Identification of Barley (Hordeum vulgare L. subsp. vulgare) Root Exudates Allelochemicals, Their Autoallelopathic Activity and Against Bromus diandrus Roth. Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Greenhouse Experiment
2.3. Total Phenolic Content According to Soil Type
2.4. Collection of Barley Root Exudates
2.5. HPLC Analysis
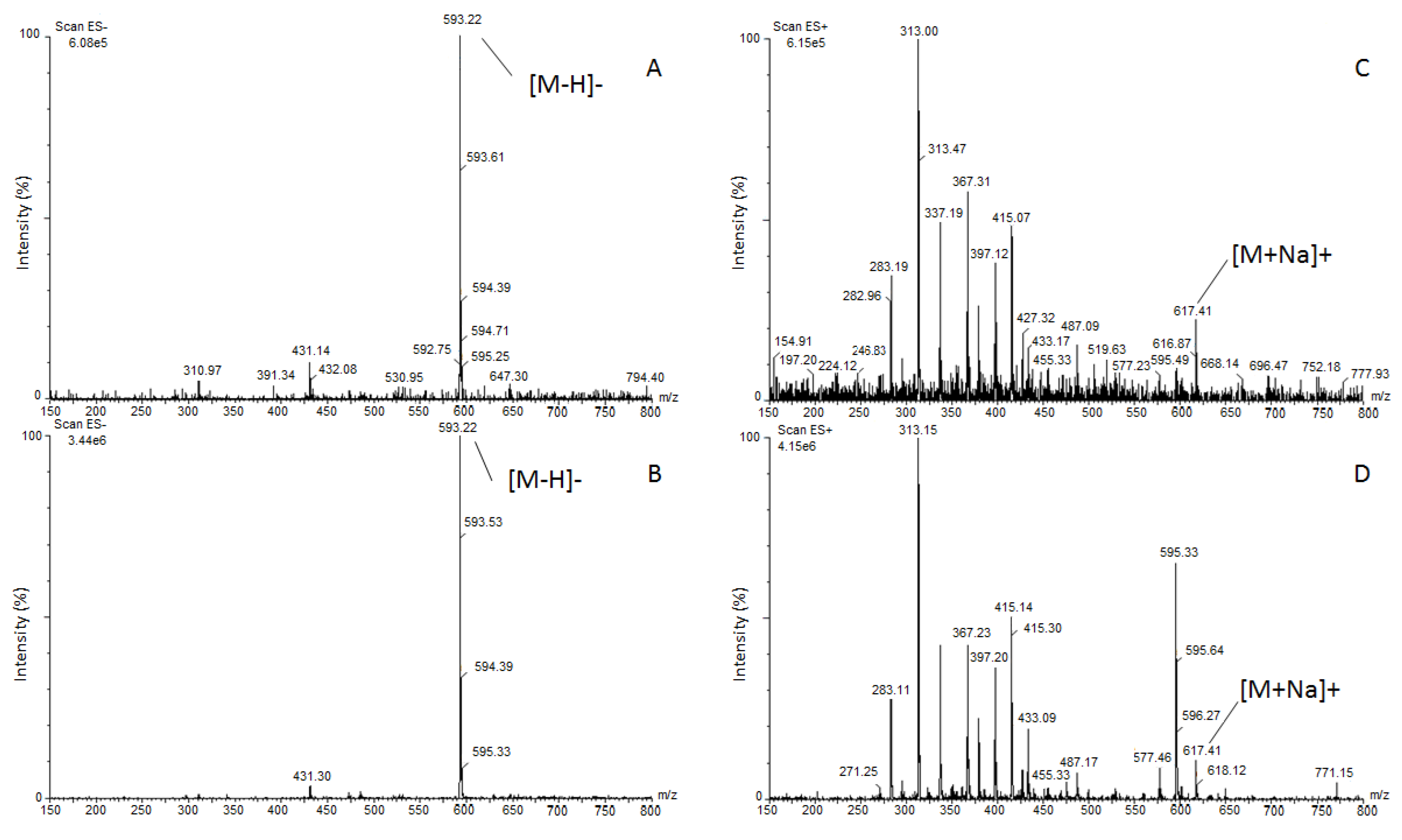
2.6. UPLC-ESI-MS Profiling of Allelochemical Compounds
2.7. Effect of Barley Allelochemicals Identified as Candidates
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic Content According to Soil Type
3.2. Identification of Barley Root Exudate Allelochemicals
3.3. Effect of Allelochemicals Identified as Candidates
4. Discussion
4.1. Variation in Total Phenolic Content According to Soil Type
4.2. Allelochemicals of Barley Root Exudates
4.3. Effect of Allelochemicals Identified as Candidates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Danner, H.; Brown, P.; Cator, E.A.; Harren, F.J.; van Dam, N.M.; Cristescu, S.M. Aboveground and belowground herbivores synergistically induce volatile organic sulfur compound emissions from shoots but not from roots. J. Chem. Ecol. 2015, 41, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Elijarrat, E.; Barcelo, D. Sample handling and analysis of allelochemical compounds in plants. Trends Anal. Chem. 2001, 20, 584–590. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Chon, S.U.; Jennings, J.A.; Nelson, C.J. Alfalfa (Medicago sativa L.) autotoxicity: Current status. Allelopath. J. 2006, 18, 57–80. [Google Scholar]
- Pedrol, N.; González, L.; Reigosa, M.J. Allelopathy and abiotic stress. In Proceedings of Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M.J., Pedrol, N., González, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 171–209. [Google Scholar]
- Souissi, T.; Belhadjsalah, H.; Latiri, K. Brome in cereal crops: Infestations and management. L’Investisseur Agricole 2001, 42, 29–32. [Google Scholar]
- Souissi, T.; Belhadjsalah, H.; Mhafdhi, M.; Latiri, K. Non chemical control of Bromus diandrus Roth. in wheat in Tunisia. In Proceedings of the XI International Conference on Weed Biology; Association Française de Protection des Plantes: Dijon, France, 2000; pp. 417–424. [Google Scholar]
- Mejri, D.; Gamalero, E.; Tombolini, R.; Musso, C.; Massa, N.; Berta, G.; Souissi, T. Biological control of great brome (Bromus diandrus) in durum wheat (Triticum durum): Specificity, physiological traits and impact on plant growth and root architecture of the fluorescent pseudomonad strain X33d. Biocontrol 2010, 55, 561–572. [Google Scholar] [CrossRef]
- Mejri, D.; Gamalero, E.; Souissi, T. Formulation development of the deleterious rhizobacterium Pseudomonas trivialis X33d for biocontrol of brome (Bromus diandrus) in durum wheat. J. Appl. Microbiol. 2013, 114, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bertholdsson, N.O. Early vigour and allelopathy–two useful traits for enhanced barley and wheat competitiveness against weeds. Weed. Res. 2005, 45, 94–102. [Google Scholar] [CrossRef]
- Christensen, S. Weed suppression ability of spring barley varieties. Weed Res. 1995, 35, 241–247. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Gatsis, T.; Eleftherohorinos, I. Competitive interactions of fifty barley cultivars with Avena sterilis and Asperugo procumbens. Field Crops Res. 2010, 117, 90–100. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Variation in allelopathic activity over 100 years of barley selection and breeding. Weed Res. 2004, 44, 78–86. [Google Scholar] [CrossRef]
- Bouhaouel, I.; Gfeller, A.; Fauconnier, M.L.; Slim Amara, H.; du Jardin, P. Allelopathic and autotoxicity effects of barley (Hordeum vulgare L. ssp. vulgare) root exudates. Biocontrol 2015, 60, 425–436. [Google Scholar] [CrossRef]
- Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 2003, 54, 1931–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, M.L.; Corcuera, L.J. Effect of environment on gramine content in barley leaves and susceptibility to the aphid Schizaphis graminum. Phytochemistry 1991, 30, 3237–3240. [Google Scholar] [CrossRef]
- Glinwood, R.; Ninkovic, V.; Pettersson, J.; Ahmed, E. Barley exposed to aerial allelopathy from thistles (Cirsium spp.) becomes less acceptable to aphids. Ecol. Entomol. 2004, 29, 188–195. [Google Scholar] [CrossRef]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Röse, U.S.R. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010, 185, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.; Ben-Hammouda, M. Allelopathic Plants. 19. Barley (Hordeum vulgare L). Allelopath. J. 2009, 24, 225–242. [Google Scholar]
- Liu, D.L.; Lovett, J.V. Biologically active secondary metabolites of barley. II. Phytotoxicity of barley allelochemicals. J. Chem. Ecol. 1993, 19, 2231–2244. [Google Scholar] [CrossRef]
- Baghestani, A.; Lemieux, C.; Leroux, G.D.; Baziramakenga, R. Determination of allelochemicals in spring cereal cultivars of different competitiveness. Weed Sci. 1999, 47, 498–504. [Google Scholar] [CrossRef]
- Hoult, A.H.C.; Lovett, J.V. Biologically active secondary metabolites of barley. III. A method for identification and quantification of hordenine and gramine in barley by high-performance liquid chromatography. J. Chem. Ecol. 1993, 19, 2245–2254. [Google Scholar] [CrossRef]
- Lovett, J.V.; Hoult, A.H.C.; Christen, O. Biologically active secondary metabolites of barley. IV. Hordenine production by different barley lines. J. Chem. Ecol. 1994, 20, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- El Gharbi, M.S.; El Felah, M. Les céréales en Tunisie: Plus d’un siècle de recherche variétale. Ann. L’INRAT 2013, 86, 45–68. [Google Scholar]
- Wu, H.; Pratley, J.; Lemerle, D.; An, M.; Liu, D.L. Autotoxicity of wheat (Triticum aestivum L.) as determined by laboratory bioassays. Plant Soil 2007, 296, 85–93. [Google Scholar] [CrossRef]
- Inderjit. Soil microorganisms: An important determinant of allelopathic activity. Plant Soil 2005, 274, 227–236. [Google Scholar] [CrossRef]
- Ben-Hammouda, M.; Ghorbal, H.; Kremer, R.J.; Oueslati, O. Autotoxicity of barley. J. Plant Nutr. 2002, 25, 1155–1161. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica I. The quantitative analysis of constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Zhang, F.J.; Guo, J.Y.; Liu, W.X.; Wan, F.H. Influence of coastal plain yellowtops (Flaveria bidentis) residues on growth of cotton seedlings and soil fertility. Arch. Agron. Soil Sci. 2012, 58, 1117–1128. [Google Scholar] [CrossRef]
- Banwart, W.L.; Porter, P.M.; Granato, T.C.; Hassett, J.J. HPLC separation and wavelength area ratios of more than 50 phenolic acids and flavonoids. J. Chem. Ecol. 1985, 11, 383–395. [Google Scholar] [CrossRef]
- Robbins, J.R.; Bean, S.R. Development of a quantitative high performance liquid chromatography-photodiode array detection measurement system for phenolic acids. J. Chromatogr. A 2004, 1038, 97–105. [Google Scholar] [CrossRef]
- Bouhaouel, I.; Gfeller, A.; Fauconnier, M.L.; Delory, B.; Slim Amara, H.; du Jardin, P. Evaluation of the allelopathic potential of water-soluble compounds of barley (Hordeum vulgare L. subsp. vulgare) and great brome (Bromus diandrus Roth.) using a modified bioassay. Biotechnol. Agron. Soc. Environ. 2016, 20, 482–494. [Google Scholar]
- Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Role of root-mediated interactions in phytotoxic interference of Ageratum conyzoides with rice (Oryza sativa). Flora 2009, 204, 388–395. [Google Scholar] [CrossRef]
