Effect of Microplastics on the Bioavailability of (Semi-)Metals in the Soil Earthworm Eisenia fetida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Microplastics
2.2. Test Organism
2.3. Soil Incubation Experiment
2.4. Exposure Experiment on the Earthworms
2.5. Physicochemical Properties and Metal(Loid) Concentration of Soil and Organisms
2.6. Modeling Toxicokinetic Processes
2.7. Statistical Analysis
3. Results
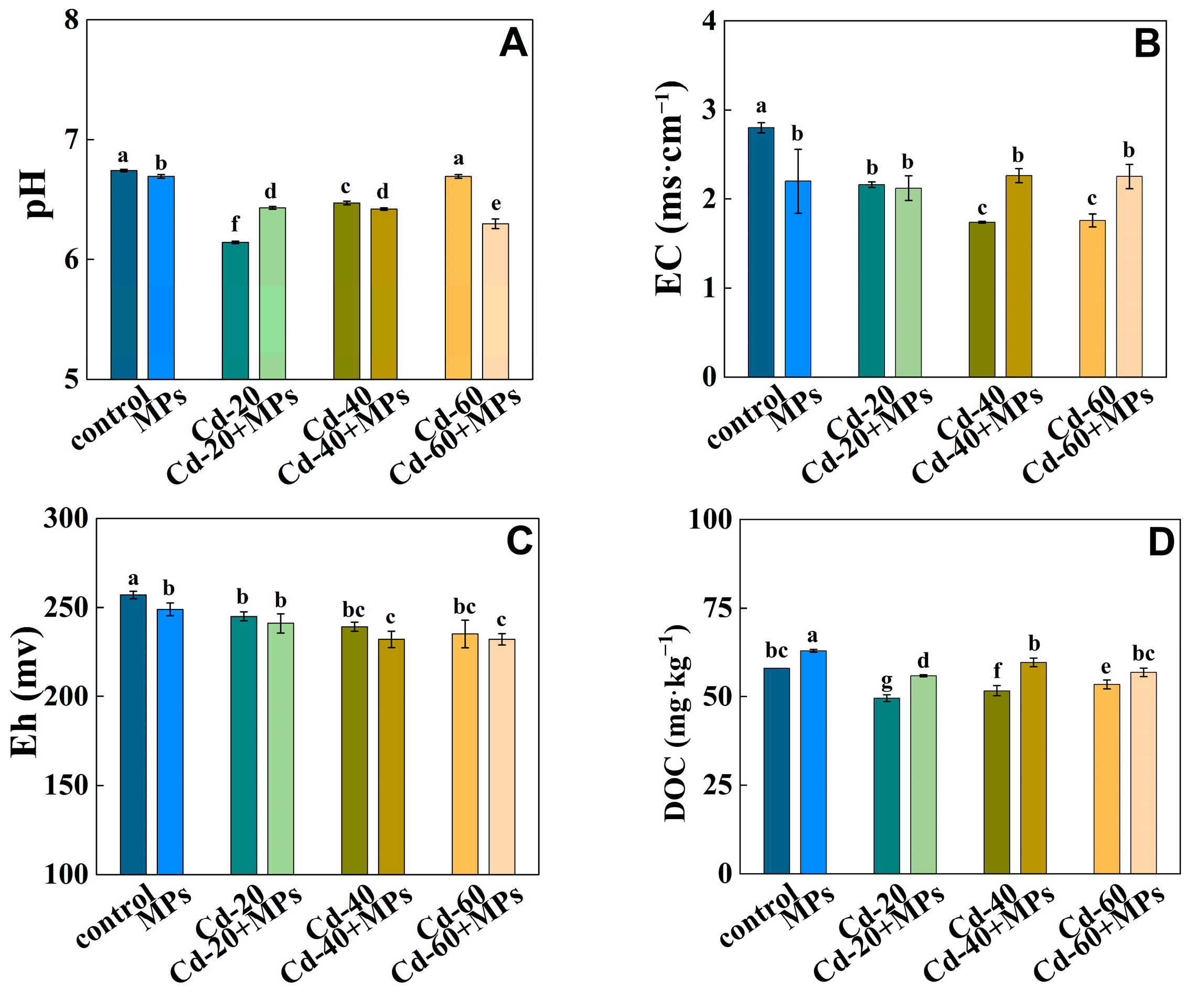
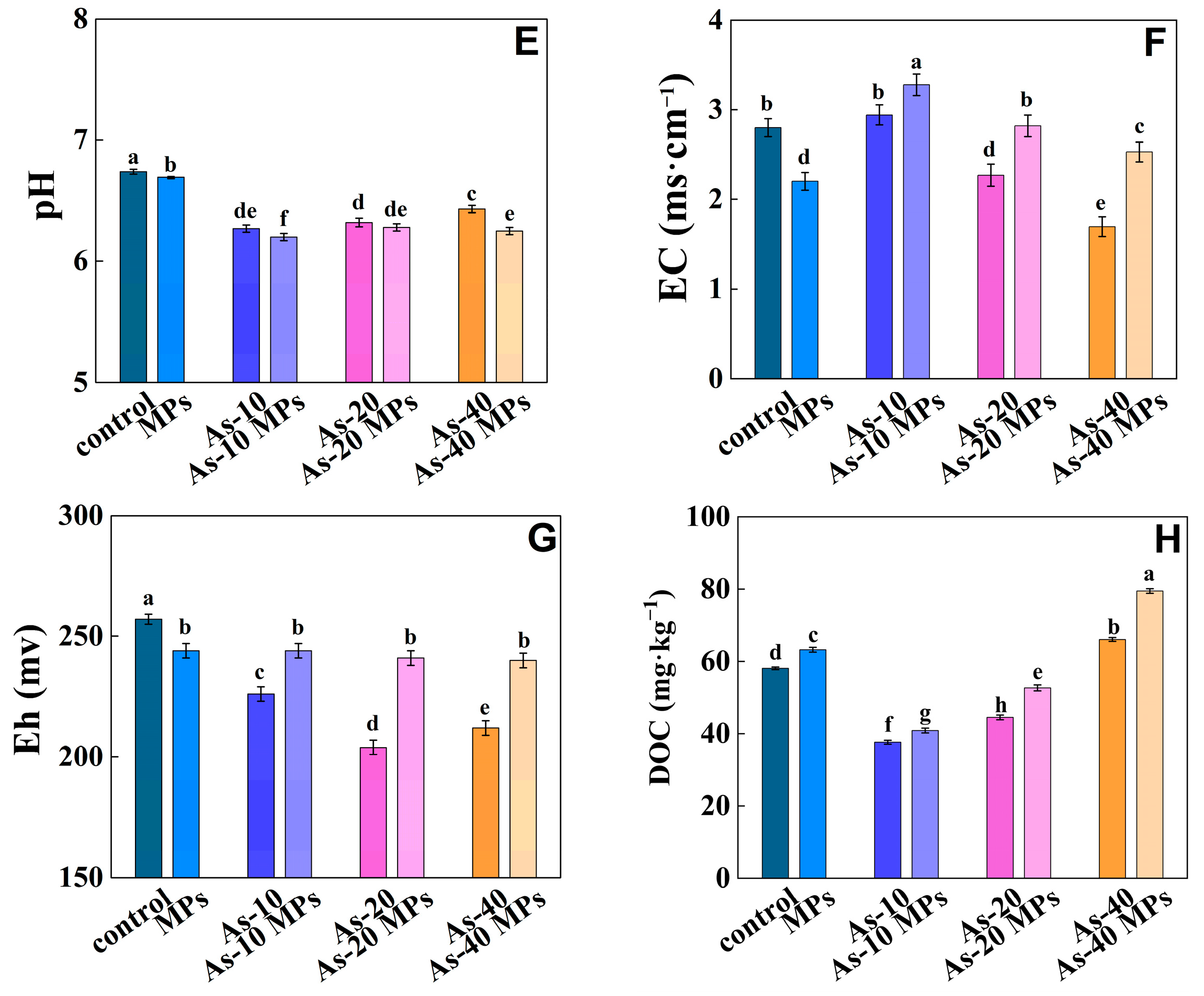
3.1. The Influence of PS-MPs on the Properties of Cd/As Contaminated Soil
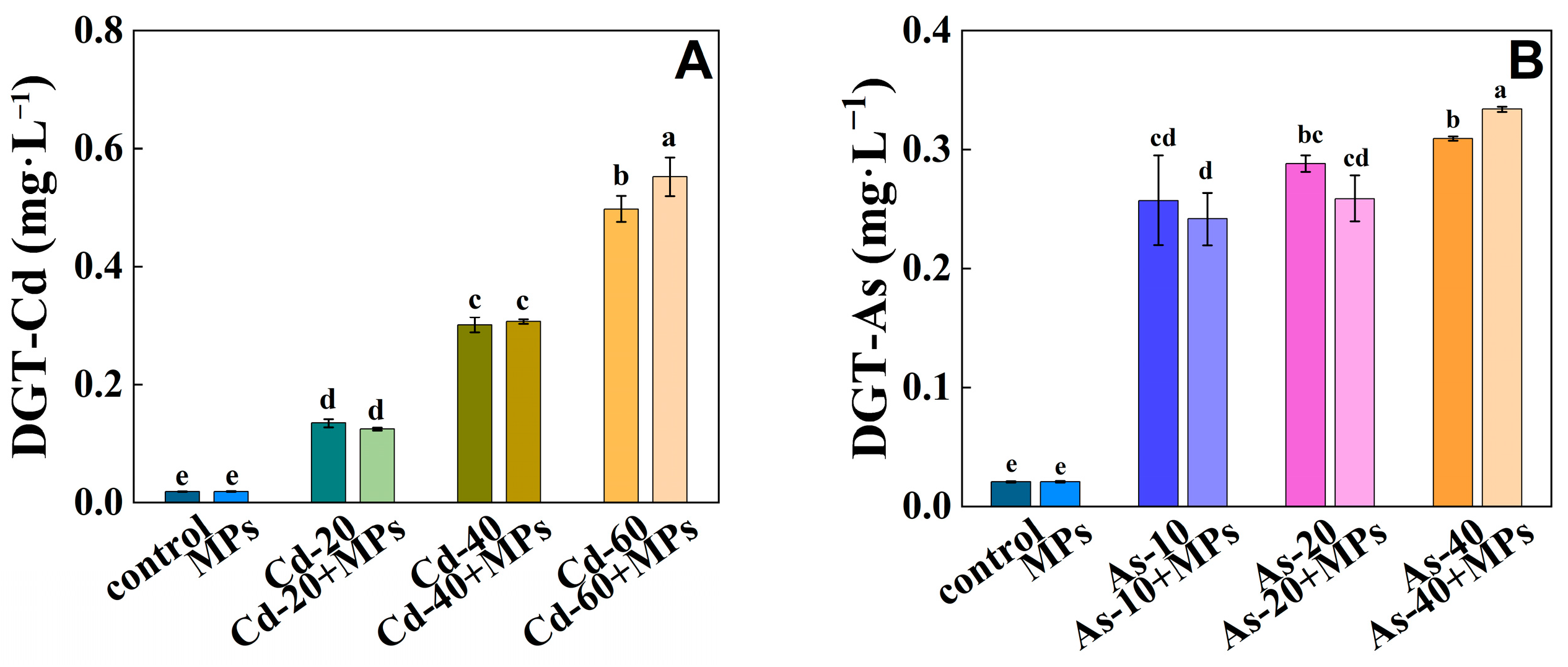
3.2. The Available Fraction of Cd/As in Soil
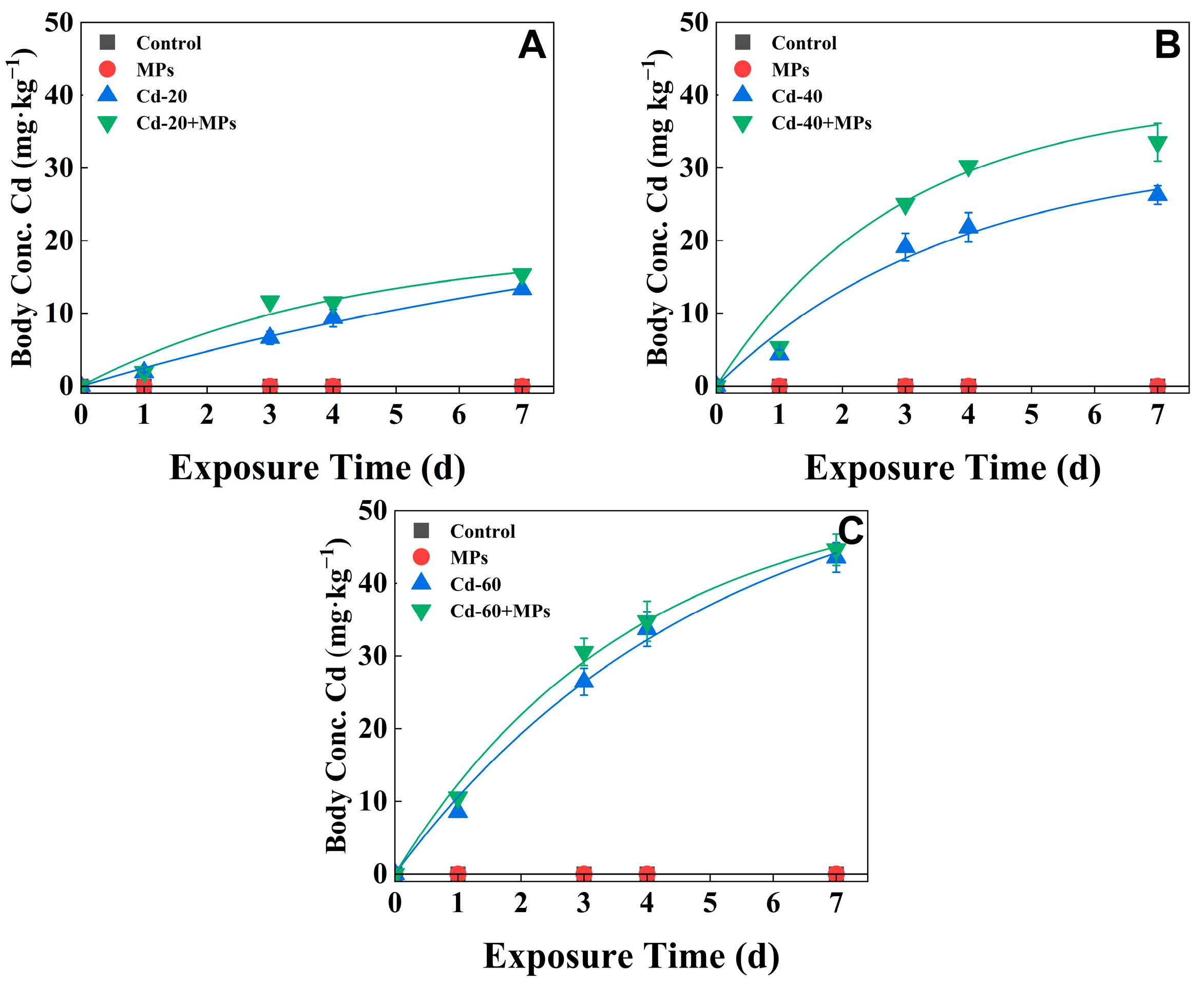
3.3. Dynamic Bioaccumulation of Cd
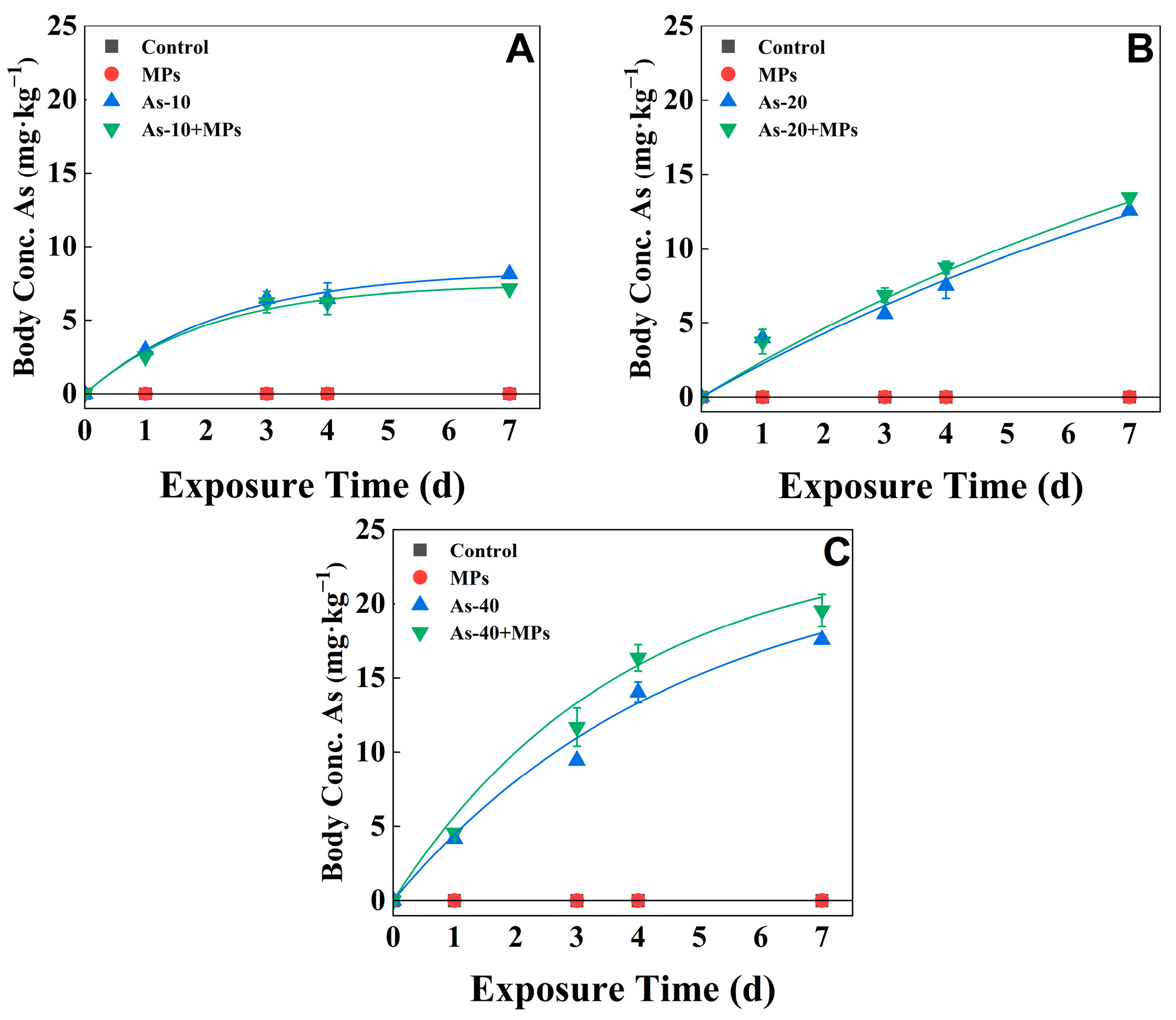
3.4. Dynamic Bioaccumulation of As
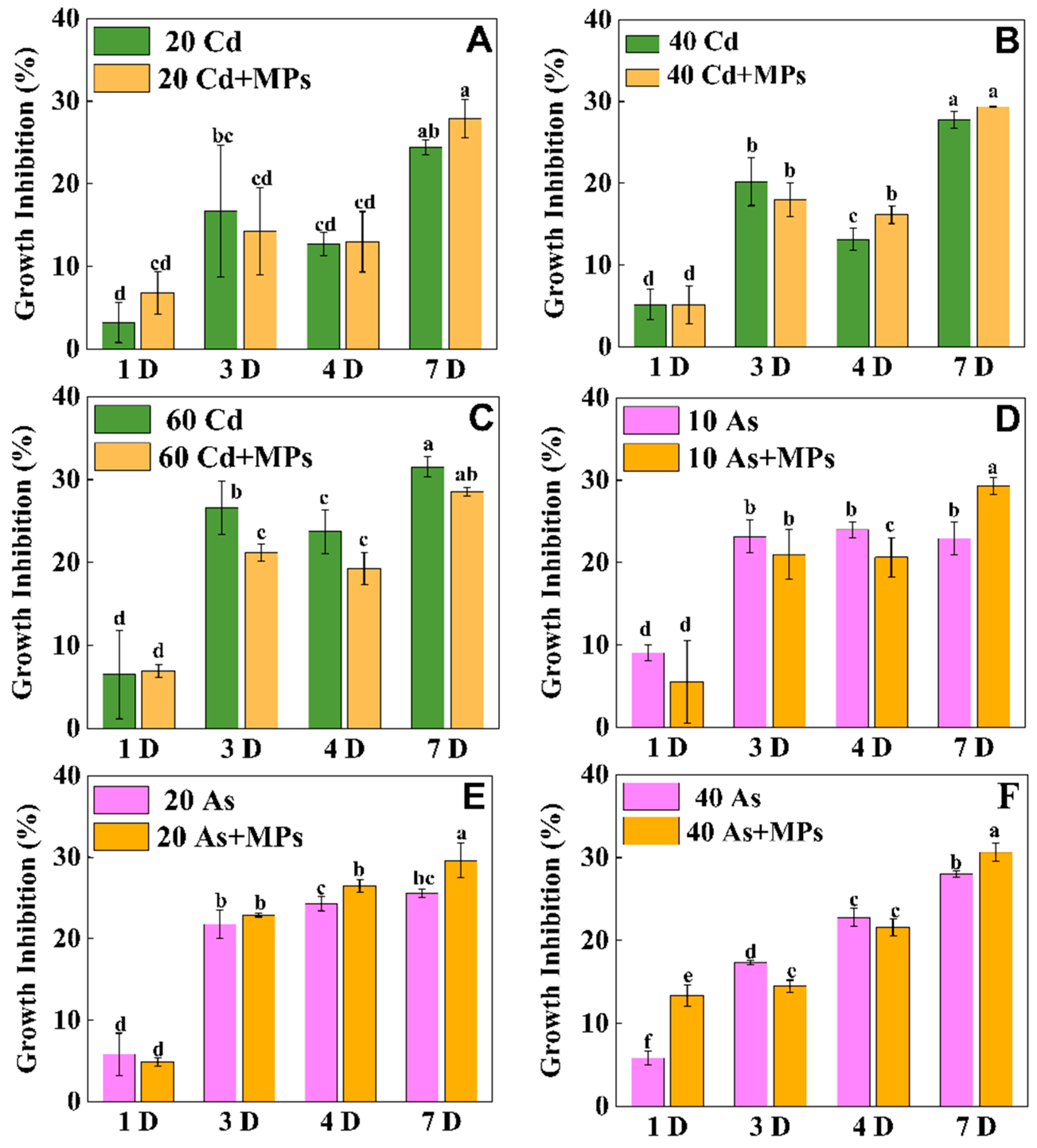
3.5. PS-MPs Affect the Growth Inhibition of Eisenia fetida in Cd/As-Contaminated Soil
4. Discussion
4.1. Effects of PS-MPs on Soil Properties
4.2. Effects of PS-MPs on Available Cd/As
4.3. Effects of PS-MPs on the Toxicokinetic Process of Cd/As in Eisenia fetida
4.4. Effects of PS-MPs on the Toxicity of Cd/As in Eisenia fetida
