Impact of Biochar on Organic Contaminants in Soil: A Tool for Mitigating Risk?
Abstract
:1. Introduction
2. Behaviour of Organic Contaminants in Soil

2.1. Impact of Soil Contact Time on Contaminant Mobility and Biota
2.1.1. Definitions of Bioavailability, Bioaccessibility and Chemical Activity
2.2. Current Uses of Biochar
2.3. Biochar Production
| Process | Liquid (bio-oil) | Solid (biochar) | Gas (syngas) |
|---|---|---|---|
| Fast pyrolysis | 75 | 12 | 13 |
| Temperature at 500 °C | |||
| Short vapour residence time (seconds) | |||
| Intermediate pyrolysis | 50 | 25 | 25 |
| Low moderate temperature | |||
| Moderate vapour residence time (hours) | |||
| Slow pyrolysis | 30 | 35 | 35 |
| Low moderate temperature (400-600 °C) | |||
| Long vapour residence time (days) | |||
| Gasification | 5 | 10 | 85 |
| High temperature >800 °C | |||
| Long vapour residence time |
| Feedstock | Temperature (°C) | Residence time | BET N2 Surface area (m2 g−1) | Total pore volume (cm3 g−1) | Ash content (%) |
|---|---|---|---|---|---|
| Orange peel | 250 | 6.0 h | 33.3 | 0.0202 | 1.05 |
| 500 | 6.0 h | 42.4 | 0.0191 | 4.27 | |
| 700 | 6.0 h | 201.0 | 0.0350 | 2.79 | |
| Switch grass | 500 | 1.5 s | 21.6 | n/a | 54.60 |
| Corn stover | 500 | 1.5 s | 7.0 | n/a | 49.70 |
| Switch grass | 500 | 2.0 h | 50.2 | n/a | 52.50 |
| Corn stover | 500 | 2.0 h | 20.9 | n/a | 32.40 |
| P. sylvestris | 300 | 1.0 h | 1.0 | 0.0017 | n/a |
| 500 | 1.0 h | 320.0 | 0.1860 | n/a | |
| B. pendula | 300 | 1.0 h | 2.3 | 0.0035 | n/a |
| 500 | 1.0 h | 6.5 | 0.0068 | n/a | |
| 700 | 1.0 h | 430.0 | 0.2530 | n/a | |
| 820 | 1.0 h | 66.0 | 0.0600 |
3. Interactions between Biochar and Soil
3.1. Biochar Adsorption and Stability

3.2. Fate and Behaviour of Organic Contaminants within Biochar-Amended Soils
3.2.1. Sorption of Organic Contaminants
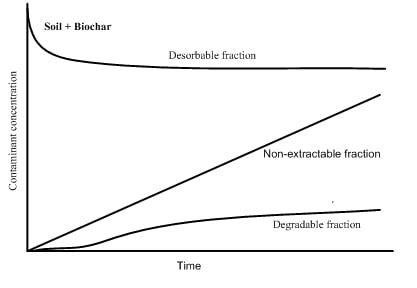
3.2.2. Desorption Mechanism Studies
3.2.3. Biodegradation


3.3. Application of Biochar in Remediation of Contaminated Soil
4. Conclusions
Acknowledgments
References
- United States Environmental Protection Agency. Available online: http://www.epa.gov/osw/hazard/wastemin/priority.htm (accessed on 28 July 2010).
