Abstract
The objective of this study was to assess the impacts of nitrogen on the physiological characteristics of the source–sink system of upper fruiting branches under various amounts of nitrogen fertilization. A two-year field experiment was conducted with a Bt cotton cultivar in the Yellow River Basin of China. The growth and yield of cotton of the upper fruiting branches were compared under four nitrogen levels: Control (N0, 0 kg ha−1), low nitrogen (N1, 120 kg ha−1), moderate nitrogen (N2, 240 kg ha−1), and high nitrogen (N3, 480 kg ha−1). The results indicated that in the subtending leaves in upper fruiting branches, chlorophyll content, protein content, and peroxidase (POD) activity dramatically increased with nitrogen application, reaching the highest under the moderate nitrogen treatment. The physiological characters in the seeds had the same trends as in the subtending leaves. Furthermore, the moderate nitrogen rate (240 kg ha−1) had a favorable yield and quality. Our results supported that a moderate nitrogen rate (240 kg ha−1) could coordinate the source–sink growth of cotton in the late stage, enhance the yield and fiber quality, and decrease the cost of fertilizer in the Yellow River Basin of China and other similar ecological areas.
1. Introduction
Nitrogen is a critical element for cotton growth and development, and it is required more consistently and in larger amounts than other elements [1]. Nitrogen is also an essential element for canopy development and photosynthesis [2]. Nitrogen fertilization has significant impacts on cotton growth, boll development, lint yield, and fiber quality [3,4,5]. In addition, nitrogen can also improve salinity tolerance, water productivity, and nitrogen use efficiency [6,7,8]. However, nitrogen management of cotton is especially difficult because of cotton’s indeterminate growth characteristics. This could lead to unbalanced growth with either inadequate or excessive rates of nitrogen [9]. Low rates of nitrogen fertilization often result in poor growth and development, and eventually low yield [10]. On the contrary, high rates of nitrogen fertilization frequently lead to a decrease in boll production as a result of excessive vegetation development and maturation of yield later in the growing season. Therefore, proper nitrogen management in cotton is very difficult and important. Thus, nitrogen is treated as one of the key factors in cotton production [11]. In recent years, with the emergence of global environmental issues, rising awareness of global warming and the increased cost of nitrogen have spurred an interest in the investigation of nitrogen fertilization. A number of studies over the past few decades have investigated the effects of nitrogen on cotton growth [12,13,14]. These studies focused on the effects of nitrogen application on cotton leaves or on lint yield. Most research has focused on the main stem leaves or on the bolls separately. However, the effects of nitrogen rate on bolls and subtending leaves of upper fruiting branches have not been investigated, although it is understood that upper fruiting branches are the most sensitive part with regards to premature senescence in cotton.
Nitrogen fertilizer has been shown to significantly impact physiologically active substances in cotton growth. Nitrogen influences the formation of various critical components of the cotton plant such as chlorophyll, protein, enzymes, and phytohormones [15]. Hence, nitrogen affects the physiological characteristics of cotton, which further significantly influence the growth and directly impact the morphology. Eventually, nitrogen will determine the final yield and quality. Optimum application of nitrogen fertilizer has been shown to increase chlorophyll [16]. Therefore, optimum nitrogen will improve photosynthetic capacity and increase leaf number, leaf area, plant height, and number of nodes [17,18,19]. The number of bolls, individual boll weight, yield, and fiber quality are also consistently affected by nitrogen [5]. Preliminary results have shown that the upper fruiting branches were more sensitive to nitrogen, which could reflect the premature senescence in cotton [20]. However, how the physiologically active substances of upper fruiting branches are changed is unknown. Therefore, it is necessary to investigate in detail the physiological and biochemical changes of the boll–leaf system in upper fruiting branches.
Crop yield formation is essentially the process of source–sink interactions. In cotton, it is especially important to coordinate the source–sink system because of the property of a long coexisting period of reproductive growth along with vegetative growth. Unbalance in the source–sink system would eventually depress the yield [21]. The boll and the subtending leaf are the major portions of the sink and source of cotton photosynthesis, which are closely linked to the synthesis, transportation, and distribution of photosynthetic material products. Excessive vegetative growth (source) produces excess photosynthesis, which decreases boll growth (sink), which in turn delays maturity [22,23,24]. However, insufficient source growth will lead to premature senescence and reduced boll production and yield [5]. Excessive vegetative growth causes excess source that will decrease the growth of the sink and in turn delay boll maturity [22,23,24]. However, insufficient source growth will lead to premature senescence. Therefore, boll production and yield will be limited [5]. The upper fruiting branches are more sensitive to the late growth of cotton and can be used as a reflection of premature senescence. Therefore, the “boll–leaf system” of upper fruiting branches is the key location to diagnose the growing conditions, but how it relates to nitrogen is yet unknown.
