH was used to prevent browning in shoot tip explants of apple (Malus pumila Mill.) [50]. However, the anti-browning agents used in ovary culture did not reduce explants darkening and had no impact on plant regeneration.
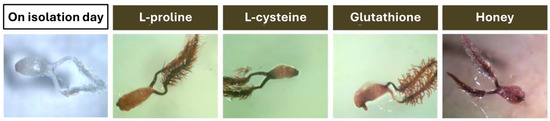
Figure 1.
Darkening of ovaries in tissue culture of Miscanthus × giganteus observed after the first week, despite supplementation of the callus-inducing medium with one of the following additives: L-proline, L-cysteine, reduced glutathione, or multifloral honey.
3.2. Flower Bud Culture
In the experiment aimed at plant regeneration in flower bud cultures, 155,200 flower buds were placed on induction media (Figure 2). The explants darkened after one week of culture, similar to the ovaries. Supplementation of the basal medium with honey, L-cysteine, L-proline, or GSH did not prevent this phenomenon (Figure 2).

Figure 2.
Darkening of flower buds in tissue culture of Miscanthus × giganteus observed after the first week, despite supplementation of the callus-inducing medium with one of the following additives: L-proline, L-cysteine, reduced glutathione, or multifloral honey.
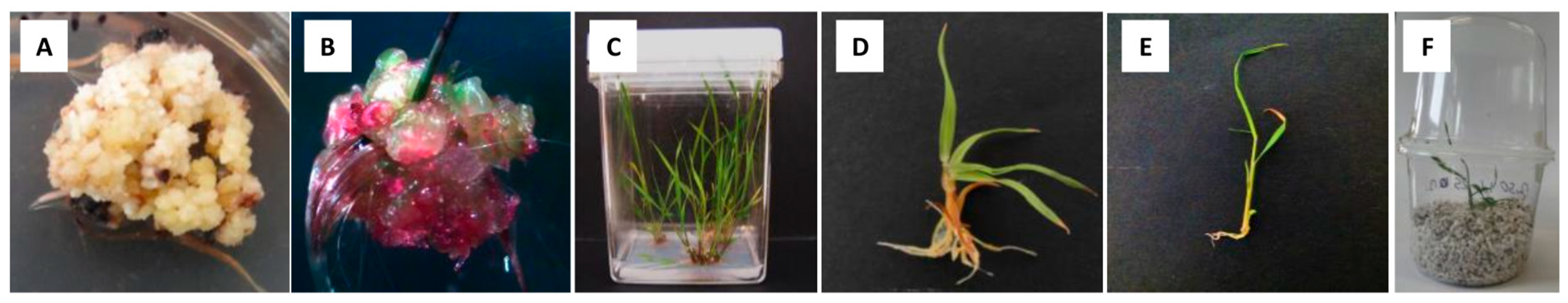
In the established cultures, only callus formation was observed. This occurred exclusively in explants taken from the MG2 clone and on media where the basal medium was MS. Two types of calli were formed: yellowish nodular callus (Figure 3A) and a semisoft, anthocyanin-colored callus (Figure 3B).

Figure 3.
Two types of calli were formed on induction media in flower bud culture of Miscanthus × giganteus: yellowish nodular callus (A) and semisoft, anthocyanin-colored callus (B). The first type formed shoots and roots on the regeneration medium (C,D), while the second type directly produced shoots on the induction medium (E). Plantlet acclimatized to greenhouse conditions (F).
The most common type was the yellowish nodular callus, which contained some soft areas and small, white, compact clusters (Figure 3A). Based on previous studies, this type of callus has been referred to as embryogenic-like callus. A similar callus, derived from fragments of immature inflorescences and leaf explants from in vitro-propagated shoots, was described by Holme and Petersen [18] and Ślusarkiewicz et al. [43]. The latter reported that somatic embryos often developed in loosely attached groups on the surface of callus tissue. Somatic embryogenesis was initiated at the very early stages of callus growth in immature inflorescences, with lobular and polarized embryos appearing 3–4 weeks after culture initiation.