- Mielke, H.W.; Wang, G.; Gonzales, C.R.; Powell, E.T.; Le, B.; Quach, V.N. PAHs and metals in soils of inner-city and suburban New Orleans, Louisiana, USA. Environ. Toxicol. Pharmaco. 2004, 18, 243–247. [Google Scholar] [CrossRef]
- Roy, S.; Labelle, S.; Mehta, P.; Mihoc, A.; Fortin, N.; Masson, C.; Leblanc, R.; Châteauneuf, G.; Sura, C.; Gallipeau, C.; Olsen, C.; Delisle, S.; Labrecque, M.; Greer, C.W. Phytoremediation of heavy metal and PAH-contaminated brownfield sites. Plant Soil 2005, 272, 277–290. [Google Scholar] [CrossRef]
- Chen, J. Rapid urbanization in China: A real challenge to soil protection and food security. Catena 2007, 69, 1–15. [Google Scholar] [CrossRef]
- Environment Act 1995 Part II A contaminated land. Section 57. Available online: http://www.legislation.gov.uk/ukpga/1995/25/section/57 (accessed on 22 March 2011).
- United Nations Economic Commission for Europe (UNECE). Protocol on Persistent Organic Pollutants under the 1979 Convention on Long-Range Transboundary Air Pollution. UNECE (ECB/EB Air/60). 1998. Available online: http://www.unece.org/fileadmin/DAM/env/lrtap/full%20text/1998.POPs.e.pdf (accessed on 12 April 2011).
- Regitano, J.B.; Koskinen, W.C.; Sadowsky, M.J. Influence of soil aging on sorption and bioavailability of simazine. J. Agric. Food Chem. 2006, 54, 1373–1379. [Google Scholar] [CrossRef]
- Dimitrov, S.; Nedelcheva, D.; Dimitrova, N.; Mekenyan, O. Development of a biodegradation model for the prediction of metabolites in soil. Sci. Total Environ. 2010, 408, 3811–3816. [Google Scholar] [CrossRef]
- Pollard, S.J.T.; Hough, R.L.; Kim, K.H.; Bellarby, J.; Paton, G.; Semple, K.T.; Coulon, F. Fugacity modelling to predict the distribution of organic contaminants in the soil:oil matrix of constructed biopiles. Chemosphere. 2008, 71, 1432–1439. [Google Scholar] [CrossRef]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Cornelissen, G.; Gustafsson, O.; Bucheli, T.D.; Jonker, M.T.O.; Koelmans, A.A.; van Noort, P.C.M. Critical review: Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005, 39, 6881–6895. [Google Scholar] [CrossRef]
- Rhodes, A.H.; McAllister, L.E.; Chen, R.; Semple, K.T. Impact of activated charcoal on the mineralization of 14C-phenanthrene in soils. Chemosphere 2010a, 79, 463–469. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications and current challenges. Global Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Rhodes, A.H.; Carlin, A.; Semple, K.T. Impact of black carbon in the extraction and mineralization of phenanthrene in soil. Environ. Sci. Technol. 2008a, 42, 740–745. [Google Scholar] [CrossRef]
- Sundelin, B.; Wiklund, A.K.E.; Lithner, G.; Gustafsson, O. Evaluation of the role of black carbon in attenuating bioaccumulation of polycyclic aromatic hydrocarbons from field-contaminated sediments. Environ. Toxicol. Chem. 2004, 23, 2611–2617. [Google Scholar] [CrossRef]
- Amonette, J.E.; Kim, J.; Russell, C.K.; Palumbo, A.V.; Daniels, W.L. Enhancement of soil carbon sequestration by amendment with fly ash. In Proceedings of International Ash Utilization Symposium, Organised by University of Kentucky Center for Applied Energy Research, The Lexington Center’s Heritage Hall and the Hyatt Regency Lexington, Lexington, KY, USA, 20–22 October 2003.
- Stroud, J.L.; Paton, G.I.; Semple, K.T. Importance of chemical structure on the development of hydrocarbon catabolism in soil. FEMS Microbiol. Lett. 2007, 272, 120–126. [Google Scholar] [CrossRef]
- Posada-Baquero, R.; Ortega-Calvo, J.J. Recalcitrance of polycyclic aromatic hydrocarbons in soil contributes to background pollution. Environ. Pollut. 2011, 159, 3692–3699. [Google Scholar] [CrossRef]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- 2006 was earth’s fifth warmest year. National Aeronautics and Space Administration. Available online: www.nasa.gov/centers/goddard/news/topstory/2006/2006_warm.html (accessed on 22 June 2010).
