Effects of Different Cultivation Substrates on the Growth of Podocarpus macrophyllus and the Rhizosphere Soil Microbial Community Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Experimental Methods
2.3. High-Throughput Sequencing of Rhizosphere Soil Microorganisms
2.4. Data Analysis
3. Results
3.1. Analysis of Basic Physicochemical Properties of Different Cultivation Substrates
3.2. Effects of Different Cultivation Substrates on the Growth Indicators of P. macrophyllus
3.3. Effects of Different Cultivation Substrates on the Physiological Indicators of P. macrophyllus
3.4. Effects of Different Cultivation Substrates on Rhizosphere Bacteria of P. macrophyllus
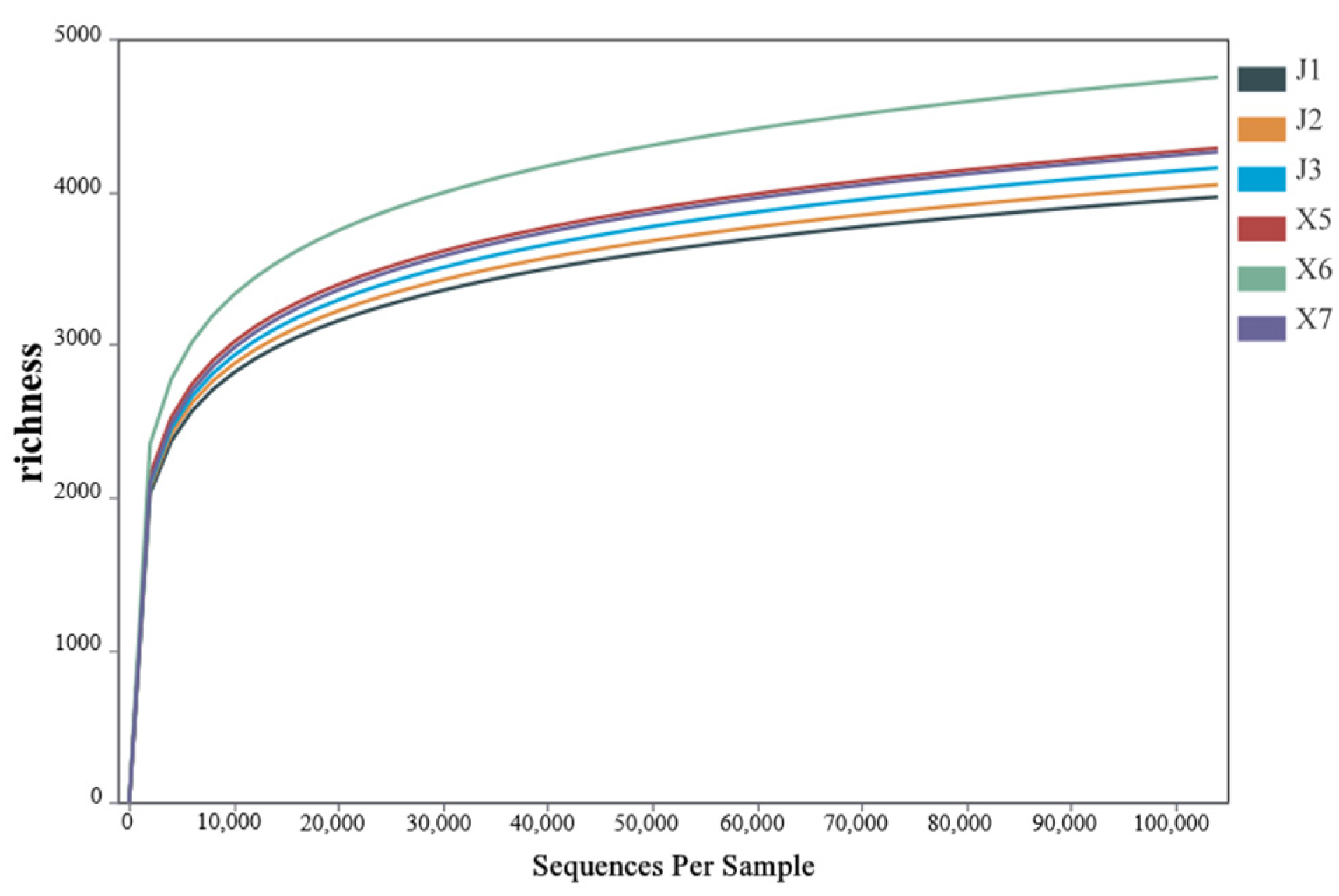
3.4.1. Rarefaction Curve Analysis of Rhizosphere Bacteria of Podocarpus macrophyllus Under Different Cultivation Substrates
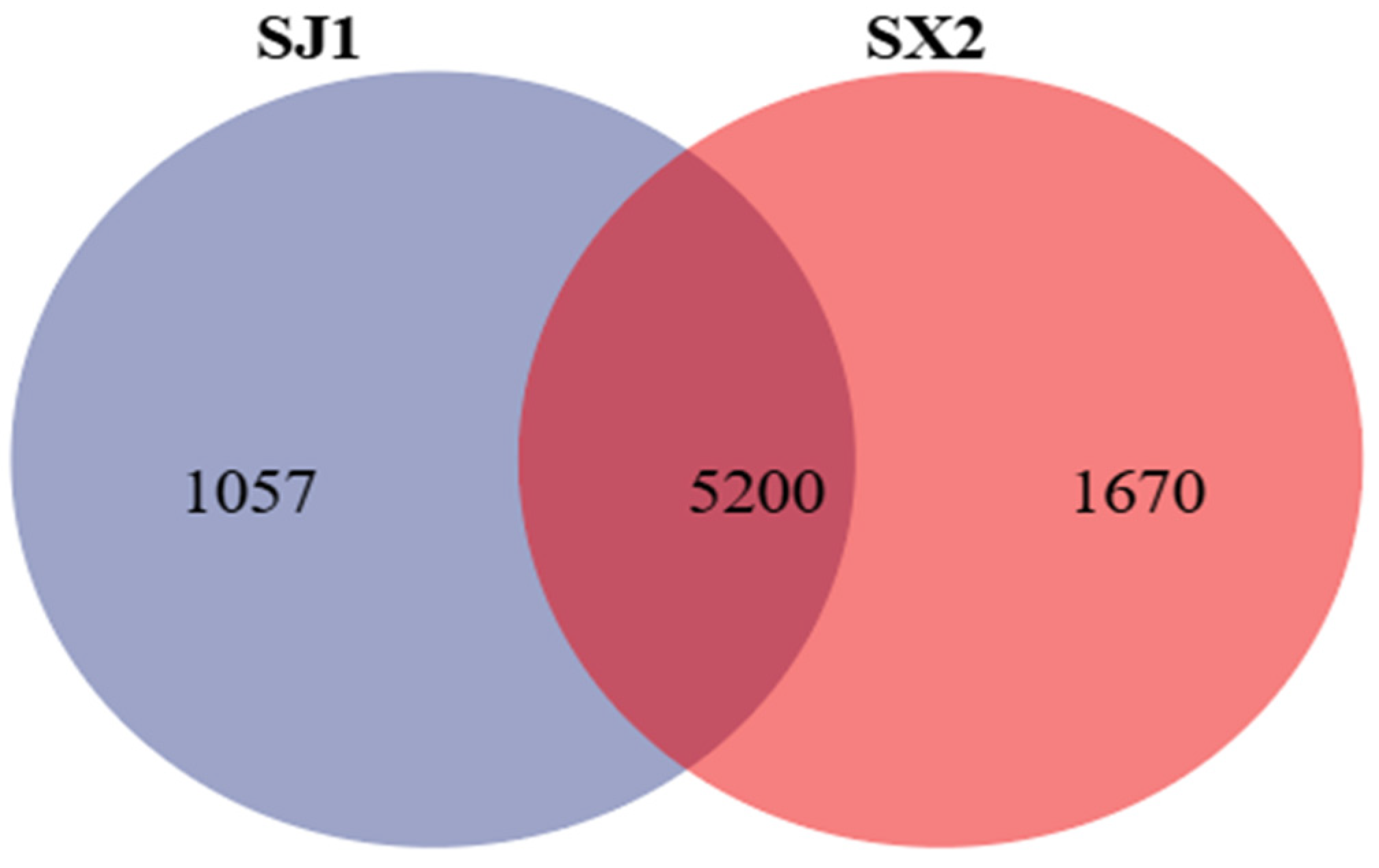
3.4.2. Venn Analysis of Rhizosphere Bacteria of P. macrophyllus Under Different Cultivation Substrates
3.4.3. Alpha Diversity Index of Rhizosphere Bacteria of P. macrophyllus Under Different Cultivation Substrates
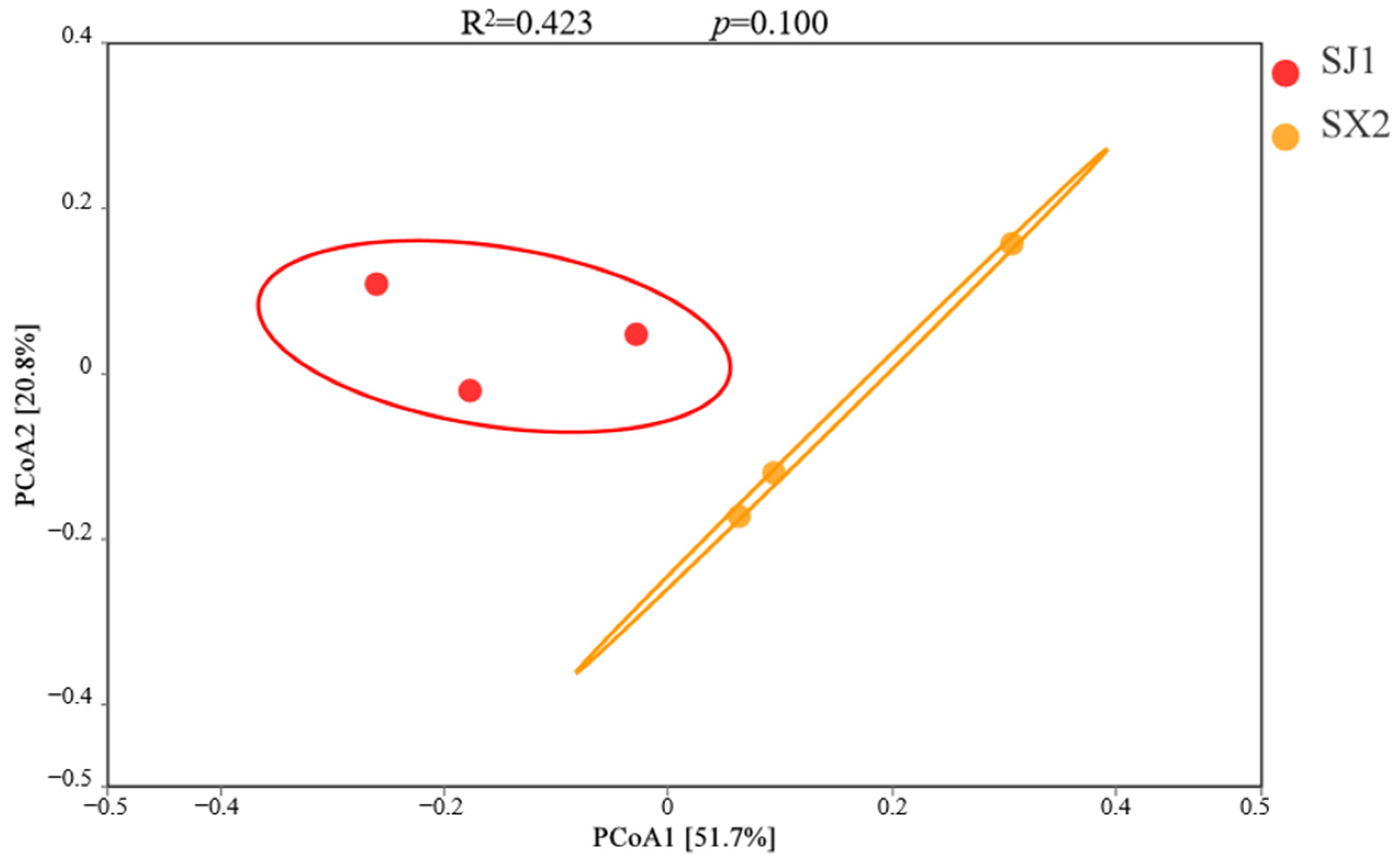
3.4.4. Beta Diversity of Rhizosphere Bacteria in P. macrophyllus Under Different Cultivation Substrates
3.4.5. Analysis of Rhizosphere Bacterial Community Composition of P. macrophyllus Under Different Cultivation Substrates
3.4.6. Effects of Environmental Factors on the Structure of Rhizosphere Bacterial Communities Under Different Cultivation Substrates
3.5. Effects of Different Cultivation Substrates on Rhizosphere Fungi of P. macrophyllus
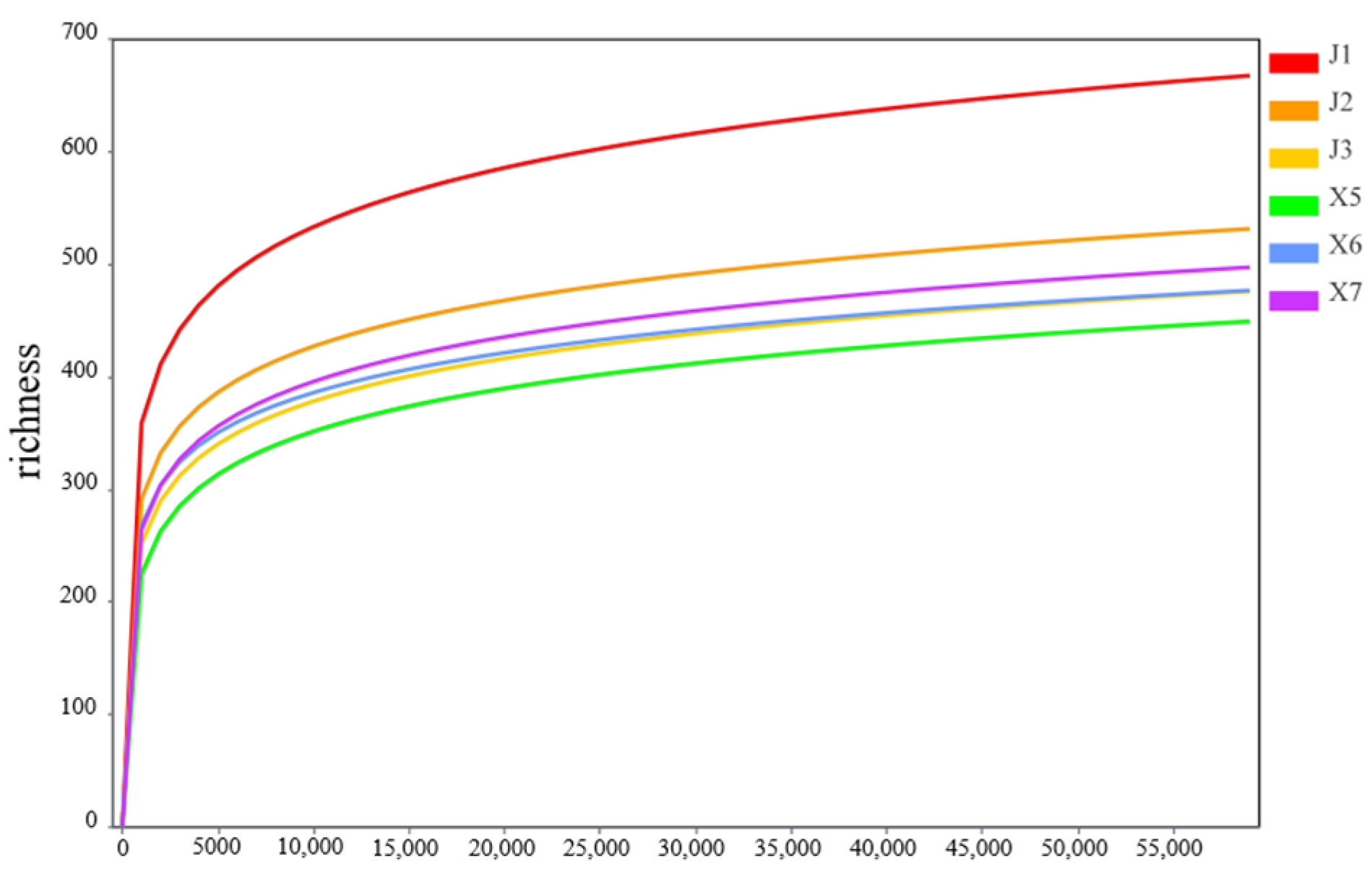
3.5.1. Rarefaction Curve Analysis of Rhizosphere Fungi of Podocarpus macrophyllus Under Different Cultivation Substrates
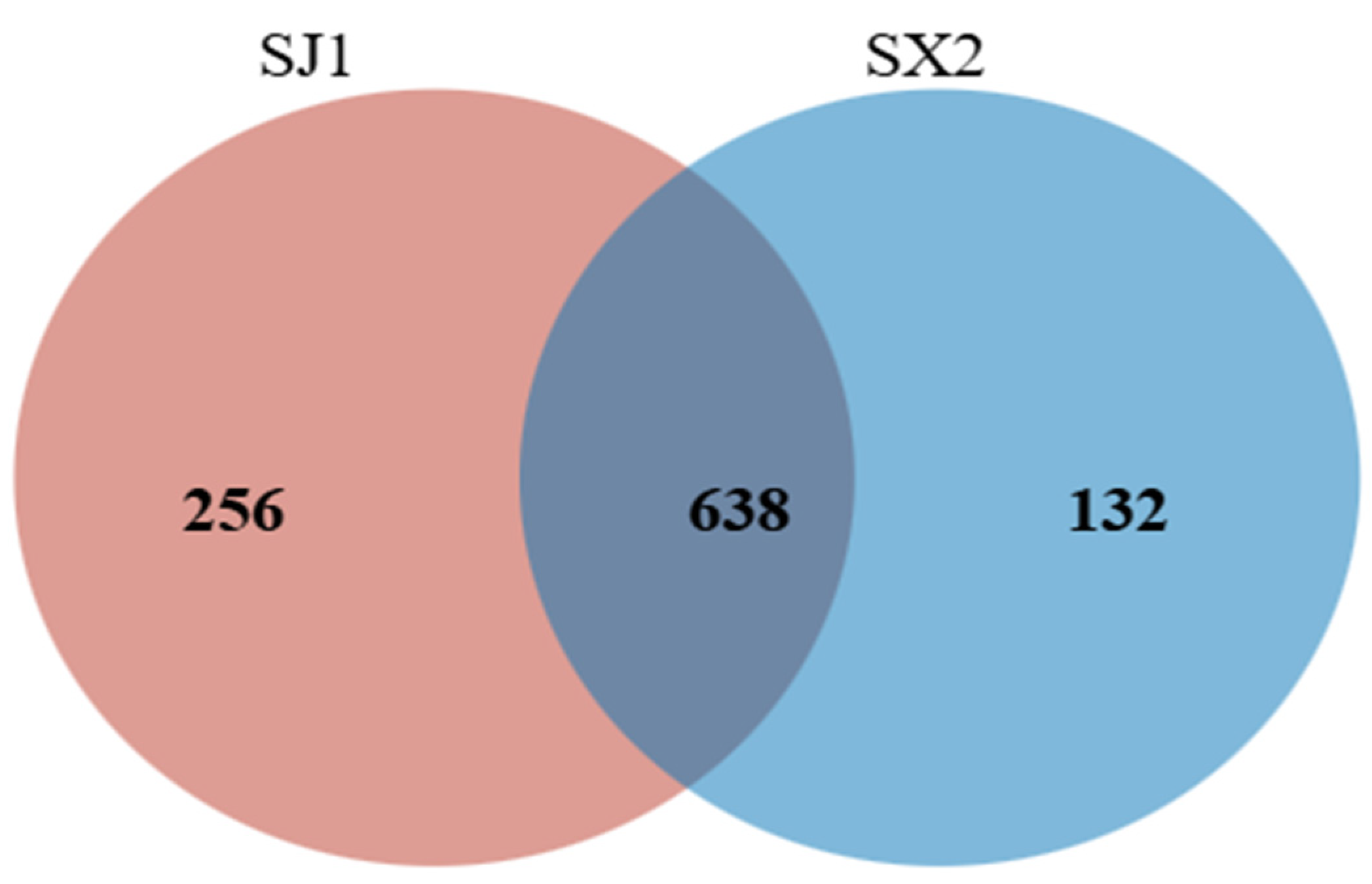
3.5.2. Venn Analysis of Rhizosphere Fungi of P. macrophyllus Under Different Cultivation Substrates
3.5.3. Alpha Diversity Index of Rhizosphere Fungi of P. macrophyllus Under Different Cultivation Substrates
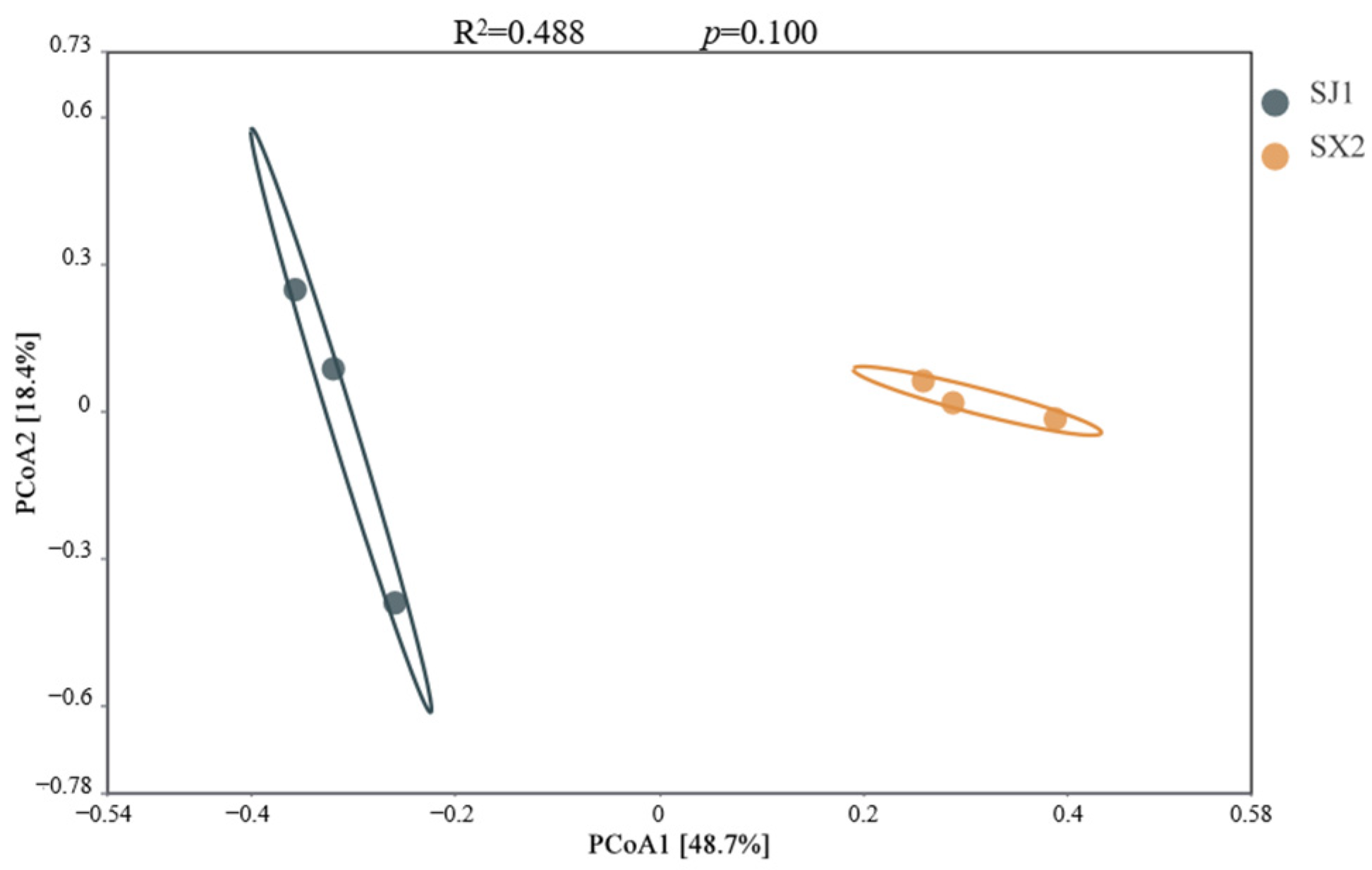
3.5.4. Beta Diversity of Rhizosphere Fungi in P. macrophyllus Under Different Cultivation Substrates
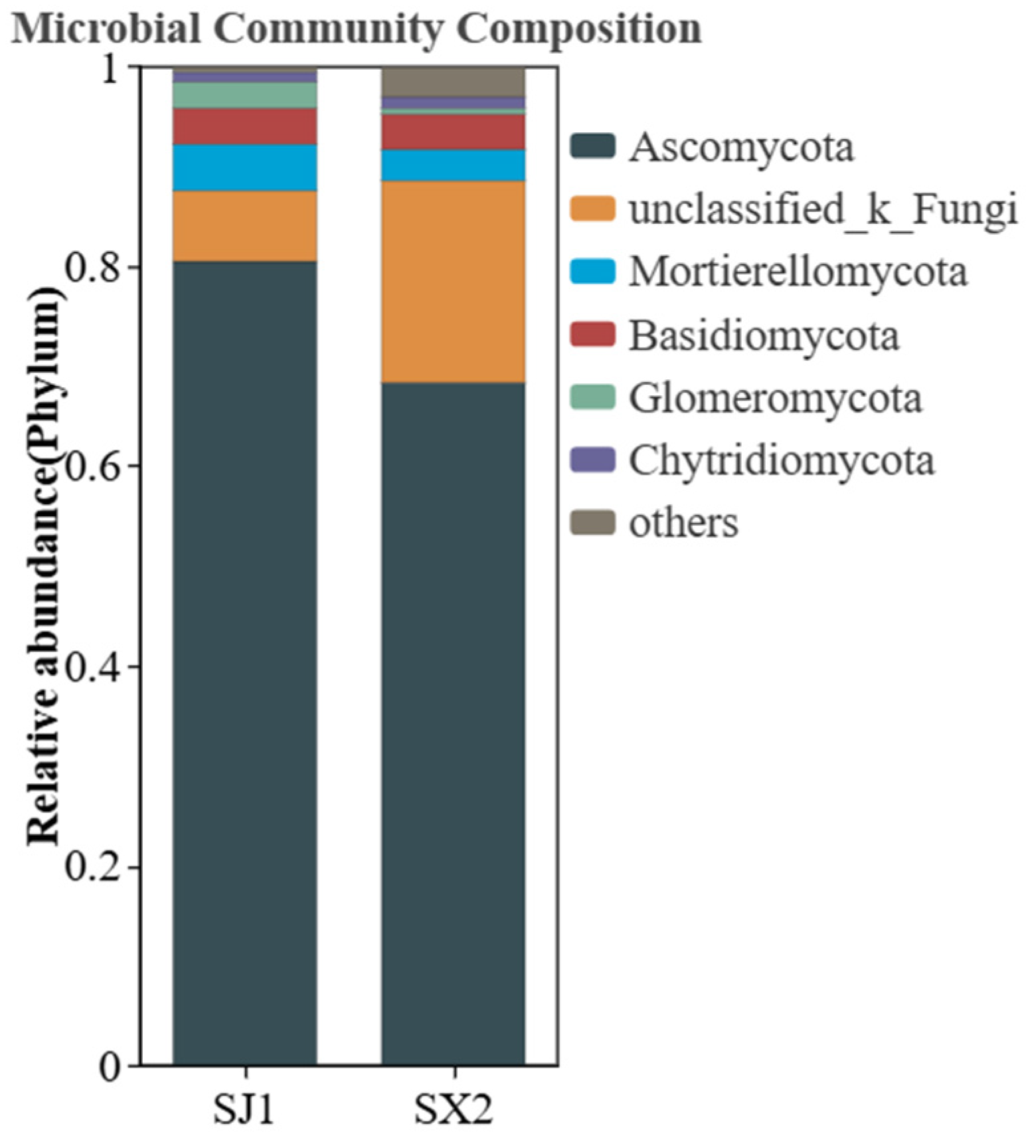
3.5.5. Analysis of the Rhizosphere Fungal Community Composition of Podocarpus macrophyllus Under Different Cultivation Substrates
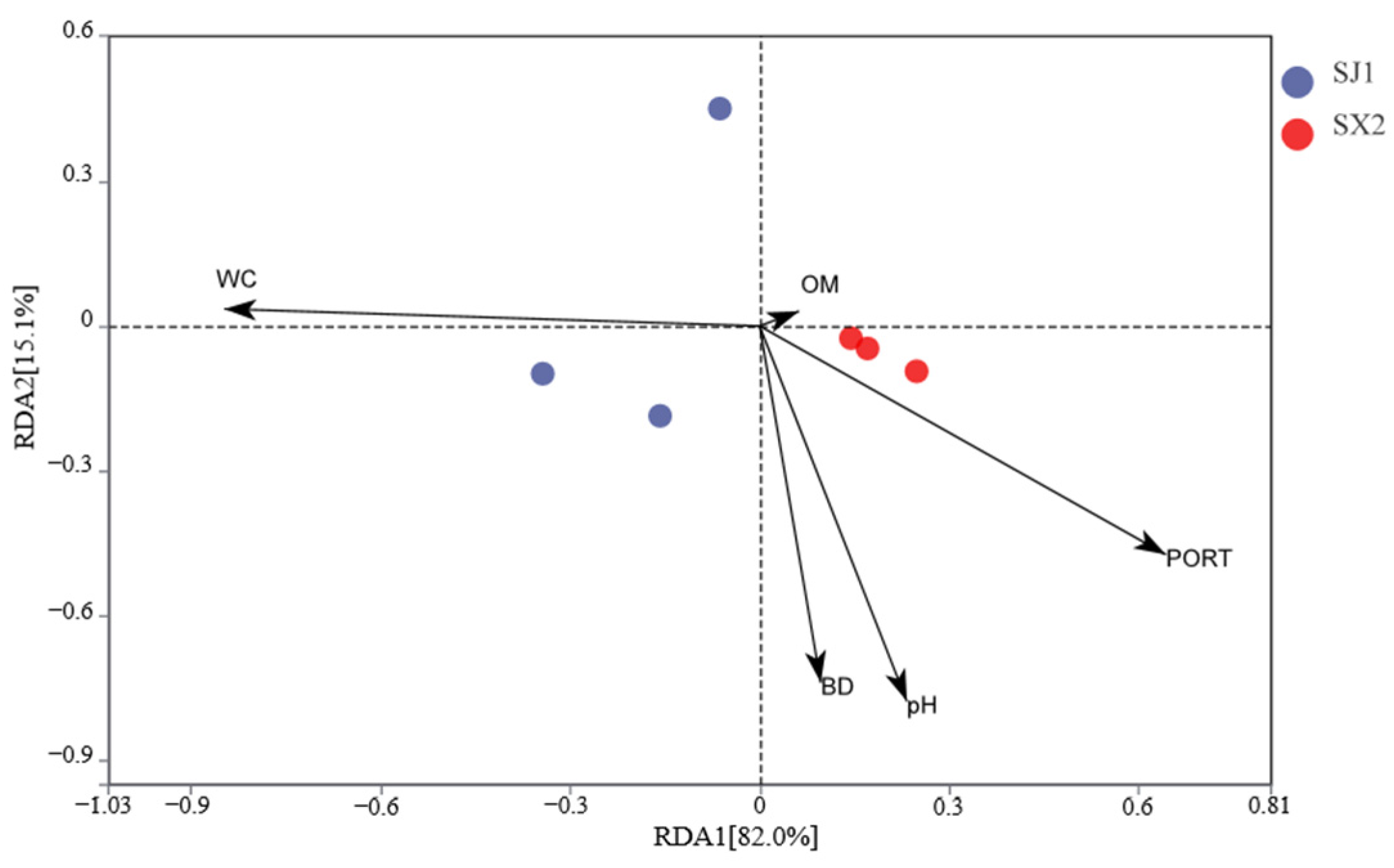
3.5.6. Effects of Environmental Factors on the Structure of Rhizosphere Fungal Communities Under Different Cultivation Substrates