- Chung, I.M.; Ahn, J.K.; Yun, S.J. Identification of allelopathic compounds from rice (Oryza sativa L.) straw and their biological activity. Can. J. Plant Sci. 2001, 81, 815–819. [Google Scholar] [CrossRef]
- Jose, S.; Gillespie, A.R. Allelopathy in black walnut (Juglans nigra L.) alley cropping. II. Effects of juglone on hydroponically grown corn (Zea mays L.) and soybean (Glycine max L. Merr.) growth and physiology. Plant Soil 1998, 203, 199–206. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. The release of root exudates as affected by the plant’s physiological status. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 41–93. [Google Scholar]
- Batchu, A.K.; Zimmermann, D.; Schulze-Lefert, P.; Koprek, T. Correlation between hordatine accumulation, environmental factors and genetic diversity in wild barley (Hordeum spontaneum C. Koch) accessions from the Near East Fertile Crescent. Genetica 2006, 127, 87–99. [Google Scholar] [CrossRef]
- Cecchi, A.M.; Koskinen, W.C.; Cheng, H.H.; Haider, K. Sorption–desorption of phenolic acids as affected by soil properties. Biol. Fert. Soils 2004, 39, 235–242. [Google Scholar] [CrossRef]
- Bhowmik, P.C. Sorption of benzoic acid onto soil colloids and its implications for allelopathy studies. Biol. Fert. Soils 2004, 40, 345–348. [Google Scholar]
- Bajpai, D.; Rajeswari, M.S. Interaction of 8-hydroxyquinoline with soil environment mediates its ecological function. PLoS ONE 2010, 5, 1–7. [Google Scholar]
- Tharayil, N.; Bhowmik, P.C.; Xing, B. Preferential sorption of phenolic phytotoxins to soil: Implications for altering the availability of allelochemicals. J. Agric. Food Chem. 2006, 54, 3033–3040. [Google Scholar] [CrossRef]
- Oueslati, O.; Ben-Hammouda, M.; Ghorbal, M.H.; El Gazzeh, M.; Kremer, R.J. Role of phenolic acids in expression of barley (Hordeum vulgare) autotoxicity. Allelopath. J. 2009, 23, 157–166. [Google Scholar]
- Haoa, M.; Beta, T. Qualitative and quantitative analysis of the major phenolic compounds as antioxidants in barley and flaxseed hulls using HPLC/MS/MS. J. Sci. Food Agric. 2012, 92, 2062–2068. [Google Scholar] [CrossRef]
- Chon, S.U.; Kim, Y.M. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agron. Crop Sci. 2004, 190, 145–150. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.B.; Valentão, P.; Gil-Izquierdo, A. Further knowledge on barley (Hordeum vulgare L.) leaves O-glycosyl-C-glycosyl flavones by liquid chromatography-UV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. A 2008, 1182, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Kleinschmidt, K.; Weissenböck, G.; Klein, M. Flavonoid biosynthesis in barley primary leaves requires the presence of the vacuole and controls the activity of vacuolar flavonoid transport. Plant Physiol. 2007, 144, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Seikel, M.K.; Geissman, T.A. The flavonoid constituents of barley (Hordeum vulgare): I. Saponarin. Arch. Biochem. Biophys. 1957, 71, 17–30. [Google Scholar] [CrossRef]
- Blume, D.E.; McClure, J.W. C-glycosylflavone accumulation in Sandoz 6706-treated barley seedlings is a photocontrolled response. Phytochemistry 1978, 17, 1549–1551. [Google Scholar] [CrossRef]
- Abou-Zaida, M.M.; Lombardoa, D.A.; Kiteb, G.C.; Grayerb, R.J.; Veitch, N.C. Acylated flavone C-glycosides from Cucumis sativus. Phytochemistry 2001, 58, 167–172. [Google Scholar] [CrossRef]
- Keasling, A.W.; Otter, R.R.; Bailey, F.C. Phytotoxicity of Passiflora incarnate extracts on germination and growth of Hordeum vulgare and Raphanus sativus. Allelopath. J. 2013, 31, 319–332. [Google Scholar]
- Keyhanian, S.; Stahl-Biskup, E. Phenolic constituents in dried flowers of Aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. 2007, 73, 599–602. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; López-Sáez, J.A.; Cobianchi, R.C. Effects of seven pure flavonoids from mosses on germination and growth of Tortula muralis HEDW. (Bryophyta) and Raphanus sativus L. (Magnoliophyta). Phytochemistry 2003, 62, 1145–1151. [Google Scholar] [CrossRef]
