Biometric Analyses of Yield, Oil and Protein Contents of Wheat (Triticum aestivum L.) Genotypes in Different Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials
2.2. Environmental Variables
2.3. Wheat Cultivars
2.4. Agronomical and Technological Traits
2.5. Statistical Analyses
3. Results
3.1. Genotypic Variations
3.2. GE Effect on Oil Content
3.3. GE Effects on Protein Content
3.4. GE Effects on Grain Yield
4. Discussion
4.1. Genotypic Variations
4.2. GE Effects on OC, PC and GY
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shewry, P.R.; Piironen, V.; Lampi, A.M.; Edelmann, M.; Kariluoto, S.; Nurmi, T.; Fernandez-Orozco, R.; Ravel, C.; Charmet, G.; Andersson, A.A.; et al. The HEALTHGRAIN wheat diversity screen: effects of genotype and environment on phytochemicals and dietary fiber components. J. Agric. Food Chem. 2010, 58, 9291–9298. [Google Scholar] [CrossRef]
- Prinsen, P.; Gutiérrez, A.; Faulds, C.B.; Del Río, J.C. Comprehensive study of valuable lipophilic phytochemicals in wheat bran. J. Agric. Food Chem. 2014, 62, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Lindemans, G.C.; Delcour, J.A. Large-scale production and characterisation of wheat bran arabinoxylooligosaccharides. J. Sci. Food Agric. 2006, 86, 1722–1731. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Bonacini, G.; Tribuzio, G.; Vittadini, E. Effect of the addition of bran fractions on bread properties. J. Cereal Sci. 2013, 57, 325–332. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT-Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Tech. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Andersson, A.A.; Dimberg, L.; Aman, P.; Landberg, D. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- De Brier, N.; Gomand, S.V.; Joye, I.J.; Pareyt, B.; Courtin, C.M.; Delcour, J.A. The impact of pearling as a treatment prior to wheat roller milling on the texture and structure of bran-rich breakfast flakes. LWT-Food Sci. Technol. 2015, 62, 668–674. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: the increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Al-Hooti, S.N.; Al-Saqer, J.M. Effect of adding wheat bran and germ fractions on the chemical composition of high-fiber toast bread. Food Chem. 1999, 67, 365–371. [Google Scholar] [CrossRef]
- Reisinger, M.; Tirpanalan, Ö.; Prückler, M.; Huber, F.; Kneifel, W.; Novalin, S. Wheat bran biorefinery–a detailed investigation on hydrothermal and enzymatic treatment. Bioresour. Technol. 2013, 144, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Winter, K.M.; Stevenson, L.; Morris, C.; Leach, D.N. Wheat bran lipophilic compounds with in vitro anticancer effects. Food Chem. 2012, 130, 156–164. [Google Scholar] [CrossRef]
