Rice-Fish Co-Culture Promotes Soil Carbon Sequestration Through Alterations in Soil Microbial Community Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil Sampling and Measurements
2.3. PLFA Analysis
2.4. Soil Enzyme Assays
2.5. Statistical Analysis
3. Results
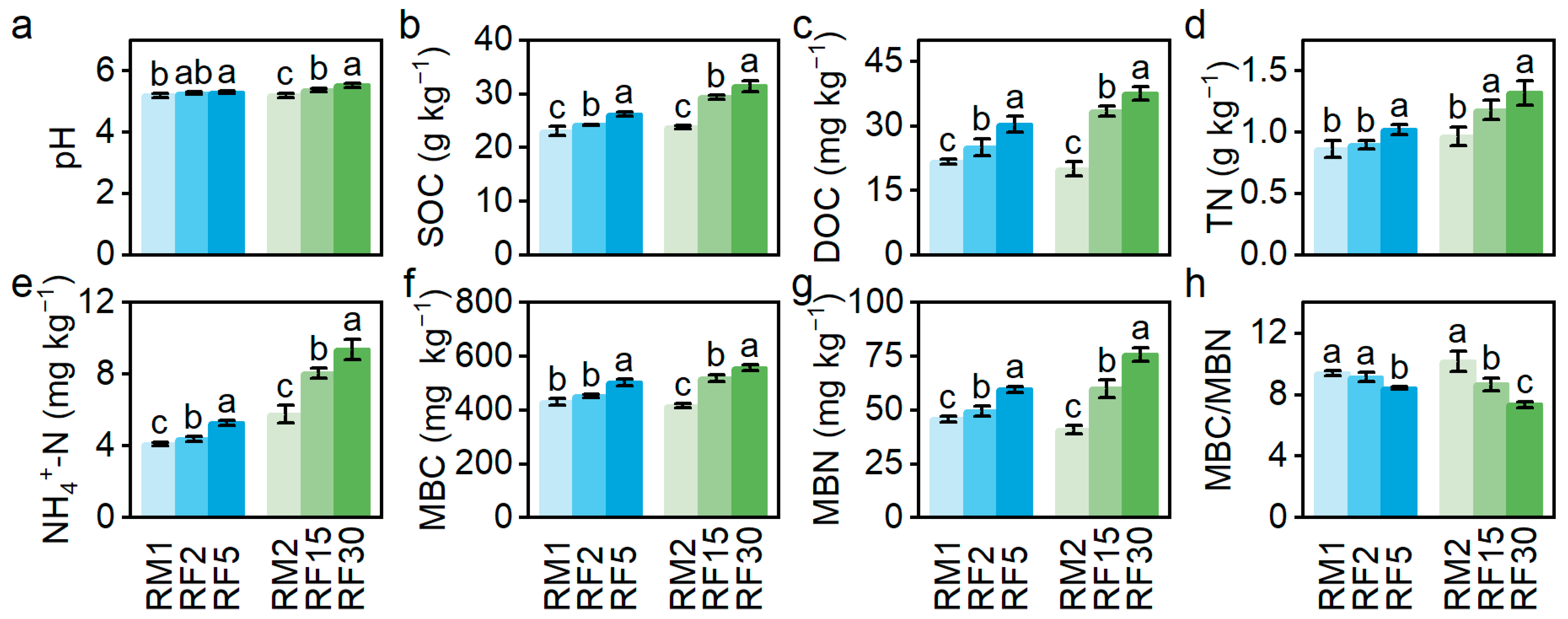
3.1. The Response of Soil Chemical Properties to RF Durations
3.2. The Effects of RF Durations on the Soil Microbial Community Structure
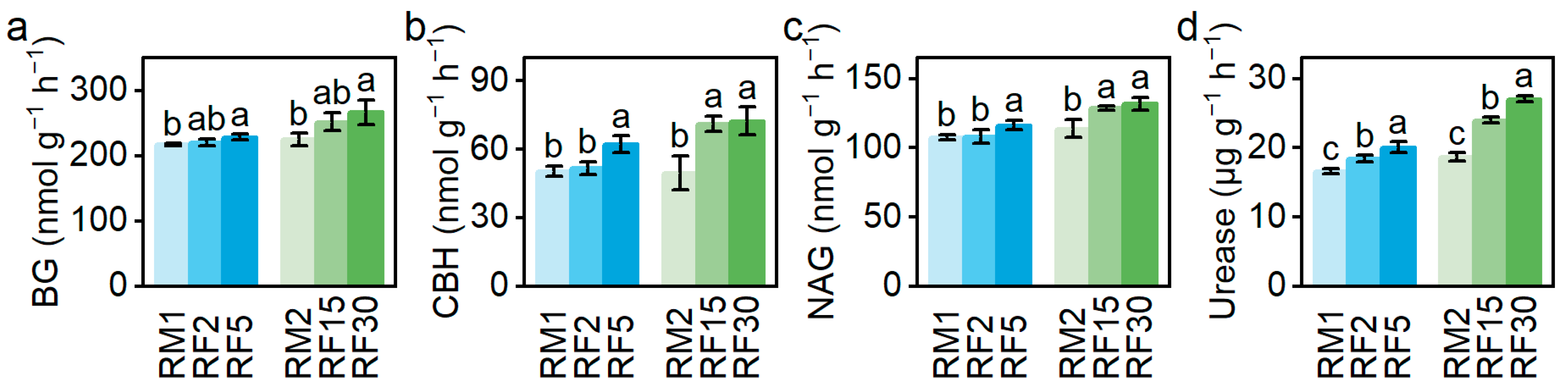
3.3. The Effects of RF Durations on Soil Enzyme Activities
3.4. Relationships of C Sequestration with Microbial Community Under Different RF Durations
4. Discussion
4.1. RF Promotes Soil Fertility
4.2. RF Changes the Soil Microbial Community Structure
4.3. RF Promotes the Soil Enzyme Activities
4.4. RF Promotes the C Sequestration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agricultural Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 February 2024).
- Mishra, A.K.; Pede, V.O.; Arouna, A.; Labarta, R.; Andrade, R.; Veettil, P.C.; Bhandari, H.; Laborte, A.G.; Balie, J.; Bouman, B. Helping feed the world with rice innovations: CGIAR research adoption and socioeconomic impact on farmers. Glob. Food Secur. 2022, 33, 100628. [Google Scholar] [CrossRef]
- Purwanto, B.H.; Alam, S. Impact of intensive agricultural management on carbon and nitrogen dynamics in the humid tropics. Soil Sci. Plant Nutr. 2020, 66, 50–59. [Google Scholar] [CrossRef]
- Chen, Z.K.; Li, X.R.; Liu, T.; Fu, H.; Yuan, X.J.; Cheng, Q.Y.; Liao, Q.; Zhang, Y.; Li, W.; Sun, Y.J.; et al. Strategies for fertilizer management to achieve higher yields and environmental and fertilizer benefits of rice production in China. Sci. Total Environ. 2023, 904, 166325. [Google Scholar] [CrossRef]
- Brooker, R.W.; George, T.S.; Homulle, Z.; Karley, A.J.; Newton, A.C.; Pakeman, R.J.; Schöb, C. Facilitation and biodiversity–ecosystem function relationships in crop production systems and their role in sustainable farming. J. Ecol. 2021, 109, 2054–2067. [Google Scholar] [CrossRef]
- Halwart, M.; Gupta, M.V. Culture of Fish in Rice Fields; FAO: Rome, Italy; WorldFish Center: Penang, Malaysia, 2004. [Google Scholar]
- Surendran, U.; Raja, P.; Jayakumar, M.; Subramoniam, S.R. Use of efficient water saving techniques for production of rice in India under climate change scenario: A critical review. J. Clean. Prod. 2021, 309, 127272. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Hu, L.L.; Zhang, J.; Ren, W.Z.; Guo, L.; Cheng, Y.X.; Li, J.Y.; Li, K.X.; Zhu, Z.W.; Zhang, J.E.; Luo, S.M.; et al. Can the co-cultivation of rice and fish help sustain rice production? Sci. Rep. 2016, 6, 28728. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q.N.; Zhang, C.; Li, X.D.; Chen, J.; Zhang, X.L.; Chen, J.J.; Liu, K.X. Rice-fish-duck system regulation of soil phosphorus fraction conversion and availability through organic carbon and phosphatase activity. Front. Envirion. Sci. 2022, 10, 979234. [Google Scholar] [CrossRef]
