Biofumigation with Brassica Species and Their Derivatives: A Comprehensive Review of an Innovative Pest Control Strategy Targeting Wireworms (Coleoptera: Elateridae)
Abstract
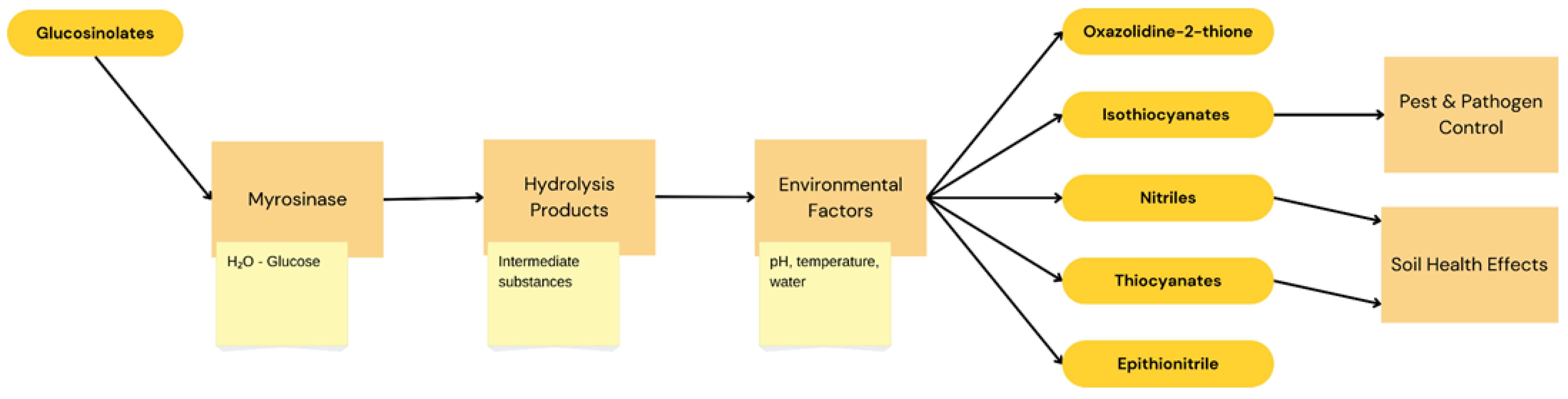
:1. Introduction
2. Biofumigation and Its Relevance as a Sustainable Alternative to Conventional Pest Control Strategies
2.1. Bridging the Gap Between Conventional and Sustainable Practices
2.2. Benefits of Biofumigation
3. Utilization Strategies
3.1. Intercropping and Crop Rotation
3.2. Plant-Based Processing By-Products
3.3. Green Manure
4. Crops of Interest and Their Biofumigant Properties
4.1. Brassica juncea (Indian Mustard)
4.2. Brassica carinata (Ethiopian Mustard)
5. Applications of B. juncea and B. carinata in Managing Wireworms
5.1. Mechanism of Control
5.2. Efficacy
5.3. Implementation Strategies
6. Global Adoption and Promising Results from Selected Case Studies
| Biofumigant Agent | Pest Species | Host Plant | Control Strategy | Experiment Type | Key Findings | Source |
|---|---|---|---|---|---|---|
| Defatted rapeseed meal (Brassica napus L.) | Wireworms (Limonius infuscatus Motschulsky) | Soil amendment study (not targeting specific host plants) | Application of rapeseed meal as a soil amendment; evaluation of Gln degradation products and their effects on wireworms | Laboratory bioassay and chemical analysis | Rapeseed meal rapidly produced IsoTs (301 nmol/g, 2 h) and thiocyanate (180 nmol/g, 8 h). Wireworms avoided treated soil within 24 h, despite no reported 17-day toxicity. Repellency was likely due to IsoT presence, with possible thiocyanate contribution. | [100] |
| Allyl isothiocyanate | Wireworms (Limonius californicus [Mannerheim]) | Not specified (study focused on soil treatment) | Soil amendment with allyl isothiocyanate derived from Glns in Brassica spp. | Laboratory bioassay | Allyl isothiocyanate was toxic to wireworms at concentrations of 150–300 nmol/g soil, with mortality rates up to 90%. Sublethal doses reduced feeding. Allyl isothiocyanate concentrations decreased rapidly, suggesting its potential use as a short-term control method. | [101] |
| Rapeseed (Brassica napus) seed meal | Wireworms (Limonius californicus) | Not specified (study focused on soil treatment) | Soil amendment with rapeseed seed meal containing Glns that decompose to IsoTs | Laboratory bioassay | Rapeseed seed meal (41.7–500 g/kg soil) caused up to 95% wireworm mortality in 7 days. LC50 dropped from 124.8 to 114.4 g/kg by 21 days. IsoTs were the primary toxins, as detoxified meal had no effect. | [38] |
| Defatted biocidal seed meals from B. carinata A. Braun (ISCI7), E. sativa Mill (cv. Nemat), Barbarea verna (Mill.) Asch. (ISCI100), Sinapis alba L. (cv. Pira), and whole/freeze-dried B. juncea (L.) Czern. (ISCI99) | Wireworms (Agriotes brevis Candèze, Agriotes sordidus (Illiger), Agriotes ustulatus [Schaller]) | Winter Wheat (Triticum aestivum L.), Maize (Zea mays L.) | Defatted biocidal seed meals and whole Brassica spp. plant materials | Laboratory bioassay and semi-natural field trials (pot trials) | B. carinata and Eruca sativa Nemat meals were highly effective against wireworms. B. juncea plants caused 100% mortality. | [27] |
| Gln seed meal (B. napus, B. juncea) | Various soilborne pests (fungi, bacteria, nematodes, and wireworms) | Various crops (focus on soil amendment effects) | Seed meals used as soil amendment to control soil-borne pathogens, nematodes, and insects | Review and experimental study (2000–2002) | Some bioactive compounds have strong antimicrobial and insecticidal effects. | [102] |
| B. juncea (ISCI20) | Soilborne fungi (Pythium spp., Rhizoctonia solani J.G. Kühn), nematodes (Meloidogyne incognita Kofold & White, Heterodera schachtii Schmidt) and wireworms (Agriotes spp.) | Potato (Solanum tuberosum L.), Winter Wheat (Triticum aestivum) | B. juncea plant material | Long-term field trials (13 years) | Biofumigation with B. juncea replaced chemical fumigants in potato-wheat rotation, improving soil fertility, reducing CO2 emissions by 700 kg/ha, and sequestering up to 3.5 t/ha of CO2 in soil organic matter. | [103] |