- Hildebrandt, A.; Larcorte, S.; Barceló, D. Occurrence and fate of organochlorinated pesticides and PAH in agricultural soils from the Ebro River Basin. Arch. Environ. Contam. Toxicol. 2009, 57, 247–255. [Google Scholar] [CrossRef]
- Northcott, G.L.; Jones, K.C. Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment. Environ. Pollut. 2000, 108, 19–43. [Google Scholar] [CrossRef]
- Bamforth, S.M.; Singleton, I. Review: Bioremediation of polycyclic aromatic hydrocarbons: Current knowledge and future directions. J. Chem. Technol. Biotechnol. 2005, 80, 723–736. [Google Scholar] [CrossRef]
- Rhodes, A.H.; Hofman, J.; Semple, K.T. Development of phenanthrene catabolism in natural and artificial soils. Environ. Pollut. 2008, 152, 424–430. [Google Scholar] [CrossRef]
- Stokes, J.D.; Paton, G.I.; Semple, K.T. Behaviour and assessment of bioavailability of organic contaminants in soil: Relevance for risk assessment and remediation. Soil Use Manage. 2005, 21, 475–486. [Google Scholar] [CrossRef]
- Rhodes, A.H.; Dew, N.M.; Semple, K.T. Relationship between cyclodextrin extraction and biodegradation of phenanthrene in soil. Environ. Toxicol. Chem. 2008, 27, 1488–1495. [Google Scholar] [CrossRef]
- Van Noort, P.C.M.; Cornelissen, G.; ten Hulscher, T.E.M.; Vrind, B.A.; Rigterink, H.; Belfroid, A. Slow and very slow desorption of organic compounds from sediment: Influence of sorbate planarity. Water Res. 2003, 37, 2317–2322. [Google Scholar]
- Kim, I.S.; Park, J.S.; Kim, K.W. Enhanced biodegradation of polycyclic aromatic hydrocarbons using nonionic surfactants in soil slurry. Appl. Geochem. 2001, 16, 1419–1428. [Google Scholar] [CrossRef]
- Semple, K.T.; Doick, K.J.; Wick, L.Y.; Harms, H. Review: Microbial interactions with organic contaminants in soil: Definitions, processes and measurement. Environ. Pollut. 2007, 150, 166–176. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. General Introduction and Sorption Processes Involving Organic Matter. In Environmental Organic Chemistry; John Wiley and Sons Inc: Hoboken, NJ, USA, 2003; p. 277. [Google Scholar]
- Accardi-Dey, A.; Gschwend, P.M. Assessing the combined roles of natural organic matter and black carbon as sorbents in sediments. Environ. Sci. Technol. 2002, 36, 21–29. [Google Scholar] [CrossRef]
- Accardi-Dey, A.; Gschwend, P.M. Reinterpreting literature sorption data considering both absorption into organic carbon and adsorption onto black carbon. Environ. Sci. Technol. 2003, 37, 99–106. [Google Scholar] [CrossRef]
- Cornelissen, G.; Kukulska, Z.; Kalaitzidis, S.; Christanis, K.; Gustafsson, Ö. Relations between environmental black carbon sorption and geochemical sorbent characteristics. Environ. Sci. Technol. 2004, 38, 3632–3640. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Xing, B. Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 1995, 30, 1–11. [Google Scholar] [CrossRef]
- Alexander, M. Aging, bioavailability, and overestimation of risk from environmental pollutant. Environ. Sci. Technol. 2000, 34, 4259–4265. [Google Scholar] [CrossRef]
- Barraclough, D.; Kearney, T.; Croxford, A. Bound residues: environmental solution or future problem? Environ. Pollut. 2005, 133, 85–90. [Google Scholar] [CrossRef]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef]
- Rhodes, A.H.; McAllister, L.E.; Semple, K.T. Linking desorption kinetics to phenanthrene biodegradation in soil. Environ. Pollut. 2010b, 158, 1348–1353. [Google Scholar] [CrossRef]