- Wan, N.f.; Li, S.x.; Li, T.; Cavalieri, A.; Weiner, J.; Zheng, X.q.; Ji, X.y.; Zhang, J.q.; Zhang, H.l.; Zhang, H.; et al. Ecological intensification of rice production through rice-fish co-culture. J. Clean. Prod. 2019, 234, 1002–1012. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Liptzin, D.; Norris, C.E.; Cappellazzi, S.B.; Bean, G.M.; Cope, M.; Greub, K.L.H.; Rieke, E.L.; Tracy, P.W.; Aberle, E.; Ashworth, A.; et al. An evaluation of carbon indicators of soil health in long-term agricultural experiments. Soil Biol. Biochem. 2022, 172, 108708. [Google Scholar] [CrossRef]
- Zhao, Z.; Chu, C.B.; Zhou, D.P.; Wang, Q.F.; Wu, S.H.; Zheng, X.Q.; Song, K.; Lv, W.G. Soil bacterial community composition in rice-fish integrated farming systems with different planting years. Sci. Rep. 2021, 11, 10855. [Google Scholar] [CrossRef]
- Guo, L.; Hu, L.L.; Zhao, L.F.; Shi, X.Y.; Ji, Z.J.; Ding, L.L.; Ren, W.Z.; Zhang, J.; Tang, J.J.; Chen, X. Coupling Rice with Fish for Sustainable Yields and Soil Fertility in China. Rice Sci. 2020, 27, 175–179. [Google Scholar] [CrossRef]
- Chen, X.Y.; Sun, W.t.; Yu, F.q.; Li, Z.q.; Yu, Y.q.; Sun, F.y. Effect of Rice-Crab Co-Culture System on Soil Fertility and Economic Benefits. Chin. J. Soil Sci. 2021, 52, 1165–1172. [Google Scholar] [CrossRef]
- He, L.y.; Lipson, D.A.; Mazza Rodrigues, J.L.; Mayes, M.; Björk, R.G.; Glaser, B.; Thornton, P.; Xu, X.f. Dynamics of Fungal and Bacterial Biomass Carbon in Natural Ecosystems: Site-Level Applications of the CLM-Microbe Model. J. Adv. Model. Earth Syst. 2021, 13, e2020MS002283. [Google Scholar] [CrossRef]
- Karhu, K.; Alaei, S.; Li, J.; Merilä, P.; Ostonen, I.; Bengtson, P. Microbial carbon use efficiency and priming of soil organic matter mineralization by glucose additions in boreal forest soils with different C:N ratios. Soil Biol. Biochem. 2022, 167, 108615. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Li, P.; Wu, G.G.; Li, Y.J.; Hu, C.; Ge, L.; Zheng, X.Q.; Zhang, J.Q.; Chen, J.; Zhang, H.L.; Bai, N.L.; et al. Long-term rice-crayfish-turtle co-culture maintains high crop yields by improving soil health and increasing soil microbial community stability. Geoderma 2022, 413, 115745. [Google Scholar] [CrossRef]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.-A. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R.; Kitayama, K.; Mo, J. Does the ratio of β-1,4-glucosidase to β-1,4-N-acetylglucosaminidase indicate the relative resource allocation of soil microbes to C and N acquisition? Soil Biol. Biochem. 2021, 160, 108363. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Sinhababu, D.P.; Roy, K.S.; Dash, P.K.; Sahu, P.K.; Dandapat, R.; Neogi, S.; Mohanty, S. Effect of fish species on methane and nitrous oxide emission in relation to soil C, N pools and enzymatic activities in rainfed shallow lowland rice-fish farming system. Agric. Ecosyst. Environ. 2013, 176, 53–62. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; Universuty of Wisconsin-Madison: Madison, WI, USA, 1973. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Wang, J.x.; Lu, X.n.; Zhang, J.e.; Wei, H.; Li, M.j.; Lan, N.; Luo, H. Intercropping perennial aquatic plants with rice improved paddy field soil microbial biomass, biomass carbon and biomass nitrogen to facilitate soil sustainability. Soil Tillage Res. 2021, 208, 104908. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Ma, J.; Zhang, Y.; Li, X.; Pang, D. Aggregate-associated carbon contributes to soil organic carbon accumulation along the elevation gradient of Helan Mountains. Soil Biol. Biochem. 2023, 178, 108926. [Google Scholar] [CrossRef]
- Liu, X.; Sun, D.; Huang, H.; Zhang, J.; Zheng, H.; Jia, Q.; Zhao, M. Rice-fish coculture without phosphorus addition improves paddy soil nitrogen availability by shaping ammonia-oxidizing archaea and bacteria in subtropical regions of South China. Sci. Total Environ. 2024, 927, 171642. [Google Scholar] [CrossRef]
- German, D.P.; Chacon, S.S.; Allison, S.D. Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 2011, 92, 1471–1480. [Google Scholar] [CrossRef]
- Sekaran, U.; Kumar, S.; Luis Gonzalez-Hernandez, J. Integration of crop and livestock enhanced soil biochemical properties and microbial community structure. Geoderma 2021, 381, 114686. [Google Scholar] [CrossRef]
- Ren, L.P.; Liu, P.P.; Xu, F.; Gong, Y.C.; Zhai, X.M.; Zhou, M.; Wang, J.J.; Wang, Z.M. Rice-fish coculture system enhances paddy soil fertility, bacterial network stability and keystone taxa diversity. Agric. Ecosyst. Environ. 2023, 348, 108399. [Google Scholar] [CrossRef]
- Chen, B.; Guo, L.; Tang, J.; Li, Y.; Li, C. Comprehensive impacts of different integrated rice-animal co-culture systems on rice yield, nitrogen fertilizer partial factor productivity and nitrogen losses: A global meta-analysis. Sci. Total Environ. 2024, 915, 169994. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, Z.J.; Zhang, J.E.; Sun, D.L.; Wei, H. Effects of integrated rice-animals co-culture on paddy soil and water properties and rice yield: A meta-analysis. Arch. Agron. Soil Sci. 2022, 69, 2187–2201. [Google Scholar] [CrossRef]
- Oehme, M.; Frei, M.; Razzak, M.A.; Dewan, S.; Becker, K. Studies on nitrogen cycling under different nitrogen inputs in integrated rice-fish culture in Bangladesh. Nutr. Cycl. Agroecosyst. 2007, 79, 181–191. [Google Scholar] [CrossRef]