- Baenziger, P.S.; Clements, R.L.; McIntosh, M.S.; Yamazaki, W.T.; Starling, T.M.; Sammons, D.J.; Johnson, J.W. Effect of cultivar, environment, and their interaction and stability analyses on milling and baking quality of soft red winter wheat. Crop Sci. 1985, 25, 5–8. [Google Scholar] [CrossRef]
- Schipper, A. Modifications of the dough physical properties of various wheat cultivars by environmental influences. Agribiol. Res. 1991, 44, 114–132. [Google Scholar]
- Rao, A.C.S.; Smith, J.L.; Jandhyala, V.K.; Papendick, R.I.; Parr, J.F. Cultivar and climatic effects on the protein content of soft white winter wheat. Agron. J. 1993, 85, 1023–1028. [Google Scholar] [CrossRef]
- Uhlen, A.K.; Hafskjold, R.; Kalhovd, A.H.; Sahlström, S.; Longva, Å.; Magnus, E.M. Effects of cultivar and temperature during grain filling on wheat protein content, composition, and dough mixing properties. Cereal Chem. 1998, 75, 460–465. [Google Scholar] [CrossRef]
- Memon, G.H.; Jamro, G.H. Influence of nitrogen fertilization on grain protein content and NPK content of straw in late sown wheat (Triticum aestivum L.). Pak. J. Sci. Ind. Res. 1988, 31, 649–650. [Google Scholar]
- Vaughan, B.; Westfall, D.G.; Barbarick, K.A. Nitrogen rate and timing effects on winter wheat grain yield, grain protein, and economics. J. Prod. Agric. 1990, 3, 324–328. [Google Scholar] [CrossRef]
- Olson, R.A.; Frank, K.D.; Deibert, E.J.; Dreier, A.F.; Sander, D.H.; Johnson, V.A. Impact of residual mineral N in soil on grain protein yields of winter wheat and corn. Agron. J. 1976, 68, 769–772. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Koregave, B.A. Effects of N and P fertilizers on wheat yield and its economics under sequence cropping. J. Maharashtra Agric. Univ. 1988, 13, 270–273. [Google Scholar]
- Smika, D.E.; Greb, B.W. Protein content of winter wheat grain as related to soil and climatic factors in the semiarid Central Great Plains. Agron. J. 1973, 65, 433–436. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Harris, M.; Wilson, J.; Haley, S. Antioxidant properties of bran extracts from “Akron” wheat grown at different locations. J. Agric. Food Chem. 2003, 51, 1566–1570. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Antioxidant properties of bran extracts from Trego wheat grown at different locations. J. Agric. Food Chem. 2004, 52, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Hurburgh, C.R.; Brumm, T.J.; Guinn, J.M.; Hartwig, R.A. Protein and oil patterns in US and world soybean markets. J. Am. Oil Chem. Soc. 1990, 67, 966–973. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Serretti, C. Influence of high protein, genotype, and environment on protein quality of soybean. Ph.D. dissertation, Kansas State University, Manhattan, Kansas, 1993. [Google Scholar]
