Exogenous DCPTA Increases the Tolerance of Maize Seedlings to PEG-Simulated Drought by Regulating Nitrogen Metabolism-Related Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Growth Conditions
2.2. Experimental Design and Sampling
2.3. Relative Growth Rate (RGR)
2.4. Ribonucleic Acid (RNA) Extraction and Sequencing
2.5. qRT-PCR
2.6. Western Blot Analysis
2.7. Enzymic Activities
2.8. The Contents of Foliar NO3–, NO2−, NH4+, Proteins, and Amino Acids
2.9. Statistical Analysis
3. Results
3.1. RGR
3.2. Transcriptome Analysis
3.3. Relative Expression Levels of Genes Encoding N Metabolism-Related Enzymes
3.4. Protein Abundance of Genes Encoding N Metabolism-Related Enzymes
3.5. Activities of N Metabolism-Related Enzymes
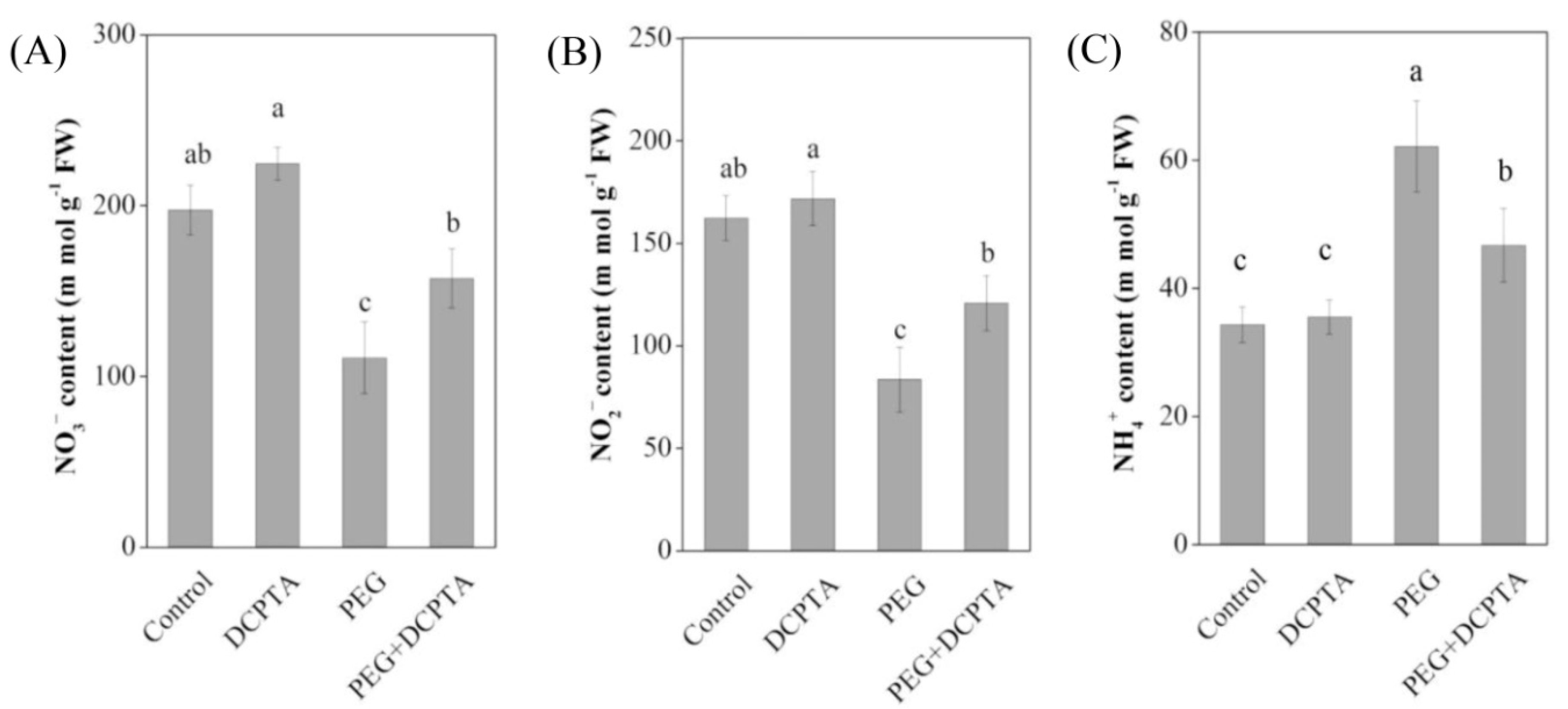
3.6. Contents of Foliar NO3−, NO2− and NH+
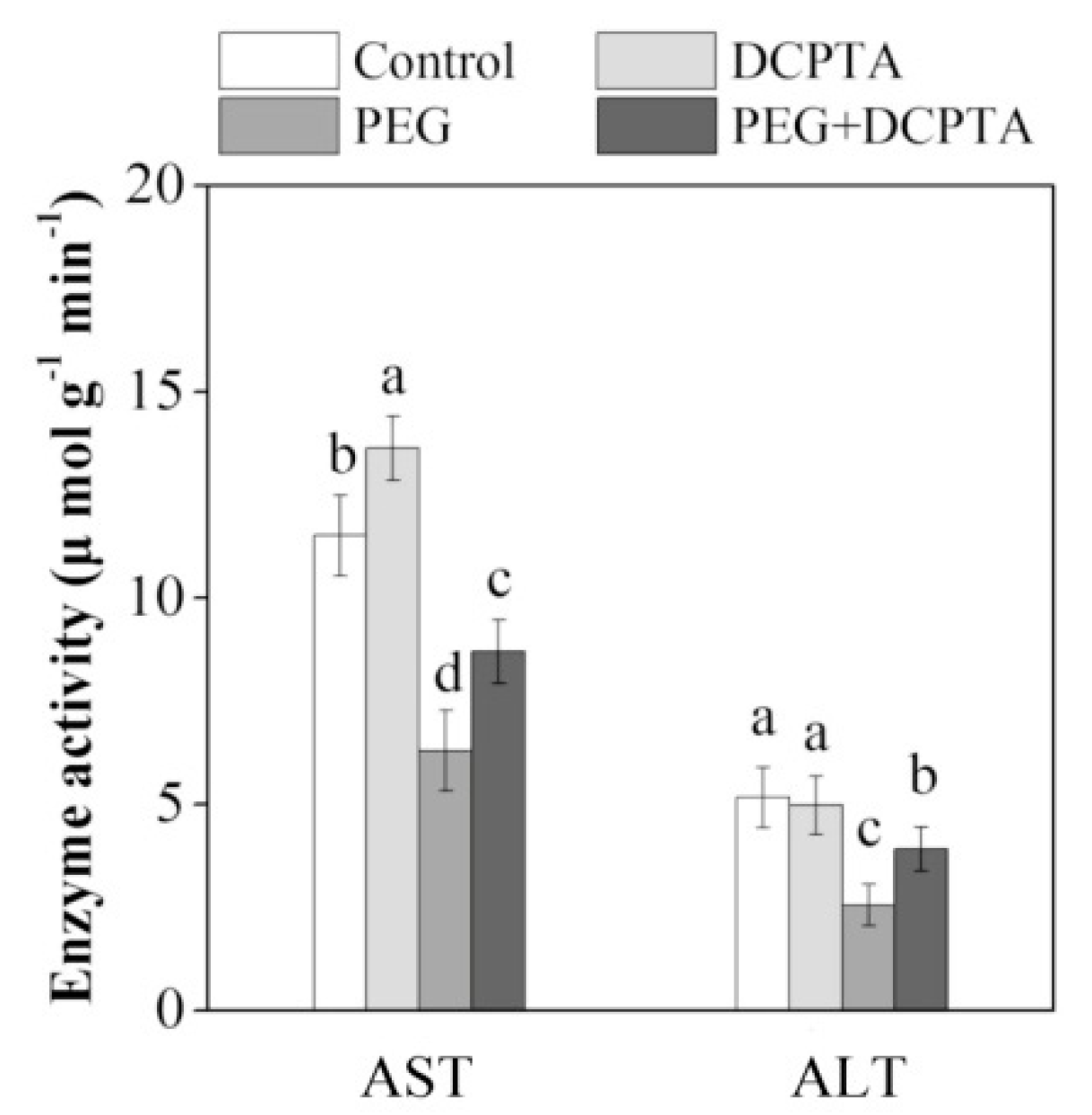
3.7. Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) Activities
3.8. Contents of Soluble Protein and Free Amino Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawson, T.P.; Perryman, A.H.; Osborne, T.M. Modelling impacts of climate change on global food security. Clim. Chang. 2016, 134, 429–440. [Google Scholar] [CrossRef]
- Ziyomo, C.; Bernardo, R. Drought tolerance in maize: Indirect selection through secondary traits versus genomewide selection. Crop Sci. 2013, 53, 1269–1275. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Q.; Jessup, K.E.; Hao, B.; Hou, X.; Marek, T.H.; Xu, W.; Evett, S.; O’Shaughnessy, S.A.; Brauer, D.K. Yield and water use of drought-tolerant maize hybrids in a semiarid environment. Field Crops Res. 2018, 216, 1–9. [Google Scholar] [CrossRef]
- Martin, A.; Belastegui-Macadam, X.; Floriot, M.; Valadier, M.H.; Pommel, B.; Andrieu, B.; Donnison, I.; Hirel, B. Nitrogen management and senescence in two maize hybrids differing in the persistence of leaf greenness: Agronomic, physiological and molecular aspects. New Phytol. 