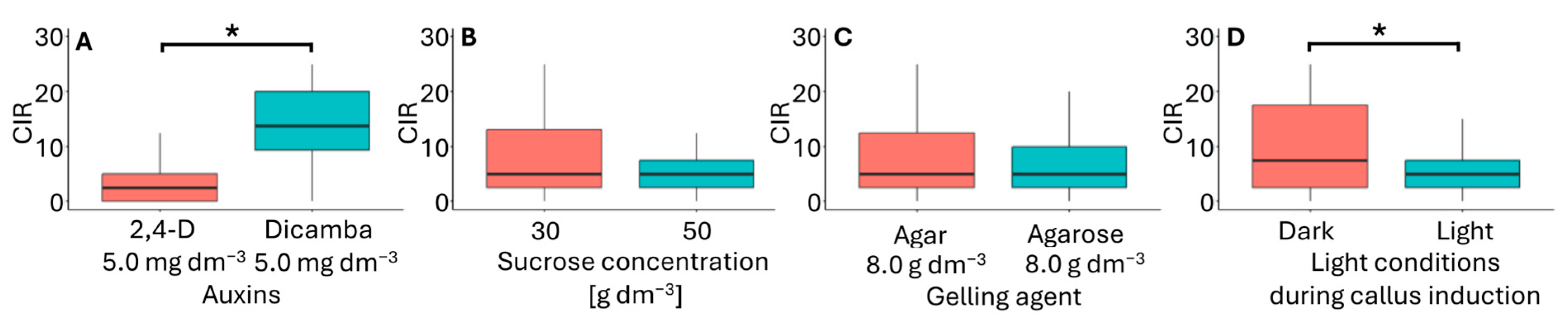
In the flower bud culture, embryogenic-like callus (Figure 3A) was obtained from explants originating from inflorescences pretreated for four days at 10 °C. Of the 16 media tested, callus formation only occurred on six (Table 3). These media used were modified MS medium supplemented with 5.0 mg dm−3 2,4-D or dicamba, 30 or 50 g dm−3 sucrose, and solidified with either agar or agarose. Embryogenic-like calli developed on these media under both light and dark. The most efficient callus development occurred on F6 medium (5.0 mg dm−3 dicamba, 30 g dm−3 sucrose, 8 g dm−3 agar) in darkness, with a CIR 21.3 (Table 2). The media that supported embryogenic-like callus formation varied in the type of auxin used (Figure 4A), sucrose concentration (Figure 4B), type of gelling agent (Figure 4C), and light conditions during callus induction (Figure 4D). In this experiment, neither sucrose concentration nor the type of gelling agent had a significant effect on CIR. However, CIR was influenced by the type of auxin and light conditions of the culture. Callus growth was the most effective in media supplemented with dicamba and cultured in darkness. These findings contradict previous studies, which have suggested that the most effective auxin for callus induction is 2,4-D at an intermediate concentration [18,20,21,43].

Table 3.
The average efficiency of embryogenic-like calli induction in flower bud culture of Miscanthus × giganteus across various media formulations and light conditions, along with the corresponding efficiency of plant regeneration. Media that did not induce callus formation were excluded from the table.
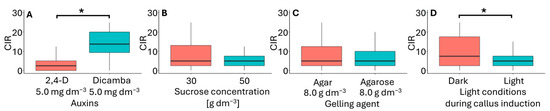
Figure 4.
Callus induction rate (CIR) in flower bud cultures of Miscanthus × giganteus, depending on media formulations, including the type of auxin supplementation (A), sucrose concentration (B), type of gelling agent (C), and light conditions (D). Differences between groups were assessed using the Mann–Whitney U test (p > 0.05) and are indicated with an asterisk. The box plots are presented in Tukey’s style. CIR values: the number of calli per 100 flower buds.
Ślusarkiewicz et al. [43] reported a CIR 517.9 in immature inflorescence cultures using MS induction medium supplemented with 5.0 mg dm−3 2,4-D, 0.5 mg dm−3 BA, 30 g dm−3 sucrose, and solidified with 7 g dm−3 agar, incubated in darkness. Płażek and Dubert [21] reported CIR higher than 60.0 value in immature inflorescence cultures conducted in darkness using MS induction medium supplemented with 6.5 mg dm−3 2,4-D, 0.25 mg dm−3 BA, 500 mg dm−3 casein hydrolysate, various sugar sources (30 g dm−3 sucrose, 30 g dm−3 honey, or 65 g dm−3 banana pulp), and solidified with 8 g dm−3 agar. The type of gelling agent added to the medium influenced embryo development in a two-step flower/ovary culture procedure of onion, ultimately leading to the regeneration of gynogenic plants. Using gellan gum instead of the commonly used agar significantly increased embryo induction, but also resulted in a higher number of abnormal regenerates [51]. This suggests that selecting appropriate gelling agents in such studies can enhance the formation of gynogenic structures while minimizing abnormalities in regenerants, which could otherwise limit their ability to acclimate to greenhouse conditions.