| B. juncea and B. carinata | Wireworms (Agriotes brevis, Agriotes sordidus, Agriotes ustulatus) | Lettuce (Lactuca sativa L.), Corn (Zea mays) | Defatted seed meals, chopped residues | Laboratory bioassay and field trials | Insecticidal effect with high larval mortality and crop protection. | [23] |
| Rapeseed (B. napus), Oilseed Radish (R. sativus L. var. oleifera), Oilseed Rape (B. napus L. var. oleifera), Kale (B. oleracea L. var. acephala), White Mustard (S. alba). | Wireworms (A. brevis, A. lineatus, A. obscurus, A. sputator, and A. ustulatus) | Potato (Solanum tuberosum) | Green Manure, chopped residues incorporated into the soil through ploughing | Field trials | No differences in insecticidal effect among the studied cruciferous plants. However, some efficacy was shown compared to the positive control. | [13] |
| Gln plants (B. juncea, B. napus, E. sativa), compost amendments, and bacterial biological control agents | Wireworms (Agriotes spp., Limonius spp.), mealybugs (Planococcus citri Risso, Phenacoccus solani Ferris) | Tomato (Solanum lycopersicum L.), Mint (Mentha spicata L.), Tarragon (Artemisia dracunculus L.) | Brassica spp., compost amendments, and bacterial biocontrol for pathogen and pest suppression | Field trials and greenhouse studies | Notable reduction in pest incidence. | [104] |
| Brassica spp. pellets, calcium cyanamide, limestone dust, propolis | Wireworms (Agriotes spp.), Colorado potato beetle (Leptinotarsa decemlineata Say), early blight (Alternaria solani Sorauer), late blight (Phytophthora infestans Montagne) | Potato (Solanum tuberosum) | Application of Brassica spp. pellets, calcium cyanamide, limestone dust, and propolis through broadcast, mix-in, spraying, and dusting. | Field trials | Calcium cyanamide reduced wireworm damage more than Brassica spp. pellets, which still showed promise. Limestone dust controlled L. decemlineata, and propolis reduced blight. Treated plots had higher yields, making these eco-friendly alternatives promising. | [105] |
| B. juncea | Wireworms (Agriotes spp.) | Potato (Solanum tuberosum) | Incorporation of B. juncea into the soil to suppress wireworm damage on potatoes | Field trials | B. juncea biofumigation significantly reduced wireworm damage in potatoes. IsoTs and their derivatives played a key role in pest suppression. | [106] |
| Brassica spp. seed meal formulations | Wireworms (Agriotes spp.) | Cereal Crops (Poaceae) | Seed meal incorporation, broadcast application in soil to suppress wireworm populations | Laboratory bioassy and field trials | Biofumigant seed meal application altered wireworm behavior, causing repellency and reduced feeding activity. | [107] |
7. Future Directions and Challenges
7.1. Advancing Biofumigation with Brassicaceae for Wireworm Control
7.2. Enhancing Efficacy and Consistency in Field Conditions
7.3. Addressing Environmental and Agronomic Trade-Offs
7.4. Overcoming Adoption Barriers in Commercial Agriculture
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Glns | Glucosinolates |
| IsoTs | Isothiocyanates |
References
- Gamliel, A.; Van Bruggen, A.H.C. Maintaining soil health for crop production in organic greenhouses. Sci. Hortic. 2016, 208, 120–130. [Google Scholar] [CrossRef]
- Dutta, T.K.; Khan, M.R.; Phani, V. Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: Current status and future prospects. Curr. Plant Biol. 2019, 17, 17–32. [Google Scholar] [CrossRef]
- Ladhalakshmi, D.; Madhubala, R.; Sundravadana, S.; Laha, G.S.; Krishnaveni, D.; Sangeetha, G.; Ragothuman, G. Biofumigation in crop disease management. In Sustainable Crop Disease Management Using Natural Products; CABI Publishing: Oxford, UK, 2015; pp. 389–402. [Google Scholar] [CrossRef]
- Parker, W.E.; Howard, J.J. The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the U.K. Agric. For. Entomol. 2001, 3, 85–98. [Google Scholar] [CrossRef]
- Vernon, R.S.; Van Herk, W.; Tolman, J. European wireworms (Agriotes spp.) in North America: Distribution, damage, monitoring, and alternative integrated pest management strategies. IOBC/WPRS Bull. 2005, 28, 73–79. [Google Scholar]
- Parker, W.E.; Clarke, A.; Ellis, S.A.; Oakley, J.N. Evaluation of insecticides for the control of wireworms (Agriotes spp.) on potato. Ann. Appl. Biol. 1990, 116, 28–29. [Google Scholar]