2005, 167, 483–492. [Google Scholar] [CrossRef]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Daniel-Vedele, F.; Krapp, A.; Kaiser, W.M. Cellular biology of nitrogen metabolism and signaling. Plant Cell Monogr. 2015, 17, 145–172. [Google Scholar]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Piwpuan, N.; Zhai, X.; Brix, H. Nitrogen nutrition of cyperus laevigatus, and phormium tenax: Effects of ammonium versus nitrate on growth, nitrate reductase activity and N uptake kinetics. Aquat. Bot. 2013, 106, 42–51. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef]
- Hirel, B.; Lea, P.J. Genomics of nitrogen use efficiency in maize: From basic approaches to agronomic applications. In The Maize Genome; Springer: Cham, Switzerland, 2018; pp. 259–286. [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, S.; Socha-Hanc, J.; Paszkowski, A. Alanine aminotransferase and glycine aminotransferase from maize (Zea mays L.) leaves. Acta Biochim. Pol. 1999, 46, 447–457. [Google Scholar] [PubMed]
- Muench, D.G.; Christopher, M.E.; Good, A.G. Cloning and expression of a hypoxic and nitrogen inducible maize alanine aminotransferase gene. Physiol. Plant. 2010, 103, 503–512. [Google Scholar] [CrossRef]
- Ferrario-Mery, S.; Valadier, M.H.; Foyer, C.H. Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol. 1998, 117, 293–302. [Google Scholar] [CrossRef]
- Fresneau, C.; Ghashghaie, J.; Cornic, G. Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): Role of leaf internal CO2. J. Exp. Bot. 2007, 58, 2983–2992. [Google Scholar] [CrossRef]
- Yanagisawa, S. Transcription factors involved in controlling the expression of nitrate reductase genes in higher plants. Plant Sci. 2014, 229, 167–171. [Google Scholar] [CrossRef]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003, 39, 137–141. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.R.; Xie, T.L.; Li, L.J.; Sun, Y.; Zhang, H.; Li, J.; Wei, S. Mixed compound of dcpta and ccc increases maize yield by improving plant morphology and up-regulating photosynthetic capacity and antioxidants. PLoS ONE 2016, 11, e0149404. [Google Scholar] [CrossRef]