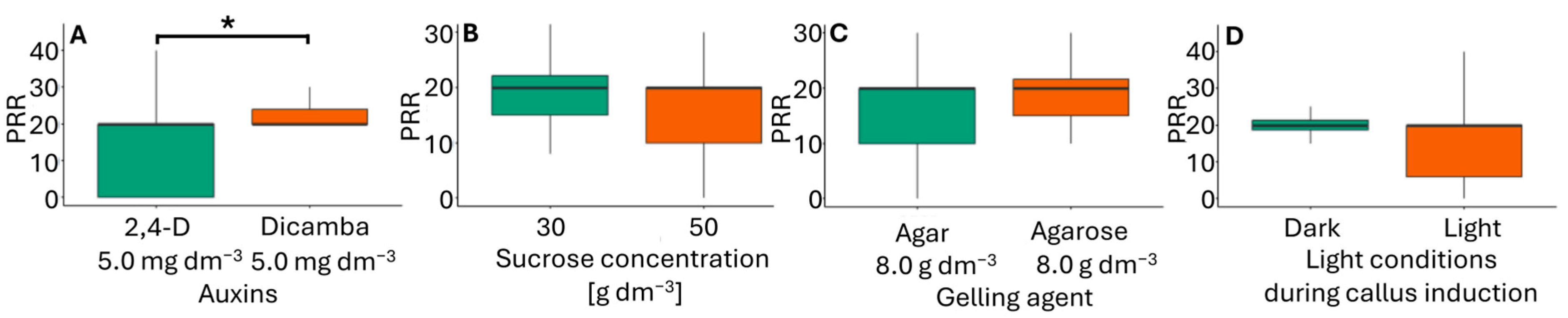
Calli were excised from the original explants and transferred to RM, where they regenerated roots and simultaneously produced one–four shoots. All shoots were green. Rooted shoots were easily separated from callus residues, forming plantlets (Figure 3C). The plantlets were then transferred to pots filled with perlite soaked in Hoagland’s medium and acclimatized to greenhouse conditions. The combination of callus induction medium type and light resulted in a similar plantlet regeneration across treatments, with values ranging from 1.8 to 2.3 (Table 3). Among the factors considered (Figure 5), only the type of auxin had an impact on the plantlet regeneration rate. In immature inflorescences, Ślusarkiewicz et al. [43] reported PPR values ranging from 146.8 to 349.7, while Płażek and Dubert [21] obtained PPR values between 33.5 and 72.8.
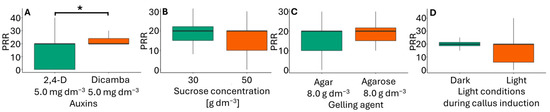
Figure 5.
Plantlet regeneration rate (PRR) in flower bud cultures of Miscanthus × giganteus, depending on induction media formulations, including the type of auxin supplementation (A), sucrose concentration (B), type of gelling agent (C), and light conditions during callus induction (D). Differences between groups were assessed using the Mann–Whitney U test (p > 0.05) and are indicated with an asterisk. The box plots are presented in Tukey’s style. PRR value: the number of individual plantlets per 100 calli.
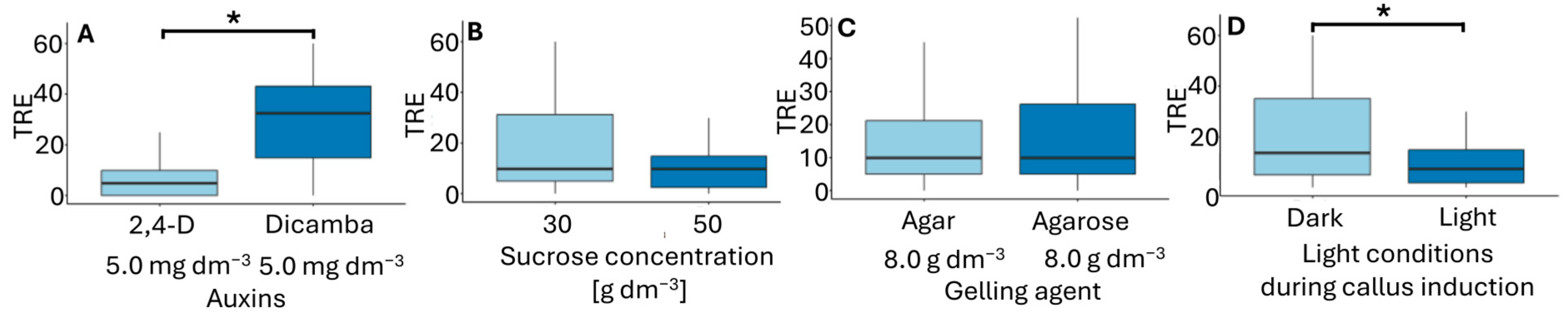
Total regeneration efficiency (TRE) reflects the combined influence of conditions of callus formation and plantlet regeneration. In flower bud culture, the highest TRE values were obtained when callus was induced on F6 and F5 media in darkness (Table 3), with TRE values of 45.8 and 41.8, respectively. The only difference between these media was the type of gelling agent used. Similarly to the CIR parameter, TRE was primarily influenced by the type of auxin and the light conditions during callus induction (Figure 6). The use of dicamba in the inducing medium and callus induction in darkness had a positive effect on regeneration efficiency. Ślusarkiewicz et al. [43] reported plant regeneration efficiency reaching a TRE of 1811.9, whereas Płażek and Dubert [21] obtained TRE values ranging from 22.5 to 56.9, depending on the composition of the inducing and regenerating medium.