- Macleod, C.J.A.; Morriss, A.W.J.; Semple, K.T. The role of microorganisms in ecological risk assessment of hydrophobic organic contaminants in soils. Adv. Appl. Microbiol. 2001, 48, 171–212. [Google Scholar] [CrossRef]
- Ortego-Calvo, J.J.; Ball, W.P.; Schulin, R.; Semple, K.T.; Wick, L.Y. Bioavailability of pollutants and soil remediation. J. Environ. Qual. 2007, 36, 1383–1384. [Google Scholar] [CrossRef]
- Chiou, W.L. The rate and extent of oral bioavailability versus the rate and extent of oral absorption: Clarification and recommendation of terminology. J. Pharmacokinet. Pharmacodyn. 2001, 28, 3–6. [Google Scholar] [CrossRef]
- Kelsey, J.W.; Kottler, B.D.; Alexander, M. Selective chemical extractants to predict bioavailability of soil-aged organic chemicals. Environ. Sci. Technol. 1996, 31, 214–217. [Google Scholar]
- White, J.C.; Kelsey, J.W.; Hatzinger, P.B.; Alexander, M. Factors affecting sequestration and bioavailability of phenanthrene in soils. Environ. Toxicol. Chem. 1997, 16, 2040–2045. [Google Scholar] [CrossRef]
- Stucki, G.; Alexander, M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl. Environ. Microbiol. 1987, 53, 292–297. [Google Scholar]
- Harms, H.; Bosma, T.N.P. Mass transfer limitation of microbial growth and pollutant degradation. J. Ind. Microbiol. Biotechnol. 1997, 18, 97–105. [Google Scholar] [CrossRef]
- Gunasekara, A.S.; Xing, B. Sorption and desorption of naphthalene by soil organic matter. J. Environ. Qual. 2003, 32, 240–246. [Google Scholar]
- Ehlers, G.A.C.; Loibner, A.P. Linking organic pollutant (bio) availability with geosorbent properties and biomimetric methodology: A review of geosorbent characterisation and (bio) availability prediction. Environ. Pollut. 2006, 141, 494–512. [Google Scholar] [CrossRef]
- Reichenberg, F.; Mayer, P. Two complementary sides of bioavailability: Accessibility and chemical activity of organic contaminants in sediments and soils. Environ. Toxicol. Chem. 2006, 25, 1239–1245. [Google Scholar] [CrossRef]
- Mayer, P.; Toräng, L.; Glæsner, N.; Jönsson, J.A. Silicone membrane equilibrator: Measuring chemical activity of nonpolar chemicals with poly(dimethylsiloxane) microtubes immersed directly in tissue and lipids. Anal. Chem. 2009, 81, 1536–1542. [Google Scholar]
- Reichenberg, F.; Smedes, F.; Jönsson, J.A.; Mayer, P. Determining the chemical activity of hydrophobic organic compounds in soil using polymer coated vials. Chem Central J. 2008, 2, 1–10. [Google Scholar] [CrossRef]
- Mayer, P.; Holmstup, M. Passive dosing of soil invertebrates with polycyclic aromatic hydrocarbons: Limited chemical activity explains toxicity cutoff. Environ. Sci. Technol. 2008, 42, 7516–7521. [Google Scholar] [CrossRef]
- Reichenberg, F.; Karlson, U.G.; Gustafsson, Ö.; Long, S.M.; Pritchard, P.M.; Mayer, P. Low accessibility and chemical activity of PAHs restrict bioremediation and risk of exposure in a manufactured gas plant soil. Environ. Pollut. 2010, 158, 1214–1220. [Google Scholar] [CrossRef]
- Wilson, S.C.; Jones, K.C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): A review. Environ. Pollut. 1993, 81, 229–249. [Google Scholar] [CrossRef]
- Giger, W. Micropollutants in the Environment. EAWAG News 40E. 1996, pp. 3–7. Available online: http://library.eawag.ch/eawag-publications/EAWAGnews/40E%281996%29.pdf (accessed on 11 May 2011).