- Gibson, L.R.; Mullen, R.E. Soybean seed composition under high day and night growth temperatures. J. Am. Oil Chem. Soc. 1996, 73, 733–737. [Google Scholar] [CrossRef]
- Piper, E.L.; Boote, K.I. Temperature and cultivar effects on soybean seed oil and protein concentrations. J. Am. Oil Chem. Soc. 1999, 76, 1233–1241. [Google Scholar] [CrossRef]
- FAO, Rome. 2014. Available online: http://www.fao.org/3/a-i3794e.pdf (accessed on 10 August 2018).
- American Association of Cereal Chemistry, Approved methods of Analysis, (11th edn); Methods (44-15.02) and (46-16.01); AACC: St. Paul, MN, USA, 2000; Available online: http://methods.aaccnet.org/toc.aspx (accessed on 15 September 2010).
- Vargas, M.; Crossa, J.; Sayre, K.; Reynolds, M.; Ramírez, M.E.; Talbot, M. Interpreting Genotype × Environment Interaction in Wheat by Partial Least Squares Regression. Crop Sci. 1998, 38, 679–689. [Google Scholar] [CrossRef]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Prediction. J. Royal Stat. Soc. (Series B) 1974, 36, 111–147. [Google Scholar] [CrossRef]
- Zorić, M.; Terzić, S.; Sikora, V.; Brdar-Jokanović, M.; Vassilev, D. Effect of environmental variables on performance of Jerusalem artichoke (Helianthus tuberosus L.) cultivars in a long term trial: A statistical approach. Euphytica 2017, 213. [Google Scholar] [CrossRef]
- R Core Team. 2017. Available online: http://www.R-project.org/ (accessed on 18 June 2018).
- Hristov, N.; Mladenov, N.; Đurić, V.; Kondić-Špika, A.; Marjanović-Jeromela, A.; Lečić, N. Genotipska varijabilnost sadržaja ulja kod pšenice. Zbornik Radova–A Period. Sci. Res. Field Veget. Crops 2009, 46, 5–10. [Google Scholar]
- Moore, C.M.; Richards, R.A.; Rebetzke, G.J. Phenotypic variation and QTL analysis for oil content and protein concentration in bread wheat (Triticum aestivum L.). Euphytica. 2015, 204, 371–382. [Google Scholar] [CrossRef]
- Marjanović Jeromela, A.; Marinković, R.; Jocković, M.; Atlagić, J.; Terzić, S.; Mikić, A.; Lečić, N. Presented at Quality of Alternative Oil and Protein Crops. In Proceedings of the 6th Central European Congress on Food, CEFood, Novi Sad, Serbia, 23–26 May 2012; pp. 437–440. [Google Scholar]
- Agudo, A.; Amiano, P.; Barcos, A.; Barricarte, A.; Beguiristain, J.M.; Chirlaque, M.D.; Dorronsoro, M.; González, C.A.; Lasheras, C.; Martínez, C.; et al. Dietary intake of vegetables and fruits among adults in five regions of Spain. EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Eur. J. Clin. Nutr. 1999, 53, 174–180. [Google Scholar] [CrossRef]
