Spent Pleurotus ostreatus Substrate Has Potential for Controlling the Plant-Parasitic Nematode, Radopholus similis in Bananas
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Multiplication and Preparation of Radopholus similis Inoculum
2.2. Spent P. ostreatus Substrate Preparation
2.3. Screening for Nematicidal Effects of Spent P. ostreatus Substrate in Banana Plantlets Inoculated with R. similis
2.4. Statistical Analysis
3. Results
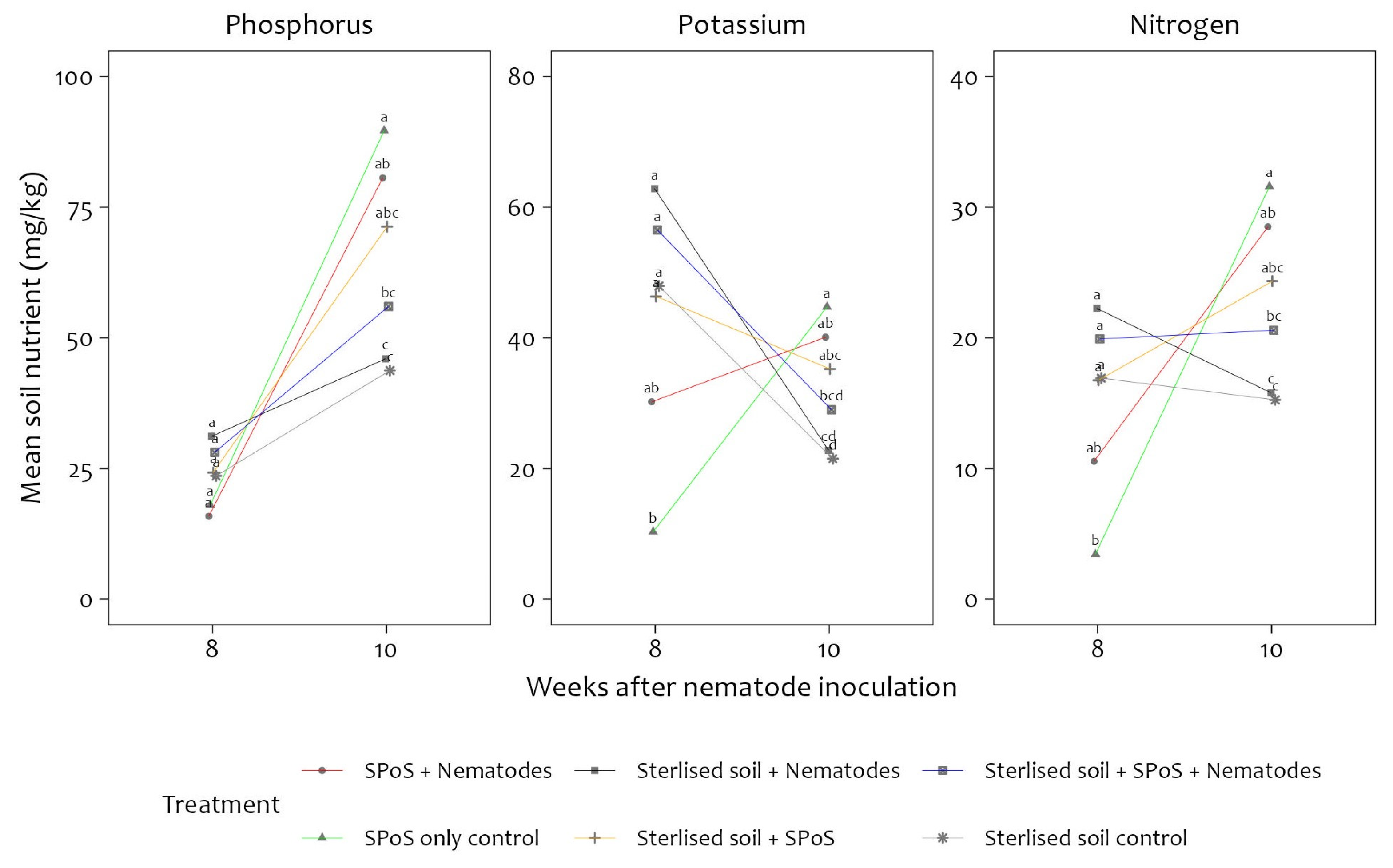
3.1. Soil Chemical Parameters for Treated Pots with Banana Plants
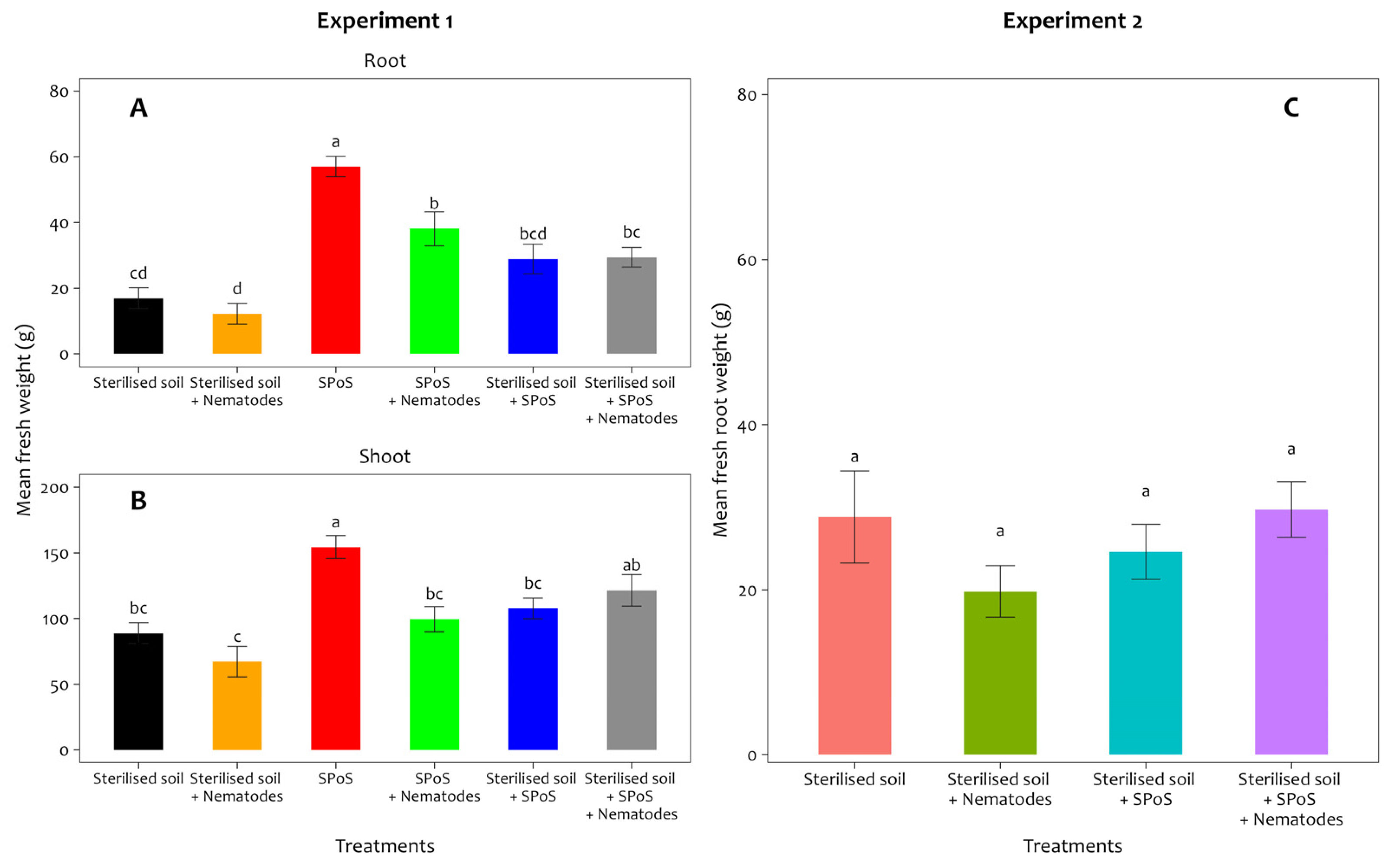
3.2. Effect of Spent P. ostreatus Substrate Treatment on Banana Root and Shoot Biomass
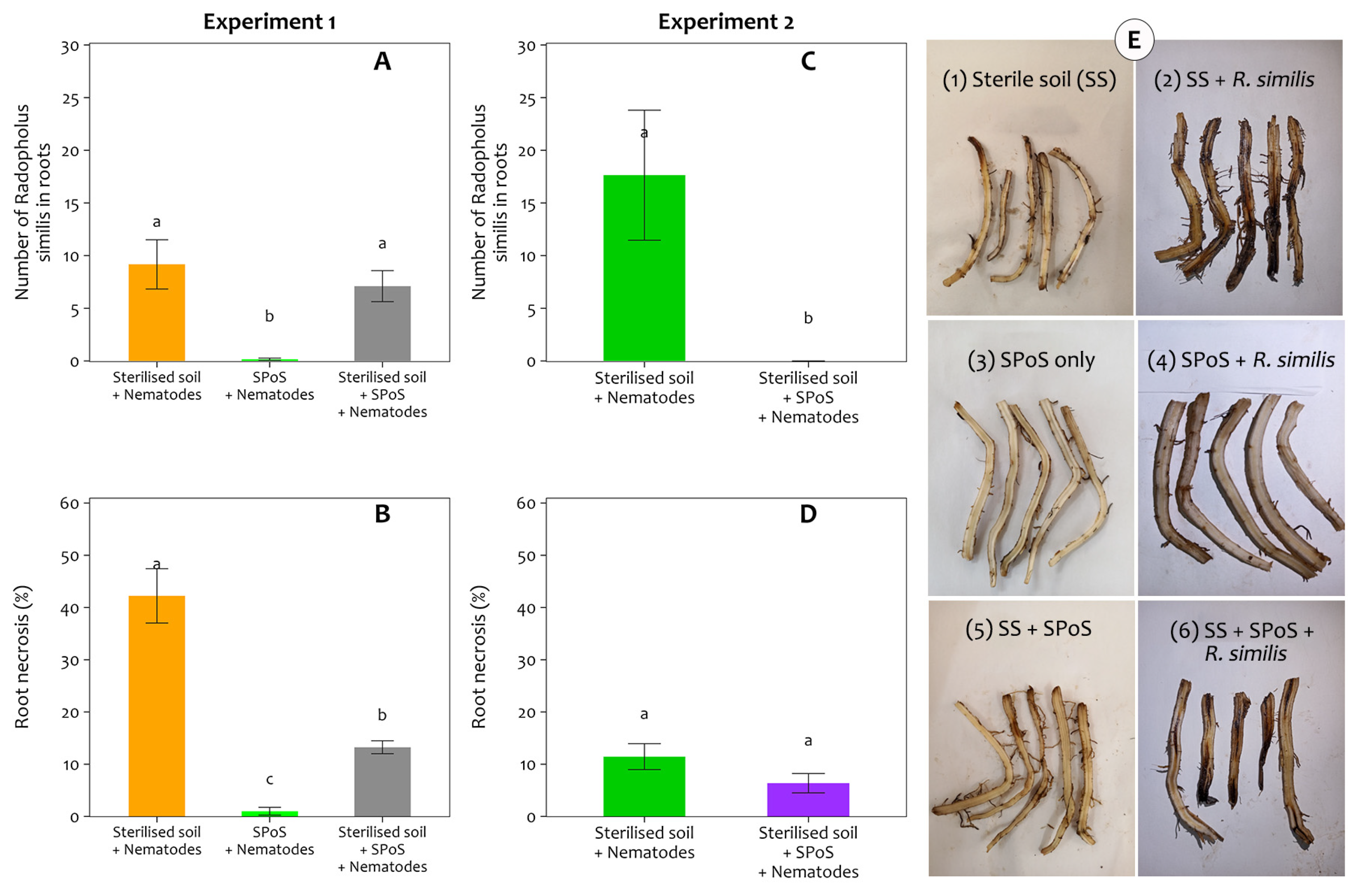
3.3. The Effect of R. similis and Spent P. ostreatus Substrate on Survival of Banana Roots
3.4. Effect of Spent P. ostreatus Spent Substrate on the Number of R. similis in Plant Tissues and Associated Root Necrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royse, D.J. A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; Volume 1, pp. 1–6. [Google Scholar]
