Marked Spatial Variability in Acidity Characteristics of Purple Soil at Field Scale Induced by Citrus Plantation
Abstract
:1. Introduction
2. Materials and Methods
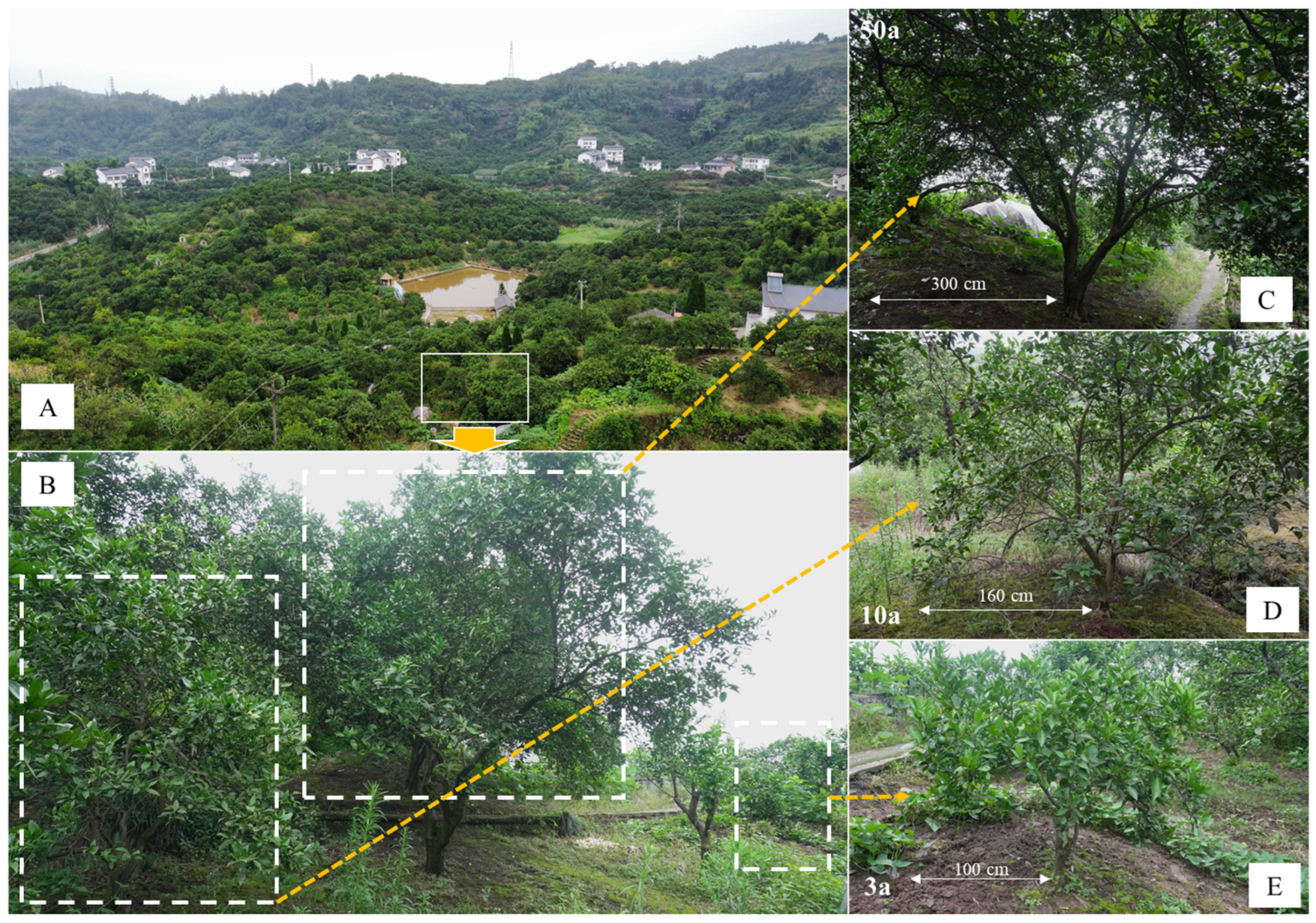
2.1. Survey and Soil Collection
2.2. Soil Analysis
2.3. Data Statistics and Analysis
3. Results and Discussion
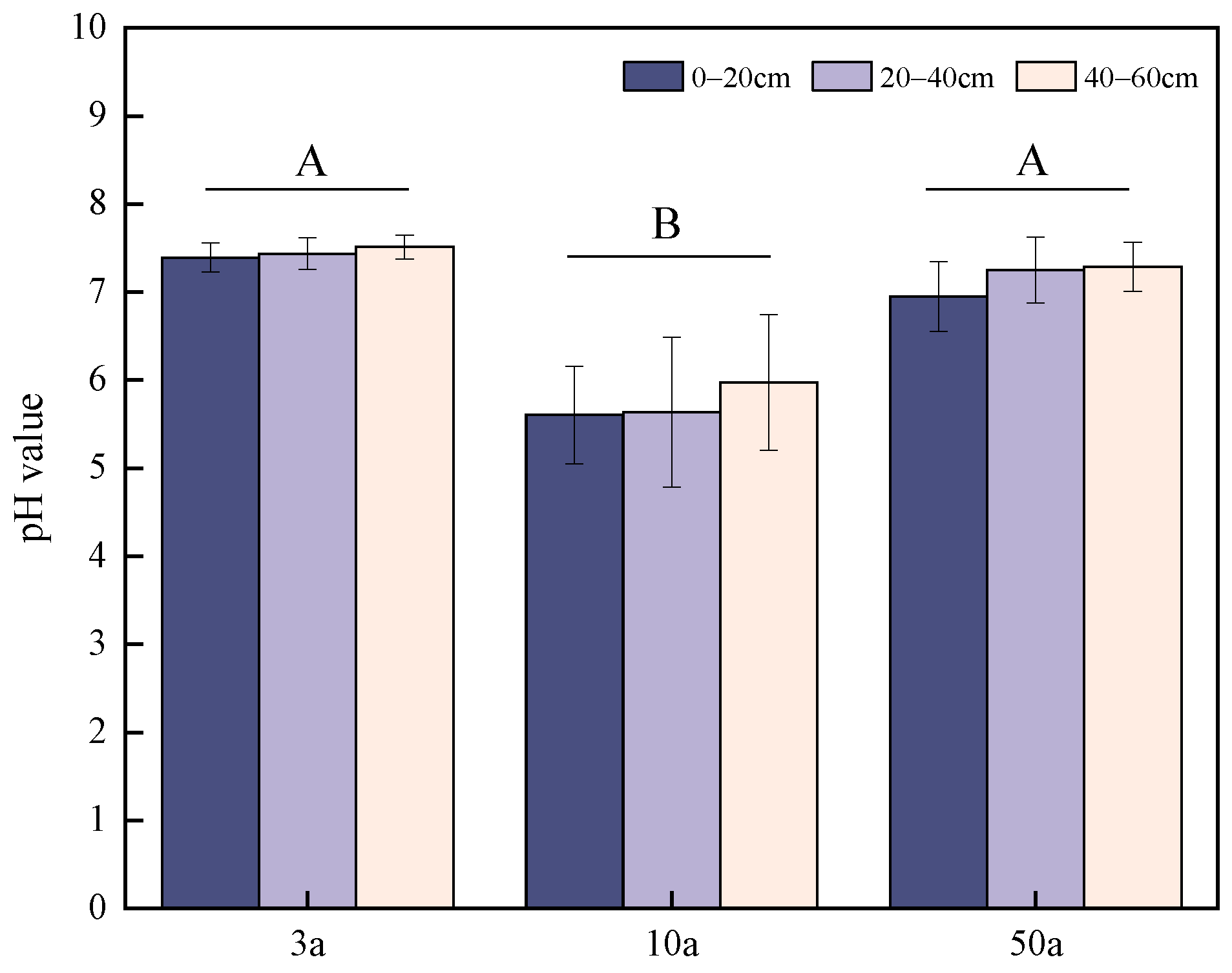
3.1. Variation in Soil Acidity After Planting Citrus for Different Years
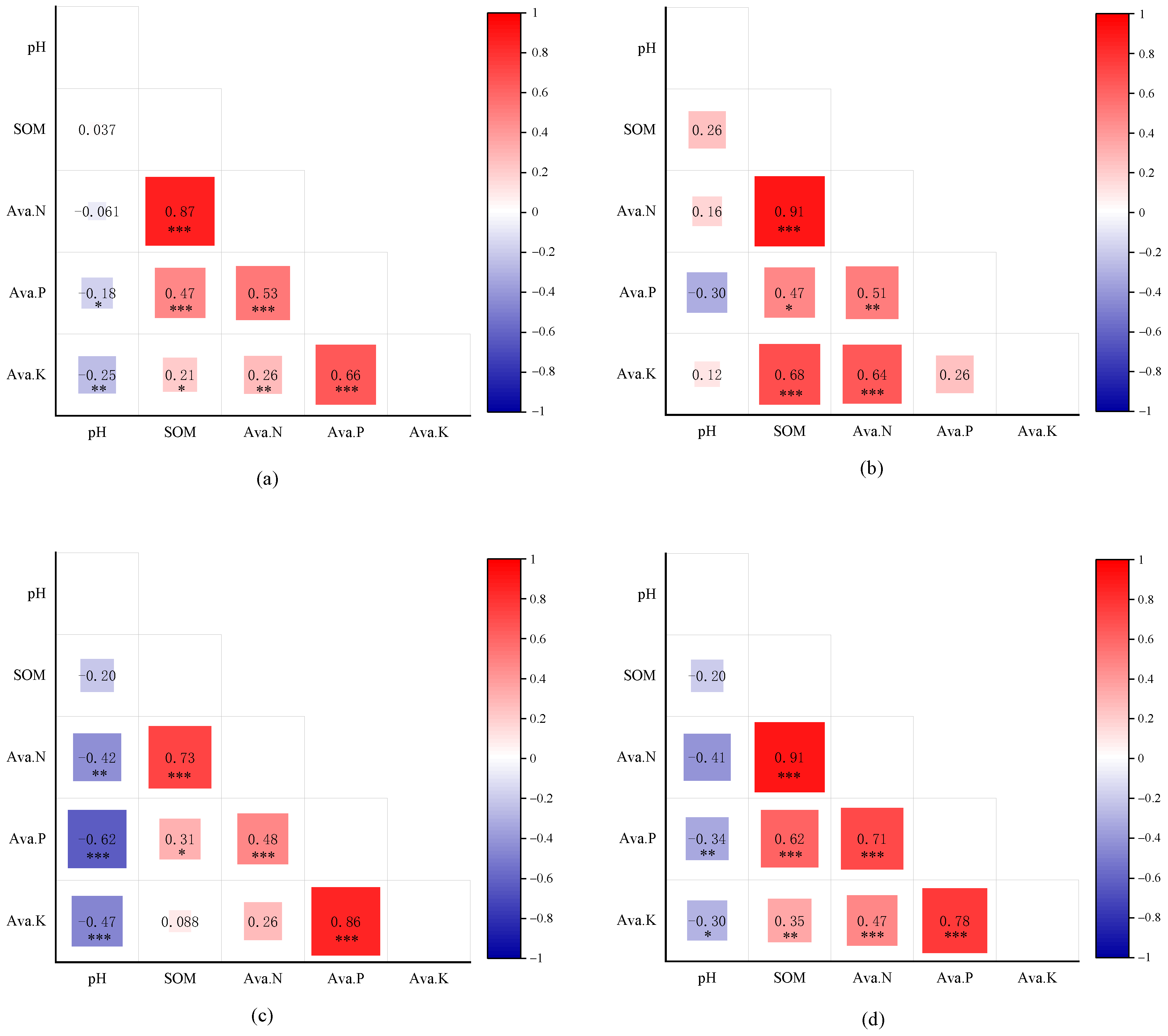
3.2. Correlation Between Soil pH and Soil Fertility Indicators
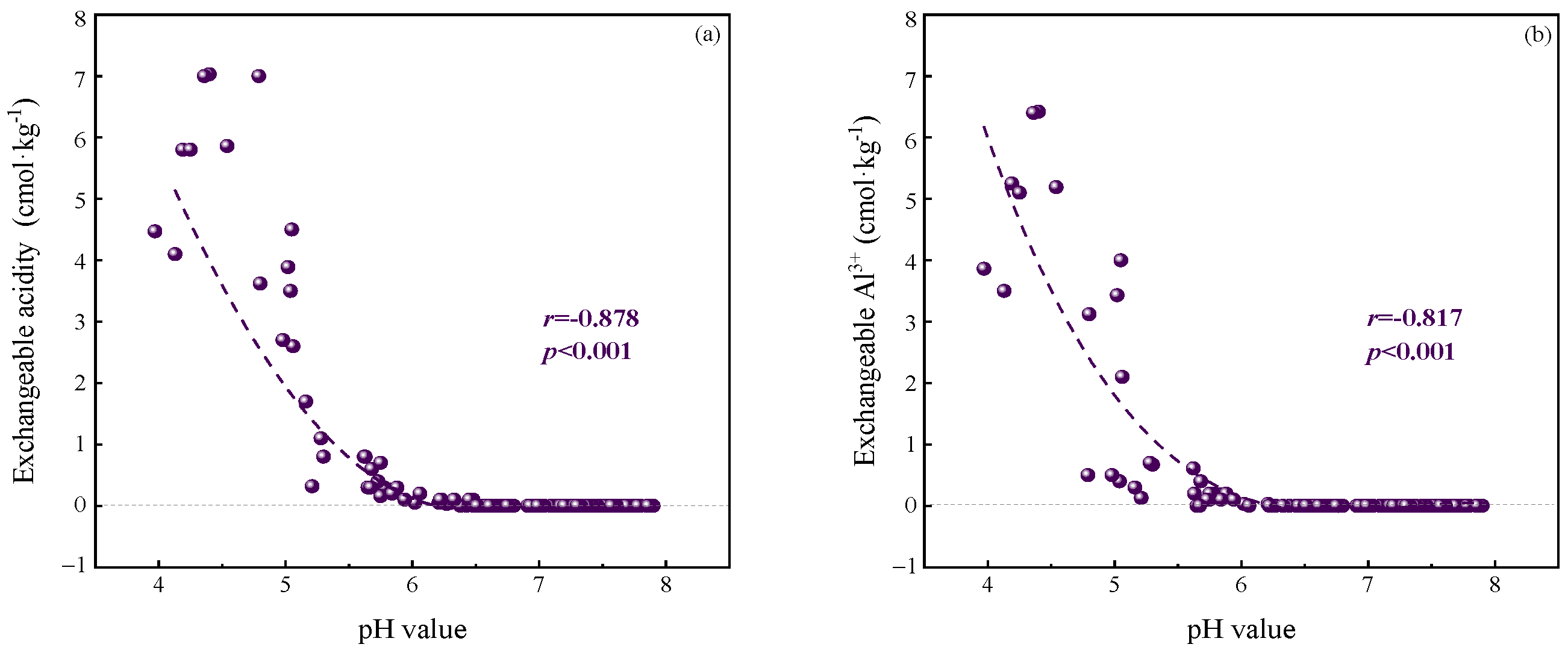
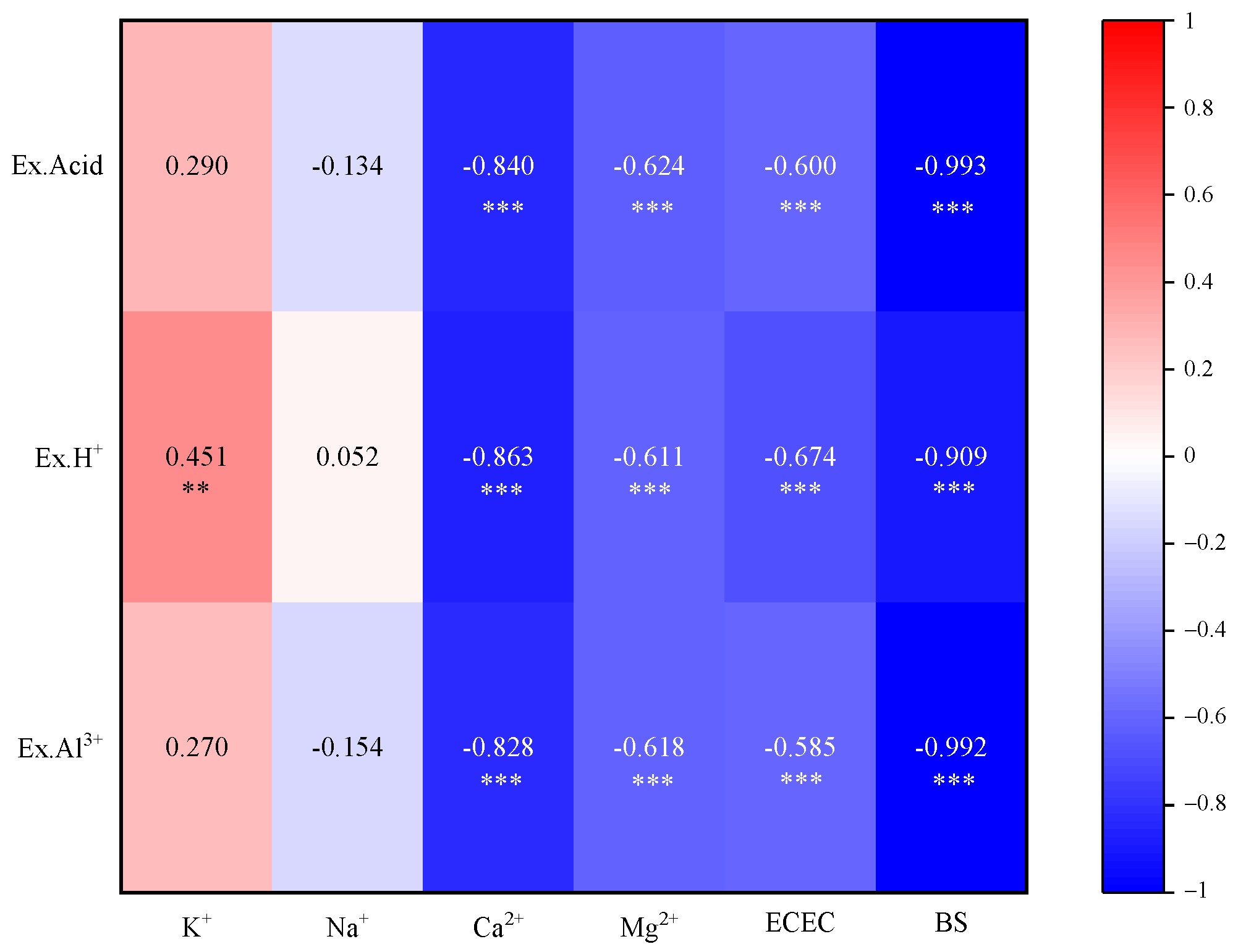
3.3. Effects of Soil Acidification on Exchangeable Base Cations
3.4. Effects of Soil Acidification on Available Metals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSGC | Chinese Soil Genetic Classification |
| SOM | soil organic matter |
| AN | available nitrogen |
| AP | available phosphorus |
| AK | available potassium |
| ECEC | effective cation exchange capacity |
| BS | base saturation |
References
- Larssen, T.; Carmichael, G.R. Acid rain and acidification in China: The importance of base cation deposition. Environ. Pollut. 2000, 110, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.C.; Liu, X.J.; Hao, T.X.; Zeng, M.F.; Shen, J.B.; Zhang, F.S.; de Vries, W. Cropland acidification increases risk of yield losses and food insecurity in China. Environ. Pollut. 2020, 256, 113145. [Google Scholar] [CrossRef]