- Pan, L.; Jiang, Z.; Zhang, W.; Zhou, J.; Liu, J.; Cai, Y.; Li, Y. Effects of straw and its biochar application on soil ammonia-oxidizing microorganisms and N cycling related enzyme activities in a Phyllostachys edulis forest. J. Zhejiang AF Univ. 2024, 41, 1–11. [Google Scholar] [CrossRef]
- Wang, A.; Dai, D.; Ma, X.; Mou, Q.; Yu, Y.; Lü, W. Effects of rice-crab culture on nitrogen leaching in rice fields in the north of China. J. Zhejiang Univ. (Agric. Life Sci.) 2019, 45, 332–342. [Google Scholar] [CrossRef]
- Yu, Q.G.; Hu, X.; Ma, J.W.; Ye, J.; Sun, W.C.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Xu, M.G.; Lou, Y.L.; Sun, X.L.; Wang, W.; Baniyamuddin, M.; Zhao, K. Soil organic carbon active fractions as early indicators for total carbon change under straw incorporation. Biol. Fertil. Soils 2011, 47, 745–752. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.Y.F.; Wang, C.; Pan, T.; Zeng, C. Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field. Soil Tillage Res. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Wang, F.L.; Bettany, J.R. Influence of Freeze-Thaw and Flooding on the Loss of Soluble Organic Carbon and Carbon Dioxide from Soil. J. Environ. Qual. 1993, 22, 709–714. [Google Scholar] [CrossRef]
- Li, W.T.; Kuzyakov, Y.; Zheng, Y.L.; Liu, M.; Wu, M.; Dong, Y.H.; Li, Z.P. Effect of long-term fertilisation on enzyme activities and microbial community composition in the rice rhizosphere. Acta Agric. Scand. Sect. B 2022, 72, 454–462. [Google Scholar] [CrossRef]
- Qaswar, M.; Jing, H.; Ahmed, W.; Abbas, M.; Li, D.C.; Khan, Z.H.; Gao, J.S.; Liu, S.J.; Zhang, H.M. Linkages between ecoenzymatic stoichiometry and microbial community structure under long-term fertilization in paddy soil: A case study in China. Appl. Soil Ecol. 2021, 161, 103860. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Tuo, D.F.; Wang, C.; Fu, B.J.; Lv, Y.H.; Liu, G.H. Land use change and stand age regulate soil respiration by influencing soil substrate supply and microbial community. Geoderma 2020, 359, 113991. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.L.; Xu, W.H.; Li, W.; Dai, W.W.; Jiang, P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef]
- Kerfahi, D.; Tripathi, B.M.; Slik, J.W.F.; Sukri, R.S.; Jaafar, S.; Adams, J.M. Distinctive Soil Archaeal Communities in Different Variants of Tropical Equatorial Forest. Microb. Ecol. 2018, 76, 215–225. [Google Scholar] [CrossRef]
- Yu, C.Q.; Han, F.S.; Fu, G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Sci. Total Environ. 2019, 655, 814–822. [Google Scholar] [CrossRef]