- Nandeha, N. Substrates for Mushroom Production. In Mushroom: The Fascinating Fungi, 1st ed.; Padamini, R., Rathour, S.K., Dange, M.M., Ali, M.U., Chavda, H., Eds.; Emerald Publishing House: New Delhi, India, 2024; pp. 77–94. [Google Scholar]
- Williams, B.C.; McMullan, J.T.; McCahey, S. An initial assessment of spent mushroom compost as a potential energy feedstock. Bioresour. Technol. 2001, 79, 227–230. [Google Scholar] [CrossRef]
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The reuse of spent mushroom compost and coal tailings for energy recovery: Comparison of thermal treatment technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Paredes, C.; Medina, E.; Bustamante, M.A.; Moral, R. Effects of spent mushroom substrates and inorganic fertilizer on the characteristics of a calcareous clayey-loam soil and lettuce production. Soil Use Manag. 2016, 32, 487–494. [Google Scholar] [CrossRef]
- Adamović, M.J.; Bočarov-Stančić, A.S.; Milenković, I.M.; Štrbac, S.S.; Adamović, I. The quality of silage of corn grain and spent P. ostreatus mushroom substrate. Zb. Matice Srp. Prir. Nauk. 2007, 113, 211–218. Available online: https://doiserbia.nb.rs/img/doi/0352-4906/2007/0352-49060713211A.pdf (accessed on 23 April 2025). [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef]
- Kwack, Y.; Song, J.H.; Shinohara, Y.; Maruo, T.; Chun, C. Comparison of six spent mushroom composts as growing media for transplant production of lettuce. Compost Sci. Util. 2012, 20, 92–96. [Google Scholar] [CrossRef]
- Ocimati, W.; Were, E.; Ogwal, G.; Dita, M.; Tazuba, A.F.; Zheng, S.J.; Blomme, G. Can edible mushrooms boost soil health in banana organic systems? Acta Hortic. 2023, 1367, 107–116. [Google Scholar] [CrossRef]
- Raj, H. Possible management of Fusarium wilt of tomato by soil amendments with composts. Indian Phytopathol. 1997, 50, 387–395. [Google Scholar]