- Kuhar, T.P.; Alvarez, J.M. Timing of injury and efficacy of soil-applied insecticides against wireworms on potato in Virginia. Crop Prot. 2008, 27, 792–798. [Google Scholar] [CrossRef]
- Johnson, S.N.; Anderson, E.A.; Dawson, G.; Griffiths, D.W. Varietal susceptibility of potatoes to wireworm herbivory. Agric. For. Entomol. 2008, 10, 167–174. [Google Scholar] [CrossRef]
- Jansson, R.K.; Lecrone, S.H. Effects of summer cover crop management on wireworm (Coleoptera: Elateridae) abundance and damage to potato. J. Econ. Entomol. 1991, 84, 581–586. [Google Scholar] [CrossRef]
- Milevoj, L.; Gomboc, S.; Bobnar, A.; Mikuš, T.; Gril, T. Efficacy of different density of pheromone traps on an attack of lined click beetles (Agriotes lineatus L.). Acta Agric. Slov. 2005, 85, 375–384. (In Slovenian) [Google Scholar]
- Noronha, C. Crop rotation as a management tool for wireworms in potatoes. IOBC/WPRS Bull. 2011, 66, 85–98. [Google Scholar]
- Bohinc, T.; Trdan, S. Alternative methods for controlling wireworms (Coleoptera, Elateridae) in the fields. Acta Agric. Slov. 2013, 101, 137–147. (In Slovenian) [Google Scholar]
- Laznik, Ž.; Trdan, S.; Vučajnk, F.; Bohinc, T.; Vidrih, M. Cruciferous plants’ use as biofumigants in potato against wireworms. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 606–614. [Google Scholar] [CrossRef]
- Piqué, J.; Eizaguirre, M.; Pons, X. Soil insecticide treatments against maize soil pests and corn borers in Catalonia under traditional crop conditions. Crop Prot. 1998, 17, 557–561. [Google Scholar] [CrossRef]
- Rashed, A.; Van Herk, W.G. Pest elaterids of North America: New insights and opportunities for management. Annu. Rev. Entomol. 2024, 69, 1–20. [Google Scholar] [CrossRef]
- Labrada, R.; Fornasari, L. (Eds.) Global Report on Validated Alternatives to the Use of Methyl Bromide for Soil Fumigation; Food & Agriculture Organization: Rome, Italy, 2001; No. 166. [Google Scholar]
- Baker, L.W.; Fitzell, D.L.; Seiber, J.N.; Parker, T.R.; Shibamoto, T.; Poore, M.W.; Duncan, D.W. Ambient air concentrations of pesticides in California. Environ. Sci. Technol. 1996, 30, 1365–1368. [Google Scholar] [CrossRef]
- Epstein, L. Fifty years since Silent Spring. Annu. Rev. Phytopathol. 2014, 52, 377–402. [Google Scholar] [CrossRef]
- Prasad, P.; Kumar, J.; Pandey, S. Biofumigation: Success and prospects in soilborne plant disease management. JAPSA 2015, 1, 47–59. [Google Scholar]
- O’Neill, T.M.; Budge, G.; Shepherd, A.; Ratcliffe, T. Evaluation of a combined dazomet and metam-sodium treatment for pre-plant soil fumigation. Acta Hortic. 2005, 698, 51. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Yang, H.; Chang, Z. Effect of biofumigation and chemical fumigation on soil microbial community structure and control of pepper Phytophthora blight. World J. Microbiol. Biotechnol. 2014, 30, 507–518. [Google Scholar] [CrossRef]
- Furlan, L.; Bonetto, C.; Finotto, A.; Lazzeri, L.; Malaguti, L.; Patalano, G.; Parker, W. The efficacy of biofumigant meals and plants to control wireworm populations. Ind. Crops Prod. 2010, 31, 245–254. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Suppression of fungal root pathogens of cereals by tissues. Plant Pathol. 1996, 45, 593–603. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Zasada, I.A.; Ferris, H. Nematode suppression with brassicaceous amendments: Application based upon glucosinolate profiles. Soil Biol. Biochem. 2004, 36, 1017–1024. [Google Scholar] [CrossRef]
- Furlan, L.; Bonetto, C.; Patalano, G.; Lazzeri, L. Potential of biocidal meals to control wireworm populations. In Proceedings of the First International Symposium on Biofumigation: A Possible Alternative to Methyl Bromide; Springer: Berlin/Heidelberg, Germany, 2004; Volume 31, pp. 313–316. [Google Scholar]
- Ploeg, A.; López-Pérez, J.A.; Bello, A. Biofumigation to manage plant-parasitic nematodes. In Management of Nematode and Insect-Borne Diseases; CRC Press: Boca Raton, FL, USA, 2024; pp. 195–204. [Google Scholar]