It is not clear how nitrogen fertilization rate affects the source–sink system of upper fruiting branches. The objectives of this study were to investigate the effects of nitrogen fertilization rate on physical biochemistry features in relation to the “boll–leaf system” of the upper fruiting branches, and most importantly to quantify the relationship between the physiological substances in the “boll–leaf system” of upper fruiting branches and premature aging or late maturity under different nitrogen treatments, and then discuss the optimal nitrogen rate for coordinated growth of source and sink at the late stage of cotton.
2. Materials and Methods
2.1. Experimental Design
The field study was conducted in 2011 and 2012 at the experimental station (38.85° N, 115.30° E) in Hebei Province, in the Yellow River Delta of China. This site has a temperate climate, and the soil, which is upland soil, was sampled at a profile depth of 20 cm before planting. This field was sown with wheat and corn before cotton. The soil type was loam with organic matter (16.4 g kg−1), with total nitrogen of 1.13 g kg−1, available N of 79.83 mg kg−1, available P of 15.36 mg kg−1, and available K of 191 mg kg−1. The soil indexes were determined before fertilization. The growth and yield of cotton was compared under four treatment fertilizer nitrogen levels: Control (N0, 0 kg ha−1), low nitrogen (N1, 120 kg ha−1), moderate nitrogen (N2, 240 kg ha−1), and high nitrogen (N3, 480 kg ha−1). The amount of nitrogen fertilizer was decided according to the local conditions and preliminary experiment [20]. Half of each N rate (Urea: 0, 130.4, 260.9, and 521.7 kg ha−1) was applied basally before planting, and the other half was furrow-dressed at the flowering stage and the peaking bolls setting stage. All plots received a basal rate of 135 kg ha−1 P2O5 and 75kg ha−1 K2O based on local practice. The field was fertilized, irrigated, harrowed, ploughed, and then sowed. A plastic film cover was used after sowing to increase soil temperature, reduce evaporation, and restrain weeds. A high-yielding commercial transgenic cotton cultivar with the Bt Cry1A gene, SCRC 28, was planted on 28 Apr. 2011 and 19 Apr. 2012 at a planting density of 45,000 plants per ha. Each plot contained ten rows of cotton, 14.5 m long with line spacings of 0.50 and 1.00 m and an inter-row spacing of 0.29 m. Fertilizer amounts and fertilizer periods are shown in Table 1. During the growing period, all the plots were irrigated once (late June of 2011 and 2012). Planting methods and cultivation management used the conventional high-yield cultivation mode.

Table 1.
Fertilizer rates under different nitrogen treatments.
2.2. Sampling and Investigation
During the mid and late cotton growth stages, the inner and outer blooms of the upper fruiting branches (12th–14th fruiting branches) in the central four rows of each plot were tagged and the dates of observation were recorded. The flower and the subtending leaf at the inner nodes of each upper fruit branch were named the inner flower and inner leaf, and those at the outer nodes were named the outer flower and outer leaf (Figure 1). Every 10 days starting from the flowering day, the subtending leaf and boll were sampled in order to reflect the changes of the boll–leaf system. About six leaves or bolls were sampled for each treatment. The dates of sampling the inner flowers were 31 Jul. 2011 and 7 Aug. 2012. The dates of sampling of the inner leaves and bolls were 8 Aug., 20 Aug., 30 Aug., and 9 Sep. in 2011; and 17 Aug., 27 Aug., and 8 Sep. in 2012. The dates of sampling the outer flowers were 19 Aug. 2011 and 16 Aug. 2012. The dates of sampling of the outer leaves and bolls were 29 Aug., 8 Sep., and 20 Sep. in 2011; and 26 Aug. and 5 Sep. in 2012.
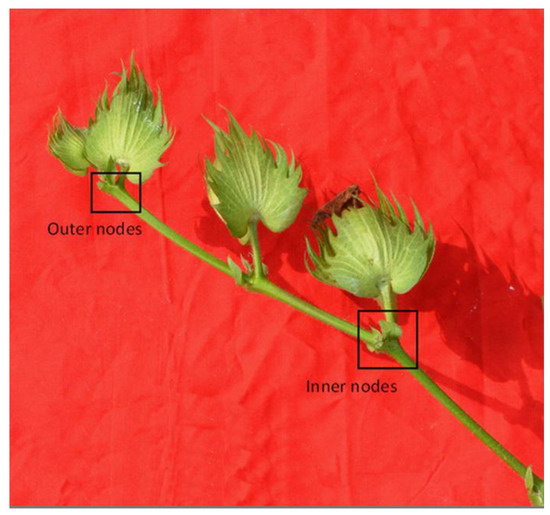
Figure 1.