- Adedeji, K.O.; Aduramigba, M.A.O. In vitro evaluation of spent mushroom compost on growth of Fusarium oxysporium f. sp lycopersici. Adv. Plants Agric. Res. 2016, 4, 332–339. [Google Scholar] [CrossRef]
- Kwak, A.M.; Min, K.J.; Lee, S.Y.; Kang, H.W. Water extract from spent mushroom substrate of Hericium erinaceus suppresses bacterial wilt disease of tomato. Mycobiology 2015, 43, 311–318. [Google Scholar] [CrossRef]
- Yusidah, I.; Istifadah, N. The abilities of spent mushroom substrate to suppress basal rot disease (Fusarium oxysporum f. sp cepae) in shallot. Int. J. Biosci. 2018, 13, 440–448. [Google Scholar]
- Ocimati, W.; Were, E.; Tazuba, A.F.; Dita, M.; Zheng, S.J.; Blomme, G. Spent Pleurotus ostreatus substrate has potential for managing Fusarium wilt of banana. J. Fungi 2021, 7, 946. [Google Scholar] [CrossRef] [PubMed]
- Sabina Aslam, S.A.; Saifullah, S. Organic management of root knot nematodes in tomato with spent mushroom compost. Sarhad J. Agric. 2013, 29, 1016–4383. Available online: https://worldveg.tind.io/record/49821/#files (accessed on 20 February 2025).