- Dai, Z.M.; Zhang, X.J.; Tang, C.; Muhammda, N.; Wu, J.J.; Brookes, P.C.; Xu, J.M. Potential role of biochars in decreasing soil acidification- A critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Zhong, S.Q.; Han, Z.; Du, J.; Ci, E.; Ni, J.P.; Xie, D.T.; Wei, C.F. Relationships between the lithology of purple rocks and the pedogenesis of purple soils in the Sichuan Basin, China. Sci. Rep. 2019, 9, 13272. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Shi, X.J.; Zheng, Y.; Qin, Z.X.; Xie, D.T.; Li, Z.L.; Guo, T. Abundance and community structure of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Eur. J. Soil Biol. 2014, 60, 24–33. [Google Scholar] [CrossRef]
- Li, Z.Y.; Cheng, Y.Y.; Yang, J.H. The acidification characteristic of neutral purple soil in Chongqing district. J. Soil Water Conserv. 2012, 6, 234–242. [Google Scholar]
- Li, Z.Y.; Wang, P.S.; Liu, L.; Zheng, Y.Y.; Xie, D.T. High negative surface charge increases the acidification risk of purple soil in China. Catena 2021, 196, 104819. [Google Scholar] [CrossRef]
- Li, Q.Q.; Li, S.; Xiao, Y.; Zhao, B.; Wang, C.Q.; Li, B.; Gao, X.S.; Li, Y.D.; Bai, G.C.; Wang, Y.D.; et al. Soil acidification and its influencing factors in the purple hilly area of Southwest China from 1981 to 2012. Catena 2019, 175, 278–285. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.; You, X.; Gao, M.R. Nutrient release from weathering of purplish rocks in the Sichuan Basin, China. Pedosphere 2008, 18, 257–264. [Google Scholar] [CrossRef]