A sample of inner nodes, outer nodes, inner diameter, and outer diameter of a fruiting branch.
2.3. Physiological Measurements and Sampling
The tagged leaves and bolls were sampled every ten days after flowering between 7 and 8 am in the morning, and the leaves were taken to the laboratory. Three fresh tagged leaves were taken to measure chlorophyll content, while other flag leaves and boll seeds were kept at −80 °C after being quickly weighed out, and were frozen by liquid nitrogen for measuring the contents of soluble protein and malondialdehyde (MDA), and enzyme activity.
The chlorophyll content was measured using triplicate 0.1 g leaves, which were extracted and placed into three test tubes [25]. The samples were dissolved in 10 cm3 of 95% ethanol for 24 h. The supernatant obtained was used to assay the chlorophyll content using colorimetric determination with a UV-2450 spectrophotometer at 665, 649, and 470 nm colorimetric wavelengths.
Soluble protein was estimated based on the method described by Read and Northcote [26]. For determination of soluble protein, 0.1 g leaves or seeds were crushed with 5.0 cm3 distilled water in ice bath conditions, and the homogenate was centrifuged at 3000 r/min for 5 min. Then, the appropriate supernatant (1 cm3) and 5 cm3 Coomassie brilliant blue G-250 were added, and after 2 min of standing, the absorbance was read at 595 nm.
MDA content was calculated using the method of Zou [27]. Cotton leaves of seeds were weighed to achieve 0.5 g samples, which were crushed in 5 cm3 10% trichloroacetic acid (TCA), then ground to form a homogenate. Then, the homogenate was centrifuged at 10,000 g/min for 10 min. Two mL supernatant and 2 mL 0.6% thiobarbituric acid (TBA) were mixed, and then the homogenate was heated in a 100 °C water bath for 15 min. Later on, after immediately cooling, the homogenate was centrifuged at 4000 r/min for 10 min. Finally, the absorbance of the supernatant was measured at 450, 532, and 600 nm.
In order to determine the superoxide dismutase (SOD) and peroxidase (POD) activities in leaves and seeds, 0.5 g samples were homogenized in 5 cm3 of 50 mM Na–phosphate buffer (pH 7.8) under chilled conditions. The crude extract was centrifuged at 10,000 g for 20 min at 4 °C, and the supernatant was used for the determination of SOD and POD activities. SOD activity was estimated based on the method of Zou, using the photochemical nitroblue tetrazolium chloride (NBT) method [27]. The reaction mixture (4 cm3) contained 1 cm3 0.03 mM/cm3 NBT, 0.5 cm3 104 mM/cm3 L-methionine, 0.5 cm3 0.8 mM cm−3 ethylene diamine tetraacetic acid (EDTA), and 0.05 cm3 0.32 mM cm−3 riboflavin in 2 cm3 50 mM cm−3 Na–phosphate buffer (pH 7.8). Absorbance was read at 560 nm. POD activity was estimated using guaiacol as the substrate in a total volume of 3 cm3 [28]. The reaction solution consisted of 0.02 cm3 crude extract, 2.91 cm3 50 mM Na phosphate buffer (pH 7.0), 0.05 cm3 20 mM/cm3 guaiacol, and 0.02 cm3 40 mM/cm3 H2O2. The change of absorbance was measured at 470 nm due to guaiacol oxidation.
2.4. Measurements of Quality and Yield
To measure the fiber quality, the inner and outer bolls of the upper fruiting branches were picked separately. Fiber quality, i.e., length (mm), uniformity (%), micronaire, elongation (%), and strength (cN tex-1181), was tested according to ASTMD5867-955HVI900 methods by the Supervision and Testing Center for Cotton Quality, Ministry of Agriculture. In order to get the seed yield and lint yield, plants in the central four rows of each plot were harvested two times (on 12 Oct. and 6 Nov. 2011, and 5 Oct. and 3 Nov. 2012). The seed yield was determined after the seed cotton was dried in the sun. Seed cotton was ginned on a hand-fed laboratory gin, and lint yield was then determined after ginning.
2.5. Statistical Analysis
The one-way ANOVA was performed, with the complete randomized analysis calculating the standard deviation (SD) and differences in measured variables between different nitrogen fertilizer rate treatments according to Duncan’s multiple range tests at the 5% probability level. All the analyses were performed separately for each year and each sampling time using the SPSS 17.0 software. Spearman’s method of SPSS was used to analyze the correlative coefficients of nitrogen treatment, seed yield, lint yield, and physiological indexes.