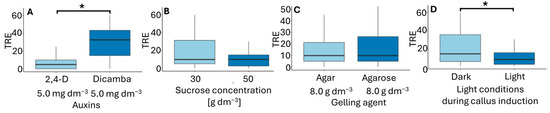
Figure 6.
Total regeneration efficiency (TRE) in flower bud cultures of Miscanthus × giganteus, depending on induction media formulations, including the type of auxin supplementation (A), sucrose concentration (B), type of gelling agent (C), and light conditions during callus induction (D). Differences between groups were assessed using the Mann–Whitney U test (p > 0.05) and are indicated with an asterisk. The box plots are presented in Tukey’s style. TRE value: the number of individual plantlets per 100 flower buds.
The second type of callus that developed in flower bud cultures, though occurring at a very low frequency, was the semisoft, anthocyanin-colored callus (Figure 3B). A similar structure was described by Holme and Petersen [18] as nodular, semisoft callus, sometimes exhibiting anthocyanin-colored spots. They observed the highest percentage of this callus type in cultures of immature inflorescences. Similar observations were reported by Głowacka et al. [20]. In flower bud cultures, this type of callus originated from explants of non-pretreated inflorescences from the MG2 clone. It was grown on four MS-inducing media containing 5 mg dm−3 2,4-D or 5 mg dm−3 dicamba and 30 or 50 g dm−3 sucrose, and solidified with agarose or agar. All cultures were incubated in light. After 12 weeks, green shoot regeneration was observed while still on the induction medium. All shoots transferred to the regeneration medium were successfully rooted and then acclimatized to greenhouse conditions. However, callus pieces transferred to RM did not regenerate green shoots and roots and died after two weeks. Holme and Petersen [18] previously reported that this type of callus could form roots upon transfer to a medium with lower 2,4-D concentrations. In the present study, the CIR, PRR, and TRE parameters describing callus induction, and plant regeneration efficiency was relatively low (Table 4). However, an intriguing observation was the emergence of shoots of unknown origin from within the callus tissues (Figure 3B). This phenomenon may suggest the regeneration of shoots from embryo sac cells.

Table 4.
The average efficiency of semisoft, anthocyanin-colored calli induction in flower bud culture of Miscanthus × giganteus across various media formulations and light conditions, along with the corresponding efficiency of plant regeneration. Media that did not induce callus formation were excluded from the table.
3.3. Ploidy and Chromosome Number of Regenerants
A total of 751 plantlets of M. × giganteus were developed from embrogenic-like calli induced in flower bud culture (Figure 3C,D,F). Additionally, ten regenerants were obtained whose shoots regenerated directly on induction media in flower bud culture (Figure 3E,F). First, the ploidy of the regenerants was analyzed by flow cytometry. The analysis confirmed that all examined plants had the same ploidy level as the MG2 clone from which they were derived (Figure 7A,B), indicating that they remained triploid. Secondly, chromosome counts were conducted on cells from root apical meristems. All plantlets possessed the characteristic chromosome number for, 2n = 57 (Figure 7C,D). In some metaphase plates, 1–2 B-chromosomes were observed. During karyotype analysis of M. × giganteus, Chramiec-Głąbik et al. [42] detected numerous metaphases with an incomplete chromosome count, along with 1–4 B-chromosomes and chromosomal bodies that were likely acentric chromosome fragments.
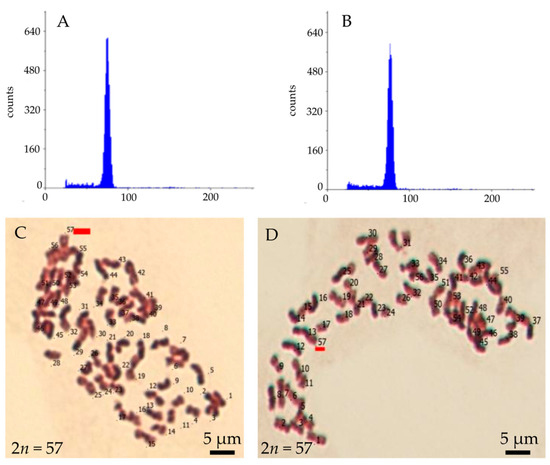
Figure 7.