- Ohkawa, M. Three new anti-oxidative saponarin analogs from young green barley leaves. Chem. Pharm. Bull. 1998, 46, 1887–1890. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mukherjee, A.; Goswami, R.; Basu, S. Hypoglycemic activity of the antioxidant saponarin, characterized as α-glucosidase inhibitor present in Tinospora cordifolia. J. Enzyme Inhib. Med. Chem. 2009, 24, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Vitcheva, V.; Simeonova, R.; Krasteva, I.; Yotova, M.; Nikolov, S.; Mitcheva, M. Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. on cocaine-induced oxidative stress in rats. Redox Rep. 2011, 16, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sprouts inhibits NF-κB and MAPK on LPS-induced RAW 264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef]
- Reuber, S.; Bornman, J.F.; Weissenböck, G. A flavonoid mutant of barley (Hordeum vulgare L.) exhibits increased sensitivity to UV-B radiation in the primary leaf. Plant Cell Environ. 1996, 19, 593–601. [Google Scholar] [CrossRef]
- Podstolski, A.; Sznajder, J.; Wichowska, G. Accumulation of phenolics and growth rate of barley seedlings (Hordeum vulgare L.). Biol. Plant PRAHA 1981, 23, 120–127. [Google Scholar] [CrossRef]
- Christen, O.; Lovett, J.V. Effects of a short-term p-hydroxybenzoic acid application on grain yield and yield components in different tiller categories of spring barley. Plant Soil 1993, 151, 279–286. [Google Scholar] [CrossRef]
- Ray, H.; Hastings, P.J. Variation within flax (Linum usitatissimum) and barley (Hordeum vulgare) in response to allelopathic chemicals. Theor. Appl. Genet. 1992, 84, 460–465. [Google Scholar] [CrossRef]
- Ruan, X.; Li, Z.H.; Wang, Q.; Pan, C.D.; Jiang, D.A.; Wang, G.G. Autotoxicity and allelopathy of 3,4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef]
- Dieterman, L.J.; Lin, C.Y.; Rohrbaugh, L.; Thiesfeld, V.; Wender, S.H. Identification and quantitative determination of scopolin and scopoletin in tobacco plants treated with 2,4-dichlorophenoxyacetic acid. Anal. Biochem. 1964, 9, 139–145. [Google Scholar] [CrossRef]
- Fay, P.K.; Duke, W.B. An assessment of allelopathic potential in Avena germplasm. Weed Sci. 1977, 25, 224–228. [Google Scholar] [CrossRef]
- Zobel, A.M.; Bialonska, D.; Silva, C.; Nighswander, J.E. Allelopathic role of phenolic compounds extruded by Medicago sativa L. leaves in response to bacterial or viral infections. Allelopath. J. 2005, 16, 131–136. [Google Scholar]
- Peterson, J.K.; Harisson, H.F.; Jacksson, D.M. Biological activities and contents of scopolin and scopoletin in sweetpotato clones. HortScience 2003, 38, 1129–1133. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Cimmino, A.; Evidente, A.; Rubiales, D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 2013, 61, 9797–9803. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, H.B.; Xiong, J.; Fang, C.X.; Wu, W.X.; He, H.B.; Lin, W.X. Regulation effect of exogenous salicylic acid on weed suppression and molecular physiological characteristics of allelopathic rice. Chin. J. Appl. Ecol. 2008, 19, 330–336. [Google Scholar]
- Glass, A.D.M. Inhibition of phosphate uptake in barley roots by hydroxy-benzoic acids. Phytochemistry 1975, 14, 2127–2130. [Google Scholar] [CrossRef]
- Kaur, H.; Kaushik, S. Cellular evidence of allelopathic interference of benzoic acid to mustard (Brassica juncea L.) seedling growth. Plant Physiol. Biochem. 2005, 43, 77–81. [Google Scholar] [CrossRef]
- US EPA. Office for Pesticides Programs Reregistration Eligibility Decision for Dicamba and Associated Salts. 2006. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/dicamba_red.pdf (accessed on 13 December 2018).