- Wilson, T.A.; Nicolosi, R.J.; Woolfrey, B.; Kritchevsky, D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypocholesterolemic hamsters. J. Nutr. Biochem. 2007, 18, 105–112. [Google Scholar] [CrossRef]
- Gray, B.; Swick, J.; Ronnenberg, A.G. Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr. Rev. 2011, 69, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Balalić, I.; Marjanović Jeromela, A.; Crnobarac, J.; Terzić, S.; Radić, V.; Miklič, V.; Jovičić, D. Variability of oil and protein content in rapeseed cultivars affected by seeding date. Emir. J. Food Agr. 2017, 29, 404–410. [Google Scholar] [CrossRef]
- Bockisch, M. Fats and Oils Handbook; Elsevier Inc.: Hamburg, Germany, 1998; pp. 174–344. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Mohdaly, A.A.A.; Elneairy, N.A.A. Wheat Germ: An Overview on Nutritional Value, Antioxidant Potential and Antibacterial Characteristics. Food Nutr. Sci. 2015, 6, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Chen, J.; Liu, Y.; Wang, L.; Zhao, G.; Chen, Z.Y. Dietary wheat bran oil is equally as effective as rice bran oil in reducing plasma cholesterol. J. Agric. Food Chem. 2018, 66, 2765–2774. [Google Scholar] [CrossRef]
- Yasukawa, K.; Akihisa, T.; Kimura, Y.; Tamura, T.; Takido, M. Inhibitory effect of cycloartenyl ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin. Biol. Pharm. Bull. 1998, 21, 1072–1076. [Google Scholar] [CrossRef]
- Talawar, S.T.; Harohally, N.V.; Ramakrishna, C.; Suresh Kumar, G. Development of Wheat Bran Oil Concentrates Rich in Bioactives with Antioxidant and Hypolipidemic Properties. J. Agric. Food Chem. 2017, 65, 9838–9848. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food. Sci. 2011, 76, 7–10. [Google Scholar] [CrossRef]
- Proulx, R.A.; Naeve, S.L. Pod removal, shade, and defoliation effects on soybean yield, protein, and oil. Agron. J. 2009, 101, 971–978. [Google Scholar] [CrossRef]
- Akond, A.G.M.; Ragin, B.; Bazzelle, R.; Kantartzi, S.K.; Meksem, K.; Kassem, M.A. Quantitative trait loci associated with moisture, protein, and oil content in soybean [Glycine max (L.) Merr.]. J. Agric. Sci. 2012, 4, 16–25. [Google Scholar] [CrossRef]
- Kaya, Y.; Akcura, M. Effects of genotype and environment on grain yield and quality traits in bread wheat (T. aestivum L.). Food Sci. Technol. 2014, 34, 386–393. [Google Scholar] [CrossRef]
- Rodrigues, J.I.; Arruda, K.M.; Cruz, C.D.; Piovesan, N.D.; Barros, E.G.; Moreira, M.A. Biometric analysis of protein and oil contents of soybean genotypes in different environments. Pesq. Agropec. Bras. 2014, 49, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Mladenov, N.; Hristov, N.; Kondic-Spika, A.; Djuric, V.; Jevtic, R.; Mladenov, V. Breeding progress in grain yield of winter wheat cultivars grown at different nitrogen levels in semiarid conditions. Breed. Sci. 2011, 61, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Hristov, N.; Mladenov, N.; Djuric, V.; Kondic-Spika, A.; Marjanovic-Jeromela, A. Improvement of wheat quality in cultivars released in Serbia during the 20th century. Cereal Res. Commun. 2010, 38, 111–121. [Google Scholar] [CrossRef]