Ploidy and chromosome number of Miscanthus × giganteus plantlet regenerated from callus induced in flower bud culture: flow cytometry histogram of a regenerant (A) and the explant source plant (B), and chromosome number observed in a metaphase of plate from the root apical meristem plants regenerate from embryogenic-like callus (C) and anthocyanin-colored callus (D).
The fact that plantlets contained 57 chromosomes suggests that they were not regenerated from embryo sac cells, indicating that gynogenesis was not induced. Gynogenesis in vitro is typically applied to species that are not amenable to androgenesis development and to overcome challenges such as albinism and inability of plants to regenerate [52,53,54]. Gynogenic plants regenerate from embryo sac cells in unfertilized flowers/flower buds, ovaries, or ovule culture. The flower buds and ovaries of M. × giganteus were collected from parts of inflorescences containing bi- and trinuclear pollen grains. In many cereal species, this stage of pollen development corresponds to the presence of a mature embryo sac. This method is used for the in vitro induction of gynogenic structures and the development of haploid or doubled haploid plants, where the chromosome number spontaneously doubled [52,55,56].
M. × giganteus produces only a few normally developed Polygonum-type embryo sacs. Megasporogenesis and female gametophyte development are severely disrupted, with degeneration occurring in the early stages of ovule development—shortly after female meiosis—as well as in later stages of female gametophyte development [14]. Abnormal organization and degeneration of the female gametophyte are common phenomena in allopolyploids, particularly in those with an odd-numbered set of chromosomes [57].
4. Conclusions
The indirect in vitro regeneration of M. × giganteus plants in flower bud cultures is the first report of its kind in the Miscanthus genus. Previous studies have primarily focused on using immature inflorescences fragments for plant propagation. Many researchers have noted that total regeneration efficiency decreases as the inflorescence matures. In our study, the flower buds used to establish cultures were taken from mature, well-formed inflorescences, which likely contributed to the low plant regeneration efficiency observed. As a result, we obtained a relatively small number of plantlets compared to the number of explants cultured.
Embryogenic-like callus was derived from explants originated from MG2 clone inflorescences that had undergone a four-day pretreating at 10 °C. The most effective medium for callus induction was a modified MS medium supplemented with 5 mg dm−3 dicamba, 0.2 mg dm−3 BA, 30 g dm−3 sucrose, and solidified with 8 g dm−3 agar or agarose. The optimal conditions for callus induction were achieved by culturing in the dark. The regenerated plants exhibited the characteristic chromosome number of the species, confirming that the regenerants did not arise through gynogenesis.
In ovary culture, neither callus development nor plant regeneration was observed. This suggests that the callus formation originated from tissues other than the ovaries. The exclusive death of explants in ovary culture may have been caused by disturbances in embryo sac development, unsuitable culture conditions, physical damage to the ovary, or the toxic effects of oxidation products from phenolic compounds released by the tissues.