- Keithly, J.H.; Yokoyama, H.; Gausman, H. Enhanced yield of tomato in response to 2-(3,4-dichlorophenoxy) triethylamine (DCPTA). Plant Growth Regul. 1990, 9, 127–136. [Google Scholar] [CrossRef]
- Hartz, T.K.; Kies, L.J.; Baameur, A.; May, D.M. DCPTA ineffective as a production aid on field-grown tomato and pepper. Hortscience 1995, 30, 78–79. [Google Scholar] [CrossRef]
- Keithly, J.H.; Yokoyama, H.; Gausman, H.W. Effect of 2-(3,4-dichlorophenoxy) triethylamine (DCPTA) on the growth and development of sugarbeet. Plant Sci. 1990, 68, 57–64. [Google Scholar] [CrossRef]
- Gausman, H.W.; Burd, J.D.; Quisenberry, J.; Yokoyama, H.; Dilbeck, R.; Chan, R.B. Effect of 2-diethylaminoethyl-3,4-dichlorophenylether (DCPTA) on cotton plant (Gossypium hirsutum) growth and phenology. Nat. Biotechnol. 1985, 3, 255–257. [Google Scholar] [CrossRef]
- Keithly, J.H.; Kobayashi, H.; Yokoyama, H. Effect of 2-(3,4-dichlorophenoxy) triethylamine (DCPTA) on the growth and development of blue spruce (picea pungens englm. var. glauca). Plant Growth Regul. Soc. Am. Q. 1990, 18, 55–61. [Google Scholar]
- Benedict, C.R.; Rosenfield, C.L.; Mahan, J.R.; Madhavan, S.; Yokoyamaet, H. The chemical regulation of carotenoid biosynthesis in citrus. Plant Sci. 1985, 41, 169–173. [Google Scholar] [CrossRef]
- Xie, T.; Gu, W.; Meng, Y.; Li, J.; Li, L.; Wang, Y.; Qu, D.; Wei, S. Exogenous DCPTA ameliorates simulated drought conditions by improving the growth and photosynthetic capacity of maize seedlings. Sci. Rep. 2017, 7, 12684. [Google Scholar] [CrossRef]
- Xie, T.; Gu, W.; Zhang, L.; Li, L.; Qu, D.; Li, C.; Meng, Y.; Li, J.; Wei, S.; Li, W. Modulating the antioxidant system by exogenous 2-(3,4-dichlorophenoxy) triethylamine in maize seedlings exposed to polyethylene glycol-simulated drought stress. PLoS ONE 2018, 13, e0203626. [Google Scholar] [CrossRef]