- Clarke, J.M.; Clarke, F.R.; Ames, N.P.; McCaig, T.N.; Knox, R.E. Evaluation of predictors of quality for use in early generation selection. In Proceedings of the Durum wheat improvement in the Mediterranean region: New challenges Zaragoza: CIHEAM. Serie A. Mediterranean Seminars, Zaragoza, Spain, 12–14 April 2000; pp. 439–446. [Google Scholar]
- Rakszegi, M.; Boros, D.; Kuti, C.; Láng, L.; Bedo, Z.; Shewry, P.R. Composition and end-use quality of 150 wheat lines selected for the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9750–9757. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Westgate, M.E. Meta-analysis of environmental effects on soybean seed composition. Field Crops Res. 2009, 110, 147–156. [Google Scholar] [CrossRef]
- Barbosa, V.D.S.; Peluzio, J.M.; Afférri, F.S.; Siqueira, D.G.B. Comportamento de cultivares de soja, emdiferentesépocas de semeaduras, visando a produção de biocombustível. Rev. Ciênc. Agron. 2011, 42, 742–749. [Google Scholar] [CrossRef]
- Pimsaen, W.; Jogloy, S.; Suriharn, B.; Kesmala, T.; Pensuk, V.; Patanothai, A. Genotype by environment (G × E) interactions for yield components of Jerusalem Artichoke (Helianthus tuberosus L.). Asian J. Plant Sci. 2010, 9, 11–19. [Google Scholar] [CrossRef]
- Janket, A.; Jogloy, S.; Vorasoot, N.; Kesmala, T.; Holbrook, C.; Patanothai, A. Genetic diversity of water use efficiency in Jerusalem artichoke (Helianthus tuberosus L.) germplasm. Aust. J. Crop Sci. 2013, 7, 1670–1681. [Google Scholar]
- Reynolds, M.P.; Trethowan, R.; Crossa, J.; Vargas, M.; Sayre, K.D. Erratum to “Physiological factors associated with genotype by environment interaction in wheat” (Field Crops Res. 75, 2002, 139–160). Field Crops Res. 2004, 85, 251. [Google Scholar] [CrossRef]
- Fisher, R.A. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agric. Sci. 1985, 108, 447–461. [Google Scholar] [CrossRef]

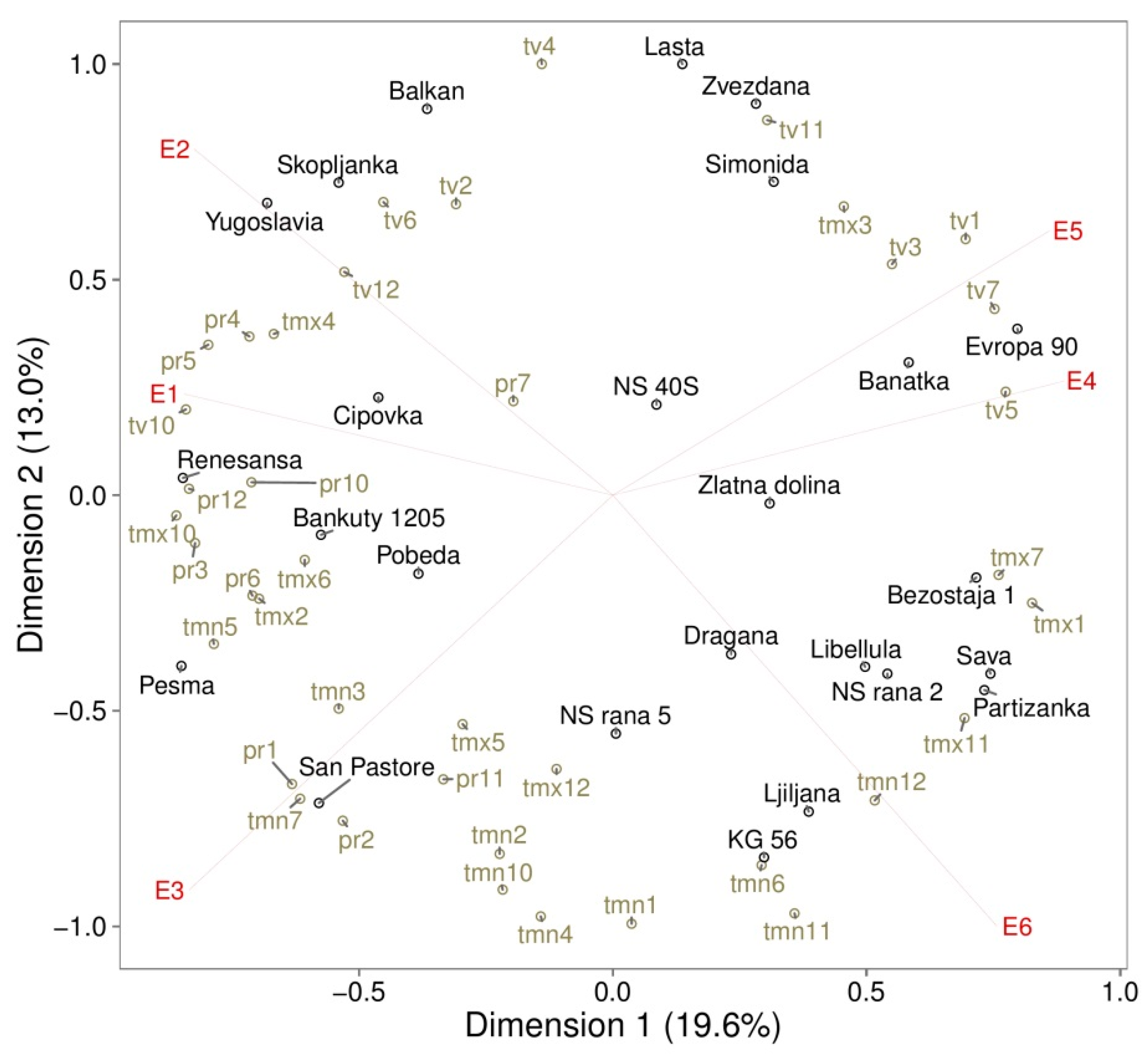

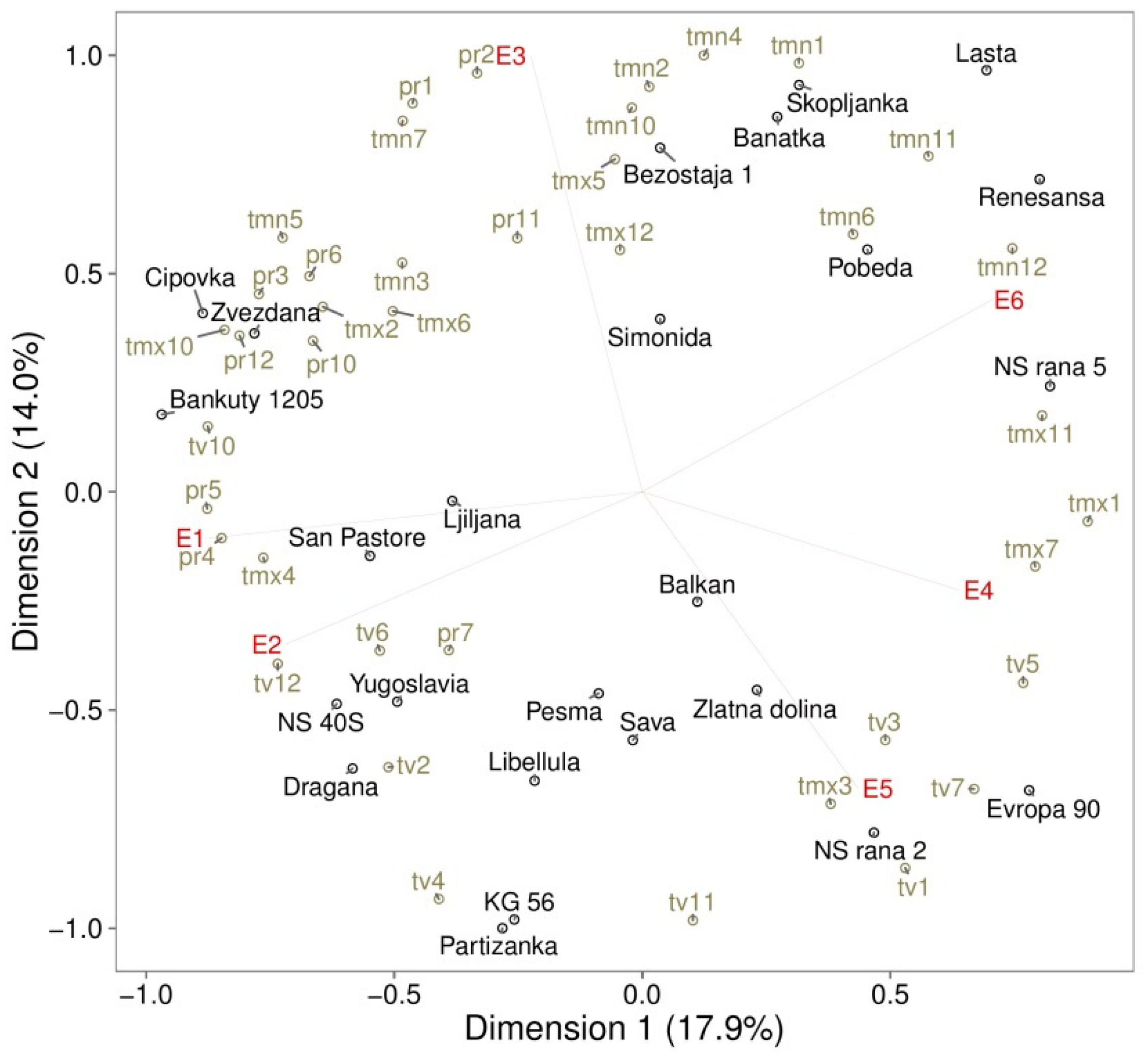

| Cultivar | Year of Release (YOR) | Country of Origin |
|---|---|---|
| Banatka | 1955 | Serbia |
| Bankuty 1205 | 1955 | Hungary |
| San Pastore | 1958 | Italy |
| Bezostaja 1 | 1959 | Russia |
| Libellula | 1962 | Italy |
| Zlatna Dolina | 1970 | Croatia |
| Sava | 1970 | Serbia |
| Partizanka | 1973 | Serbia |
| NS rana 2 | 1975 | Serbia |