Author Contributions
Conceptualization, P.K. and A.P.; investigation, P.K., A.P. and K.L.; formal analysis P.K.; writing, P.K.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by project No 810/NCOST/2010/0 financed by the Polish Ministry of Science and Higher Education under COST Action FA 0903, “Harnessing Plant Reproduction for Crop Improvement (HAPRECI)”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to acknowledge Adam Kula for supporting in cytological analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ben Fradj, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European bio-economy: A network analysis. Ind. Crops Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- Lewandowski, I. Micropropagation of Miscanthus × giganteus. In High-Tech and Micropropagation V. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 39, pp. 239–255. [Google Scholar]
- Jensen, E.; Robson, P.; Farrar, K.; Thomas Jones, S.; Clifton-Brown, J.; Payne, R.; Donnison, I. Towards Miscanthus combustion quality improvement: The role of flowering and senescence. GCB Bioenergy 2017, 9, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Bilandzija, N.; Jurisic, V.; Voca, N.; Leto, J.; Matin, A.; Sito, S.; Kricka, T. Combustion properties of Miscanthus x giganteus biomass—Optimization of harvest time. J. Energy Inst. 2017, 90, 528–533. [Google Scholar] [CrossRef]
- Ma, J.Y.; Sun, W.; Koteyeva, N.K.; Voznesenskaya, E.; Stutz, S.S.; Gandin, A.; Smith-Moritz, A.M.; Heazlewood, J.L.; Cousins, A.B. Influence of light and nitrogen on the photosynthetic efficiency in the C4 plant Miscanthus × giganteus. Photosynth. Res. 2017, 131, 1–13. [Google Scholar] [CrossRef]
- Nakajima, T.; Yamada, T.; Anzoua, K.G.; Kokubo, R.; Noborio, K. Carbon sequestration and yield performances of Miscanthus × giganteus and Miscanthus sinensis. Carbon Manag. 2018, 9, 415–423. [Google Scholar] [CrossRef]
- Hanssen, S.V.; Daioglou, V.; Steinmann, Z.J.N.; Doelman, J.C.; Van Vuuren, D.P.; Huijbregts, M.A.J. The climate change mitigation potential of bioenergy with carbon capture and storage. Nat. Clim. Chang. 2020, 10, 1023–1029. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Chase, M.W.; Takahashi, C.; Leitch, I.J.; Bennett, M.D.; Renvoize, S.A. The use of DNA sequencing (ITS and TRNL-F), AFLP, and fluorescent in situ hybridization to study allopolyploid Miscanthus (Poaceae). Am. J. Bot. 2002, 89, 279–286. [Google Scholar] [CrossRef]
- Nishiwaki, A.; Mizuguti, A.; Kuwabara, S.; Toma, Y.; Ishigaki, G.; Miyashita, T.; Yamada, T.; Matuura, H.; Yamaguchi, S.; Lane Rayburn, A.; et al. Discovery of natural Miscanthus (Poaceae) triploid plants in sympatric populations of Miscanthus sacchariflorus and Miscanthus sinensis in southern Japan. Am. J. Bot. 2011, 98, 154–159. [Google Scholar] [CrossRef]
- Sacks, E.J.; Juvik, J.A.; Lin, Q.; Ryan Stewart, J.; Yamada, T. The gene pool of Miscanthus species and its improvement. In Genomics of the Saccharinae; Springer: New York, NY, USA, 2013; pp. 73–101. [Google Scholar]
- Clifton-Brown, J.C.; Chiang, Y.C.; Hodkinson, T.R. Miscanthus: Genetic Resources and Breeding Potential to Enhance Bioenergy Production; Vermerris, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Danielewicz, D.; Surma-Ślusarska, B. Miscanthus × giganteus stalks as a potential non-wood raw material for the pulp and paper industry. Influence of pulping and beating conditions on the fibre and paper properties. Ind. Crops Prod. 2019, 141, 111744. [Google Scholar] [CrossRef]
- Kopeć, P.; Płażek, A. An attempt to restore the fertility of Miscanthus × giganteus. Agronomy 2023, 13, 323. [Google Scholar] [CrossRef]
- Słomka, A.; Kuta, E.; Płazek, A.; Dubert, F.; Zur, I.; Dubas, E.; Kopeć, P.; Zurek, G. Sterility of Miscanthus × giganteus results from hybrid incompatibility. Acta Biol. Cracoviensia Ser. Bot. 2012, 54, 113–120. [Google Scholar] [CrossRef]
- Dwiyanti, M.S.; Rudolph, A.; Swaminathan, K.; Nishiwaki, A.; Shimono, Y.; Kuwabara, S.; Matuura, H.; Nadir, M.; Moose, S.; Stewart, J.R.; et al. Genetic analysis of putative triploid Miscanthus hybrids and tetraploid M. sacchariflorus collected from sympatric populations of Kushima, Japan. Bioenergy Res. 2013, 6, 486–493. [Google Scholar] [CrossRef]
- Płazek, A.; Dubert, F.; Kopeć, P.; Krepski, T.; Kacorzyk, P.; Micek, P.; Kurowska, M.; Szarejko, I.; Zurek, G. In vitro-propagated Miscanthus × giganteus plants can be a source of diversity in terms of their chemical composition. Biomass Bioenergy 2015, 75, 142–149. [Google Scholar] [CrossRef]