- Lodhi, M.A.K. Allelopathic effects of hackberry in a bottomland forest community. J. Chem. Ecol. 1975, 1, 171–182. [Google Scholar] [CrossRef]
- Stupnicka-Rodzynkiewicz, E.; Dabkowska, T.; Stoklosa, A.; Hura, T.; Dubert, F.; Lepiarczyk, A. The effect of selected phenolic compounds on the initial growth of four weed species. J. Plant Dis. Protect. 2006, 20, 479–486. [Google Scholar]
- Hura, T.; Dubert, F.; Dabkowska, T.; Stupnicka-Rodzynkiewicz, E.; Stoklosa, A.; Lepiarczyk, A. Quantitative analysis of phenolics in selected crop species and biological activity of these compounds evaluated by sensitivity of Echinochloa crus-galli. Acta Physiol. Plant. 2006, 28, 537–545. [Google Scholar] [CrossRef]
- He, H.; Shen, L.; Song, B.; Guo, Y.; Liang, Y.; Liang, K.; Lin, W. Interactive effects between allelochemical substitutes. Chin. J. Appl. Ecol. 2005, 16, 890–894. [Google Scholar]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]

| Factors | Increase Rate of Total Phenolic Content (%) |
|---|---|
| Genotype (G) | |
| Manel | 37.8 a |
| Tej | 39.8 a |
| Rihane | 32.6 a |
| Arbi | 117.1 b,c |
| Ardhaoui | 71.9 a,b |
| Saudi | 120.6 c |
| df | 5 |
| F | 16.4 |
| P | <0.001 |
| Substrate (S) | |
| Sandy | 94.2 b |
| Sandy-clay-loam | 45.7 a |
| df | 1 |
| F | 35.1 |
| P | <0.001 |
| G × S | |
| df | 5 |
| F | 6.66 |
| P | <0.001 |
| Compounds. | tR (min) | λmax (nm) | df | F | P |
|---|---|---|---|---|---|
| p-hydroxybenzoic acid | 13.85 | 258 | 5 | 12.1 | <0.001 |
| Gentisic acid | 14.24 | 330 | 5 | 84.4 | <0.001 |
| Vanillic acid | 17.42 | 258 | 5 | 8.6 | <0.001 |
| Caffeic acid | 18.37 | 330 | ND | ND | ND |
| Syringic acid | 19.47 | 258 | 5 | 23.8 | <0.001 |
| p-coumaric acid | 24.61 | 330 | 5 | 7.1 | 0.001 |
| Saponarin | 25.61 | 335 | 5 | 17.8 | <0.001 |
| Scopoletin | 27.33 | 330 | 5 | 11.0 | <0.001 |
| Ferulic acid | 28.14 | 330 | 5 | 0.7 | 0.644 |
| m-coumaric acid | 30.04 | 330 | ND | ND | ND |
| Benzoic acid | 31.23 | 258 | 5 | 47.4 | <0.001 |
| Salicylic acid | 33.69 | 245 | 5 | 200.8 | <0.001 |
| o-coumaric acid | 34.88 | 330 | 5 | 68.4 | <0.001 |
| IS | 44.23 | 258 | – | – | – |
| trans-cinnamic acid | 46.68 | 258 | 5 | 22.2 | <0.001 |
| Dependent Variables | Variable Chosen | R2 |
|---|---|---|
| Inhibition rate of root length | Benzoic acid Benzoic acid, o-coumaric acid Benzoic acid, o-coumaric acid, Saponarin | 0.88 *** 0.93 *** 0.97 *** |
| Inhibition rate of shoot length | Benzoic acid Benzoic acid, p-hydroxybenzoic acid Benzoic acid, p-hydroxybenzoic acid, o-coumaric acid Benzoic acid, p-hydroxybenzoic acid, o-coumaric acid, Vanillic acid Benzoic acid, p-hydroxybenzoic acid, o-coumaric acid, Vanillic acid, Salicylic acid | 0.71 *** 0.74 *** 0.79 *** 0.83 *** 0.86 *** |
| Inhibition rate of root dry weight | Benzoic acid Benzoic acid, o-coumaric acid Benzoic acid, o-coumaric acid, Saponarin Benzoic acid, o-coumaric acid, Saponarin, Vanillic acid | 0.90 *** 0.92 *** 0.95 *** 0.96 *** |