3. Results
3.1. Influences of Nitrogen on Physiological Indexes in Subtending Leaves of Upper Fruiting Branches
As shown in Figure 2 and Figure 3, the application of nitrogen fertilizer in the field increased the chlorophyll (a+b) content in the subtending leaves of the upper fruiting branches. Compared to no nitrogen, chlorophyll (a+b) content in subtending leaves with moderate nitrogen fertilizer was significantly increased by more than 12.6% and 20.8% on the inner leaves in 2011 and 2012, respectively (Figure 2A,B). The chlorophyll (a+b) contents with a moderate nitrogen rate in the outer subtending leaves were also higher than those with no nitrogen (Figure 3A,B).
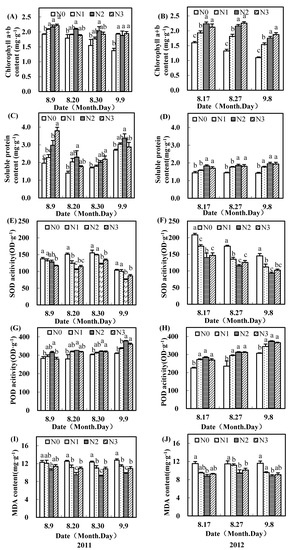
Figure 2.
Effects of nitrogen fertilizer on chlorophyll a+b (A,B), soluble protein content (C,D), superoxide dismutase (SOD) activity (E,F), peroxidase (POD) activity (G,H), and malondialdehyde (MDA) content (I,J) in inner subtending leaves of cotton upper fruiting branches surveyed in the Yellow River Basin of China in 2011 and 2012.
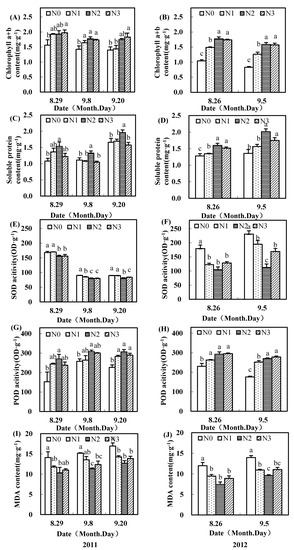
Figure 3.
Effects of nitrogen fertilizer on chlorophyll a+b (A,B), soluble protein content (C,D), SOD activity (E,F), POD activity (G,H), and MDA content (I,J) in outer subtending leaves of cotton upper fruiting branches surveyed in the Yellow River Basin of China in 2011 and 2012.
Soluble protein contents of upper fruiting branches’ leaves with a moderate nitrogen rate were significantly higher than those of no nitrogen. The inner ones improved soluble protein content by more than 2.9% and 27.0% separately in 2011 and 2012, respectively, and the outer ones had 19.5% and 24.9% higher soluble protein content separately in 2011 and 2012, respectively. POD activities in the subtending leaves of upper fruiting branches also first increased and then decreased with an increase in nitrogen rate (Figure 2G,H and Figure 3G,H). The POD activities in subtending leaves of upper fruiting branches with a moderate nitrogen rate were significantly greater than those with no nitrogen.
SOD activities and MDA content in subtending leaves of upper fruiting branches were reduced by moderate and high nitrogen fertilizer (Figure 2E,F,I,J; Figure 3E,F,I,J). Compared to no nitrogen, SOD activities with a moderate nitrogen rate were decreased by more than 6.7% and 7.2% in 2011, and 41.6% and 32.8% in 2012 for inner ones and outer subtending leaves, respectively. SOD activities with a moderate nitrogen rate mostly reached significant levels, except for in the inner subtending leaves at 9 Aug. in 2011. Compared to no nitrogen, MDA contents with a moderate nitrogen rate were significantly lower.
3.2. Influences of Nitrogen on Physiological Indexes in Seeds of Upper Fruiting Branches
The application of nitrogen fertilizer increased the soluble protein contents in upper fruiting branch seeds. The soluble protein contents under the moderate nitrogen rate were significantly higher than those under no nitrogen (Figure 4A,B; Figure 5A,B). Compared to N0, N2 treatment improved soluble protein contents in inner seeds by more than 22.0% and 16.3% separately in 2011 and 2012, respectively, and soluble protein contents in outer seeds were 27.8% and 14.0% higher separately in 2011 and 2012, respectively.
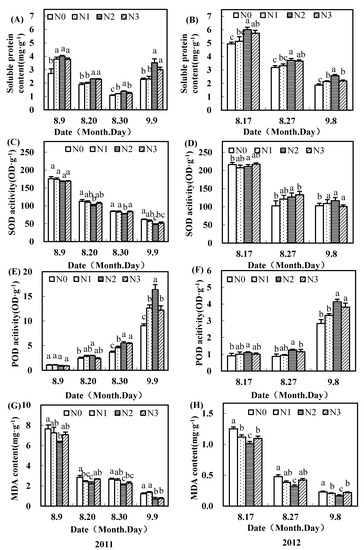
Figure 4.