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar]
- Conant, R.T.; Drijber, R.A.; Haddix, M.L.; Paton, W.J.; Paul, E.A.; Plante, A.F.; Six, J.; Steinweg, J.M. Sensitivity of organic matter decomposition to warming varies with its quality. Global Change 2008, 14, 868–877. [Google Scholar] [CrossRef]
- Bellamy, P.H.; Loveland, P.J.; Bradley, R.I.; Lark, R.M.; Kirk, G.J.D. Carbon losses from all soils across England and Wales 1978-2003. Nature 2005, 437, 245–248. [Google Scholar]
- Bosma, T.; Harms, H. Bioavailability of organic pollutants. EAWAG News 40E. 1996, pp. 28–31. Available online: http://library.eawag.ch/eawag-publications/EAWAGnews/40E%281996%29.pdf (accessed on 11 May 2011).
- Nkana, J.C.V.; Demeyer, A.; Verloo, M.G. Chemical effects of wood ash on plant growth in tropical acid soils. Bioresour. Technol. 1998, 63, 251–260. [Google Scholar]
- Demeyer, A.; Nkana, J.C.V.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Review paper. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–29. [Google Scholar]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 85–105. [Google Scholar]
- Bushnaf, K.M.; Puricelli, S.; Saponaro, S.; Werner, D. Effect of biochar on the fate of volatile petroleum hydrocarbons in an aerobic sandy soil. J. Contam. Hydrol. 2011, 126, 208–215. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Rhodes, A.H.; Riding, M.J.; McAllister, L.E.; Lee, K.; Semple, K.T. Influence of activated charcoal on desorption kinetics and biodegradation of phenanthrene in soil. Environ. Sci. Technol. 2012, 46, 12445–12451. [Google Scholar] [CrossRef]
- Lohmann, R.; Macfarlane, K.J.; Gschwend, P.M. Importance of black carbon to sorption of native PAHs, PCBs, and PCDDs in Boston and New York Harbor sediment. Environ. Sci. Technol. 2005, 39, 141–148. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management, 1st; Lehmann, J., Ed.; Earthscan: London, UK, 2009; pp. 1–9. [Google Scholar]
- Winsley, P. Biochar and bioenergy production for climate change mitigation. New Zealand Sci. Rev. 2007, 64, 5–10. [Google Scholar]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Steiner, C.; Garcia, M.; Zech, W. Effects of charcoal as slow release nutrient carrier on N-P-K dynamics and soil microbial population: Pot experiments with ferralsol substrate. In Amazonian Dark Earths, Wim Sombroek’s Vision, 1st; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A., Rebellato, L., Eds.; Springer: Berlin, Germany, 2009; p. 325. [Google Scholar]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef]
- Gaunt, J.L.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Lavoué, D.; Liousse, C.; Cachier, H.; Stocks, B.J.; Goldammer, J.G. Modeling of carbonaceous particles emitted by boreal and temperate wildfires at northern latitudes. J. Geophy. Res. 2000, 105, 26871–26890. [Google Scholar] [CrossRef]
- Ishii, T.; Kadoya, K. Effects of charcoal as a soil conditioner on citrus growth and vesicular-arbuscular mycorrhizal development. J. Jpn. Soc. Hort. Sci. 1994, 63, 529–535. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strat. Global Change 2006, 11, 403–427. [Google Scholar]
- Windeisen, E.; Wegener, G. Behaviour of lignin during thermal treatments of wood. Ind. Crops Products 2008, 27, 157–162. [Google Scholar] [CrossRef]
- Maiti, S.; Dey, S.; Purakayastha, S.; Ghosh, B. Physical and thermochemical characterization of rice husk char as a potential biomass source. Bioresour. Technol. 2006, 97, 2065–2070. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, Climate Change and Soil: A Review to Guide Future Research; CSIRO Land and Water Science Report 05/09; CSIRO Publishing: Melbourne, Australia, 2009; pp. 5–6. [Google Scholar]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils. A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; EUR 240099 EN; Office for the Official Publications of the European Communities: Luxembourg, 2009; pp. 1–149.