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An Overview of Microplastic and Nanoplastic Pollution in Agroecosystems. Sci. Total. Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural Plastic Mulching as a Source of Microplastics in the Terrestrial Environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total. Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic Mulching in Agriculture. Trading Short-Term Agronomic Benefits for Long-Term Soil Degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Identification of Microplastic in Effluents of Waste Water Treatment Plants Using Focal Plane Array-Based Micro-Fourier-Transform Infrared Imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric Transport Is a Major Pathway of Microplastics to Remote Regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic Fertilizer as a Vehicle for the Entry of Microplastic into the Environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Machado, A.A.D.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and Effects of Microplastics with Heavy Metals in Aquatic and Terrestrial Environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Zhang, Y.; Fan, P.; Xi, B.; Tan, W. Metal Type and Aggregate Microenvironment Govern the Response Sequence of Speciation Transformation of Different Heavy Metals to Microplastics in Soil. Sci. Total. Environ. 2021, 752, 141956. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A Review of Soil Cadmium Contamination in China Including a Health Risk Assessment. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of Aging on Adsorption Behavior of Polystyrene Microplastics for Pharmaceuticals: Adsorption Mechanism and Role of Aging Intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A Critical Review on the Interactions of Microplastics with Heavy Metals: Mechanism and Their Combined Effect on Organisms and Humans. Sci. Total. Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled Effects of Microplastics and Heavy Metals on Plants: Uptake, Bioaccumulation, and Environmental Health Perspectives. Sci. Total. Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics Influence the Adsorption and Desorption Characteristics of Cd in an Agricultural Soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological Effects of Microplastics and Cadmium on the Earthworm Eisenia fetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef]
- Wang, H.-T.; Ding, J.; Xiong, C.; Zhu, D.; Li, G.; Jia, X.-Y.; Zhu, Y.-G.; Xue, X.-M. Exposure to Microplastics Lowers Arsenic Accumulation and Alters Gut Bacterial Communities of Earthworm Metaphire californica. Environ. Pollut. 2019, 251, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent Advances on Ecological Effects of Microplastics on Soil Environment. Sci. Total. Environ. 2021, 798, 149338. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.; Wang, X.; Yang, X.; Zhang, C.; Qi, Y.; Guo, X. The Occurrence and Distribution Characteristics of Microplastics in the Agricultural Soils of Shaanxi Province, in North-Western China. Sci. Total. Environ. 2020, 720, 137525. [Google Scholar] [CrossRef]
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia Fetida/Eisenia Andrei); OECD: Paris, France, 2016. [Google Scholar] [CrossRef]
- Dongqin, L. Effect Mechanisms of Fe and Dom on Cd Lability in High Heavymetal Background Soil under Water Regulations; South China Agriculture University: Guangzhou, China, 2018. [Google Scholar]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics Change Soil Properties, Heavy Metal Availability and Bacterial Community in a Pb-Zn-Contaminated Soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yin, L.; Zhou, Z.; Kang, Z.; Sun, Q.; Zhang, Y.; Long, Y.; Nie, X.; Wu, Z.; Jiang, C. Microplastics Can Affect Soil Properties and Chemical Speciation of Metals in Yellow-Brown Soil. Ecotoxicol. Environ. Saf. 2022, 243, 113958. [Google Scholar] [CrossRef]
- Li, B.; Zhu, H.; Sun, H.; Xu, J. Effects of the Amendment of Biochars and Carbon Nanotubes on the Bioavailability of Hexabromocyclododecanes (HBCDs) in Soil to Ecologically Different Species of Earthworms. Environ. Pollut. 2017, 222, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of Polyester Microfibers on Soil Physical Properties: Perception from a Field and a Pot Experiment. Sci. Total. Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Polyethylene Microplastics Increase Cadmium Uptake in Lettuce (Lactuca sativa L.) by Altering the Soil Microenvironment. Sci. Total. Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; Machado, A.A.D. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of Soil Dissolved Organic Matter to Microplastic Addition in Chinese Loess Soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Huang, C.; Ge, Y.; Yue, S.; Zhao, L.; Qiao, Y. Microplastics Aggravate the Joint Toxicity to Earthworm Eisenia fetida with Cadmium by Altering Its Availability. Sci. Total. Environ. 2021, 753, 142042. [Google Scholar] [CrossRef]
- Peng, L.; Liu, P.; Feng, X.; Wang, Z.; Cheng, T.; Liang, Y.; Lin, Z.; Shi, Z. Kinetics of Heavy Metal Adsorption and Desorption in Soil: Developing a Unified Model Based on Chemical Speciation. Geochim. Cosmochim. Acta 2018, 224, 282–300. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Xu, G.; Wang, Y.; Yu, Y. Impacts of Polyethylene Microplastics on Bioavailability and Toxicity of Metals in Soil. Sci. Total. Environ. 2021, 760, 144037. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of Microplastics on the Accumulation and Chronic Toxic Effects of Cadmium in Zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Jager, T.; Gudmundsdottir, E.M.; Cedergreen, N. Dynamic Modeling of Sublethal Mixture Toxicity in the Nematode Caenorhabditis elegans. Environ. Sci. Technol. 2014, 48, 7026–7033. [Google Scholar] [CrossRef]
- Melignani, E.; Faggi, A.M.; de Cabo, L.I. Growth, Accumulation and Uptake of Eichhornia crassipes Exposed to High Cadmium Concentrations. Environ. Sci. Pollut. Res. 2019, 26, 22826–22834. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, E.; Qiu, H.; Zhang, L.; Tang, Y.; Zhao, C.; Li, M.; Xiao, X.; Qiu, R. Do Toxicokinetic and Toxicodynamic Processes Hold the Same for Light and Heavy Rare Earth Elements in Terrestrial Organism Enchytraeus crypticus? Environ. Pollut. 2020, 262, 114234. [Google Scholar] [CrossRef]
- Argasinski, K.; Bednarska, A.; Laskowski, R. The Toxicokinetics Cell Demography Model to Explain Metal Kinetics in Terrestrial Invertebrates. Ecotoxicology 2012, 21, 2186–2194. [Google Scholar] [CrossRef]
- Wang, W.-X.; Fisher, N.S. Modeling Metal Bioavailability for Marine Mussels. In Reviews of Environmental Contamination and Toxicology; Metcalfe, C.D., Bennett, E., Eds.; Springer: New York, NY, USA, 1997; pp. 39–65. [Google Scholar]
- Crommentuijn, T.; Doodeman, C.J.A.M.; Doornekamp, A.; Van Der Pol, J.J.C.; Van Gestel, C.A.M.; Bedaux, J.J.M. Lethal Body Concentrations and Accumulation Patterns Determine Time-Dependent Toxicity of Cadmium in Soil Arthropods. Environ. Toxicol. Chem. 1994, 13, 1781–1789. [Google Scholar] [CrossRef]