- Zhang, Y.T.; de Vries, W.; Thomas, B.W.; Hao, X.Y.; Shi, X.J. Impacts of long-term nitrogen fertilization on acid buffering rates and mechanisms of a slightly calcareous clay soil. Geoderma 2017, 305, 92–95. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Xu, H.; Ma, C.B.; Xue, Y.D.; Wang, C.J.; Xu, M.G.; Zhang, W.J. Change of soil fertility and productivity of purple soil in Western China in recent 30 years. J. Plant Nutr. Fertil. 2018, 24, 1492–1499. [Google Scholar]
- Chen, J.J.; Yu, J.F.; Li, Z.Y.; Zhou, J.; Zhan, L.Q. Ameliorating Effects of Biochar, Sheep Manure and Chicken Manure on Acidified Purple Soil. Agronomy 2023, 13, 1142. [Google Scholar] [CrossRef]
- Wang, Y.H.; Long, Q.; Li, Y.Y.; Kang, F.R.; Fan, Z.H.; Xiong, H.Y.; Zhao, H.Y.; Luo, Y.Y.; Guo, R.; He, X.H.; et al. Mitigating magnesium deficiency for sustainable citrus production: A case study in Southwest China. Sci. Hortic. 2022, 295, 110832. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Xiong, H.Y.; Zhao, H.Y.; Hu, B.; Yan, C.Q.; Yao, T.S.; Tang, X.D.; Zhao, J.K.; Zhang, Y.Q.; Shi, X.J.; et al. Accumulated temperature rather than nitrogen fertilization is the main factor determining growth of young citrus trees in the field. Sci. Hortic. 2023, 323, 112511. [Google Scholar] [CrossRef]
- Yang, M.; Long, Q.; Li, W.L.; Wang, Z.C.; He, X.H.; Wang, J.; Wang, X.Z.; Xiong, H.Y.; Guo, C.Y.; Zhang, G.C.; et al. Mapping the environmental cost of a typical citrus-producing county in China: Hotspot and optimization. Sustainability 2020, 12, 1827. [Google Scholar] [CrossRef]