- Kirkegaard, J.A. Biofumigation—Using Brassica species to control pests and diseases in horticulture and agriculture. In Proceedings of the 9th Australian Research Assembly on Brassicas; Wratten, N., Mailer, R.J., Eds.; The Assembly: Wagga Wagga, Australia, 1993; pp. 77–82. [Google Scholar]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Lazzeri, L.; Leoni, O.; Manici, L.M. Biocidal plant dried pellets for biofumigation. Ind. Crops Prod. 2004, 20, 59–65. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Kirkegaard, J. Biofumigation for plant disease control–From the fundamentals to the farming system. In Disease Control in Crops: Biological and Environmentally Friendly Approaches; Wiley: Hoboken, NJ, USA, 2009; pp. 172–195. [Google Scholar]
- Szczygłowska, M.; Piekarska, A.; Konieczka, P.; Namieśnik, J. Use of Brassica plants in the phytoremediation and biofumigation processes. Int. J. Mol. Sci. 2011, 12, 7760. [Google Scholar] [CrossRef]
- Kumar, G.K.; Jayasudha, S.M.; Kirankumar, K.C. Disease management by biofumigation in organic farming systems. J. Pharmacogn. Phytochem. 2018, 7, 676–679. [Google Scholar]
- Ziedan, E.S.H. A review of the efficacy of biofumigation agents in the control of soil-borne plant diseases. J. Plant Prot. Res. 2022, 1, 1–11. [Google Scholar]
- Van Bruggen, A.H.C.; Finckh, M.R. Plant diseases and management approaches in organic farming systems. Annu. Rev. Phytopathol. 2016, 54, 25–54. [Google Scholar] [CrossRef]
- Elberson, L.R.; Borek, V.; McCaffrey, J.P.; Morra, M.J. Toxicity of rapeseed meal-amended soil to wireworms, Limonius californicus (Coleoptera: Elateridae). J. Agric. Entomol. 1996, 13, 323–330. [Google Scholar]
- Karavina, C.; Mandumbu, R. Biofumigation for crop protection: Potential for adoption in Zimbabwe. J. Anim. Plant Sci. 2012, 14, 1996–2005. [Google Scholar]
- Lazzeri, L.; Malaguti, L.; Cinti, S.; Ugolini, L.; De Nicola, G.R.; Bagatta, M.; Patalano, G. The Brassicaceae biofumigation system for plant cultivation and defense: An Italian twenty-year experience of study and application. Acta Hortic. 2013, 1005, 375–382. [Google Scholar] [CrossRef]
- Morra, M.J.; Kirkegaard, J.A. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002, 34, 1683–1690. [Google Scholar] [CrossRef]
- Borek, V.; Elberson, L.R.; McCaffrey, J.P.; Morra, M.J. Toxicity of isothiocyanates produced by glucosinolates in Brassicaceae species to black vine weevil eggs. J. Agric. Food Chem. 1998, 46, 5318–5323. [Google Scholar] [CrossRef]
- Jensen, J.; Styrishave, B.; Gimsing, A.L.; Bruun Hansen, H.C. The toxic effects of benzyl glucosinolate and its hydrolysis product, the biofumigant benzyl isothiocyanate, to Folsomia fimetaria. Environ. Toxicol. Chem. 2010, 29, 359–364. [Google Scholar] [CrossRef]
- Brennan, R.J.B.; Glaze-Corcoran, S.; Wick, R.; Hashemi, M. Biofumigation: An alternative strategy for the control of plant parasitic nematodes. J. Integr. Agric. 2020, 19, 1680–1690. [Google Scholar] [CrossRef]
- Wieczorek, R.; Zydlik, Z.; Zydlik, P. Biofumigation treatment using Tagetes patula, Sinapis alba and Raphanus sativus changes the biological properties of replanted soil in a fruit tree nursery. Agriculture 2024, 14, 1023. [Google Scholar] [CrossRef]
- Castellá-Lorenzo, G.; Savigliano, R.; Pizano, M. Breaking the bondage to methyl bromide in agriculture—UNIDO experience. In Proceedings of the VIII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation, Torino, Italy, 13 July 2014; ISHS: Leuven, Belgium, 2014; Volume 1044, pp. 281–287. [Google Scholar]
- Larregla, S.; Gandariasbeitia, M.; Ojinaga, M.; Mendarte, S.; Guerrero, M.M.; Ortiz-Barredo, A. Gases released during soil biodisinfestation of pepper greenhouses reduce survival of Phytophthora capsici oospores in Northern Spain. Front. Sustain. Food Syst. 2021, 5, 663915. [Google Scholar] [CrossRef]
- FITO-INFO. Slovenian Information System for Plant Protection; Ministry of Agriculture, Forestry and Food, Phytosanitary Administration of the Republic of Slovenia: Ljubljana, Slovenia, 2025. [Google Scholar]
- Clarkson, J.; Michel, V.; Neilson, R. Mini-Paper—Biofumigation for the Control of Soil-Borne Diseases. EPI-AGRI, Soil-Borne Diseases, 2015, p. 7. Available online: https://ec.europa.eu/eip/agriculture/sites/agri-eip/files/9_eip_sbd_mp_biofumigation_final_0.pdf (accessed on 23 January 2025).