- Hale, L.; Hendratna, A.; Scott, N.; Gao, S. Biochar enhancement of nitrification processes varies with soil conditions. Sci. Total Environ. 2023, 887, 164146. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, W.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; He, P. Distribution of soil nutrients, extracellular enzyme activities and microbial communities across particle-size fractions in a long-term fertilizer experiment. Appl. Soil Ecol. 2015, 94, 59–71. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Denef, K.; Roobroeck, D.; Manimel Wadu, M.C.W.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar] [CrossRef]
- Si, G.; Peng, C.L.; Yuan, J.F.; Xu, X.Y.; Zhao, S.J.; Xu, D.B.; Wu, J.S. Changes in soil microbial community composition and organic carbon fractions in an integrated rice-crayfish farming system in subtropical China. Sci. Rep. 2017, 7, 2856. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xing, J.; Brookes, P.C.; Xu, J. Soil available phosphorus content drives the spatial distribution of archaeal communities along elevation in acidic terrace paddy soils. Sci. Total Environ. 2019, 658, 723–731. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Chen, S.; Li, J.; Sun, R.; Liu, B.; Hu, C. Response of Nitrifier and Denitrifier Abundance and Microbial Community Structure to Experimental Warming in an Agricultural Ecosystem. Front. Microbiol. 2018, 9, 474. [Google Scholar] [CrossRef]
- Potthast, K.; Hamer, U.; Makeschin, F. Land-use change in a tropical mountain rainforest region of southern Ecuador affects soil microorganisms and nutrient cycling. Biogeochemistry 2012, 111, 151–167. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Ramos-Zapata, J.A.; Marrufo-Zapata, D.; Guadarrama, P.; Carrillo-Sanchez, L.; Hernandez-Cuevas, L.; Caamal-Maldonado, A. Impact of weed control on arbuscular mycorrhizal fungi in a tropical agroecosystem: A long-term experiment. Mycorrhiza 2012, 22, 653–661. [Google Scholar] [CrossRef]
- Zheng, W.; Gong, Q.L.; Zhao, Z.Y.; Liu, J.S.; Zhai, B.N.; Wang, Z.H.; Li, Z.Y. Changes in the soil bacterial community structure and enzyme activities after intercrop mulch with cover crop for eight years in an orchard. Eur. J. Soil Biol. 2018, 86, 34–41. [Google Scholar] [CrossRef]
- Bihari, P.; Nayak, A.K.; Gautam, P.; Lal, B.; Shahid, M.; Raja, R.; Tripathi, R.; Bhattacharyya, P.; Panda, B.B.; Mohanty, S.; et al. Long-term effect of rice-based farming systems on soil health. Environ. Monit. Assess. 2015, 187, 296. [Google Scholar] [CrossRef]
- Bell, J.M.; Robinson, C.A.; Schwartz, R.C. Changes in soil properties and enzymatic activities following manure applications to a rangeland. Rangel. Ecol. Manag. 2006, 59, 314–320. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.; Nannipieri, P.; Kästner, M.; Schmidt, B.; Botterweck, J. From humic substances to soil organic matter–microbial contributions. In honour of Konrad Haider and James P. Martin for their outstanding research contribution to soil science. J. Soils Sediments 2015, 15, 1865–1881. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Khatri-Chhetri, U.; Thompson, K.A.; Quideau, S.A.; Boyce, M.S.; Chang, S.X.; Kaliaskar, D.; Bork, E.W.; Carlyle, C.N. Adaptive multi-paddock grazing increases soil nutrient availability and bacteria to fungi ratio in grassland soils. Appl. Soil Ecol. 2022, 179, 104590. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Srinivasa Rao, C.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Chapter One—Soil organic carbon dynamics: Impact of land use changes and management practices: A review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 156, pp. 1–107. [Google Scholar]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Glaser, B.; Turrión, M.a.-B.; Alef, K. Amino sugars and muramic acid—Biomarkers for soil microbial community structure analysis. Soil Biol. Biochem. 2004, 36, 399–407. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Schroeder, J.; Dămătîrcă, C.; Bölscher, T.; Chenu, C.; Elsgaard, L.; Tebbe, C.C.; Skadell, L.; Poeplau, C. Liming effects on microbial carbon use efficiency and its potential consequences for soil organic carbon stocks. Soil Biol. Biochem. 2024, 191, 109342. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Ruan, H. Increased microbial carbon and nitrogen use efficiencies under drought stress in a poplar plantation. For. Ecol. Manag. 2022, 519, 120341. [Google Scholar] [CrossRef]
- Ullah, M.R.; Carrillo, Y.; Dijkstra, F.A. Drought-induced and seasonal variation in carbon use efficiency is associated with fungi:bacteria ratio and enzyme production in a grassland ecosystem. Soil Biol. Biochem. 2021, 155, 108159. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Zheng, H.; Shi, Z.; Zhang, J.; Jia, Q.; Liu, X.; Zhao, M.; Chen, Y.; Chen, Q.; Luo, M. Rice-Fish Co-Culture Promotes Soil Carbon Sequestration Through Alterations in Soil Microbial Community Structure. Agronomy 2025, 15, 1054. https://doi.org/10.3390/agronomy15051054
Sun D, Zheng H, Shi Z, Zhang J, Jia Q, Liu X, Zhao M, Chen Y, Chen Q, Luo M. Rice-Fish Co-Culture Promotes Soil Carbon Sequestration Through Alterations in Soil Microbial Community Structure. Agronomy. 2025; 15(5):1054. https://doi.org/10.3390/agronomy15051054
Chicago/Turabian StyleSun, Daolin, Hongjun Zheng, Zhaoji Shi, Jiaen Zhang, Qi Jia, Xing Liu, Min Zhao, Yuting Chen, Qi Chen, and Mingzhu Luo. 2025. "Rice-Fish Co-Culture Promotes Soil Carbon Sequestration Through Alterations in Soil Microbial Community Structure" Agronomy 15, no. 5: 1054. https://doi.org/10.3390/agronomy15051054
APA StyleSun, D., Zheng, H., Shi, Z., Zhang, J., Jia, Q., Liu, X., Zhao, M., Chen, Y., Chen, Q., & Luo, M. (2025). Rice-Fish Co-Culture Promotes Soil Carbon Sequestration Through Alterations in Soil Microbial Community Structure. Agronomy, 15(5), 1054. https://doi.org/10.3390/agronomy15051054