- Leonard, S.S.; Harris, G.K.; Shi, X. Metal-Induced Oxidative Stress and Signal Transduction. Free. Radic. Biol. Med. 2004, 37, 1921–1942. [Google Scholar] [CrossRef]
- Fattorini, D.; Regoli, F. Arsenic Speciation in Tissues of the Mediterranean polychaete Sabella spallanzanii. Environ. Toxicol. Chem. 2004, 23, 1881–1887. [Google Scholar] [CrossRef]
- Ghosh, D.; Datta, S.; Bhattacharya, S.; Mazumder, S. Long-Term Exposure to Arsenic Affects Head Kidney and Impairs Humoral Immune Responses of Clarias batrachus. Aquat. Toxicol. 2007, 81, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.G.; Brouwer, T.M.H.; Brouwer, M.; Lopez, G.R. Alterations in Prey Capture and Induction of Metallothioneins in Grass Shrimp Fed Cadmium-Contaminated Prey. Environ. Toxicol. Chem. 2000, 19, 962–971. [Google Scholar] [CrossRef]
| Treatment | ku (L·kg−1·d−1) | ke (d−1) | |
|---|---|---|---|
| Cd | Cd-20 | 31.1 (±4.0) | 0.57 (±0.043) * |
| Cd-20+MPs | 48.7 (±3.5) * | 0.49 (±0.037) | |
| Cd-40 | 28.2 (±8.3) | 0.26 (±0.062) | |
| Cd-40+MPs | 44.2 (±6.3) * | 0.34 (±0.14) | |
| Cd-60 | 23.5 (±6.2) | 0.20 (±0.094) | |
| Cd-60+MPs | 25.5 (±3.0) | 0.26 (±0.075) | |
| As | As-10 | 14.2 (±0.7) | 0.44 (±0.19) |