- Li, W.L.; Yang, M.; Wang, J.; Wang, Z.C.; Fan, Z.H.; Kang, F.R.; Wang, Y.H.; Luo, Y.Y.; Kuang, D.J.; Chen, Z.H.; et al. Agronomic Responses of Major Fruit Crops to Fertilization in China: A Meta-Analysis. Agronomy 2019, 10, 15. [Google Scholar] [CrossRef]
- Fan, Z.H.; Xiong, H.Y.; Luo, Y.Y.; Wang, Y.H.; Zhao, H.Y.; Li, W.L.; He, X.H.; Wang, J.; Shi, X.J.; Zhang, Y.Q. Fruit Yields Depend on Biomass and Nutrient Accumulations in New Shoots of Citrus Trees. Agronomy 2020, 10, 1988. [Google Scholar] [CrossRef]
- Zhang, S.W.; Chen, X.H.; Ji, Z.J.; Yan, X.J.; Kong, K.P.; Cao, Y.Y.; Zhu, Q.C.; Muneer, M.A.; Zhang, F.S.; Wu, L.Q. Reducing aluminum is the key nutrient management strategy for ameliorating soil acidification and improving root growth in an acidic citrus orchard. Land Degrad. Dev. 2022, 34, 1681–1693. [Google Scholar] [CrossRef]
- Hao, T.X.; Liu, X.J.; Zhu, Q.C.; Zeng, M.F.; Chen, X.J.; Yang, L.S.; Shen, J.B.; Shi, X.J.; Zhang, F.S.; de Vries, W. Quantifying drivers of soil acidification in three Chinese cropping systems. Soil Tillage Res. 2022, 215, 105230. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding KW, T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Guo, J.; Yang, J.; Zhang, L.; Chen, H.; Jia, Y.; Wang, Z.; Wang, D.; Liao, W.; Chen, L.S.; Li, Y. Lower soil chemical quality of pomelo orchards compared with that of paddy and vegetable fields in acidic red soil hilly regions of southern China. J. Soils Sediments 2019, 19, 2752–2763. [Google Scholar] [CrossRef]