| Inhibition rate of shoot dry weight | Benzoic acid Benzoic acid, o-coumaric acid Benzoic acid, o-coumaric acid, Scopoletin | 0.70 *** 0.83 *** 0.86 *** |
| Factors | Inhibition Rate of Radicle Length (%) | Inhibition Rate of Coleoptile Length (%) | ||||
|---|---|---|---|---|---|---|
| df | F | P | Df | F | P | |
| Compound (Comp.) | 8 | 138.3 | <0.001 | 8 | 58.8 | <0.001 |
| Concentration (Conc.) | 2 | 1004.6 | <0.001 | 2 | 366.1 | <0.001 |
| Species (Sp.) | 2 | 17.8 | <0.001 | 2 | 62.3 | <0.001 |
| Comp. × Conc. | 16 | 61.9 | <0.001 | 16 | 31.9 | <0.001 |
| Comp. × Sp. | 16 | 7.3 | <0.001 | 16 | 3.1 | <0.001 |
| Sp. × Conc. | 4 | 9.4 | <0.001 | 4 | 5.5 | <0.001 |
| Comp. × Conc. × Sp. | 34 | 1.9 | 0.001 | 34 | 1.3 | 0.133 |
| Concentrations | Benzoic Acid | o-coumaric Acid | Saponarin | Vanillic Acid | Salicylic Acid | Scopoletin | p-hydroxybenzoic Acid | Gentisic Acid | Mixture | |
|---|---|---|---|---|---|---|---|---|---|---|
| Brome | Control | 9.48 ± 0.54 c | 9.48 ± 0.54 c | 9.48 ± 0.54 c | 9.48 ± 0.54 b | 9.48 ± 0.54 c | 9.48 ± 0.54 c | 9.48 ± 0.54 ab | 9.48 ± 0.54 bc | 9.48 ± 0.54 c |
| 10−5 M | 9.19 ± 0.48 bc | 8.06 ± 0.45 b | 9.88 ± 0.28 c | 9.13 ± 0.32 ab | 7.77 ± 0.36 b | 9.06 ± 0.41 c | 10.16 ± 0.39 b | 10.74 ± 0.40 c | 9.24 ± 0.27 c | |
| (3.1) | (14.9) | (−4.2) | (3.8) | (18.1) | (4.4) | (−7.2) | (−10.2) | (2.5) | ||
| 10−4 M | 8.19 ± 0.33 b | 7.13 ± 0.40 b | 8.16 ± 0.25 b | 8.97 ± 0.45 ab | 7.04 ± 0.45 b | 4.55 ± 0.26 b | 9.05 ± 0.40 a | 8.56 ± 0.29 ab | 3.51 ± 0.19 b | |
| (13.6) | (24.8) | (13.9) | (5.4) | (25.7) | (58.4) | (4.6) | (9.7) | (62.9) | ||
| 10−3 M | 2.17 ± 0.33 a | 5.81 ± 0.39 a | 1.83 ± 0.11 a | 8.03 ± 0.26 a | 2.89 ± 0.22 a | 1.34 ± 0.23 a | 8.42 ± 0.38 a | 7.84 ± 0.49 a | 0.54 ± 0.09 a | |
| (77.1) | (38.7) | (80.7) | (15.3) | (69.5) | (85.6) | (11.2) | (17.3) | (94.3) | ||
| Average | (31.62) | (26.13) | (30.13) | (8.16) | (37.76) | (49.46) | (2.86) | (5.6) | (53.23) | |
| Manel | Control | 11.07 ± 0.46 c | 11.07 ± 0.46 c | 11.07 ± 0.46 b | 11.07 ± 0.46 b | 11.07 ± 0.46 d | 11.07 ± 0.46 c | 11.07 ± 0.46 ab | 11.07 ± 0.46 b | 11.07 ± 0.46 c |
| 10−5 M | 11.74 ± 0.54 c | 9.35 ± 0.41 b | 12.03 ± 0.24 c | 8.93 ± 0.32 a | 9.13 ± 0.41 c | 10.52 ± 0.43 c | 11.97 ± 0.36 b | 11.19 ± 0.41 b | 12.24 ± 0.34 d | |
| (−6.1) | (15.6) | (−8.7) | (19.7) | (17.5) | (4.9) | (−8.2) | (−1.1) | (−10.6) | ||
| 10−4 M | 9.65 ± 0.26 b | 8.37 ± 0.62 b | 10.34 ± 0.21 b | 8.68 ± 0.34 a | 7.72 ± 0.39 b | 5.36 ± 0.38 b | 10.70 ± 0.23 a | 9.40 ± 0.34 a | 5.87 ± 0.16 b | |
| (12.9) | (24.4) | (6.6) | (21.7) | (30.3) | (51.6) | (3.3) | (15.1) | (46.9) | ||
| 10−3 M | 6.58 ± 0.34 a | 6.95 ± 0.40 a | 3.03 ± 0.22 a | 8.29 ± 0.34 a | 5.44 ± 0.30 a | 2.65 ± 0.29 a | 9.88 ± 0.60 a | 9.38 ± 0.46 a | 1.58 ± 0.10 a | |
| (40.6) | (37.2) | (72.6) | (25.1) | (50.9) | (76.1) | (10.8) | (15.3) | (85.7) | ||
| Average | (15.8) | (25.73) | (23.5) | (22.16) | (32.9) | (44.2) | (1.96) | (9.76) | (40.66) | |