Effects of nitrogen fertilizer on soluble protein content (A,B), SOD activity (C,D), POD activity (E,F), and MDA content (G,H) in inner seeds of cotton upper fruiting branches surveyed in the Yellow River Basin of China in 2011 and 2012.
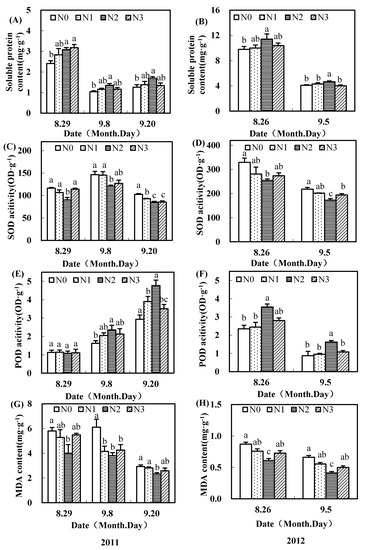
Figure 5.
Effects of nitrogen fertilizer on soluble protein content (A,B), SOD activity (C,D), POD activity (E,F), and MDA content (G,H) in outer seeds of upper fruiting branches surveyed in the Yellow River Basin of China in 2011 and 2012.
Compared to N0, N2 treatment significantly increased the POD activities in upper fruiting branch seeds, except in inner seeds 10 days after flowering (9 Aug. 2011 and 17 Aug. 2012) (Figure 4E,F; Figure 5E,F). After flowering, the POD activities of inner seeds increased, while those of outer seeds decreased.
In each sampling time, compared to N0, N2 treatment significantly decreased the SOD activities and MDA contents in seeds of upper fruiting branches (Figure 4C,G,H; Figure 5C,D,G,H). Meanwhile, the changes of SOD activities in inner seeds in 2012 had various changes (Figure 4D). The diversity of SOD activities of outer seeds was greater than in the inner seeds, and SOD activities of outer seeds were higher than those in the inner ones. With the increase of nitrogen fertilizer, MDA content of upper fruiting branches’ seeds first decreased and then increased, and MDA content with a moderate nitrogen rate was significantly lower than with no nitrogen. MDA content of inner upper fruiting branches’ seeds decreased more than the outer ones with increasing time.
3.3. Influences of Nitrogen on Yield and Quality
Fiber uniformity, micronaires, and strength both at inner and outer nodes of upper fruiting branches increased with the rate of nitrogen fertilizer (Table 2). Fiber uniformity was the highest with the moderate nitrogen and high nitrogen rates. Additionally, micronaires first increased and then decreased with increasing nitrogen fertilizer rates. Compared to no nitrogen fertilization, micronaires of outer nodes in 2011 and of outer nodes in 2012 under N2 treatment reached a significant level. Fiber strength in both inner and outer nodes with a moderate nitrogen rate was significantly enhanced in comparison with no nitrogen fertilization at two years. These results indicated that fiber uniformity, micronaire, and fiber strength could be obviously improved with an appropriate quantity of nitrogen fertilizer, and the moderate nitrogen and high nitrogen rates had the best fiber uniformity and fiber strength. The moderate nitrogen rate led to the best micronaires.

Table 2.
Fiber quality in cotton fertilization rate studies conducted in the Yellow River Basin of China in 2011 and 2012.
Compared to no nitrogen fertilization, N2 treatment resulted in higher seed yield and lint yield (Table 3). The seed yield and lint yield separately increased by 18.9% and 49.3% in 2011, respectively, and 34.5% and 39.8% in 2012, respectively.

Table 3.
Seed yield and lint yield in cotton fertilization rate studies conducted in the Yellow River Basin of China in 2011 and 2012.
3.4. Correlation Coefficients between Nitrogen Treatment, Seed Yield, Lint Yield, and Physiological Indexes
The correlative coefficients among nitrogen treatment, seed yield, lint yield, and physiological indexes were analyzed (Table 4 and Table 5). Nitrogen treatment, seed yield, and lint yield were significantly correlated with outer leaves’ protein, inner leaves’ SOD, inner leaves’ MDA, inner seeds’ POD, and outer seeds’ MDA.

Table 4.
The correlation coefficients between nitrogen treatment, seed yield, lint yield, and physiological indexes in leaves of upper fruiting branches.

Table 5.
The correlation coefficients between nitrogen treatment, seed yield, lint yield, and physiological indexes in seeds of upper fruiting branches.