- Mawar, R.; Lodha, S. Suppression of soilborne plant pathogens by cruciferous residues. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Insam, F., Franke-Whittle, S., Goberna, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 413–433. [Google Scholar]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for fighting replant disease—A review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant-parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Lord, J.S.; Lazzeri, L.; Atkinson, H.J.; Urwin, P.E. Biofumigation for control of pale potato cyst nematodes: Activity of Brassica leaf extracts and green manures on Globodera pallida in vitro and in soil. J. Agric. Food Chem. 2011, 59, 7882–7890. [Google Scholar] [CrossRef]
- De Nicola, G.R.; D’Avino, L.; Curto, G.; Malaguti, L.; Ugolini, L.; Cinti, S.; Lazzeri, L. A new biobased liquid formulation with biofumigant and fertilizing properties for drip irrigation distribution. Ind. Crops Prod. 2013, 42, 113–118. [Google Scholar] [CrossRef]
- Curto, G.; Dallavalle, E.; Matteo, R.; Lazzeri, L. Biofumigant effect of new defatted seed meals against the southern root-knot nematode, Meloidogyne incognita. Ann. Appl. Biol. 2016, 169, 17–26. [Google Scholar] [CrossRef]
- Wei, F.; Passey, T.; Xu, X. Effects of individual and combined use of biofumigation-derived products on the viability of Verticillium dahliae microsclerotia in soil. Crop Prot. 2016, 79, 170–176. [Google Scholar] [CrossRef]
- Serrano-Pérez, P.; Palo, C.; Rodríguez-Molina, M.C. Efficacy of Brassica carinata pellets to inhibit mycelial growth and chlamydospores germination of Phytophthora nicotianae at different temperature regimes. Sci. Hortic. 2017, 216, 126–133. [Google Scholar] [CrossRef]
- Roncato, S.C.; Stangarlin, J.R.; Kuhn, O.J.; Gonçalves Júnior, A.C.; Dildey, O.D.F.; Gonçalves, E.D.V.; Rissato, B.B.; Broetto, L.; Faria, V.O. Control of Meloidogyne incognita in tomato by Crambe extract using different application forms. Summa Phytopathol. 2018, 44, 261–266. [Google Scholar] [CrossRef]
- Gamliel, A.; Katan, J. Control of plant disease through soil solarization. In Disease Control in Crops; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 196–220. [Google Scholar] [CrossRef]
- Ros, M.; Garcia, C.; Hernandez, M.T.; Lacasa, A.; Fernandez, P.; Pascual, J.A. Effects of biosolarization as a methyl bromide alternative for Meloidogyne incognita control on quality of soil under pepper. Biol. Fertil. Soils 2008, 45, 37–44. [Google Scholar] [CrossRef]
- Domínguez, P.; Miranda, L.; Soria, C.; de los Santos, B.; Chamorro, M.; Romero, F.; Daugovish, O.; López-Aranda, J.M.; Medina, J.J. Soil biosolarization for sustainable strawberry production. Agron. Sustain. Dev. 2014, 34, 821–829. [Google Scholar] [CrossRef]
- Agerbirk, N.; De Vos, M.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M. Biofumigation potential of brassicas. Plant Soil 1998, 201, 71–89. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Yim, B.; Winkelmann, T.; Smalla, K.; Schreiner, M. Degradation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. PLoS ONE 2015, 10, e0132931. [Google Scholar] [CrossRef]
- Bellostas, N.; Sørensen, J.C.; Sørensen, H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 2007, 87, 1586–1594. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. The effects of plant breeding on soil microbes. In Soil Microbiology and Sustainable Crop Production; Dixon, G.R., Tilston, E.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 297–314. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation potential of brassicas. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Bindumadhavi, G.; Gopi, R. Exploitation of biofumigation and biocontrol agents for the management of soil-borne diseases. In Innovative Approaches in Diagnosis and Management of Crop Diseases; Apple Academic Press: Cambridge, MA, USA, 2021; pp. 409–435. [Google Scholar]
- Monaci, E.; Casucci, C.; De Bernardi, A.; Marini, E.; Landi, L.; Toscano, G.; Romanazzi, G.; Vischetti, C. Brassica carinata seed meal as soil amendment and potential biofumigant. Crops 2022, 2, 17. [Google Scholar] [CrossRef]