- Mostafa, D.M.; Allah, S.F.A.; Awad-Allah, E.F. Potential of Pleurotus sajor-caju compost for controlling Meloidogyne incognita and improve nutritional status of tomato plants. J. Plant Sci. Phytopathol. 2019, 3, 118–127. [Google Scholar]
- Nyangwire, B.; Ocimati, W.; Tazuba, A.F.; Blomme, G.; Alumai, A.; Onyilo, F. Pleurotus ostreatus is a potential biological control agent of root-knot nematodes in eggplant (Solanum melongena). Front. Agron. 2024, 6, 1464111. [Google Scholar] [CrossRef]
- Stadler, M.; Sheldrick, W.S.; Dasen-brock, J.; Steglich, W.; Anke, H. Antibiotics from the nematode-trapping basidiomycete Nematoctonus robustus. Nat. Prod. Lett. 1994, 4, 209–216. [Google Scholar] [CrossRef]
- Kwok, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A nematicidal toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 1992, 18, 127–136. [Google Scholar] [CrossRef]
- Genier, H.L.A.; de Freitas Soares, F.E.; de Queiroz, J.H.; de Souza Gouveia, A.; Araújo, J.V.; Braga, F.R.; Pinheiro, I.R.; Kasuya, M.C.M. Activity of the fungus Pleurotus ostreatus and of its proteases on Panagrellus sp. larvae. Afr. J. Biotech. 2015, 14, 1496–1503. [Google Scholar]
- Barron, G.L.; Thorn, R.G. Destruction of nematodes by species of Pleurotus. Can. J. Bot. 1987, 65, 774–778. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, H.W.; Yang, C.T.; Wali, N.; Shie, J.J.; Hsueh, Y.P. Sensory cilia as the Achilles heel of nematodes when attacked by carnivorous mushrooms. Proc. Natl. Acad. Sci. USA 2020, 117, 6014–6022. [Google Scholar] [CrossRef]
- Sasser, J.N.; Freckman, D.W. A World Perspective of Nematology: The Role of Society; Veech, J.A., Dickson, D.W., Eds.; Society of Nematologists: Hyattsville, MD, USA, 1987; pp. 7–14. [Google Scholar]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Nyaku, S.T.; Affokpon, A.; Danquah, A.; Brentu, C. Harnessing Useful Rhizosphere Microorganisms for Nematode Control. In Nematology; Shah, M.M., Ed.; InTech.: London, UK, 2017; ISBN 9789535155270. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Gowen, S.R.; Quénéhervé, P.; Fogain, R. Nematode parasites of bananas and plantains. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R.A., Bridge, J., Eds.; CAB International: Wallingford, UK, 2005; pp. 611–643. [Google Scholar]
- Coyne, D.L.; Cortada, L.; Dalzell, J.J.; Claudius-Cole, A.O.; Haukeland, S.; Luambano, N.; Talwana, H. Plant-parasitic nematodes and food security in Sub-Saharan Africa. Ann. Rev. Phytopathol. 2018, 56, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Sarah, J.L.; Pinochet, J.; Stanton, J.M. The burrowing nematode of bananas, Radopholus similis Cobb, 1913. In Musa Pest Fact Sheet No. 1; International Network for the Improvement of Banana and Plantain: Montpellier, France, 1996; Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/db166405-8887-4da5-b949-1082c64173a5/content (accessed on 24 February 2025).