4. Discussion
4.1. Effects of Nitrogen Rate on Physiological Features of Upper Fruiting Branches
In this paper, we found that chlorophyll, protein, and MDA content, and SOD and POD activities in the inner and outer subtending leaves of upper fruiting branches were significantly affected by nitrogen fertilization rate. The moderate nitrogen rate generally had the highest chlorophyll and protein content, while it had the lowest MDA content. The activities of the protecting enzyme system in the leaves were different. SOD activity under the moderate nitrogen rate was lower than under the other treatments, but the POD activity was the highest. The decrease of chlorophyll and protein reflected the beginning of leaf senescence, while the rise of SOD activity is closely related to the generation of reactive oxygen species [29]. The results indicated that nitrogen deficiency decreased the nutritive substance content and caused senescence in the source system of upper fruiting branches. This is consistent with the changes in stem leaves [30]. In addition, nitrogen deficiency destroyed the balance of the formation and removal of reactive oxygen species (ROS) in the leaves, which caused the amassing of MDA, which inactivates the proteins and damages the membrane structure and physiological function. Excess ROS resulted in the changes of SOD and POD activities, which are the important enzymes included in the enzyme protection system [31]. Our results for SOD and POD were different from previous research, whose SOD and POD had the same trend [32]. This could be explained by the fact that too many reactive oxygen radicals in the subtending leaves of upper fruiting branches with a nitrogen deficiency caused the high SOD activity and brought about too much hydrogen peroxide, thus poisoning the cotton. Therefore, the POD activity could be inhibited. Compared to no nitrogen treatment, we can clearly know the conclusion that the source system of upper fruiting branches was the best with the moderate nitrogen rate.
The physiological differences in cotton seeds were similar to the ones in the subtending leaves of upper fruiting branches. In this study, the moderate nitrogen rate generally gave the highest protein content, and significantly decreased the MDA content and SOD activity in both inner and outer cotton seeds of upper fruiting branches. This indicated that the moderate nitrogen rate could ensure the enzyme metabolism in seeds, hence relieving the nitrogen stress and preventing the generation of large amounts of ROS.
In a word, nitrogen rate affected the metabolic processes of physiological activators both in the source and sink systems of upper fruiting branches. The growths of the source and sink systems were inhibited by nitrogen deficiency. Cotton grown with a low level of nitrogen has particularly low physiologically active substance content [11], and the metabolizing enzymes also decreased, resulting in a decrease in photosynthetic products and less vigorous growth. In addition, estrogen metabolism will affect the allocation of photosynthetic products [32] and will bring about a reduction of nutrition in the sink system, resulting in a decrease of related enzyme content, and the sink system may in turn affect the source system. Additionally, too much nitrogen may sometimes increase the chlorophyll content, but it could not enhance the other physiological activators in the source nor the sink systems and eventually resulted in an unbalance of source and sink. Thus, this study could provide favorable results that a moderate treatment (240 kg ha−1) turned out to be the most optimal to improve the physiological metabolism in the source–sink system of upper fruiting branches and accelerate the growth balance.
4.2. Effects of Nitrogen Rate on Boll Weight, Fiber Quality, and Yield
Our results indicated that the moderate nitrogen fertilization rate gave the best fiber quality on both the inner and outer bolls of upper fruiting branches. It also gave the highest seed yield and lint yield. On the contrary, a lack of nitrogen fertilization or excessive nitrogen fertilization both inhibited boll and fiber yield and quality formation, because the accumulation of the cotton boll process is the process of storage and redistribution of photosynthate as well as the synthesis results of various physiological processes, which are constrained by nitrogen [33].
Our results showed that a lack of nitrogen fertilization or an excess of nitrogen fertilization inhibited yield and fiber quality formation. Furthermore, seed yield and lint yield were significantly correlated with inner or outer leaves’ protein and MDA contents. There are many reasons for this inhibition caused by a lack of nitrogen fertilization. For example, with a lack of nitrogen fertilization, the nitrogen concentration in the subtending leaf is decreased, and the chlorophyll content is decreased, resulting in a decline in the photosynthesis and the physiologically active substances such that the carbohydrates supplying the bolls is decreased [11]. In addition, the deficiency of nitrogen could also contribute to metabolic disorders of carbon and nitrogen [34], and then the resulting increase of membrane lipid peroxidation results in MDA accumulation. A decrease in boll weight and fiber quality is the result of simultaneous inhibition of vegetative and reproductive growth. On the other hand, excessive nitrogen fertilization is beneficial for chlorophyll accumulation in leaves, and so the growth of the source system becomes a priority. This eventually results in an imbalance of vegetative and reproductive growth. Besides, the carbon and nitrogen metabolism are affected by excessive nitrogen fertilization, and nitrogen content in boll shells, seeds, and physiological metabolism would rise [35], which would inhibit the photosynthetic products’ transportation to the boll. This could cause a decrease in boll weight and fiber quality.