| KG 56 | 1975 | Serbia |
| Balkan | 1979 | Serbia |
| Yugoslavia | 1980 | Serbia |
| Skopljanka | 1982 | Macedonia |
| Lasta | 1987 | Serbia |
| Evropa 90 | 1990 | Serbia |
| NS rana 5 | 1992 | Serbia |
| Pobeda | 1990 | Serbia |
| Renesansa | 1994 | Serbia |
| Pesma | 1995 | Serbia |
| Ljiljana | 2000 | Serbia |
| Cipovka | 2002 | Serbia |
| Dragana | 2002 | Serbia |
| Simonida | 2003 | Serbia |
| Zvezdana | 2005 | Serbia |
| NS 40S | 2006 | Serbia |
| OIL CONTENT (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | Avg | LSD (0.05)a | |
| Banatka | 3.56 | 3.82 | 3.54 | 3.80 | 3.90 | 4.08 | 3.8 | cb |
| Bankut 1205 | 3.47 | 3.31 | 3.86 | 4.29 | 3.70 | 3.85 | 3.7 | cd |
| San Pastore | 3.84 | 4.05 | 3.76 | 3.65 | 3.90 | 3.39 | 3.8 | cd |
| Bezostaja 1 | 4.29 | 4.12 | 4.06 | 4.16 | 3.76 | 3.51 | 4.0 | bc |
| Libelula | 3.36 | 3.90 | 3.46 | 4.01 | 4.03 | 2.97 | 3.6 | d |
| Zlatna dolina | 2.49 | 2.98 | 2.40 | 3.49 | 3.84 | 3.55 | 3.1 | cd |
| Sava | 4.01 | 2.49 | 4.51 | 4.03 | 3.47 | 3.51 | 3.7 | cd |
| Partizanka | 3.82 | 3.59 | 4.37 | 3.88 | 3.73 | 3.53 | 3.8 | c |
| NS rana 2 | 4.10 | 4.66 | 5.13 | 3.41 | 2.93 | 2.81 | 3.8 | c |
| KG 56 | 5.00 | 5.04 | 3.62 | 3.69 | 3.10 | 2.63 | 3.8 | c |
| Balkan | 3.23 | 3.24 | 3.55 | 3.00 | 2.62 | 3.11 | 3.1 | d |
| Jugoslavija | 3.57 | 2.75 | 4.01 | 3.97 | 3.45 | 3.88 | 3.6 | cd |
| Skopljanka | 2.78 | 5.03 | 4.43 | 3.20 | 3.42 | 3.21 | 3.7 | cd |
| Lasta | 3.61 | 3.67 | 3.47 | 3.50 | 3.44 | 4.34 | 3.7 | cd |
| Evropa 90 | 3.59 | 4.32 | 3.49 | 4.13 | 3.87 | 4.04 | 3.9 | bc |
| NS rana 5 | 3.41 | 4.65 | 3.97 | 4.01 | 3.89 | 4.36 | 4.0 | abc |
| Pobeda | 3.43 | 4.44 | 3.74 | 4.38 | 4.39 | 3.59 | 4.0 | abc |
| Renesansa | 5.30 | 4.98 | 4.44 | 4.42 | 3.23 | 4.76 | 4.5 | ab |
| Pesma | 4.17 | 4.12 | 4.65 | 3.81 | 2.82 | 4.41 | 4.0 | abc |
| Ljiljana | 4.36 | 2.75 | 3.80 | 4.21 | 4.12 | 4.53 | 4.0 | bc |
| Cipovka | 4.33 | 3.60 | 3.31 | 4.39 | 3.40 | 4.43 | 3.9 | bc |
| Dragana | 3.57 | 2.72 | 4.11 | 4.62 | 4.39 | 4.50 | 4.0 | bc |
| Simonida | 3.80 | 3.24 | 4.16 | 4.72 | 4.07 | 4.48 | 4.1 | abc |
| NS 40S | 3.76 | 3.77 | 3.79 | 3.88 | 3.96 | 4.67 | 4.0 | bc |
| Zvezdana | 3.64 | 4.10 | 5.49 | 4.32 | 4.73 | 5.57 | 4.6 | a |
| LSD (0.05) | a | a | a | a | a | a | ||
| Max | 5.3 | 5.0 | 5.5 | 4.7 | 4.7 | 5.6 | ||
| Min | 2.5 | 2.5 | 2.4 | 3.0 | 2.6 | 2.6 | ||
| Avg | 3.8 | 3.8 | 4.0 | 4.0 | 3.7 | 3.9 | ||
| StDev | 0.60 | 0.76 | 0.63 | 0.43 | 0.50 | 0.70 | ||
| CV | 15.97 | 19.82 | 15.77 | 10.87 | 13.69 | 18.02 | ||
| PROTEIN CONTENT (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | Avg | LSD (0.05)a | |
| Banatka | 16.7 | 15.3 | 17.4 | 15.4 | 14.2 | 16.5 | 15.9 | ab |
| Bankut 1205 | 16.2 | 15.5 | 17.8 | 15.6 | 13.5 | 15.6 | 15.7 | ab |