4. Discussion
4.1. Physicochemical Properties of Different Cultivation Substrates
4.2. Effects of Different Cultivation Substrates on the Physicochemical Properties of P. macrophyllus
4.3. Effects of Different Cultivation Substrates on the Rhizosphere Microbial Community Structure of P. macrophyllus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Syahfrina, A.R.M.; AlThiabat, M.G.; Nogawa, T.; Futamura, Y.; Okano, A.; Wahab, H.A. Naturally Occurring 8ß, 13ß-kaur-15-en-17-al and Anti-Malarial Activity from Podocarpus polystachyus Leaves. Pharmaceuticals 2022, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.R.; Zhang, M.J.; Chen, F.M. Occurrence of Pestalotiopsis lushanensis causing leaf blight on Buddhist pine in China. Eur. J. Plant Pathol. 2022, 162, 655–665. [Google Scholar] [CrossRef]
- Nabil, Z.M.; Lamis, S.; Samia, S.; Ashraf, S.A.E. Phytochemical and metabolic profiling of the different Podocarpus species in Egypt: Potential antimicrobial and antiproliferative activities. Heliyon 2023, 9, e20034. [Google Scholar]
- Deng, Z.; Sheng, F.Y.; Yang, S.Y.; Liu, Y.; Zou, L.; Zhang, L.-L. A comprehensive review on the medicinal usage of Podocarpus species: Phytochemistry and pharmacology. J. Ethnopharmacol. 2023, 310, 116401. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Huang, X.L.; Zhu, L.Q.; Su, X.Y.; Wei, L.W.; Wang, R.D.; Zhao, L.J. Effects of different fertilizer ratios on the growth of Podocarpus macrophyllus seedlings. Guangxi For. Sci. 2018, 47, 285–289. [Google Scholar]
- Li, Y.C.; Yang, Y.; He, J.L.; Guo, S.; An, X.J.; Li, Y.; Guo, R.; Lin, Y.P.; Zhang, R.P. Effects of different water and fertilizer treatments on the matrix properties and plant growth of tailings waste. Sci. Rep. 2025, 15, 3231. [Google Scholar] [CrossRef]
- Qiu, M.H.; Zhang, R.F.; Xue, C.; Zhang, S.S.; Li, S.Q.; Zhang, N.; Shen, Q.R. Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol. Fertil. Soils 2012, 48, 807–816. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, S.F.; Wang, L.L.; Shi, J.X.; Zhao, J.; Shen, B.; Shen, Q.R. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol. Fertil. Soils 2014, 50, 961–971. [Google Scholar] [CrossRef]
- Davide, B.; Klaus, S.; Stijn, S.; Emiel, V.L.V.T.; Paul, S.L. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Buragienė, S.; Lekavičienė, K.; Adamavičienė, A.; Vaiciukevičius, E.; Šarauskis, E. The Influence of an Innovative Bioproduct on Soil and Substrate Characteristics during Strawberry Cultivation. Agriculture 2024, 14, 537. [Google Scholar] [CrossRef]