5. Conclusions
This study demonstrated that a moderate nitrogen rate (240 kg ha−1) guaranteed the coordination of the boll–leaf system of upper fruiting branches, and achieved the highest yield and better fiber quality. The results from our study imply that a rational application of nitrogen fertilizer (240 kg ha−1) is vitally important to coordinate the source–sink system, increase the yield and fiber quality, and decrease the cost of fertilizer.
Author Contributions
Conceptualization, L.L. and C.L.; Methodology, L.L.; Software, J.C.; Validation, J.C., L.L. and Z.W.; Formal Analysis, Z.L.; Investigation, J.C.; Resources, C.L.; Data Curation, S.S.; Writing—Original Draft Preparation, J.C.; Writing—Review & Editing, J.C. and C.L.; Visualization, Y.Z.; Supervision, H.S.; Project Administration, Z.B.; Funding Acquisition, C.L.
Funding
This study was supported by the National Natural Science Foundation of China, project no. 31171495 and 31301270, the Natural Science Foundation of Hebei, project no. C2016204088, and The Fund for Team building of the Crop Science Hebei Agricultural University, project no. TD2016C318.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hou, Z.; Li, P.; Li, B.; Gong, Z.; Wang, Y. Effects of fertigation scheme on N uptake and N use efficiency in cotton. Plant Soil 2007, 290, 115–126. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Oosterhuis, D.M. Canopy development and photosynthesis of cotton as influenced by nitrogen nutrition. J. Plant Nutr. 1990, 13, 1141–1154. [Google Scholar] [CrossRef]
- Ali, M.A.; Ali, M.; Yamin, M. Effect of nitrogen and plant population levels on seed cotton yield of newly introduced cotton variety CIM-497. J. Agric. Res. 2007, 45, 289–298. [Google Scholar]
- Luo, Z.; Liu, H.; Li, W.P.; Zhao, Q.; Dai, J.H.; Tian, L.W.; Dong, H.Z. Effects of reduced nitrogen rate on cotton yield and nitrogen use efficiency as mediated by application mode or plant density. Field Crops Res. 2018, 218, 150–157. [Google Scholar] [CrossRef]
- Zhou, G.S.; Lin, Y.; Tong, C.; An, L.L.; Liu, G.J. Effects of nitrogen application amount on growth characteristics, boll development and lint yield of high quality cotton. Agric. Sci. Technol. 2011, 12, 1667–1670. [Google Scholar]
- Devkota, M.; Martius, C.; Lamers, J.P.A.; Sayre, K.D.; Devkota, K.P.; Gupta, R.K.; Egamberdiev, O.; Vlek, P.L.G. Combining permanent beds and residue retention with nitrogen fertilization improves crop yields and water productivity in irrigated arid lands under cotton, wheat and maize. Field Crops Res. 2013, 149, 105–114. [Google Scholar] [CrossRef]
- Devkota, M.; Martius, C.; Lamers, J.P.A.; Sayre, K.D.; Devkota, K.P.; Vlek, P.L.G. Tillage and nitrogen fertilization effects on yield and nitrogen use efficiency of irrigated cotton. Soil Tillage Res. 2013, 134, 72–82. [Google Scholar] [CrossRef]
- Polychronaki, E.; Douma, C.; Giourga, C.; Loumou, A. Assessing nitrogen fertilization strategies in winter wheat and cotton crops in northern Greece. Pedosphere 2012, 22, 689–697. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Mikkelsen, D.S. Nitrogen source-sink relationship in cotton. J. Plant Nutr. 1989, 12, 1417–1433. [Google Scholar] [CrossRef]
- Yang, G.Z.; Tang, H.Y.; Nie, Y.C.; Zhang, X.L. Responses of cotton growth, yield, and biomass to nitrogen split application ratio. Eur. J. Agron. 2011, 35, 164–170. [Google Scholar] [CrossRef]
- Bondada, B.R.; Oosterhuis, D.M. Canopy photosynthesis, specific leaf weight, and yield components of cotton under varying nitrogen supply. J. Plant Nutr. 2001, 24, 469–477. [Google Scholar] [CrossRef]
- Muharam, F.M.; Bronson, K.F.; Maas, S.J.; Ritchie, G.L. Inter-relationships of cotton plant height, canopy width, groundcover and plant nitrogen status indicators. Field Crops Res. 2014, 169, 58–69. [Google Scholar] [CrossRef]
- Chen, Z.K.; Tao, X.P.; Khan, A.; Daniel, K.Y.; Luo, H.H. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci. 2018, 9, 173. [Google Scholar] [CrossRef]
- Rochester, I.J. Using seed nitrogen concentration to estimate crop N use efficiency in high-yielding irrigated cotton. Field Crops Res. 2012, 127, 140–145. [Google Scholar] [CrossRef]