- Chen, X.H.; Yan, X.J.; Wang, M.K.; Cai, Y.Y.; Weng, X.F.; Su, D.; Guo, J.X.; Wang, W.Q.; Hou, Y.; Ye, D.L.; et al. Longterm excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Ouyang, W.J.; Li, Z.; Liu, J.; Gao, J.S.; Fang, F.; Xiao, Y.; Lu, L.H. Inventory of apparent nitrogen and phosphorus balance and risk of potential pollution in typical sloping cropland of purple soil in China-A case study in the Three Gorges Reservoir region. Ecol. Eng. 2017, 106, 620–628. [Google Scholar] [CrossRef]
- Lu, R.K. The Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Zhou, M.H.; Butterbach-bahl, K. Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 2014, 374, 977–991. [Google Scholar] [CrossRef]
- Nogueirol, R.C.; Monteiro, F.A.; Gratao, P.L.; Borgo, L.; Azevedo, R.A. Tropical soils with high aluminum concentrations cause oxidative stress in two tomato genotypes. Environ. Monit. Assess. 2015, 187, 73. [Google Scholar] [CrossRef]
- Jiang, D.X.; Ou, Y. Melatonin-priming ameliorates aluminum accumulation and toxicity in rice through enhancing aluminum exclusion and maintaining redox homeostasis. Plant Physiol. Biochem. 2025, 219, 109433. [Google Scholar] [CrossRef]
- Rengel, Z. Aluminium cycling in the soil-plant-animal-human continuum. Biometals 2004, 17, 669–689. [Google Scholar] [CrossRef]
- Butchee, K.; Arnall, D.B.; Sutradhar, A.; Godsey, C.; Zhang, H.; Penn, C. Determining critical soil pH for grain sorghum production. Int. J. Agron. 2012, 2012, 130254. [Google Scholar] [CrossRef]