| San Pastore | 14.0 | 14.9 | 14.2 | 12.0 | 13.5 | 14.2 | 13.8 | cde |
| Bezostaja 1 | 15.3 | 14.8 | 16.0 | 13.6 | 13.8 | 15.2 | 14.8 | abcd |
| Libelula | 14.3 | 13.8 | 15.3 | 12.5 | 13.6 | 15.2 | 14.1 | cde |
| Zlatna dolina | 13.7 | 14.5 | 13.5 | 12.8 | 14.0 | 14.5 | 13.8 | cde |
| Sava | 14.8 | 15.4 | 15.6 | 13.3 | 14.8 | 15.9 | 15.0 | abc |
| Partizanka | 14.6 | 14.8 | 15.1 | 13.1 | 13.5 | 14.9 | 14.3 | cde |
| NS rana 2 | 15.2 | 14.1 | 15.5 | 13.3 | 14.2 | 15.4 | 14.6 | bcde |
| KG 56 | 15.8 | 14.8 | 15.1 | 12.2 | 14.9 | 14.1 | 14.5 | bcde |
| Balkan | 15.4 | 14.5 | 14.9 | 13.0 | 14.2 | 14.2 | 14.3 | cde |
| Jugoslavija | 15.9 | 14.4 | 15.2 | 12.5 | 14.5 | 14.4 | 14.5 | bcde |
| Skopljanka | 15.5 | 15.3 | 15.3 | 12.6 | 13.8 | 14.7 | 14.5 | bcde |
| Lasta | 14.7 | 15.0 | 14.4 | 11.7 | 13.9 | 14.0 | 14.0 | cde |
| Evropa 90 | 14.5 | 14.3 | 14.5 | 12.3 | 13.3 | 14.1 | 13.9 | cde |
| NS rana 5 | 15.4 | 15.1 | 14.6 | 11.8 | 14.3 | 14.5 | 14.3 | cde |
| Pobeda | 16.1 | 14.7 | 15.3 | 12.4 | 13.7 | 14.4 | 14.4 | bcde |
| Renesansa | 16.3 | 15.0 | 15.4 | 11.7 | 13.9 | 14.1 | 14.4 | cde |
| Pesma | 16.1 | 14.7 | 14.6 | 12.3 | 13.9 | 14.7 | 14.4 | cde |
| Ljiljana | 14.9 | 14.6 | 14.0 | 11.8 | 13.7 | 14.4 | 13.9 | cde |
| Cipovka | 15.6 | 14.4 | 14.3 | 12.3 | 14.4 | 15.0 | 14.4 | cde |
| Dragana | 13.7 | 15.4 | 14.1 | 11.5 | 14.3 | 14.1 | 13.8 | cde |
| Simonida | 14.9 | 14.0 | 13.1 | 11.2 | 14.0 | 13.3 | 13.4 | e |
| NS 40S | 13.7 | 14.7 | 13.3 | 12.0 | 13.8 | 14.3 | 13.6 | de |
| Zvezdana | 15.2 | 14.8 | 14.2 | 11.9 | 14.9 | 13.8 | 14.2 | cde |
| LSD (0.05) | a | ab | ab | d | c | b | ||
| Max | 16.7 | 15.5 | 17.8 | 15.6 | 14.9 | 16.5 | ||
| Min | 13.7 | 13.8 | 13.1 | 11.2 | 13.3 | 13.3 | ||
| Avg | 15.1 | 14.8 | 14.9 | 12.6 | 14.0 | 14.6 | ||
| StDev | 0.86 | 0.44 | 1.09 | 1.05 | 0.44 | 0.71 | ||
| CV | 5.66 | 2.98 | 7.32 | 8.35 | 3.16 | 4.86 | ||
| GRAIN YIELD (t/ha) | ||||||||
|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | Average | LSD (0.05)a | |
| Banatka | 4.23 | 2.28 | 2.66 | 5.47 | 5.44 | 2.91 | 3.8 | eb |
| Bankut 1205 | 4.31 | 2.36 | 2.81 | 5.49 | 5.40 | 2.87 | 3.9 | e |
| San Pastore | 5.26 | 3.58 | 4.99 | 7.46 | 5.94 | 4.46 | 5.3 | de |
| Bezostaja 1 | 5.89 | 3.86 | 4.66 | 7.37 | 6.58 | 4.13 | 5.4 | cde |
| Libelula | 5.85 | 4.59 | 4.90 | 7.86 | 6.87 | 4.59 | 5.8 | bcd |
| Zlatna dolina | 5.79 | 5.18 | 6.15 | 8.39 | 7.30 | 5.09 | 6.3 | abcd |
| Sava | 5.85 | 5.01 | 5.81 | 8.42 | 7.41 | 5.01 | 6.3 | abcd |
| Partizanka | 6.23 | 5.27 | 5.69 | 8.33 | 7.25 | 5.35 | 6.4 | abcd |
| NS rana 2 | 6.60 | 5.36 | 5.80 | 8.83 | 7.43 | 5.51 | 6.6 | abcd |
| KG 56 | 6.80 | 5.31 | 5.42 | 8.83 | 7.81 | 5.41 | 6.6 | abcd |