- Xie, T.; Gu, W.; Wang, M.; Zhang, L.; Li, C.; Li, C.; Li, W.; Li, L.; Wei, S. Exogenous 2-(3,4-dichlorophenoxy) triethylamine ameliorates the soil drought effect on nitrogen metabolism in maize during the pre-female inflorescence emergence stage. BMC Plant Biol. 2019, 19, 107. [Google Scholar] [CrossRef]
- Kingsbury, R.W.; Epstein, E.; Pearcy, R.W. Physiological responses to salinity in selected lines of wheat. Plant Physiol. 1984, 74, 417–423. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yuan, Y.; Liu, Y.Y.; Liu, Y.; Fu, J.J.; Zheng, J.; Wang, G.Y. Gene families of maize glutathione–ascorbate redox cycle respond differently to abiotic stresses. J. Plant Physiol. 2012, 169, 183–192. [Google Scholar] [CrossRef]
- Barro, F.; Fontes, A.G.; Maldonado, J.M. Organic nitrogen content and nitrate and nitrite reductase activities in tritordeum and wheat grown under nitrate or ammonium. Plant Soil 1991, 135, 251–256. [Google Scholar] [CrossRef]
- Ida, S.; Morita, Y. Purification and general properties of spinach leaf nitrite reductase. Plant Cell Physiol. 1973, 14, 661–671. [Google Scholar]
- O’Neal, D.; Joy, K.W. Glutamine synthetase of pea leaves. I. purification, stabilization, and PH optima. Arch. Biochem. Biophys. 1973, 159, 113–122. [Google Scholar] [CrossRef]
- Groat, R.G.; Vance, C.P. Root nodule enzymes of ammonia assimilation in alfalfa (Medicago sativa L.) developmental patterns and response to applied nitrogen. Plant Physiol. 1979, 64, 1198–1203. [Google Scholar]
- Jia, Y.; Zou, D.; Wang, J.; Liu, H.; Inayat, M.A.; Sha, H.; Zheng, H.; Sun, J.; Zhao, H. Effect of low water temperature at reproductive stage on yield and glutamate metabolism of rice (Oryza sativa L.) in China. Field Crop Res. 2015, 175, 16–25. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicylic-acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Bräutigam, A.; Gagneul, D.; Weber, A.P. High-throughput colorimetric method for the parallel assay of glyoxylic acid and ammonium in a single extract. Anal. Biochem. 