- AbdulahaAl, B.M.; Li, J.Y.; Xu, C.Y.; Mehmood, K.; Xu, R.K. Determination of critical pH and Al concentration of acidic Ultisols for wheat and canola crops. Solid Earth 2017, 8, 149–159. [Google Scholar]
- Chen, X.H.; Yu, W.H.; Cai, Y.Y.; Zhang, S.W.; Muneer, M.A.; Zhu, Q.C.; Xu, D.H.; Ma, C.C.; Yan, X.J.; Li, Y.; et al. How to identify and adopt cleaner strategies to improve the continuous acidification in orchard soils? J. Clean. Prod. 2022, 330, 129826. [Google Scholar] [CrossRef]
- Qin, W.; Assinck, F.B.T.; Heinen, M.; Oenema, O. Water and nitrogen use efficiencies in citrus production: A meta-analysis. Agr. Ecosyst. Environ. 2016, 222, 103–111. [Google Scholar] [CrossRef]
- Liu, L.; Xie, D.T.; Li, Z.Y.; Liu, F. Cations exchange and its effect on acid buffering capacity of acid purple soil. Acta Pedol. Sin. 2020, 57, 887–897. [Google Scholar]
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Adv. Agron. 2003, 78, 215–272. [Google Scholar]
- Yan, P.; Wu, L.Q.; Wang, D.H.; Fu, J.Y.; Shen, C.; Li, X.; Zhang, L.P.; Zhang, L.; Fan, L.C.; Han, W.Y. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Liao, P.; Huang, S.; Zeng, Y.J.; Shao, H.; Zhang, J.; van Groenigen, K.J. Liming increases yield and reduces grain cadmium concentration in rice paddies: A meta-analysis. Plant Soil 2021, 465, 157–169. [Google Scholar] [CrossRef]
- Li, L.Z.; Wu, H.F.; van Gestel, C.A.M.; Peijnenburg, W.J.G.M. Soil acidification increases metal extractability and bioavailability in old orchard soils of Northeast Jiaodong Peninsula in China. Environ. Pollut. 2014, 188, 144–152. [Google Scholar] [CrossRef]