- Asri, F.O.; Sonmez, S. Reflection of Different Application of Potassium and Iron Fertilization on Tomato Yield and Fruit Quality in Soilless Medium. J. Food Agric. Environ. 2010, 8, 426–429. [Google Scholar]
- Qu, P.; Cao, Y.; Wu, G.F.; Tang, Y.X.; Xia, L.R. Preparation and properties of coir-based substrate bonded by modified urea formaldehyde resins for seedlings. Bioresources 2018, 13, 4332–4345. [Google Scholar] [CrossRef]
- Khalil, S.; Panda, P.; Ghadamgahi, F.; Barreiro, A.; Rosberg, A.K.; Karlsson, M.; Vetukuri, R.R. Microbial potential of spent mushroom compost and oyster substrate in horticulture: Diversity, function, and sustainable plant growth solutions. J. Environ. Manag. 2024, 357, 120654. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.N.; Lima, L.K.S.; Aguiar, F.S.; De Jesus, O.N. Cost reduction and quality enhancement in passion fruit seedlings using alternative substrate. Semin. Cienc. Agrar. 2021, 42, 1549–1565. [Google Scholar] [CrossRef]
- Thepnamdit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Anzaline, A.; Mosca, A.; DImaria, G.; Nicotra, D.; Tessitoti, M.; Privitera, G.F.; Pulvirenti, A.; Leonardi, C.; Catara, V. Soil and Soilless Tomato Cultivation Promote Different Microbial Communities That Provide New Models for Future Crop Interventions. Int. J. Mol. Sci. 2022, 23, 8820. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Du, Y.Q.; Zhang, D.; Tang, Z.H. Nutrients Regulate the Effects of Arbuscular Mycorrhizal Fungi on the Growth and Reproduction of Cherry Tomato. Front. Microbiol. 2022, 13, 843010. [Google Scholar] [CrossRef]
- Geng, X.M.; Zhu, Z.L.; Li, M.; Luo, F.X.; Ding, Y.F. Research Progress on Cutting Propagation of Rhododendron. Chin. Wild Plant Resour. 2011, 30, 1–6. [Google Scholar]
- Xu, L.; Zhang, X.; Zhang, D.H.; Wei, H.X.; Guo, J. Using morphological attributes for the fast assessment of nutritional responses of Buddhist pine (Podocarpus macrophyllus [Thunb.] D. Don) seedlings to exponential fertilization. PLoS ONE 2019, 14, e0225708. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.Z. Effects of different light qualities and substrates on photosynthesis of Podocarpus nagi. For. Prospect. Des. 2019, 39, 70–72. [Google Scholar]
- Jing, S.M.; Lin, Z.D.; Huang, X.M.; Chen, J.F.; Peng, D.H. Study on Substrate Selection for Soilless Culture of Podocarpus macrophyllus Seedlings. Mod. Agric. Equip. 2020, 41, 54–60. [Google Scholar]