- Ashworth, D.J.; Yates, S.R.; Wang, D.; Luo, L. Natural and synthetic isothiocyanates for pest control in soil. In Biopesticides: State of the Art and Future Opportunities; American Chemical Society: Washington, DC, USA, 2014; pp. 159–177. [Google Scholar]
- Subbarao, K.V.; Kabir, Z.; Martin, F.N.; Koike, S.T. Management of soilborne diseases in strawberry using vegetable rotations. Plant Dis. 2007, 91, 964–972. [Google Scholar] [CrossRef]
- Rudolph, R.E.; Sams, C.; Steiner, R.; Thomas, S.H.; Walker, S.; Uchanski, M.E. Biofumigation performance of four Brassica crops in a green chile pepper (Capsicum annuum) rotation system in southern New Mexico. HortScience 2015, 50, 247–253. [Google Scholar] [CrossRef]
- Milosavljević, I.; Esser, A.D.; Murphy, K.M.; Crowder, D.W. Effects of imidacloprid seed treatments on crop yields and economic returns of cereal crops. Crop Prot. 2019, 119, 166–171. [Google Scholar] [CrossRef]
- Morris, E.K.; Fletcher, R.; Veresoglou, S.D. Effective methods of biofumigation: A meta-analysis. Plant Soil 2020, 446, 379–392. [Google Scholar] [CrossRef]
- Snyder, A.; Morra, M.J.; Johnson-Maynard, J.; Thill, D.C. Seed meals from Brassicaceae oilseed crops as soil amendments: Influence on carrot growth, microbial biomass nitrogen, and nitrogen mineralization. HortScience 2009, 44, 354. [Google Scholar] [CrossRef]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 2001, 91, 673–679. [Google Scholar] [CrossRef]
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. Phytopathology 2015, 105, 460–469. [Google Scholar] [CrossRef]
- Serrano-Pérez, P.; De Santiago, A.; Rodríguez-Molina, M.C. Biofumigation with pellets of defatted seed meal of Brassica carinata: Factors affecting performance against Phytophthora nicotianae in pepper crops. Front. Sustain. Food Syst. 2021, 5, 664531. [Google Scholar] [CrossRef]
- Gouws, R.; Wehner, F.C. Biofumigation as an alternative control measure for common scab on seed potatoes in South Africa. Agroindustria 2004, 3, 309–312. [Google Scholar]
- Sukovata, L.; Jaworski, T.; Kolk, A. Efficacy of Brassica juncea granulated seed meal against Melolontha grubs. Ind. Crops Prod. 2015, 70, 260–265. [Google Scholar] [CrossRef]
- McGuire, A.M. Mustard green manures replace fumigant and improve infiltration in potato cropping systems. Crop Manag. 2003, 2, 1–6. [Google Scholar] [CrossRef]
- Larkin, R.P. Long-term effects of compost amendments and Brassica green manures in potato cropping systems on soil and crop health and productivity. Agronomy 2022, 12, 2804. [Google Scholar] [CrossRef]
- Riga, E. The effects of Brassica green manures on plant-parasitic and free-living nematodes used in combination with reduced rates of synthetic nematicides. J. Nematol. 2011, 43, 119–121. [Google Scholar]
- Cochran, K.A.; Rothrock, C.S. Brassica green manure amendments for management of Rhizoctonia solani in two annual ornamental crops in the field. HortScience 2015, 50, 555–558. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Santo, G.S.; Ingham, R.E. Suppression of Meloidogyne chitwoodi with Sudangrass cultivars as green manure. J. Nematol. 1993, 25, 303–311. [Google Scholar]
- Harding, R.B.; Wicks, T.J. In vitro suppression of soil-borne potato pathogens by volatiles released from Brassica residues. In Proceedings of the 2nd Australasian Soil-Borne Diseases Symposium, Lorne, Australia, 5–8 March 2001; pp. 148–149. [Google Scholar]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Walker, B.A.; Powell, S.M.; Tegg, R.S.; Doyle, R.B.; Hunt, I.G.; Wilson, C.R. Ten years of green manuring and biofumigation alters soil characteristics and microbiota. Appl. Soil Ecol. 2023, 187, 104836. [Google Scholar] [CrossRef]
- Arroyo, J.M.; Soler, J.; Linares, R.; Palmero, D. Strategies for selecting potentially effective biofumigant species for optimal biofumigation outcomes. Agriculture 2025, 15, 147. [Google Scholar] [CrossRef]