- CABI Compendium. Radopholus Similis (Burrowing Nematode); CABI Head Office: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Haegeman, A.; Elsen, A.; De Waele, D.; Gheysen, G. Emerging molecular knowledge on Radopholus similis, an important nematode pest of banana. Mol. Plant Pathol. 2010, 11, 315. [Google Scholar] [CrossRef]
- Brooks, F.E. Burrowing nematode. Plant Health Instr. 2008, 8. [Google Scholar] [CrossRef]
- Back, M.A.; Haydock, P.P.J.; Jenkinson, P. Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathol. 2002, 51, 683–697. [Google Scholar] [CrossRef]
- Roth, M.G.; Noel, Z.A.; Wang, J.; Warner, F.; Byrne, A.M.; Chilvers, M.I. Predicting soybean yield and sudden death syndrome development using at-planting risk factors. Phytopathology 2019, 109, 1710–1719. [Google Scholar] [CrossRef]
- Parrado, L.M.; Quintanilla, M. Plant-parasitic nematode disease complexes as overlooked challenges to crop production. Front. Plant Sci. 2024, 15, 1439951. [Google Scholar] [CrossRef]
- Orion, D.; Levy, Y.; Israeli, Y.; Fischer, E. Scanning electron microscope observations on spiral nematode (Helicotylenchus multicinctus)-infested banana roots. Nematropica 1999, 29, 179–183. [Google Scholar]
- Rocha, A.D.J.; Ferreira, M.D.S.; Rocha, L.D.S.; Oliveira, S.A.; Amorim, E.P.; Mizubuti, E.S.; Haddad, F. Interaction between Fusarium oxysporum f. sp. cubense and Radopholus similis can lead to changes in the resistance of banana cultivars to Fusarium wilt. Euro. J. Plant Pathol. 2020, 158, 403–417. [Google Scholar] [CrossRef]
- Coyne, D.L.; Nicol, J.M.; Claudius-Cole, B. Practical Plant Nematology: A Field and Laboratory Guide, 2nd ed; SP-IPM Secretariat, International Institute of Tropical Agriculture (IITA): Cotonou, Benin, 2014. [Google Scholar]
- Coyne, D.L.; Adewuyi, O.; Mbiru, E. Protocol for In Vitro Culturing of Lesion Nematodes: Radopholus Similis and Pratylenchus spp. on Carrot Discs; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2014; 15p. [Google Scholar]
- Roy, S.; Roy, K.K.; Sarkar, S.; Rathod, A. Intra-specific morphological and morphometric variability of Radopholus similis (Cobb 1893) Thorne, 1949. J. Appl. Nat. Sci. 2018, 10, 841–846. [Google Scholar] [CrossRef]
- Gold, C.S.; Karamura, E.B.; Kiggundu, A.; Bagamba, F.; Abera, A.M. Geographic shifts in the highland cooking banana (Musa spp., group AAA-EA) production in Uganda. Int. J. Sustain. Dev. World Ecol. 1999, 6, 45–59. [Google Scholar] [CrossRef]
- Kajumba, C.; Speijer, P.R. Yield loss from plant parasitic nematodes in East African highland banana (Musa spp. AAA). In Proceedings of the I International Symposium on Banana: International Conference on Banana and Plantain for Africa, Kampala, Uganda, 14 October 1996; pp. 453–459. [Google Scholar]
- Rife, T.W.; Poland, J.A. Field Book: An open-source application for field data collection on android. Crop Sci. 2014, 54, 1624–1627. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2024; Available online: https://www.rstudio.com (accessed on 23 July 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 23 July 2024).