- Quan, J.H.; Zhao, L.J.; Zhu, L.Q.; Qiu, G.L. Physiological Responses of Podocarpus macrophyllus Seedlings to Nitrogen Addition under Different Phosphorus Conditions. Chin. J. Trop. Crops 2023, 44, 1817–1828. [Google Scholar]
- Lin, T.; Zhao, L.J.; Zhu, L.Q.; Huang, X.L.; Wei, G.Y. Effects of Nitrogen, Phosphorus, and Potassium Addition on the Functional Diversity of Soil Microorganisms in Podocarpus macrophyllus. Guihaia 2024, 44, 895–906. [Google Scholar]
- Zhang, C.L.; Li, X.J.; Xie, C.; Chen, Z.H.; Lu, Y.F.; Wang, B.T.; Ge, Y. Effects of Different Slope Aspects on the Physicochemical Properties and Microbial Communities of Rhizosphere Soil in Coffea arabica. J. South. Agric. 2023, 54, 3527–3537. [Google Scholar]
- Fu, S.; Lei, T.; Jin, W.; Li, Y.; Chen, X.J.; Chen, M.L.; Chen, L. Effects of salt stress on growth and physiological indexes of tomato seedlings. J. Hubei Norm. Univ. Nat. Sci. 2023, 43, 9–15. [Google Scholar]
- Li, Y.M.; Zheng, Y.J.; Liu, H.C.; Zhang, Y.T.; Gao, Y.W.; Song, S.W.; Lei, B.F. Effect of supplemental blue light intensity on the growth and quality of Chinese kale. Hortic. Environ. Biotechnol. 2019, 60, 49–57. [Google Scholar] [CrossRef]
- Sweety, J. A Comparative Study of Oven Drying Method and Microstrip Moisture Sensor for Determination of Moisture Content of Soil and Analysis of Bulking of Sand Phenomenon. Wirel. Pers. Commun. 2024, 139, 1659–1667. [Google Scholar]
- Chen, H.X.; Du, Z.L.; Guo, W.; Zhang, Q.Z. Effects of biochar application on soil bulk density, cation exchange capacity and particulate organic matter content in farmland of North China Plain. Chin. J. Appl. Ecol. 2011, 22, 930–2934. [Google Scholar]
- Zhu, X.A.; Liu, W.J.; Jiang, X.J.; Wang, P.Y.; Li, W.X. Effects of land-use changes on runoff and sediment yield: Implications for soil conservation and forest management in Xishuangbanna, Southwest China. Land Degrad. Dev. 2018, 29, 2962–2974. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed; China Agriculture Press: Beijing, China, 2000; pp. 25–114. [Google Scholar]
- Kontaxakis, E.; Papadimitriou, D.; Daliakopoulos, I.; Sabathianakis, I.; Stavropoulou, A.; Manios, T. Water Availability in Pumice, Coir, and Perlite Substrates Regulates Grapevine Growth and Grape Physicochemical Characteristics in Soilless Cultivation of Sugraone and Prime Cultivars (Vitis vinifera L.). Agriculture 2023, 13, 1690. [Google Scholar] [CrossRef]