2007, 362, 151–153. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Du, J.; Shu, S.; An, Y.; Zhou, H.; Guo, S.; Sun, J. Influence of exogenous spermidine on carbon-nitrogen metabolism under Ca(NO3)2, stress in cucumber root. Plant Growth Regul. 2016, 81, 1–13. [Google Scholar] [CrossRef]
- Tarazona, S.; Fernando, G.; Ferrer, A.; Joaquín, D.; Conesa, A. NOIseq: A RNA-seq differential expression method robust for sequencing depth biases. Univ. Southampt. 2012, 17, 18. [Google Scholar] [CrossRef]
- Tarazona, S.; Furió-Tarí, P.; Turrà, D.; Pietro, A.D.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Z.Y.; Sun, X.; Lu, S.B.; Xu, W.J.; Zhao, Q.; Peng, J. Identification of Changes in Gene expression of rats after Sensory and Motor Nerves Injury. Sci. Rep. 2016, 6, 26579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.S.; Song, J.T.; Seo, H.S. Arabidopsis nitrate reductase activity is stimulated by the E3 SUMO ligase AtSIZ1. Nat. Commun. 2011, 2, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.S.; Wang, Y.J.; Wang, D.Z. Response of phytoplankton to nitrogen addition in the Taiwan strait upwelling region: Nitrate reductase and glutamine synthetase activities. Cont. Shelf Res. 2011, 31, 57–66. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; del Mar Rubio-Wilhelmi, M.; Ríos, J.J.; Blasco, B.; Rosales, M.Á.; Melgarejo, R.; Romero, L.; Ruiz, J.M. Ammonia production and assimilation: Its importance as a tolerance mechanism during moderate water deficit in tomato plants. J. Plant Physiol. 2011, 168, 816–823. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress. Acta Physiol. Plant. 2018, 40, 206. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, C.; Su, L.; Li, Y.; Zhao, Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ. Exp. Bot. 2016, 123, 78–87. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.A. Physiological responses of forest trees to heat and drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Beyzaei, Z.; Sherbakov, R.A.; Averina, N.G. Response of nitrate reductase to exogenous application of 5-aminolevulinic acid in barley plants. J. Plant Growth Regul. 2014, 33, 745–750. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Zhu, T.; Li, W.; Zhang, L. Carbon and nitrogen metabolism in leaves and roots of dwarf bamboo (Fargesia denudataYi) subjected to drought for two consecutive years during sprouting period. J. Plant Growth Regul. 2014, 33, 243–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.H.; Shi, Y.; Zou, Z.R.; Yan, F.; Zhao, Y.Y.; Zhang, H.; Zhao, J.Z. Beneficial role of exogenous spermidine on nitrogen metabolism in tomato seedlings exposed to saline-alkaline stress. J. Am. Soc. Hortic. Sci. 2013, 138, 38–49. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.T.; Abbaraju, H.K.R.; Fallis, L.P.; Liu, H.; Lee, M.; Dhugga, K.S. Biochemical and genetic analyses of N metabolism in maize testcross seedlings:2. Roots. Theor. Appl. Genet. 2018, 131, 1191–1205. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Erdal, S.; Turk, H. Cysteine-induced upregulation of nitrogen metabolism-related genes and enzyme activities enhance tolerance of maize seedlings to cadmium stress. Environ. Exp. Bot. 2016, 132, 92–99. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ. Exp. Bot. 2011, 71, 399–408. [Google Scholar] [CrossRef]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Title: Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Debouba, M.; Gouia, H.; Suzuki, A.; Ghorbel, M.H. NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedlings. J. Plant Physiol. 2006, 163, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Cui, L.; Liu, F.; Zhang, M.; Shan, L.; Yang, S.; Deng, X. Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015, 47, 49–56. [Google Scholar]
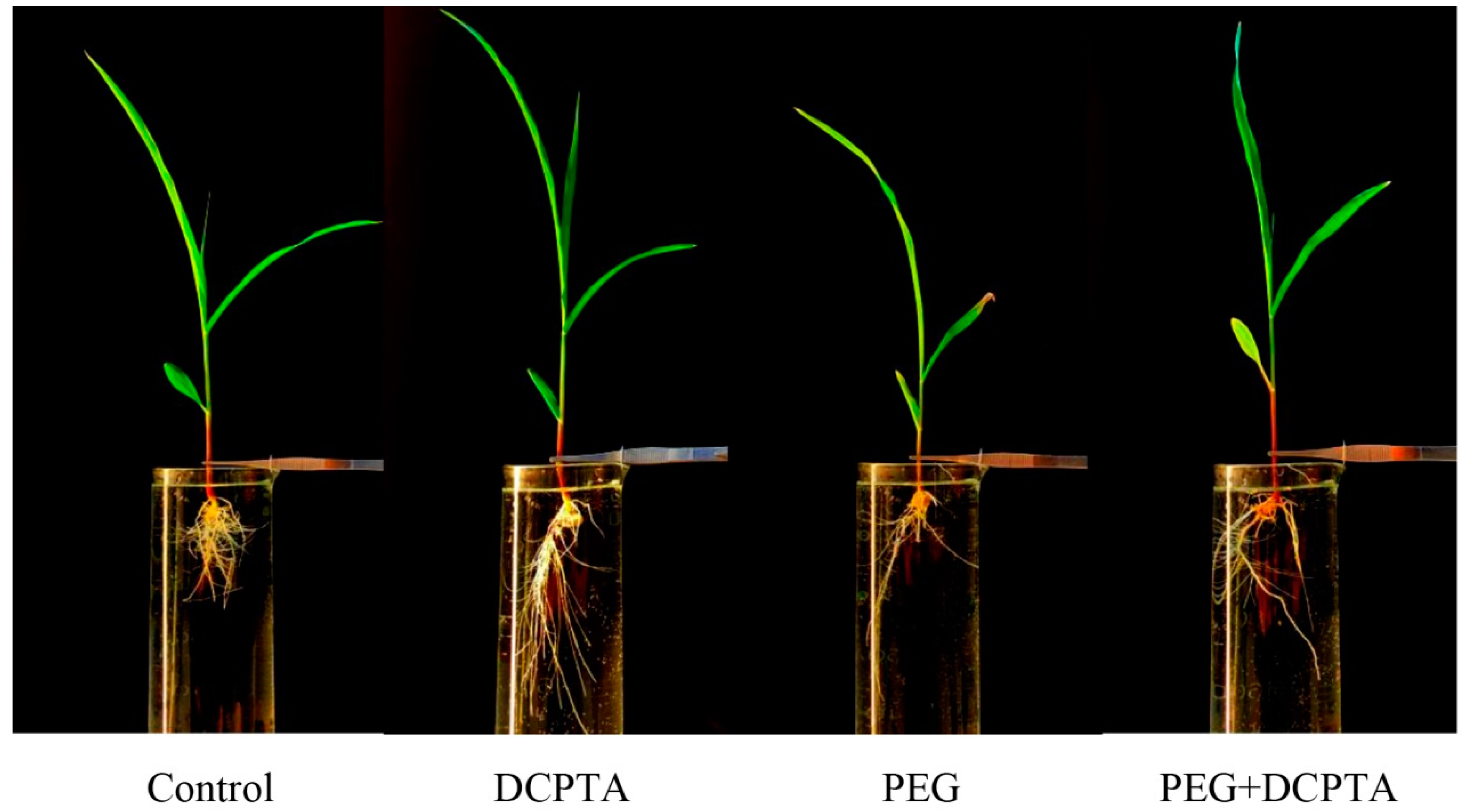
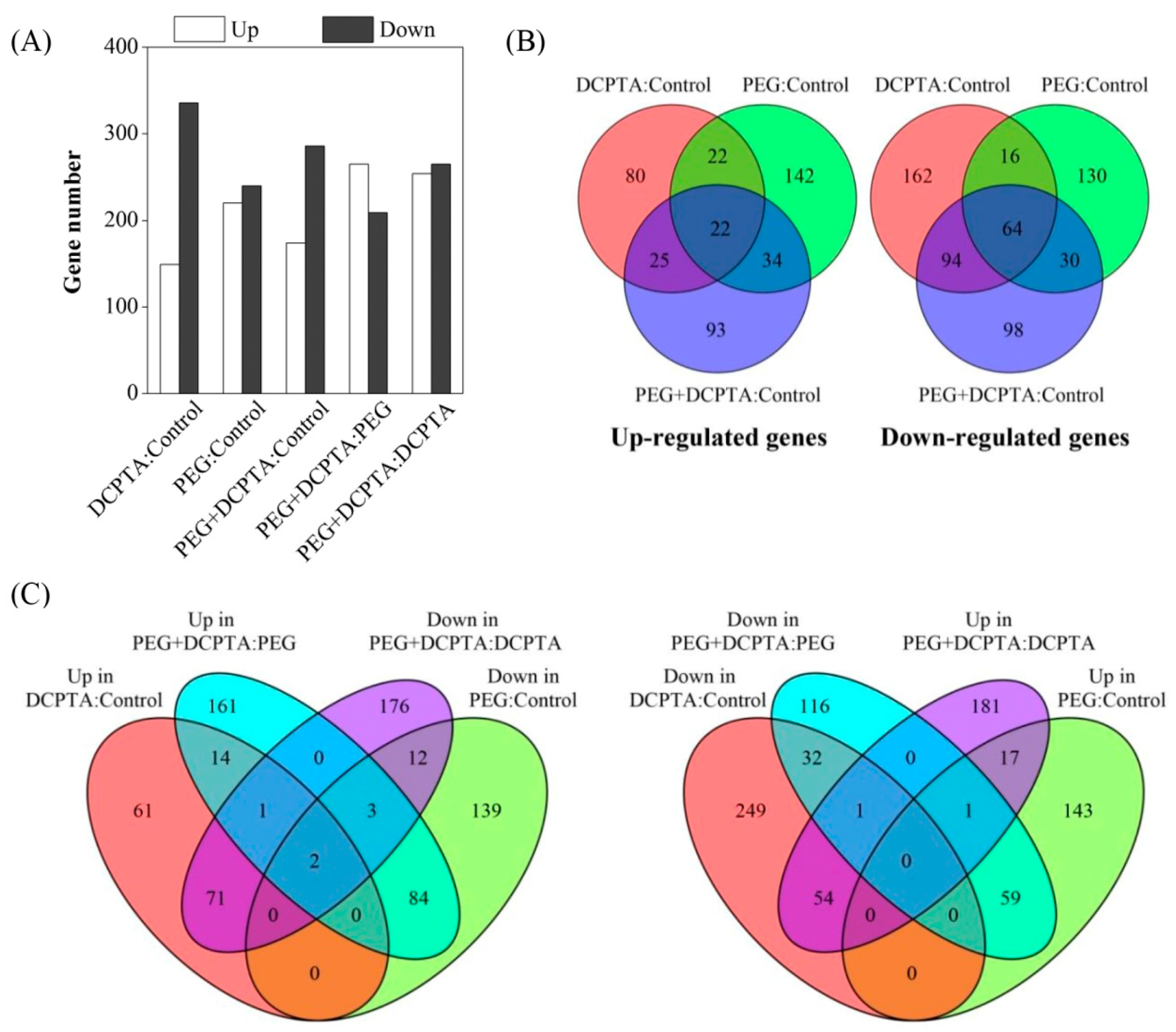