- Elhakeem, A.; van der Werf, W.; Bastiaans, L. Radiation interception and radiation use efficiency in mixtures of winter cover crops. Field Crops Res. 2021, 264, 108034. [Google Scholar] [CrossRef]
- Kuai, J.; Sun, Y.; Zhou, M.; Zhang, P.; Zuo, Q.; Wu, J.; Zhou, G. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crops Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef]
- Ugrenović, V.; Filipović, V.; Jevremović, S.; Marjanović-Jeromela, A.; Popović, V.; Buntić, A.; Delić, D.I. Effect of Brassicaceae as cover crops. Selekcija Semenarstvo 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Ngala, B.M.; Haydock, P.P.; Woods, S.; Back, M.A. Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest Manag. Sci. 2015, 71, 759–769. [Google Scholar] [CrossRef]
- Barsics, F.; Haubruge, E.; Verheggen, F.J. Wireworms’ management: An overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects 2013, 4, 117. [Google Scholar] [CrossRef]
- Bourdon, P.-A.; Zottele, M.; Baxter, I.; Myrta, A.; Midthassel, A.; Wechselberger, K.F.; Khoja, S.; Bull, J.C.; Hermann, S.; Butt, T.M. Fumigation of three major soil pests (Agriotes lineatus, Diabrotica virgifera virgifera, Phyllopertha horticola) with 3-octanone and 1-octen-3-ol enantiomers. Biocontrol Sci. Technol. 2022, 32, 863–876. [Google Scholar] [CrossRef]
- Vidrih, M.; Laznik, Ž.; Rupnik, J.; Vučajnk, F.; Bohinc, T.; Trdan, S. Biofumigation as a control method against soil insect pests. In Proceedings of the 10th Slovenian Conference on Plant Protection with International Participation, Podčetrtek, Slovenia, 1–2 March 2011; Plant Protection Society of Slovenia: Ljubljana, Slovenia, 2011; pp. 327–331. (In Slovenian). [Google Scholar]
- Brown, P.D.; Morra, M.J.; McCaffrey, J.P.; Auld, D.L.; Williams, L. Allelochemicals produced during glucosinolate degradation in soil. J. Chem. Ecol. 1991, 17, 2021–2034. [Google Scholar] [CrossRef]
- Williams, L.; Morra, M.J.; Brown, P.D.; McCaffrey, J.P. Toxicity of allyl isothiocyanate-amended soil to Limonius californicus (Mann.) (Coleoptera: Elateridae) wireworms. J. Chem. Ecol. 1993, 19, 1033–1046. [Google Scholar] [CrossRef]
- Brown, J.; Morra, M.J. Glucosinolate-Containing Seed Meal as a Soil Amendment to Control Plant Pests: 2000–2002 (NREL/SR-510-35254); National Renewable Energy Lab. (NREL): Golden, CO, USA, 2005. [Google Scholar] [CrossRef]
- Lazzeri, L.; D’Avino, L.; Gies, D. Additional benefits of the efficacy in containing soilborne pest and pathogens with biofumigant plants and materials. In Proceedings of the VII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation, Leuven, Belgium, 13 September 2009; Volume 883, pp. 323–329. [Google Scholar]
- Shaltiel-Harpaz, L.; Masaphy, S.; Tsror, L.; Palevsky, E. Biorational, environmentally safe methods for the control of soil pathogens and pests in Israel. In Agriculturally Important Microorganisms: Commercialization and Regulatory Requirements in Asia; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; Springer: Singapore, 2016; pp. 273–291. [Google Scholar]
- Bohinc, T.; Vučajnk, F.; Trdan, S. The efficacy of environmentally acceptable products for the control of major potato pests and diseases. Zemdirbyste-Agriculture 2019, 106, 135–142. [Google Scholar] [CrossRef]
- Furlan, L.; Bonetto, C.; Costa, B.; Finotto, A.; Lazzeri, L.; Malaguti, L.; Patalano, G.; Parker, W. Biofumigation as a tool for a holistic approach to integrated wireworm population management. In Proceedings of the Biofumigation 7 Symposium 2021; Michel, V., Gfeller, A., Lutz, M., Back, M., Besri, M., Eds.; Agroscope: Conthey, Switzerland, 2021; Available online: https://youtu.be/NXUfDQpi3qo (accessed on 15 February 2025).
- Lazzeri, L.; Pagnotta, E.; Ugolini, L.; Casadei, N.; Malaguti, I.; Cinti, S.; Nanetti, A.; Matteo, R. New tools and new application sectors for biofumigant cropping system. In Biofumigation 7 Symposium 2021; Michel, V., Gfeller, A., Lutz, M., Back, M., Besri, M., Eds.; Agroscope: Conthey, Switzerland, 2021; Available online: https://youtu.be/hH7VdLUE3XE (accessed on 15 February 2025).