- Jankauskiene, J.; Lauzike, K.; Kavaliauskaite, D. Effects of Vermicompost on Quality and Physiological Parameters of Cucumber (Cucumis sativus L.) Seedlings and Plant Productivity. Horticulturae 2022, 8, 1009. [Google Scholar] [CrossRef]
- Wang, G.B.; Liu, B.H.; Henderson, M.; Zhang, Y.; Zhang, Z.; Chen, M.Y.; Guo, H.X.; Huang, W.W. Effect of Terracing on Soil Moisture of Slope Farmland in Northeast China’s Black Soil Region. Agriculture 2023, 13, 1876. [Google Scholar] [CrossRef]
- Tran, T.; Thi, Q.V.C.; Duong, V.D.; Trung, L.D.; Trang, N.T.Q.; Hong, L.T.A. Optimizing the C/N ratio in composting fresh coconut husk and shell waste for organic fertilizer production. IOP Conf. Ser. Earth Environ. Sci. 2024, 1419, 012019. [Google Scholar] [CrossRef]
- Tan, S.M.; Wang, B.; Yun, Q.; Yan, W.R.; Xiao, T.B.; Zhao, Z.X. Enhancing the Growth of Chili Plants and Soil Health: Synergistic Effects of Coconut Shell Biochar and Bacillus sp. Strain Ya-1 on Rhizosphere Microecology and Plant Metabolism. Int. J. Mol. Sci. 2024, 25, 11231. [Google Scholar] [CrossRef]
- Zhu, Y.; Duan, Y.J.; Liu, Z.G.; Liu, M.J.; Liu, P. Growth and Physiological Characteristics of Sour Jujube Seedlings in Different Substrate Formulations. Agronomy 2023, 13, 1797. [Google Scholar] [CrossRef]
- Yang, B.H.; Li, W.J.; Li, W.Z.; Zhang, Y.J.; Liu, M.H.; Peng, J.; Xiao, X.C. Study on container seedling technology of Alniphyllum fortunei leaves. J. Sichuan For. Sci. Technol. 2025, 46, 137–143. [Google Scholar]
- Luan, M.D.; Tang, R.J.; Tang, Y.M.; Tian, W.; Hou, C.G.; Zhao, F.G.; Lan, W.Z.; Luan, S. Transport and homeostasis of potassium and phosphate: Limiting factors for sustainable crop production. J. Exp. Bot. 2017, 68, 3091–3105. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, X.; Zhang, X.W.; Wan, L.; Wang, Z.H. Effects of biochar application on soil nitrogen and phosphorous leaching loss and oil peony growth. Agric. Water Manag. 2021, 255, 107022. [Google Scholar] [CrossRef]
- Erro, J.; Urrutia, O.; Baigorri, R.; García Mina, J.M. The primary role of phosphate-metal-natural organic matter (humic) ternary complexes as drivers of long-term phosphorus plant nutrition in acidic soils. Environ. Res. 2025, 274, 121261. [Google Scholar] [CrossRef]