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. Bioresour. 2009, 4, 370–404. [Google Scholar]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Oshiki, T.; Kishimoto, T. Low-temperature catalytic hydrothermal treatment of wood biomass: Analysis of liquid products. Chem. Eng. J. 2005, 108, 127–137. [Google Scholar]
- Fushimi, C.; Araki, K.; Yamaguchi, Y.; Tsutsumi, A. Effect of heating rate on steam gasification biomass. 2. Thermogravimetric-mass spectrometric (TG-MS) analysis of gas evolution. Ind. Eng. Chem. Res. 2003, 42, 3929–2936. [Google Scholar] [CrossRef]
- Gundale, M.J.; Deluca, T.H. Temperature and source material influence ecological attributes of Ponderosa pine and Douglas-fir charcoal. Forest Ecol. Manag. 2006, 231, 86–93. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L.; Shen, Y. Sorption characteristics and mechanisms of organic contaminant to carbonaceous biosorbents in aqueous solution. Sci. China Ser. B Chem. 2008, 51, 464–472. [Google Scholar] [CrossRef]
- International Energy Agency 2007 Annual Report. Available online: http://www.ieabioenergy.com/LibItem.aspx?id=5761 (accessed on 18 June 2010).
- Tjeerdsma, B.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Charaterization of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh Werkst 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Sivonen, H.; Maunu, S.; Sundholm, F.; Jämsä, S.; Viitaniemi, P. Magnetic resonance studies of thermally modified wood. Holzforschung 2002, 56, 648–654. [Google Scholar]
- Nuopponen, M.; Vuorinen, T.; Jamsa, S.; Viitaniemi, P. Thermal modifications in softwood studied by FT-IR and UV resonance Raman spectroscopies. J. Wood Chem. Technol. 2004, 24, 13–26. [Google Scholar]
- Weiland, J.J.; Guyonnet, R. Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh Werkst 2003, 61, 216–220. [Google Scholar]
- Bourgois, J.; Guyonnet, R. Characterization and analysis of torrefied wood. Wood Sci. Technol. 1988, 22, 143–155. [Google Scholar] [CrossRef]
- James, G.; Sabatini, D.A.; Chiou, C.T.; Rutherford, D.; Scott, A.C.; Karapanagioti, H.K. Evaluating phenanthrene sorption on various wood chars. Water Res. 2005, 39, 549–558. [Google Scholar] [CrossRef]
- Bornemann, L.C.; Kookana, R.S.; Welp, G. Differential sorption behaviour of aromatic hydrocarbons on charcoals prepared at different temperatures from grass and wood. Chemosphere 2007, 67, 1033–1042. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, K.; Wang, H.; Gan, J. Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environ. Pollut. 2010, 158, 2821–2825. [Google Scholar] [CrossRef]
- Fan, M.; Marhsall, W.; Daugaard, D.; Brown, R. Steam activation of chars produced from oat hulls and corn stover. Bioresour. Technol. 2004, 93, 103–107. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustainable Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Allardice, D.J. The adsorption of oxygen on brown coal char. Carbon 1966, 4, 255–266. [Google Scholar] [CrossRef]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Kawamoto, K.; Ishimaru, K.; Imamura, Y. Reactivity of wood charcoal with ozone. J. Wood Sci. 2005, 51, 66–72. [Google Scholar] [CrossRef]
- Hammes, K.; Schmidt, M.W.I. Changes of biochar in soil. In Biochar for Environmental Management, 1st; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 169–181. [Google Scholar]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, C.; Zech, W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive x-ray spectrometry. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cho, H.H.; Poster, D.L.; Ball, W.P. Evidence for a pore- filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char. Environ. Sci. Technol. 2007, 41, 1212–1217. [Google Scholar] [CrossRef]