- Bohinc, T.; Ban, G.S.; Ban, D.; Trdan, S. Glucosinolates in plant protection strategies: A review. Arch. Biol. Sci. 2012, 64, 821–828. [Google Scholar] [CrossRef]
- Poggi, S.; Le Cointe, R.; Lehmhus, J.; Plantegenest, M.; Furlan, L. Alternative strategies for controlling wireworms in field crops: A review. Agriculture 2021, 11, 436. [Google Scholar] [CrossRef]
- Bohinc, T.; Trdan, S. Environmental factors affecting the glucosinolate content in Brassicaceae. J. Food Agric. Environ. 2012, 10, 357. [Google Scholar]
- Bohinc, T.; Devetak, M.; Trdan, S. Quantity of glucosinolates in 10 cabbage genotypes and their impact on the feeding of Mamestra brassicae caterpillars. Arch. Biol. Sci. 2014, 66, 867–876. [Google Scholar] [CrossRef]
- Ahmed, N.A.-K.; Dechamp-Guillaume, G.; Seassau, C. Biofumigation to protect oilseed crops: Focus on management of soilborne fungi of sunflower. OCL 2020, 27, 59. [Google Scholar] [CrossRef]
- Jung, J.; Racca, P.; Schmitt, J.; Kleinhenz, B. SIMAGRIO-W: Development of a prediction model for wireworms in relation to soil moisture, temperature and type. J. Appl. Entomol. 2014, 138, 183–194. [Google Scholar] [CrossRef]
- Chen, D.; Zebarth, B.J.; Goyer, C.; Comeau, L.-P.; Nahar, K.; Dixon, T. Effect of biofumigation on population densities of Pratylenchus spp. and Verticillium spp. and potato yield in Eastern Canada. Am. J. Potato Res. 2022, 99, 229–242. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Fang, W.; Li, Y.; Yan, D.; Cao, A.; Wang, Q. A review of biofumigation effects with plant materials. New Plant Prot. 2024, 1, e21. [Google Scholar] [CrossRef]
- Donald, D.; Villalta, O.; Pung, H. Managing Soilborne Diseases in Vegetables: Rotation with Green Manure and Biofumigant Crops Shows Disease Control & Yield Benefits. Department of Primary Industries. 2010. Available online: https://ausveg.com.au/app/data/technical-insights/docs/VG07125_Soilborne_Diseases_brochure.pdf (accessed on 14 February 2025).
- Nikoukar, A.; Rashed, A. Integrated pest management of wireworms (Coleoptera: Elateridae) and the rhizosphere in agroecosystems. Insects 2022, 13, 769. [Google Scholar] [CrossRef]
- Curk, M.; Trdan, S. Benefiting from complexity: Exploring enhanced biological control effectiveness via the simultaneous use of various methods for combating pest pressure in agriculture. Agronomy 2024, 14, 199. [Google Scholar] [CrossRef]
- van Niekerk, A.; Auerbach, R.; Lamprecht, S. Biological and chemical soil fumigation and pest and disease management comparisons in the Western Cape. In Organic Food Systems: Meeting the Needs of Southern Africa; CABI: Oxford, UK, 2020; pp. 264–283. [Google Scholar] [CrossRef]
- Poggi, S.; Le Cointe, R.; Riou, J.-B.; Larroudé, P.; Thibord, J.-B.; Plantegenest, M. Relative influence of climate and agro-environmental factors on wireworm damage risk in maize crops. J. Pest Sci. 2018, 91, 585–599. [Google Scholar] [CrossRef]
- Saguez, J.; Latraverse, A.; De Almeida, J.; Van Herk, W.G.; Vernon, R.S.; Légaré, J.-P.; Moisan-De Serres, J.; Fréchette, M.; Labrie, G. Wireworm in Quebec field crops: Specific community composition in North America. Environ. Entomol. 2017, 46, 814–825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batistič, L.; Bohinc, T.; Trdan, S. Biofumigation with Brassica Species and Their Derivatives: A Comprehensive Review of an Innovative Pest Control Strategy Targeting Wireworms (Coleoptera: Elateridae). Agronomy 2025, 15, 967. https://doi.org/10.3390/agronomy15040967
Batistič L, Bohinc T, Trdan S. Biofumigation with Brassica Species and Their Derivatives: A Comprehensive Review of an Innovative Pest Control Strategy Targeting Wireworms (Coleoptera: Elateridae). Agronomy. 2025; 15(4):967. https://doi.org/10.3390/agronomy15040967
Chicago/Turabian StyleBatistič, Luka, Tanja Bohinc, and Stanislav Trdan. 2025. "Biofumigation with Brassica Species and Their Derivatives: A Comprehensive Review of an Innovative Pest Control Strategy Targeting Wireworms (Coleoptera: Elateridae)" Agronomy 15, no. 4: 967. https://doi.org/10.3390/agronomy15040967
APA StyleBatistič, L., Bohinc, T., & Trdan, S. (2025). Biofumigation with Brassica Species and Their Derivatives: A Comprehensive Review of an Innovative Pest Control Strategy Targeting Wireworms (Coleoptera: Elateridae). Agronomy, 15(4), 967. https://doi.org/10.3390/agronomy15040967