- Wu, D.; Lu, Y.A.; Liang, H.W.; Ma, L.T.; Huang, T.W.; Li, R.R. Differences in humic acid structure extracted from different types of peat. Int. J. Coal Prep. Util. 2024, 1–16. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.; Ahabala, S.; Zhou, M.X.; Zhao, C.C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Shah, I.H.; Wu, J.; Li, X.Y.; Hameed, M.K.; Manzoor, M.A.; Li, P.L.; Zhang, Y.D.; Niu, Q.L.; Chang, L.Y. Exploring the role of nitrogen and potassium in photosynthesis implications for sugar: Accumulation and translocation in horticultural crops. Sci. Hortic. 2024, 327, 112832. [Google Scholar] [CrossRef]
- Chi, Z.F.; Wang, W.J.; Li, H.; Wu, H.T.; Yan, B.X. Soil organic matter and salinity as critical factors affecting the bacterial community and function of Phragmites australis dominated riparian and coastal wetlands. Sci. Total Environ. 2020, 762, 143156. [Google Scholar] [CrossRef] [PubMed]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The Role of Peat-Free Organic Substrates in the Sustainable Management of Soilless Cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Murphy, V.; Moore, K.; Griffith, M.P.; Husby, C. Improving Conservation through Cultivation: Nine Container Substrates Influence Growth of a Rare Cycad, Zamia pumila L. HortScience 2013, 48, 1168–1172. [Google Scholar] [CrossRef]
- Ye, W.; Li, Y.C.; Yu, W.W.; Ye, X.M.; Qian, Y.T.; Dai, W.S. Microbial biodiversity in rhizospheric soil of Torreya grandis ‘Merrillii’ relative to cultivation history. J. Appl. Ecol. 2018, 29, 3783–3792. [Google Scholar]
- Zhou, X.; Xiao, C.D.; Zhang, B.W.; Yang, X.F. Depth-dependent response of soil microbial community and greenhouse gas efflux to polylactic acid microplastics and tidal cycles in a mangrove ecosystem. J. Hazard. Mater. 2025, 489, 137664. [Google Scholar] [CrossRef]
- Boss, B.L.; Wanees, A.E.; Zaslow, S.J.; Normile, T.G.; Izquierdo, J.A. Comparative genomics of the plant-growth promoting bacterium Sphingobium sp. strain AEW4 isolated from the rhizosphere of the beachgrass Ammophila breviligulata. BMC Genom. 2022, 23, 508. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, B.X.; Li, T.T.; Lin, H.; Lin, Z.X.; Lu, G.D.; Liu, Y.L.; Lin, B.S.; Lin, D.M. Isolation of rhizobacteria from the Cenchrus fungigraminus rhizosphere and characterization of their nitrogen-fixing performance and potential role in plant growth promotion. Plant Soil. 2023, 489, 405–421. [Google Scholar] [CrossRef]
- Jiao, J.L.; Shi, R.; Wang, Y.G.; Wang, H.L.; Zhang, A.; Yan, X.R.; Wang, S.; He, X.H. Study on the differences of metabolites in ginseng rhizosphere soil under different cultivation modes. Chin. Agric. Sci. Bull. 2024, 40, 46–53. [Google Scholar]