| Ardhaoui | Control | 12.24 ± 0.62 b | 12.24 ± 0.62 b | 12.24 ± 0.62 b | 12.24 ± 0.62 b | 12.24 ± 0.62 c | 12.24 ± 0.62 d | 12.24 ± 0.62 a | 12.24 ± 0.62 b | 12.24 ± 0.62 c |
| 10−5 M | 12.14 ± 0.31 b | 10.93 ± 0.49 b | 13.57 ± 0.68 b | 10.15 ± 0.63 a | 10.77 ± 0.43 b | 10.45 ± 0.53 c | 12.63 ± 0.43 a | 10.29 ± 0.35 a | 12.87 ± 0.73 c | |
| (0.9) | (10.8) | (−10.9) | (17.2) | (12.0) | (14.7) | (−4.2) | (15.9) | (−5.1) | ||
| 10−4 M | 11.89 ± 0.40 b | 10.66 ± 0.46 b | 12.18 ± 0.80 b | 9.87 ± 0.42 a | 9.93 ± 0.48 b | 6.87 ± 0.43 b | 11.80 ± 0.35 a | 10.24 ± 0.65 a | 5.90 ± 0.22 b | |
| (2.9) | (12.9) | (0.43) | (19.4) | (18.9) | (43.9) | (3.7) | (16.4) | (51.7) | ||
| 10−3 M | 8.71 ± 0.39 a | 7.72 ± 0.64 a | 3.49 ± 0.18 a | 9.49 ± 0.36 a | 6.85 ± 0.37 a | 3.59 ± 0.25 a | 11.64 ± 0.31 a | 9.71 ± 0.46 a | 2.24 ± 0.11 a | |
| (28.9) | (36.9) | (71.5) | (22.6) | (44.0) | (70.7) | (4.9) | (20.7) | (81.7) | ||
| Average | (10.9) | (20.2) | (20.34) | (19.73) | (24.96) | (43.1) | (1.46) | (17.66) | (42.76) |
| Concentrations | Benzoic Acid | o-coumaric Acid | Saponarin | Vanillic Acid | Salicylic Acid | Scopoletin | p-hydroxybenzoic Acid | Gentisic Acid | Mixture | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brome | Control | 6.95 ± 0.30 b | 6.95 ± 0.30 a | 6.95 ± 0.30 c | 6.95 ± 0.30 b | 6.95 ± 0.30 c | 6.95 ± 0.30 c | 6.95 ± 0.30 c | 6.95 ± 0.30 bc | 6.95 ± 0.30 d | |
| 10−5 M | 6.59 ± 0.21 b | 7.03 ± 0.36 a | 6.15 ± 0.29 b | 6.61 ± 0.21 ab | 6.54 ± 0.22 bc | 6.90 ± 0.19 c | 6.39 ± 0.29 bc | 7.27 ± 0.24 c | 6.14 ± 0.35 c | ||
| (5.2) | (−1.2) | (11.4) | (4.9) | (5.9) | (0.7) | (8.1) | (−4.6) | (11.6) | |||
| 10−4 M | 6.09 ± 0.19 b | 6.97 ± 0.29 a | 5.88 ± 0.27 b | 6.47 ± 0.20 ab | 6.12 ± 0.24 b | 4.06 ± 0.31 b | 5.77 ± 0.36 ab | 6.15 ± 0.32 ab | 5.03 ± 0.16 b | ||
| (12.4) | (−0.2) | (15.4) | (6.9) | (11.9) | (41.6) | (17.1) | (11.5) | (27.5) | |||
| 10−3 M | 3.98 ± 0.42 b | 6.22 ± 0.21 a | 2.44 ± 0.19 a | 5.91 ± 0.30 a | 4.30 ± 0.34 a | 2.45 ± 0.28 a | 5.44 ± 0.24 a | 5.82 ± 0.28 a | 2.12 ± 0.17 a | ||
| (42.7) | (10.5) | (64.9) | (14.9) | (38.1) | (64.8) | (21.7) | (16.3) | (69.4) | |||
| Average | (20.1) | (3.03) | (30.56) | (8.9) | (18.63) | (35.7) | (15.63) | (7.73) | (36.17) | ||
| Manel | Control | 9.26 ± 0.26 b | 9.26 ± 0.26 a | 9.26 ± 0.26 b | 9.26 ± 0.26 b | 9.26 ± 0.26 b | 9.26 ± 0.26 c | 9.26 ± 0.26 a | 9.26 ± 0.26 b | 9.26 ± 0.26 c | |
| 10−5 M | 9.57 ± 0.42 b | 8.83 ± 0.27 a | 9.45 ± 0.31 b | 8.04 ± 0.31 a | 9.04 ± 0.26 b | 8.95 ± 0.30 c | 9.09 ± 0.31 a | 8.83 ± 0.27 ab | 9.49 ± 0.31 c | ||
| (−3.3) | (4.6) | (−2.0) | (13.2) | (2.4) | (3.3) | (1.8) | (4.7) | (−2.5) | |||
| 10−4 M | 9.49 ± 0.28 b | 8.73 ± 0.24 a | 9.06 ± 0.29 b | 7.96 ± 0.29 a | 8.77 ± 0.36 ab | 7.35 ± 0.29 b | 8.79 ± 0.26 a | 8.42 ± 0.21 ab | 7.17 ± 0.23 b | ||
| (−2.4) | (5.7) | (2.1) | (14.0) | (5.3) | (20.6) | (5.1) | (9.1) | (22.5) | |||