- Obst, M.; Grathwohl, P.; Kappler, A.; Eibl, O.; Peranio, N.; Gocht, T. Quantitative high-resolution mapping of phenanthrene sorption to black carbon particles. Environ. Sci. Technol. 2011, 45, 7314–7322. [Google Scholar] [CrossRef]
- Werner, D.; Karapanagioti, H.K. Comment on “modeling maximum adsorption capacities of soot and soot-like materials for PAHs and PCBs”. Environ. Sci. Technol. 2005, 39, 381–382. [Google Scholar] [CrossRef]
- Huang, W.; Peng, P.; Yu, Z.; Fu, J. Effects of organic matter heterogeneity on sorption and desorption of organic contaminants by soils and sediments. Appl. Geochem. 2003, 18, 955–972. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralization. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Global Change Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Shneour, E.A. Oxidation of graphitic carbon in certain soils. Science 1966, 151, 991–992. [Google Scholar]
- Mašek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manage. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Kluser, S.; Richard, J.P.; Giuliani, G.; De Bono, A.; Peduzzi, P. Illegal Oil Discharge in European Seas; European Alert Bulletin No 7. United Nations Environmental Protection (UNEP): Geneva, Switzerland, 2006; pp. 1–4. Available online: http://www.grid.unep.ch/products/3_Reports/ew_oildischarge.en.pdf (accessed on 22 November 2009).
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminant by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M. Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J. Soils Sediments 2011, 11, 62–71. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods -A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Chai, Y.; Currie, R.J.; Davis, J.W.; Wilken, M.; Martin, G.D.; Fishman, V.N.; Ghosh, U. Effectiveness of activated carbon and biochar in reducing the availability of polychlorinated dibenzo-p-dioxins/dibenzofurans in soils. Environ. Sci. Technol. 2012, 46, 1035–1043. [Google Scholar] [CrossRef]
- Yang, H.; Sheng, K. Characterization of biochar properties affected by different pyrolysis temperatures using visible-near-infrared spectroscopy. ISRN Spectroscopy 2012, 2012, 1–7. [Google Scholar]
- Zheng, W.; Guo, M.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef]
- Song, J.; Peng, P. Characterisation of black carbon materials by pyrolysis-gas chromatography-mass spectrometry. J. Anal. Appl. Pyrolysis 2010, 87, 129–137. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; DuSaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef]
- Hale, S.E.; Hanley, K.; Lehmann, J.; Zimmerman, A.R.; Cornelissen, G. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol. 2011, 45, 10445–10453. [Google Scholar] [CrossRef]
- Semer, R.; Reddy, K.R. Evaluation of soil washing process to remove mixed contaminants from a sandy loam. J. Hazard. Mater. 1996, 45, 45–57. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Wang, X.; Sato, T.; Xing, B. Competitive sorption of pyrene on wood chars. Environ. Sci. Technol. 2006, 40, 3267–3272. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, H.; Zhang, W. Desorption of polycyclic aromatic hydrocarbons from aged and unaged charcoals with and without modification of humic acids. Environ. Pollut. 2010, 158, 1916–1921. [Google Scholar] [CrossRef]
- Jonker, M.T.O.; Koelmans, A.A. Extraction of polycyclic aromatic hydrocarbons from soot and sediment: Solvent evaluation and implication for sorption mechanism. Environ. Sci. Technol. 2002, 36, 4107–4113. [Google Scholar] [CrossRef]