- Liu, H.H.; Yu, Q.G.; Wang, H.; Li, L.P.; Zhang, M.; Li, X.; Zhang, Z.F. Soil fungal community structure and functional groups under different moisture gradients in Bitahai Wetland, Southwest China. Acta Microbiol. Sin. 2022, 62, 3007–3023. [Google Scholar]
- Chen, Q.; Hui, C.M.; Tao, H.; Qin, W.L.; Wang, Y.M.; Xiao, Z.K.; Liu, J.L.; Liu, W.Y. Soil fungal community structure and functional diversity of sweet bamboo (Dendrocalamus latiflorus) from different geographical origins. Soil 2024, 56, 1294–1303. [Google Scholar]
- Tan, W.D.; Shen, M.J.; Qiu, H.J.; Zeng, F.L.; Huang, J.H.; Huang, R.S.; Luo, W.G.; Liu, Y.X. Effects of different phosphorus application rates on arbuscular mycorrhizal formation, growth, and artemisinin production in Artemisia annua. J. South. Agric. 2013, 44, 1303–1307. [Google Scholar]



| Sequencing Type | Primer Name | Primer Sequences | Sequencing Target Role |
|---|---|---|---|
| 16S | 338F | 5′-ACTCCTACGGGAGGCAGCA-3′ | Combined with the conserved region of the 16S gene, amplification of the V3 region was initiated. |
| 806R | 5′-GGACTACHVGGGTWTCTAAT-3′ | Combined with the conserved sequence of the V4 region, reverse amplification was performed. | |
| ITS | ITS3-F | 5′-GCATCGATGAAGAACGCAGC-3′ | Combined with the starting end of the ITS2 region, amplification was initiated. |
| ITS4-R | 5′-TCCTCCGCTTATTGATATGC-3′ | Reverse amplification of the end of the ITS2 region, paired with ITS3, to ensure highly specific amplification. |
| Substrates | pH | WC (%) | BD (g/cm3) | PORT (%) | OM (g/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) | AN (g/kg) | AP (g/kg) | AK (g/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SJ1 | 7.07 ±0.09 | 22.04 ±2.18 | 1.10 ±0.08 | 36.77 ±3.25 | 29.56 ±0.35 | 1.57 ±0.10 | 1.08 ±0.06 | 18.88 ±0.32 | 93.33 ±1.17 | 74.48 ±1.07 | 70.55 ±3.26 |
| SX2 | 7.17 ±0.3 | 7.28 ±1.04 | 1.16 ±0.06 | 55.74 ±2.34 | 31.90 ±8.43 | 1.07 ±0.20 | 0.73 ±0.09 | 16.12 ±0.37 | 82.83 ±4.21 | 90.67 ±2.65 | 143.45 ±20.41 |
| p | 0.349 | 0.004 | 0.596 | 0.009 | 0.808 | 0.091 | 0.032 | 0.005 | 0.074 | 0.005 | 0.024 |
| Significance | ns | ** | ns | ** | ns | ns | * | ** | ns | ** | * |
| Treatment | Ground Diameter (mm) | Plant Height (cm) |
|---|---|---|
| SJ1 | 22.52 ± 1.20 | 104.33 ± 4.84 |
| SX2 | 29.67 ± 0.74 | 175.69 ± 3.41 |
| p | 0.007 | <0.001 |
| Significance | ** | *** |
| Substrates | Chl (mg/g) | SS (mg/g) | SP (mg/g) | C (g/kg) | N (g/kg) | P (g/kg) | K (g/kg) |