- Perera, D.; Barnes, D.J.; Baldwin, B.S.; Reichert, N.A. Direct and indirect in vitro regeneration of Miscanthus × giganteus cultivar Freedom: Effects of explant type and medium on regeneration efficiency. Vitr. Cell. Dev. Biol.—Plant 2015, 51, 294–302. [Google Scholar] [CrossRef]
- Holme, I.B.; Petersen, K.K. Callus induction and plant regeneration from different explant types of Miscanthus x ogiformis Honda “Giganteus”. Plant Cell Tissue Organ Cult. 1996, 45, 43–52. [Google Scholar] [CrossRef]
- Holme, I.B.; Krogstrup, P.; Hansen, J. Embryogenic callus formation, growth and regeneration in callus and suspension cultures of Miscanthus x ogiformis Honda Giganteus’ as affected by proline. Plant Cell Tissue Organ Cult. 1997, 50, 203–210. [Google Scholar] [CrossRef]
- Głowacka, K.; Jeżowski, S.; Kaczmarek, Z. The effects of genotype, inflorescence developmental stage and induction medium on callus induction and plant regeneration in two Miscanthus species. Plant Cell Tissue Organ Cult. 2010, 102, 79–86. [Google Scholar] [CrossRef]
- Płażek, A.; Dubert, F. Improvement of medium for Miscanthus × giganteus callus induction and plant regeneration. Acta Biol. Cracoviensia Ser. Bot. 2010, 52, 105–110. [Google Scholar] [CrossRef]
- Rambaud, C.; Arnoult, S.; Bluteau, A.; Mansard, M.C.; Blassiau, C.; Brancourt-Hulmel, M. Shoot organogenesis in three Miscanthus species and evaluation for genetic uniformity using AFLP analysis. Plant Cell Tissue Organ Cult. 2013, 113, 437–448. [Google Scholar] [CrossRef]
- San Noem, L.H. In vitro induction of gynogenesis in higher plants. In Broadening the Genetic Base of Crops; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1979; pp. 327–329. [Google Scholar]
- Tulecke, W. A haploid tissue culture from the female gametophyte of Gingko biloba L. Nature 1964, 2003, 94–95. [Google Scholar] [CrossRef]
- Uchimiya, H.; Toshiaki, K.; Norindo, T. In vitro culture of unfertilized ovules in Solanum melongena and ovaries in Zea mays. Jpn. J. Breed. 1971, 21, 247–250. [Google Scholar] [CrossRef]
- San Noem, L.H. Haploides d’Hordeum vulgare L. par culture in vitro non fécondés. Ann. Amélior. Plantes 1976, 26, 751–754. [Google Scholar]
- Żur, I.; Dubas, E.; Słomka, A.; Dubert, F.; Kuta, E.; Płażek, A. Failure of androgenesis in Miscanthus × giganteus in vitro culture of cytologically unbalanced microspores. Plant Reprod. 2013, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zwierzykowski, Z.; Zwierzykowska, E.; Slusarkiewicz-Jarzina, A.; Ponitka, A. Regeneration of anther-derived plants from pentaploid hybrids of Festuca arundinacea x Lolium multiflorum. Euphytica 1999, 105, 191–195. [Google Scholar] [CrossRef]
- Zare, A.G.; Humphreys, M.W.; Rogers, J.W.; Mortimer, A.M.; Collin, H.A. Androgenesis in a Lolium multiflorum x Festuca arundinacea hybrid to generate genotypic variation for drought resistance. Euphytica 2002, 125, 1–11. [Google Scholar] [CrossRef]
- Głowacka, K.; Jezowski, S.; Kaczmarek, Z. In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind. Crops Prod. 2010, 32, 88–96. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kim, H.S.; Rayburn, A.L.; Widholm, J.M.; Juvik, J.A. Chromosome doubling of the bioenergy crop, Miscanthus × giganteus. GCB Bioenergy 2009, 1, 404–412. [Google Scholar] [CrossRef]
- Chae, W.B.; Hong, S.J.; Gifford, J.M.; Lane Rayburn, A.; Widholm, J.M.; Juvik, J.A. Synthetic polyploid production of Miscanthus sacchariflorus, Miscanthus sinensis, and Miscanthus x giganteus. GCB Bioenergy 2013, 5, 338–350. [Google Scholar] [CrossRef]
- Petersen, K.K.; Hagberg, P.; Kristiansen, K. Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult. 2003, 73, 137–146. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef]
- Baduel, P.; Bray, S.; Vallejo-Marin, M.; Kolář, F.; Yant, L. The “Polyploid Hop”: Shifting challenges and opportunities over the evolutionary lifespan of genome duplications. Front. Ecol. Evol. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Hollister, J.D. Polyploidy: Adaptation to the genomic environment. New Phytol. 2015, 205, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. Preparing the nutrient solution. The Water-Culture Method for Growing Plants without Soil. In California Agricultural Experiment Station Circular; University of Michigan Library: Ann Arbor, MI, USA, 1950; Volume 347, pp. 29–31. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tabacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sibi, M.L.; Kobaissi, A.; Shekafandeh, A. Green haploid plants from unpollinated ovary culture in tetraploid wheat (Triticum durum Defs.). Euphytica 2001, 122, 351–359. [Google Scholar] [CrossRef]