- Shen, Q.R. Crop nitrogen nutrition. In General Theory of Soil Fertilizer Science; Shen, Q.R., Ed.; Higher Education Press: Beijing, China, 2001; pp. 178–181. [Google Scholar]
- Radin, J.W.; Mauney, J.R. The nitrogen stress syndrome. In Cotton Physiology, the Cotton Foundation; Mauney, J.R., Stewart, J.M., Eds.; The Cotton Foundation: Memphis, TN, USA, 1986; pp. 91–105. [Google Scholar]
- Alitabar, R.A.; Salimbeck, R.; Alishah, O.; Seyed, A.A. Interactive effects of nitrogen and row spacing on growth and yield of cotton varieties. Int. J. Biol. Sci. 2012, 4, 120–125. [Google Scholar] [CrossRef]
- Bondada, B.R.; Oosterhuis, D.M.; Norman, R.J.; Baker, W.H. Canopy photosynthesis, growth, yield, and boll 15N accumulation under nitrogen stress in cotton. Crop Sci. 1996, 36, 127–133. [Google Scholar] [CrossRef]
- Christos, D. Dry matter, nitrogen and phosphorus accumulation, partitioning and remobilization as affected by N and P fertilization and source-sink relations. Eur. J. Agron. 2009, 30, 129–139. [Google Scholar]
- Liu, L.T.; Li, C.D.; Sun, H.C.; Lu, W.J.; Feng, L.X. Physiological effects of nitrogen nutrition on the senescence of cotton leaves at different positions. J. Plant Nutr. Fertil. 2007, 5, 910–914. [Google Scholar]
- Gwathmey, C.O.; Clement, J.D. Alteration of cotton source-sink relations with plant population density and mepiquat chloride. Field Crops Res. 2010, 116, 101–107. [Google Scholar] [CrossRef]
- Jackson, B.S.; Gerik, T.J. Boll shedding and boll load in nitrogen-stressed cotton. Agron. J. 1990, 82, 483–488. [Google Scholar] [CrossRef]
- Wahid, A.; Bukhari, S.; Rasul, E. Inter-specific differences in cotton for nutrient partitioning from subtending leaves to reproductive parts at various developmental stages, consequences for fruit growth and yield. Biol. Plant. 2003, 47, 379–385. [Google Scholar] [CrossRef]
- Wright, P.R. Premature senescence of cotton (Gossypium hirsutum L.) predominantly a potassium disorder caused by an imbalance of source and sink. Plant Soil 1999, 211, 231–239. [Google Scholar] [CrossRef]
- Zhao, S.J. Determination of chlorophyll content determination in plant tissue. In Plant Physiology Lab Guide; Zhang, S.J., Ed.; China Agriculture Press: Beijing, China, 2002; pp. 72–75. [Google Scholar]
- Read, S.M.; Northcote, D.N. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 1981, 116, 53–64. [Google Scholar] [CrossRef]
- Zou, Q. Determination of malonaldehyde content and superoxide dismutase content determination in plant tissue. In Plant Physiology Experiment Guide; Zou, Q., Ed.; Higher Education Press: Beijing, China, 2003; pp. 161–165, 168–169. [Google Scholar]
- Zhang, Z.L. Determination of superoxide dismutase activity determination in plant tissue. In Experimental Instruction for Plant Physiology; Zhang, Z.L., Ed.; People’s Education Press: Beijing, China, 1980; pp. 34–36. [Google Scholar]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef]
- Dong, H.Z.; Li, W.J.; Eneji, A.E.; Zhang, D.M. Nitrogen rate and plant density effects on yield and late-season leaf senescenc of cotton raised on a saline field. Field Crops Res. 2012, 126, 137–144. [Google Scholar] [CrossRef]
- Kotchoni, S.O.; Gachomo, E.W. The reactive oxygen species network pathways, an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J. Biosci. 2006, 31, 389–404. [Google Scholar] [CrossRef]
- Daud, M.K.; Quiling, H.; Lei, M.; Ali, B.; Zhu, S.J. Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere 2015, 120, 309–320. [Google Scholar] [CrossRef]
- Boquet, D.J.; Breitenbeck, G.A. Nitrogen rate effect on partitioning of nitrogen and dry matter by cotton. Crop Sci. 2000, 40, 1685–1693. [Google Scholar] [CrossRef]
- Egelkraut, T.M.; Kissel, D.E.; Cabrera, M.L.; Gascho, G.J.; Adkins, W. Nitrogen concentration in cotton seed as an indicator of N availability. Nutr. Cycl Agroecosyst. 2004, 68, 235–242. [Google Scholar] [CrossRef]
- Ferrari, S.; Junior, E.F.; Ferrari, J.V.; Pereira, G.A. Cotton development and yield according to nitrogen application and cover crops. Semina 2011, 32, 1405–1416. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).