- Prabu, M.; Jeyanthi, C.; Kumuthakalavalli, R. Spent mushroom substrate: An enriched organic manure for improving the yield of Vigna unguiculata [L] Walp (Cowpea) leguminous crop. Scrut. Int. Res. J. Agric. Plant Biotechnol. Bioprod. 2015, 1, 7–14. [Google Scholar]
- Stewart, D.P.C.; Cameron, K.C.; Cornforth, I.S. Effects of spent mushroom substrate on soil chemical conditions and plant growth in an intensive horticultural system: A comparison with inorganic fertiliser. Soil Res. 1998, 36, 185–198. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.Q.; Wang, L.J.; Hu, G.L.; Gong, X.Q.; Bai, Q.; Su, S.C.; Qi, J.X. Short-term effects of spent mushroom substrate mulching thickness on the soil environment, weed suppression, leaf nutrients, and nut characteristics in a hazelnut orchard. Agronomy 2021, 11, 1122. [Google Scholar] [CrossRef]
- Qorri, R.A. Enzyme activities in the spent mushroom substrate from Pleurotus ostreatus. World J. Adv. Res. Rev. 2022, 16, 942–947. [Google Scholar] [CrossRef]
- Stewart, D.P.C.; Cameron, K.C.; Cornforth, I.S. Inorganic-N release from spent mushroom compost under laboratory and field conditions. Soil Biol. Biochem. 1998, 30, 1689–1699. [Google Scholar] [CrossRef]
- Khalil, S.; Panda, P.; Ghadamgahi, F.; Barreiro, A.; Karin, A.; Karlsson, M.; Vetukuri, R.R. Microbial potential of spent mushroom compost and oyster substrate in horticulture: Diversity, function, and sustainable plant growth solutions. J. Environ. Manag. 2024, 357, 120654. [Google Scholar] [CrossRef]
- Barekye, A.; Kashaija, I.N.; Adipala, E.; Tushemereirwe, W.K. Comparison of damage levels caused by Radopholus similis and Helicotylenchus multicinctus on bananas in Uganda. Ann. Appl. Biol. 2000, 137, 273–278. [Google Scholar] [CrossRef]
- Inácio, F.D.; Ferreira, R.O.; Araujo, C.A.V.; De Brugnari, T.; Castoldi, R.; Peralta, R.M.; De Souza, C.G.M. Proteases of wood rot fungi with emphasis on the genus Pleurotus. BioMed Res. Int. 2015, 2015, 290161. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Maňasová, M.; Zouhar, M.; Ryšánek, P. Comparative virulence assessment of different nematophagous fungi and chemicals against northern root-knot nematodes, Meloidogyne hapla, on carrots. Pak. J. Zool. 2020, 52, 199–206. [Google Scholar] [CrossRef]
- Lopes, A.D.; de Melo Santana Gomes, S.; Schwengber, R.P.; Carpi, M.C.G.; Dias-Arieira, C.R. Control of Meloidogyne javanica with Pleurotus djamor spent mushroom substrate. Chem. Biol. Technol. Agric. 2023, 10, 13. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, X.J.; Cao, Y.; Dong, Q.E.; Tong, J.Y.; Mo, M.H. Vermicomposting of Pleurotus eryngii spent mushroom substrates and the possible mechanisms of vermicompost suppressing nematode disease caused by Meloidogyne incognita. Heliyon 2023, 9, e15111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazuba, A.F.; Ocimati, W.; Ogwal, G.; Nyangwire, B.; Onyilo, F.; Blomme, G. Spent Pleurotus ostreatus Substrate Has Potential for Controlling the Plant-Parasitic Nematode, Radopholus similis in Bananas. Agronomy 2025, 15, 1040. https://doi.org/10.3390/agronomy15051040
Tazuba AF, Ocimati W, Ogwal G, Nyangwire B, Onyilo F, Blomme G. Spent Pleurotus ostreatus Substrate Has Potential for Controlling the Plant-Parasitic Nematode, Radopholus similis in Bananas. Agronomy. 2025; 15(5):1040. https://doi.org/10.3390/agronomy15051040
Chicago/Turabian StyleTazuba, Anthony Fredrick, Walter Ocimati, Geofrey Ogwal, Betty Nyangwire, Francis Onyilo, and Guy Blomme. 2025. "Spent Pleurotus ostreatus Substrate Has Potential for Controlling the Plant-Parasitic Nematode, Radopholus similis in Bananas" Agronomy 15, no. 5: 1040. https://doi.org/10.3390/agronomy15051040
APA StyleTazuba, A. F., Ocimati, W., Ogwal, G., Nyangwire, B., Onyilo, F., & Blomme, G. (2025). Spent Pleurotus ostreatus Substrate Has Potential for Controlling the Plant-Parasitic Nematode, Radopholus similis in Bananas. Agronomy, 15(5), 1040. https://doi.org/10.3390/agronomy15051040