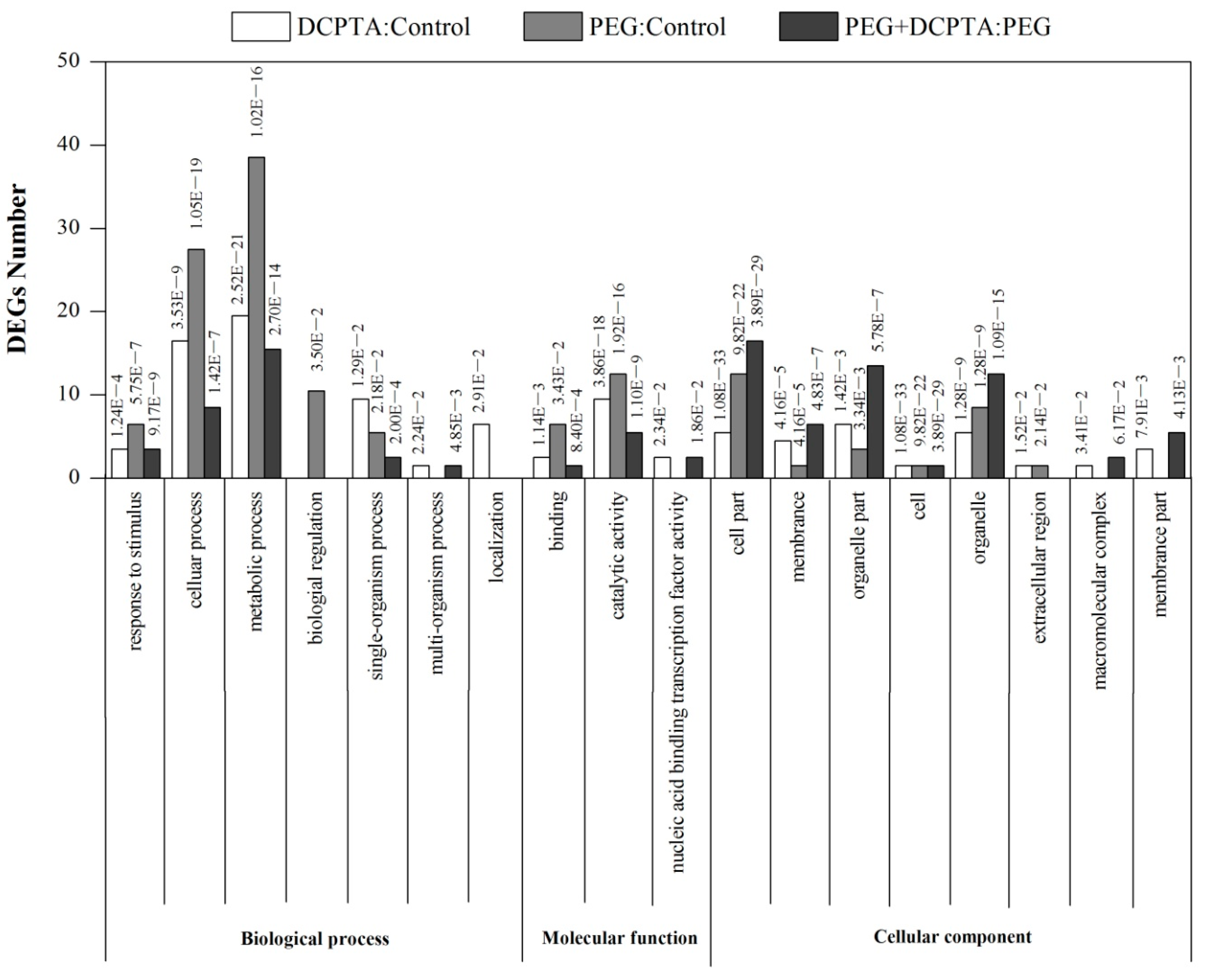
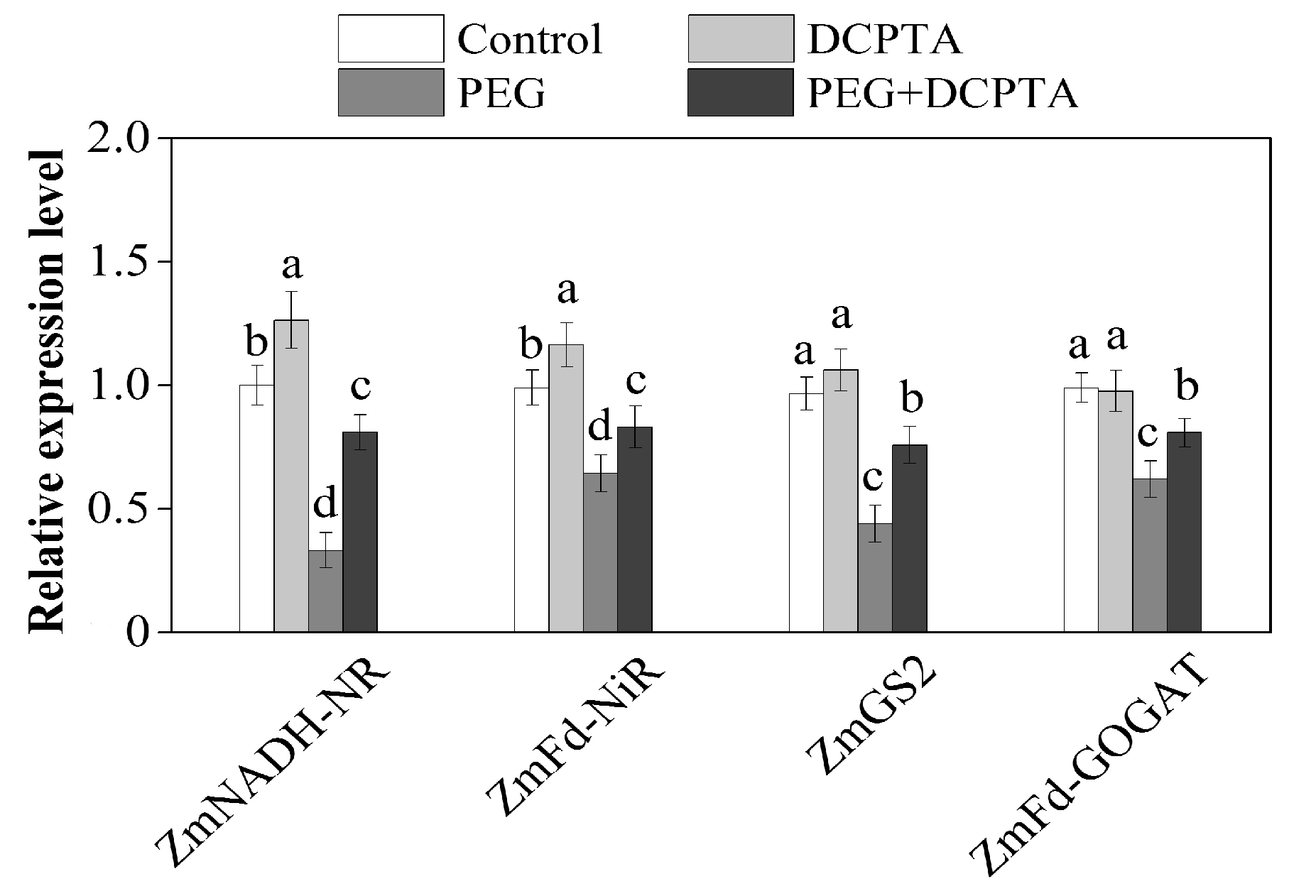



| Sample | Length | Total Reads | Total Mapped | Uniquely Mapped | Mapped (%) | Unique Mapped (%) | Seq Depth |
|---|---|---|---|---|---|---|---|
| Control | 142.98 | 28,503,138 | 23,512,659 | 21,635,820 | 82.49% | 75.91% | 2.57 |
| DCPTA | 141.32 | 26,180,040 | 21,689,777 | 19,780,924 | 82.85% | 75.56% | 2.30 |
| PEG | 140.78 | 28,759,526 | 23,719,008 | 21,795,481 | 82.47% | 75.79% | 2.57 |
| PEG + DCPTA | 141.45 | 34,278,670 | 28,164,459 | 25,444,256 | 82.16% | 74.23% | 2.03 |
| Gene | Gene ids | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| ZmNADH- NR | XM_008680224.1 | 5′-CTACCATTTCAAGGACAACCG-3′ | 5′-GCGTCGTTATCACCGAGTTT-3′ |
| ZmFd-NiR | XM_008648080.1 | 5′-CAAGTGGCTCGGCCTCT-3′ | 5′-GCGTCTGCTCGCTCGTC-3′ |
| ZmFd-GOGAT | NM_001112223.1 | 5′-ACGCAGCACATTGATCTTGGATAC-3′ | 5′-TGTCTCCATACTTCTTGGCAATCA-3′ |
| ZmGS2 | NM_001111973.1 | 5′-CAGGAAGGGGCAGAACATA-3′ | 5′-CTGAGGGCCAGGGTAGC-3′ |
| ZmActin | NC_008332.1 | 5′-ACATGCGCCTAAGGAGAAATAG-3′ | 5′-ACCTCCATGCTCACTGGTACTT-3′ |
| Treatment | Shoots | Roots |
|---|---|---|
| Control | 3.98 ± 0.20 a | 3.95 ± 0.17 b |
| DCPTA | 4.03 ± 0.17 a | 4.24 ± 0.13 a |
| PEG | 2.58 ± 0.26 c | 2.81 ± 0.17 d |
| PEG + DCPTA | 3.12 ± 0.14 b | 3.28 ± 0.14 c |
| Amino Acid (mg g−1 DW) | Treatment | |||
|---|---|---|---|---|
| Control | DCPTA | PEG | PEG + DCPTA | |
| Alanine | 14.34 ± 0.95 b | 15.44 ± 0.45 a | 7.85 ± 1.05 d | 12.03 ± 0.94 c |
| Arginine | 11.52 ± 0.53 b | 11.74 ± 0.83 b | 13.58 ± 0.74 a | 11.93 ± 1.03 b |
| Aspartate | 18.48 ± 1.51 b | 19.85 ± 0.45 a | 10.63 ± 1.01 d | 13.33 ± 1.05 c |
| Cysteine | 2.64 ± 0.21 a | 2.52 ± 0.27 a | 1.63 ± 0.20 c | 2.10 ± 0.11 b |
| Glutamate | 29.82 ± 0.69 b | 32.10 ± 1.02 a | 20.71 ± 0.95 d | 26.18 ± 1.23 c |
| Glycine | 11.67 ± 0.89 b | 11.39 ± 1.09 b | 18.85 ± 0.64 a | 12.24 ± 0.58 b |
| Histidine | 4.98 ± 1.03 b | 5.34 ± 1.02 b | 8.38 ± 1.05 a | 5.37 ± 1.05 b |