- Wremerth, E.; Levall, M.W. Doubled haploid production of sugar beet (Beta vulgaris L.). In Doubled Haploid Production in Crop Plants; Maluszysnki, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer Science+Business Media: New York, NY, USA, 2003; pp. 255–263. [Google Scholar]
- Martinez, L. In vitro gynogenesis induction and doubled haploid production in onion (Allium cepa L.). In Doubled Haploid Production in Crop Plants; Maluszynski, M., Krasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer Science+Business Media: New York, NY, USA, 2003; pp. 275–279. [Google Scholar]
- Chramiec-Głabik, A.; Grabowska-Joachimiak, A.; Sliwinska, E.; Legutko, J.; Kula, A. Cytogenetic analysis of Miscanthus × giganteus and its parent forms. Caryologia 2012, 65, 234–242. [Google Scholar] [CrossRef]
- Ślusarkiewicz-Jarzina, A.; Ponitka, A.; Cerazy-Waliszewska, J.; Wojciechowicz, M.K.; Sobańska, K.; Jeżowski, S.; Pniewski, T. Effective and simple in vitro regeneration system of Miscanthus sinensis, M. × giganteus and M. sacchariflorus for planting and biotechnology purposes. Biomass Bioenergy 2017, 107, 219–226. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Fundation for Statistical Computing: Vienna, Austria, 2023; Available online: www.r-project.org (accessed on 28 December 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D.; dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://github.com/tidyverse/dplyr (accessed on 28 December 2024).
- Wieckham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Permadi, N.; Akbari, S.I.; Prismantoro, D.; Indriyani, N.N.; Nurzaman, M.; Alhasnawi, A.N.; Doni, F.; Julaeha, E. Traditional and next-generation methods for browning control in plant tissue culture: Current insights and future directions. Curr. Plant Biol. 2024, 38, 100339. [Google Scholar] [CrossRef]
- Płażek, A.; Hura, K.; Hura, T.; Słomka, A.; Hornyák, M.; Sychta, K. Synthesis of heat-shock proteins HSP-70 and HSP-90 in flowers of common buckwheat (Fagopyrum esculentum) under thermal stress. Crop Pasture Sci. 2020, 71, 760. [Google Scholar] [CrossRef]
- Gubišová, M.; Gubiš, J.; Žofajová, A.; Mihálik, D.; Kraic, J. Enhanced in vitro propagation of Miscanthus × giganteus. Ind. Crops Prod. 2013, 41, 279–282. [Google Scholar] [CrossRef]
- Nomura, K.; Matsumoto, S.; Masuda, K.; Inoue, M. Reduced glutathione promotes callus growth and shoot development in a shoot tip culture of apple root stock M26. Plant Cell Rep. 1998, 17, 597–600. [Google Scholar] [CrossRef]
- Jakše, M.; Bohanec, B.; Ihan, A. Effect of media components on the gynogenic regeneration of onion (Allium cepa L.) cultivars and analysis of regenerants. Plant Cell Rep. 1996, 15, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.M.; Cistué, L. Production of gynogenic haploids of Hordeum vulgare L. Plant Cell Rep. 1993, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Getahun, T.; Feyissa, T.; Gugsa, L. Regeneration of plantlets from unpollinated ovary cultures of Ethiopian wheat (Triticum turgidum and Triticum aestivum). Afr. J. Biotechnol. 2013, 12, 5754–5760. [Google Scholar]
- Warchoł, M.; Czyczyło-Mysza, I.; Marcińska, I.; Dziurka, K.; Noga, A.; Kapłoniak, K.; Pilipowicz, M.; Skrzypek, E. Factors inducing regeneration response in oat (Avena sativa L.) anther culture. Vitr. Cell. Dev. Biol.—Plant 2019, 55, 595–604. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhou, C. In vitro induction of haploid plants from unpollinated ovaries and ovules. Theor. Appl. Genet. 1982, 63, 97–104. [Google Scholar] [CrossRef]
- Gugsa, L.; Sarial, A.K.; Lörz, H.; Kumlehn, J. Gynogenic plant regeneration from unpollinated flower explants of Eragrostis tef (Zuccagni) Trotter. Plant Cell Rep. 2006, 25, 1287–1293. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Hu, C.Y.; Lu, Y.G.; Li, J.Q.; Liu, X.D. Abnormalities occurring during female gametophyte development result in the diversity of abnormal embryo sacs and leads to abnormal fertilization in indica/japonica hybrids in rice. J. Integr. Plant Biol. 2009, 51, 3–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).