| As-10+MPs | 15.1 (±2.1) | 0.49 (±0.17) | |
| As-20 | 8.9 (±5.3) | 0.23 (±0.33) | |
| As-20+MPs | 9.7 (±0.056) | 0.088 (±0.003) | |
| As-40 | 16.0 (±1.3) | 0.21 (±0.024) | |
| As-40+MPs | 19.3 (±1.5) * | 0.27 (±0.061) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Li, J.-L.; Rao, W.-L.; Zhao, C.-M.; He, E.-K.; Tang, Y.-T.; Chen, H.-Y.; Qiu, R.-L. Effect of Microplastics on the Bioavailability of (Semi-)Metals in the Soil Earthworm Eisenia fetida. Agronomy 2025, 15, 1052. https://doi.org/10.3390/agronomy15051052
Xiao X, Li J-L, Rao W-L, Zhao C-M, He E-K, Tang Y-T, Chen H-Y, Qiu R-L. Effect of Microplastics on the Bioavailability of (Semi-)Metals in the Soil Earthworm Eisenia fetida. Agronomy. 2025; 15(5):1052. https://doi.org/10.3390/agronomy15051052
Chicago/Turabian StyleXiao, Xue, Jia-Ling Li, Wan-Li Rao, Chun-Mei Zhao, Er-Kai He, Ye-Tao Tang, Hua-Yi Chen, and Rong-Liang Qiu. 2025. "Effect of Microplastics on the Bioavailability of (Semi-)Metals in the Soil Earthworm Eisenia fetida" Agronomy 15, no. 5: 1052. https://doi.org/10.3390/agronomy15051052
APA StyleXiao, X., Li, J.-L., Rao, W.-L., Zhao, C.-M., He, E.-K., Tang, Y.-T., Chen, H.-Y., & Qiu, R.-L. (2025). Effect of Microplastics on the Bioavailability of (Semi-)Metals in the Soil Earthworm Eisenia fetida. Agronomy, 15(5), 1052. https://doi.org/10.3390/agronomy15051052