| Isoleucine | 8.19 ± 0.89 c | 8.59 ± 0.89 c | 10.95 ± 0.90 a | 9.79 ± 0.47 b |
| Leucine | 13.94 ± 0.92 c | 14.73 ± 0.93 c | 18.41 ± 0.91 a | 16.20 ± 1.20 b |
| Lysine | 7.33 ± 0.79 c | 8.02 ± 0.51 c | 16.84 ± 0.60 a | 12.01 ± 0.98 b |
| Methionine | 3.82 ± 0.66 b | 3.69 ± 0.47 b | 8.01 ± 0.27 a | 4.49 ± 0.30 b |
| Phenylalanine | 11.79 ± 0.89 b | 12.61 ± 0.88 b | 15.10 ± 0.88 a | 14.11 ± 0.88 a |
| Proline | 10.35 ± 0.90 c | 11.06 ± 1.01 c | 20.06 ± 0.94 b | 24.31 ± 1.17 a |
| Serine | 10.95 ± 0.90 c | 11.57 ± 1.00 bc | 14.22 ± 0.47 a | 12.50 ± 1.18 b |
| Threonine | 7.17 ± 0.99 c | 7.96 ± 1.05 bc | 10.03 ± 1.10 a | 9.20 ± 1.05 ab |
| Tyrosine | 4.67 ± 0.54 b | 5.21 ± 0.35 b | 10.08 ± 0.94 a | 4.27 ± 1.05 b |
| Valine | 10.70 ± 0.88 c | 10.63 ± 0.65 c | 16.65 ± 2.23 a | 14.27 ± 0.72 b |
| Total | 182.37 ± 5.12 d | 192.46 ± 4.26 c | 221.96 ± 5.24 a | 204.33 ± 5.25 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Gu, W.; Li, C.; Li, J.; Wei, S. Exogenous DCPTA Increases the Tolerance of Maize Seedlings to PEG-Simulated Drought by Regulating Nitrogen Metabolism-Related Enzymes. Agronomy 2019, 9, 676. https://doi.org/10.3390/agronomy9110676
Xie T, Gu W, Li C, Li J, Wei S. Exogenous DCPTA Increases the Tolerance of Maize Seedlings to PEG-Simulated Drought by Regulating Nitrogen Metabolism-Related Enzymes. Agronomy. 2019; 9(11):676. https://doi.org/10.3390/agronomy9110676
Chicago/Turabian StyleXie, Tenglong, Wanrong Gu, Congfeng Li, Jing Li, and Shi Wei. 2019. "Exogenous DCPTA Increases the Tolerance of Maize Seedlings to PEG-Simulated Drought by Regulating Nitrogen Metabolism-Related Enzymes" Agronomy 9, no. 11: 676. https://doi.org/10.3390/agronomy9110676
APA StyleXie, T., Gu, W., Li, C., Li, J., & Wei, S. (2019). Exogenous DCPTA Increases the Tolerance of Maize Seedlings to PEG-Simulated Drought by Regulating Nitrogen Metabolism-Related Enzymes. Agronomy, 9(11), 676. https://doi.org/10.3390/agronomy9110676