- Liu, C.A.; Liang, M.Y.; Nie, Y.; Tang, J.W.; Siddique, K.H.M. The conversion of tropical forests to rubber plantations accelerates soil acidification and changes the distribution of soil metal ions in topsoil layers. Sci. Total Environ. 2019, 696, 134082. [Google Scholar] [CrossRef]


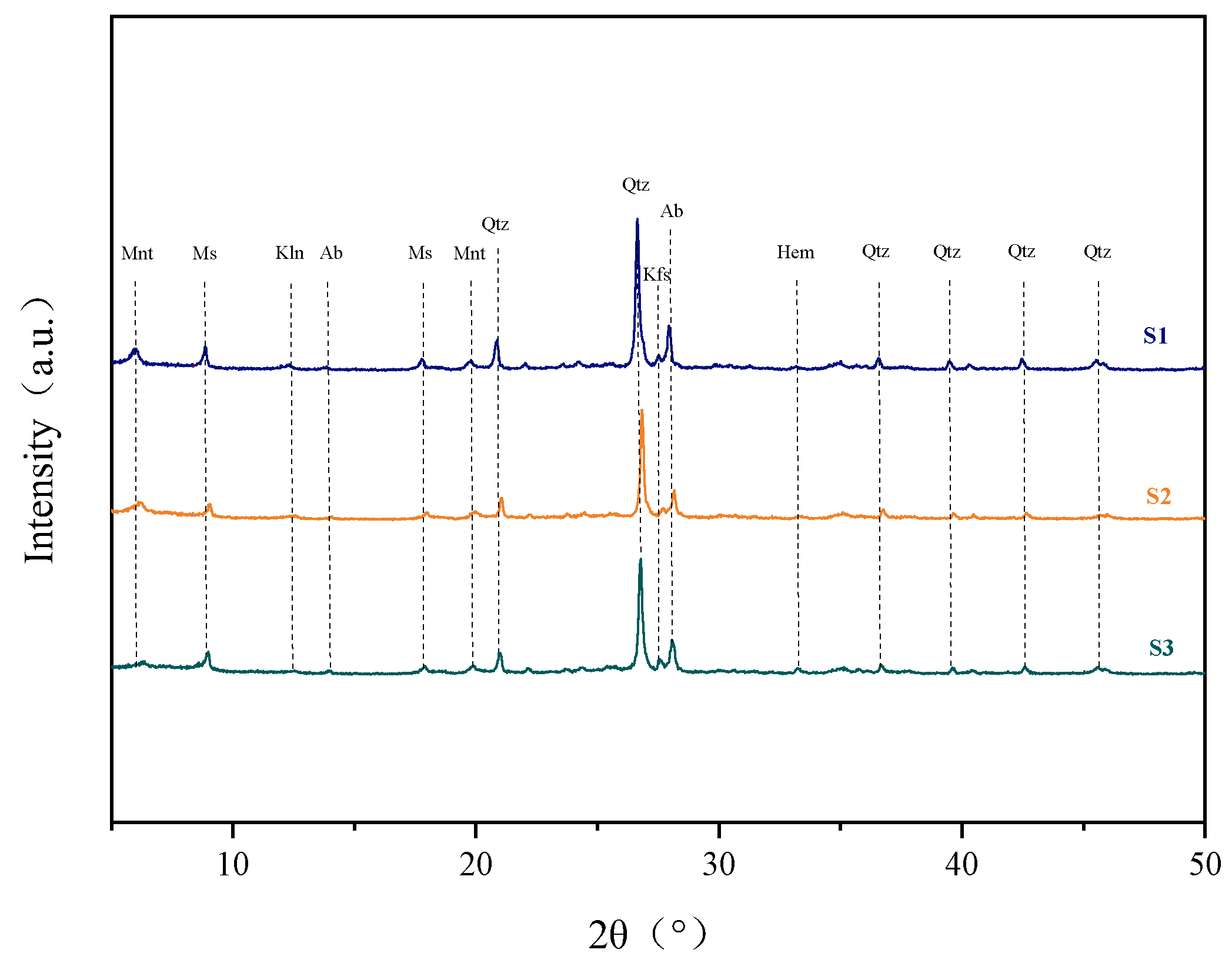


| Years | Depth | Ava. Fe | Ava. Mn | Ava. Cu | Ava. Zn | Ava. Pb | Ava. Cd | Ava. Ni |
|---|---|---|---|---|---|---|---|---|
| 3 | 0–20 cm | 17.19 ± 9.42 a | 19.33 ± 3.72 a | 1.21 ± 0.64 a | 2.58 ± 0.99 a | 0.95 ± 0.20 a | 0.07 ± 0.02 a | 0.30 ± 0.06 a |
| 20–40 cm | 15.11 ± 11.03 ab | 20.32 ± 4.07 a | 0.70 ± 0.29 b | 1.07 ± 0.42 b | 0.76 ± 0.12 b | 0.05 ± 0.02 b | 0.33 ± 0.10 a | |
| 40–60 cm | 8.65 ± 3.33 b | 21.85 ± 2.19 a | 0.48 ± 0.10 b | 0.67 ± 0.37 b | 0.71 ± 0.08 b | 0.03 ± 0.01 c | 0.28 ± 0.02 a | |
| 10 | 0–20 cm | 39.38 ± 13.70 a | 39.63 ± 4.65 a | 1.33 ± 0.49 a | 2.40 ± 0.58 a | 1.49 ± 0.31 a | 0.10 ± 0.02 a | 0.83 ± 0.26 a |
| 20–40 cm | 32.00 ± 18.25 ab | 40.05 ± 9.68 a | 0.68 ± 0.13 b | 0.85 ± 0.26 b | 1.00 ± 0.32 b | 0.04 ± 0.01 b | 0.98 ± 0.40 a | |
| 40–60 cm | 21.73 ± 14.41 b | 35.48 ± 7.79 a | 0.52 ± 0.14 b | 0.56 ± 0.32 b | 0.74 ± 0.31 c | 0.02 ± 0.01 c | 0.73 ± 0.59 a | |
| 50 | 0–20 cm | 21.56 ± 11.80 a | 39.50 ± 8.16 a | 1.61 ± 0.49 a | 3.16 ± 0.80 a | 1.15 ± 0.21 a | 0.14 ± 0.02 a | 0.55 ± 0.28 a |
| 20–40 cm | 12.29 ± 11.34 b | 26.05 ± 7.65 b | 0.62 ± 0.18 b | 0.72 ± 0.29 b | 0.64 ± 0.20 b | 0.04 ± 0.01 b | 0.36 ± 0.36 ab | |