| 10−3 M | 8.12 ± 0.28 a | 8.64 ± 0.44 a | 3.72 ± 0.30 a | 7.52 ± 0.23 a | 8.21 ± 0.23 a | 3.79 ± 0.40 a | 8.58 ± 0.47 a | 8.01 ± 0.55 a | 3.39 ± 0.20 a | ||
| (13.7) | (6.7) | (59.8) | (18.8) | (11.3) | (59.1) | (7.3) | (13.5) | (63.4) | |||
| Average | (2.66) | (5.66) | (19.96) | (15.33) | (6.33) | (27.66) | (4.73) | (9.1) | (27.8) | ||
| Ardhaoui | Control | 9.80 ± 0.29 a | 9.80 ± 0.29 ab | 9.80 ± 0.29 b | 9.80 ± 0.29 a | 9.80 ± 0.29 b | 9.80 ± 0.29 c | 9.80 ± 0.29 a | 9.80 ± 0.29 a | 9.80 ± 0.29 c | |
| 10−5 M | 9.97 ± 0.35 a | 9.67 ± 0.23 ab | 10.08 ± 0.44 b | 9.86 ± 0.27 a | 9.56 ± 0.21 b | 9.44 ± 0.26 c | 9.69 ± 0.27 a | 9.97 ± 0.15 a | 10.16 ± 0.47 c | ||
| (−1.6) | (1.4) | (−2.9) | (−0.5) | (2.5) | (3.7) | (1.1) | (−1.7) | (−3.7) | |||
| 10−4 M | 9.73 ± 0.26 a | 10.31 ± 0.33 b | 9.94 ± 0.47 b | 9.66 ± 0.29 a | 9.19 ± 0.40 b | 8.07 ± 0.34 b | 9.54 ± 0.36 a | 9.61 ± 0.34 a | 8.34 ± 0.51 b | ||
| (0.8) | (1.9) | (−1.42) | (1.5) | (6.3) | (17.8) | (2.7) | (2.0) | (14.8) | |||
| 10−3 M | 9.49 ± 0.44 a | 9.47 ± 0.26 a | 5.22 ± 0.34 a | 9.29 ± 0.23 a | 8.24 ± 0.36 a | 5.01 ± 0.26 a | 9.12 ± 0.38 a | 9.41 ± 0.35 a | 4.56 ± 0.21 a | ||
| (3.2) | (9.9) | (46.7) | (5.3) | (16.0) | (48.9) | (7.0) | (4.1) | (53.4) | |||
| Average | (0.8) | (4.4) | (14.13) | (2.1) | (8.26) | (23.46) | (3.6) | (1.46) | (21.5) | ||
| Average | (7.8) | (4.4) | (21.5) | (8.8) | (11.1) | (28.9) | (8.00) | (6.10) | (28.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhaouel, I.; Richard, G.; Fauconnier, M.-L.; Ongena, M.; Franzil, L.; Gfeller, A.; Slim Amara, H.; du Jardin, P. Identification of Barley (Hordeum vulgare L. subsp. vulgare) Root Exudates Allelochemicals, Their Autoallelopathic Activity and Against Bromus diandrus Roth. Germination. Agronomy 2019, 9, 345. https://doi.org/10.3390/agronomy9070345
Bouhaouel I, Richard G, Fauconnier M-L, Ongena M, Franzil L, Gfeller A, Slim Amara H, du Jardin P. Identification of Barley (Hordeum vulgare L. subsp. vulgare) Root Exudates Allelochemicals, Their Autoallelopathic Activity and Against Bromus diandrus Roth. Germination. Agronomy. 2019; 9(7):345. https://doi.org/10.3390/agronomy9070345
Chicago/Turabian StyleBouhaouel, Imen, Gaëtan Richard, Marie-Laure Fauconnier, Marc Ongena, Laurent Franzil, Aurélie Gfeller, Hajer Slim Amara, and Patrick du Jardin. 2019. "Identification of Barley (Hordeum vulgare L. subsp. vulgare) Root Exudates Allelochemicals, Their Autoallelopathic Activity and Against Bromus diandrus Roth. Germination" Agronomy 9, no. 7: 345. https://doi.org/10.3390/agronomy9070345
APA StyleBouhaouel, I., Richard, G., Fauconnier, M.-L., Ongena, M., Franzil, L., Gfeller, A., Slim Amara, H., & du Jardin, P. (2019). Identification of Barley (Hordeum vulgare L. subsp. vulgare) Root Exudates Allelochemicals, Their Autoallelopathic Activity and Against Bromus diandrus Roth. Germination. Agronomy, 9(7), 345. https://doi.org/10.3390/agronomy9070345