| Balkan | 6.64 | 5.59 | 5.97 | 8.97 | 7.77 | 5.54 | 6.7 | abcd |
| Jugoslavija | 7.61 | 5.68 | 5.67 | 9.11 | 8.07 | 5.85 | 7.0 | abc |
| Skopljanka | 7.31 | 5.75 | 5.64 | 8.97 | 8.61 | 5.72 | 7.0 | abc |
| Lasta | 7.60 | 5.35 | 6.26 | 9.28 | 8.30 | 6.17 | 7.2 | ab |
| Evropa 90 | 7.72 | 5.87 | 5.82 | 9.45 | 8.67 | 6.10 | 7.3 | ab |
| NS rana 5 | 7.60 | 5.48 | 5.86 | 9.28 | 8.35 | 6.09 | 7.1 | ab |
| Pobeda | 7.27 | 5.79 | 5.66 | 9.76 | 8.48 | 6.23 | 7.2 | ab |
| Renesansa | 7.70 | 6.03 | 5.49 | 9.36 | 8.69 | 6.27 | 7.3 | ab |
| Pesma | 8.16 | 6.21 | 5.93 | 9.58 | 8.65 | 5.69 | 7.4 | ab |
| Ljiljana | 7.88 | 5.92 | 5.99 | 9.76 | 8.14 | 6.26 | 7.3 | ab |
| Cipovka | 8.07 | 5.73 | 6.14 | 9.36 | 8.65 | 6.50 | 7.4 | ab |
| Dragana | 8.03 | 6.03 | 6.34 | 9.24 | 8.33 | 6.20 | 7.4 | ab |
| Simonida | 8.38 | 6.35 | 6.76 | 9.81 | 9.45 | 6.62 | 7.9 | a |
| NS 40S | 8.58 | 6.19 | 6.35 | 9.76 | 9.74 | 6.70 | 7.9 | a |
| Zvezdana | 8.66 | 6.22 | 6.70 | 9.58 | 9.29 | 6.54 | 7.8 | a |
| LSD (0.05) | c | d | d | a | b | d | ||
| Max | 8.7 | 6.4 | 6.8 | 9.8 | 9.7 | 6.7 | ||
| Min | 4.2 | 2.3 | 2.7 | 5.5 | 5.4 | 2.9 | ||
| Avg | 7.0 | 5.2 | 5.6 | 8.7 | 7.9 | 5.5 | ||
| StDev | 1.26 | 1.11 | 0.99 | 1.19 | 1.15 | 1.04 | ||
| CV | 18.05 | 21.18 | 17.73 | 13.66 | 14.62 | 18.95 | ||
| E1 | E2 | E3 | E4 | E5 | E6 | Average | |
|---|---|---|---|---|---|---|---|
| OC-GY | 0.19 | 0.05 | 0.17 | 0.13 | 0.08 | 0.44* | 0.34 |
| PC-GY | −0.14 | −0.36 | −0.88 ** | −0.86 ** | 0.22 | −0.74 ** | −0.74 ** |
| PC-OC | 0.31 | −0.03 | 0.11 | −0.21 | −0.11 | −0.34 | −0.09 |
| OC-YOR | 0.511 ** | ||||||
| PC-YOR | −0.603 ** | ||||||
| GY-YOR | 0.914 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondić-Špika, A.; Mladenov, N.; Grahovac, N.; Zorić, M.; Mikić, S.; Trkulja, D.; Marjanović-Jeromela, A.; Miladinović, D.; Hristov, N. Biometric Analyses of Yield, Oil and Protein Contents of Wheat (Triticum aestivum L.) Genotypes in Different Environments. Agronomy 2019, 9, 270. https://doi.org/10.3390/agronomy9060270
Kondić-Špika A, Mladenov N, Grahovac N, Zorić M, Mikić S, Trkulja D, Marjanović-Jeromela A, Miladinović D, Hristov N. Biometric Analyses of Yield, Oil and Protein Contents of Wheat (Triticum aestivum L.) Genotypes in Different Environments. Agronomy. 2019; 9(6):270. https://doi.org/10.3390/agronomy9060270
Chicago/Turabian StyleKondić-Špika, Ankica, Novica Mladenov, Nada Grahovac, Miroslav Zorić, Sanja Mikić, Dragana Trkulja, Ana Marjanović-Jeromela, Dragana Miladinović, and Nikola Hristov. 2019. "Biometric Analyses of Yield, Oil and Protein Contents of Wheat (Triticum aestivum L.) Genotypes in Different Environments" Agronomy 9, no. 6: 270. https://doi.org/10.3390/agronomy9060270