| 40–60 cm | 10.31 ± 5.93 b | 25.57 ± 13.75 b | 0.39 ± 0.16 c | 0.57 ± 0.48 b | 0.50 ± 0.16 c | 0.02 ± 0.02 c | 0.25 ± 0.28 b |
| Upper Limit | Grade 1 (Highest) | Grade 2 (High) | Grade 3 (Medium) | Grade 4 (Low) | Grade 5 (Lowest) | |
|---|---|---|---|---|---|---|
| Ava. Fe mg kg−1 | - | >20.0 | 10.0–20.0 | 5.0–10.0 | 3.0–5.0 | ≤3.0 |
| Ava. Mn mg kg−1 | - | >30.0 | 15.0–30.0 | 5.0–15.0 | 1.0–5.0 | ≤1.0 |
| Ava. Cu mg kg−1 | 15.0 | >2.0 | 1.0–2.0 | 0.5–1.0 | 0.2–0.5 | ≤0.2 |
| Ava. Zn mg kg−1 | 10.0 | >3.0 | 1.0–3.0 | 0.5–1.0 | 0.3–0.5 | ≤0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Zhao, J.; Zhou, J.; Li, Z. Marked Spatial Variability in Acidity Characteristics of Purple Soil at Field Scale Induced by Citrus Plantation. Agronomy 2025, 15, 1022. https://doi.org/10.3390/agronomy15051022
Luo J, Zhao J, Zhou J, Li Z. Marked Spatial Variability in Acidity Characteristics of Purple Soil at Field Scale Induced by Citrus Plantation. Agronomy. 2025; 15(5):1022. https://doi.org/10.3390/agronomy15051022
Chicago/Turabian StyleLuo, Jiayi, Jingkun Zhao, Jia Zhou, and Zhongyi Li. 2025. "Marked Spatial Variability in Acidity Characteristics of Purple Soil at Field Scale Induced by Citrus Plantation" Agronomy 15, no. 5: 1022. https://doi.org/10.3390/agronomy15051022
APA StyleLuo, J., Zhao, J., Zhou, J., & Li, Z. (2025). Marked Spatial Variability in Acidity Characteristics of Purple Soil at Field Scale Induced by Citrus Plantation. Agronomy, 15(5), 1022. https://doi.org/10.3390/agronomy15051022