- Marchal, G.; Smith, K.E.C.; Rein, A.; Winding, A.; Trapp, S.; Karlson, U.G. Comparing the desorption and biodegradation of low concentrations of phenanthrene sorbed to activated carbon, biochar and compost. Chemosphere 2013, 90, 1767–1778. [Google Scholar] [CrossRef]
- Yu, X.; Pan, L.; Ying, G.; Kookana, R.S. Enhanced and irreversible sorption of pesticide pyrimethanil by soil amended with biochars. J. Environ. Sci. 2010, 22, 615–620. [Google Scholar] [CrossRef]
- Yang, X.B.; Ying, G.G.; Peng, P.A.; Wang, L.; Zhao, J.L.; Zhang, L.J.; Yuan, P.; He, H.P. Influence of biochars on plant uptake and dissipation of two pesticides in an agricultural soil. J. Agric. Food Chem. 2010, 58, 7915–7921. [Google Scholar]
- Reid, B.J.; Stokes, J.D.; Jones, K.C.; Semple, K.T. Nonexhaustive cyclodextrin-based extraction technique for the evaluation of PAH bioavailability. Environ. Sci. Technol. 2000, 34, 3174–3179. [Google Scholar] [CrossRef]
- Doick, K.J.; Dew, N.M.; Semple, K.T. Linking catabolism to cyclodextrin extractability: Determination of the microbial availability of PAHs in soil. Environ. Sci. Technol. 2005, 39, 8858–8864. [Google Scholar] [CrossRef]
- Semple, K.T.; Dew, N.M.; Doick, K.J.; Rhodes, A.H. Can microbial mineralization be used to estimate microbial availability of organic contaminants in soil? Environ. Pollut. 2006, 140, 164–172. [Google Scholar]
- Bending, G.D.; Lincoln, S.D.; Edmondson, R.N. Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environ. Pollut. 2006, 139, 279–287. [Google Scholar] [CrossRef]
- Anderson, D.R.; Fisher, R. Sources of dioxin in the United Kingdom: the steel industry and other source. Chemosphere 2002, 46, 371–381. [Google Scholar] [CrossRef]
- Long, M.; Bonefeld-Jørgensen, S.C. Dioxin-like activity in environmental and human samples from Greenland and Denmark. Chemosphere 2012, 89, 919–928. [Google Scholar]
- Sun, K.; Gao, B.; Zhang, Z.; Zhang, G.; Zhao, Y.; Xing, B. Sorption of atrazine and phenanthrene by organic matter fraction in soil and sediment. Environ. Pollut. 2010, 158, 3520–3526. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.; Hunter, M.; Chen, W.; Ward, C.H.; Tomson, M.B. Irreversible sorption of neutral hydrocarbons to sediments: Experimental observations and model predictions. Environ. Sci. Technol. 1998, 32, 892–902. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Brooks, P. Characterization of carbonaceous combustion residues: II. Nonpolar organic compounds. Chemosphere 2003, 53, 447–458. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J. Anal. Appl. Pyrolysis 2012, in press. [Google Scholar]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef]
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.P.; Bucheli, T.D. Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem. 2012, 60, 3042–3050. [Google Scholar]
- Keiluweit, M.; Kleber, M.; Sparrow, M.A.; Simoneit, B.R.T.; Prahl, F.G. Solvent-extractable polycyclic aromatic hydrocarbons in biochar: influence of pyrolysis temperature and feedstock. Environ. Sci. Technol. 2012, 46, 9333–9341. [Google Scholar]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogbonnaya, U.; Semple, K.T. Impact of Biochar on Organic Contaminants in Soil: A Tool for Mitigating Risk? Agronomy 2013, 3, 349-375. https://doi.org/10.3390/agronomy3020349
Ogbonnaya U, Semple KT. Impact of Biochar on Organic Contaminants in Soil: A Tool for Mitigating Risk? Agronomy. 2013; 3(2):349-375. https://doi.org/10.3390/agronomy3020349
Chicago/Turabian StyleOgbonnaya, Uchenna, and Kirk T. Semple. 2013. "Impact of Biochar on Organic Contaminants in Soil: A Tool for Mitigating Risk?" Agronomy 3, no. 2: 349-375. https://doi.org/10.3390/agronomy3020349