|---|---|---|---|---|---|---|---|
| SJ1 | 10.93 ± 2.06 | 3.31 ± 0.35 | 1.10 ± 0.04 | 849.26 ± 9.62 | 17.37 ± 1.41 | 2.08 ± 0.15 | 10.87 ± 0.75 |
| SX2 | 18.04 ± 1.35 | 2.79 ± 0.08 | 1.33 ± 0.06 | 867.99 ± 10.92 | 18.36 ± 1.56 | 1.97 ± 0.15 | 7.55 ± 1.13 |
| p | 0.041 | 0.225 | 0.045 | 0.267 | 0.664 | 0.621 | 0.071 |
| Significance | * | ns | * | ns | ns | ns | ns |
| Treatment | Chao1 | ACE | Simpson | Shannon_2 | Goods_Coverage |
|---|---|---|---|---|---|
| SJ1 | 4320.53 ± 82.09 | 5054.37 ± 86.47 | 0.0034 ± 0.0002 | 9.79 ± 0.03 | 0.9882 ± 0.0070 |
| SX2 | 4842.00 ± 186.81 | 5526.88 ± 160.60 | 0.0036 ± 0.0006 | 9.85 ± 0.14 | 0.9901 ± 0.0073 |
| P | 0.063 | 0.703 | 0.061 | 0.698 | 0.128 |
| Significance | ns | ns | ns | ns | ns |
| Treatment | Chao1 | ACE | Simpson | Shannon_2 | Goods_Coverage |
|---|---|---|---|---|---|
| SJ1 | 571.40 ± 57.99 | 632.27 ± 49.10 | 0.0297 ± 0.0031 | 6.43 ± 0.03 | 0.9983 ± 0.0002 |
| SX2 | 496.70 ± 11.03 | 572.17 ± 8.46 | 0.0557 ± 0.0239 | 5.84 ± 0.39 | 0.9984 ± 0.0001 |
| p | 0.269 | 0.854 | 0.294 | 0.272 | 0.854 |
| Significance | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Zhong, D.; Zhang, C.; Pan, Y.; Zhang, C.; Guo, H.; Zhu, X.; Li, X.; He, Y.; Huang, S.; et al. Effects of Different Cultivation Substrates on the Growth of Podocarpus macrophyllus and the Rhizosphere Soil Microbial Community Structure. Agronomy 2025, 15, 1055. https://doi.org/10.3390/agronomy15051055
Liang X, Zhong D, Zhang C, Pan Y, Zhang C, Guo H, Zhu X, Li X, He Y, Huang S, et al. Effects of Different Cultivation Substrates on the Growth of Podocarpus macrophyllus and the Rhizosphere Soil Microbial Community Structure. Agronomy. 2025; 15(5):1055. https://doi.org/10.3390/agronomy15051055
Chicago/Turabian StyleLiang, Xiaomin, Donghua Zhong, Congyu Zhang, Yongfang Pan, Chenning Zhang, Herong Guo, Xiaoling Zhu, Xiaocong Li, Yuxuan He, Shaopeng Huang, and et al. 2025. "Effects of Different Cultivation Substrates on the Growth of Podocarpus macrophyllus and the Rhizosphere Soil Microbial Community Structure" Agronomy 15, no. 5: 1055. https://doi.org/10.3390/agronomy15051055
APA StyleLiang, X., Zhong, D., Zhang, C., Pan, Y., Zhang, C., Guo, H., Zhu, X., Li, X., He, Y., Huang, S., Tu, J., Gao, T., & Feng, Y. (2025). Effects of Different Cultivation Substrates on the Growth of Podocarpus macrophyllus and the Rhizosphere Soil Microbial Community Structure. Agronomy, 15(5), 1055. https://doi.org/10.3390/agronomy15051055





