The Nitrification Inhibitor Vizura® Reduces N2O Emissions When Added to Digestate before Injection under Irrigated Maize in the Po Valley (Northern Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. N2O-N Emissions
2.2.1. N2O-N Field Sampling
2.2.2. N2O-N Emissions Calculation
2.3. Bulk Density, Soil Nitrate Content, and Water-Filled Pore Space (WFPS)
2.4. Statistical Analysis
3. Results
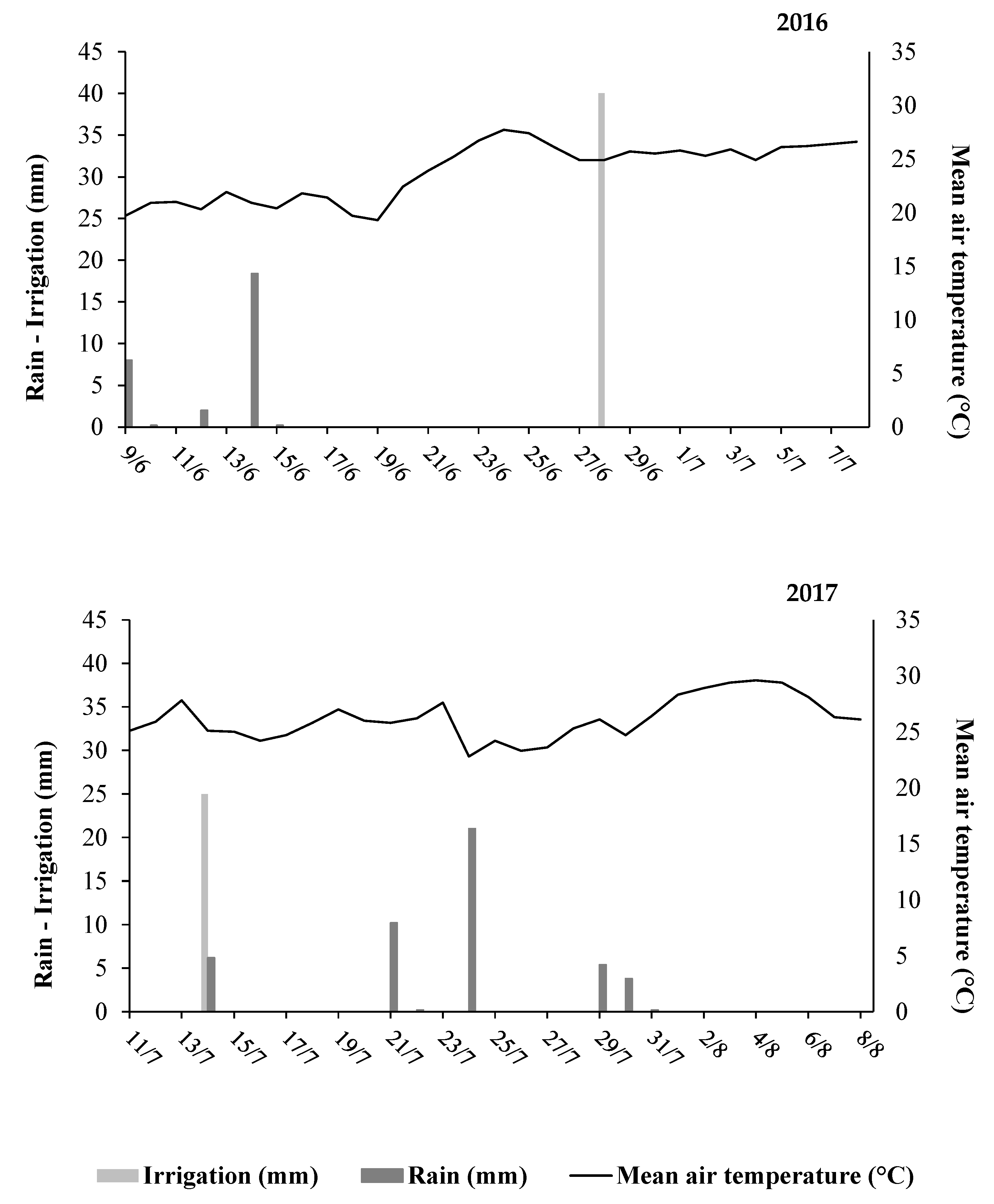
3.1. Weather Conditions
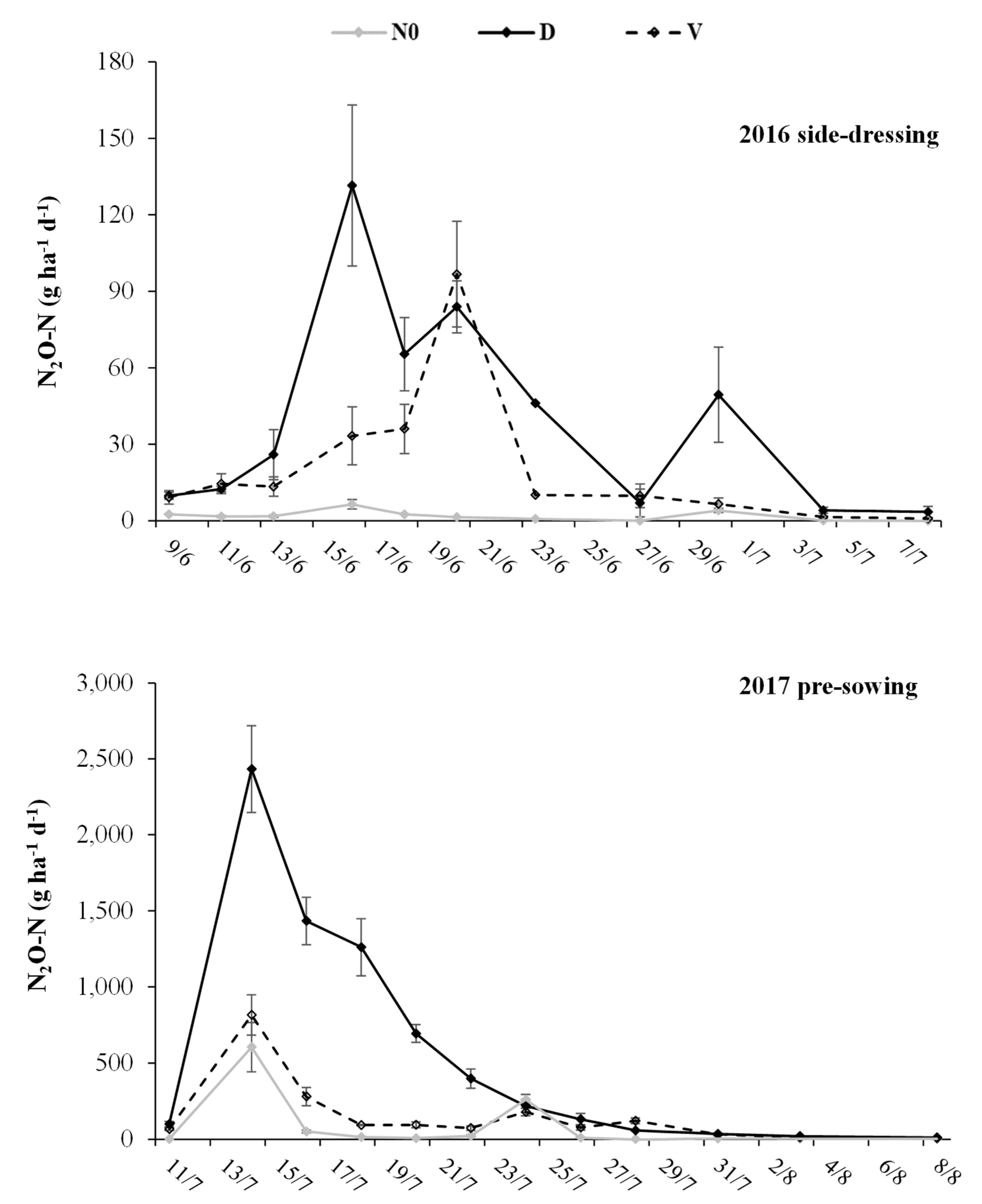
3.2. N2O-N Emissions
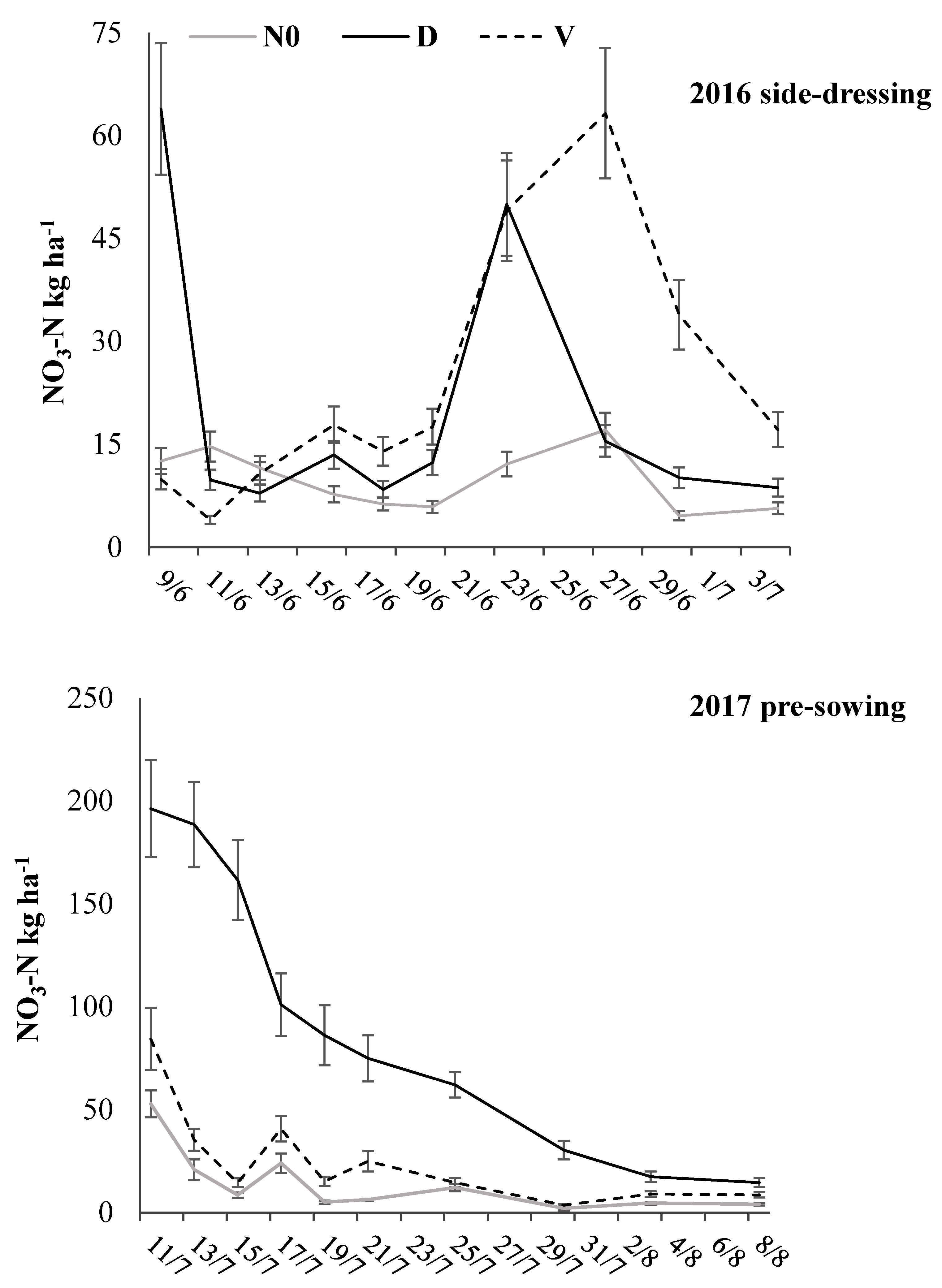
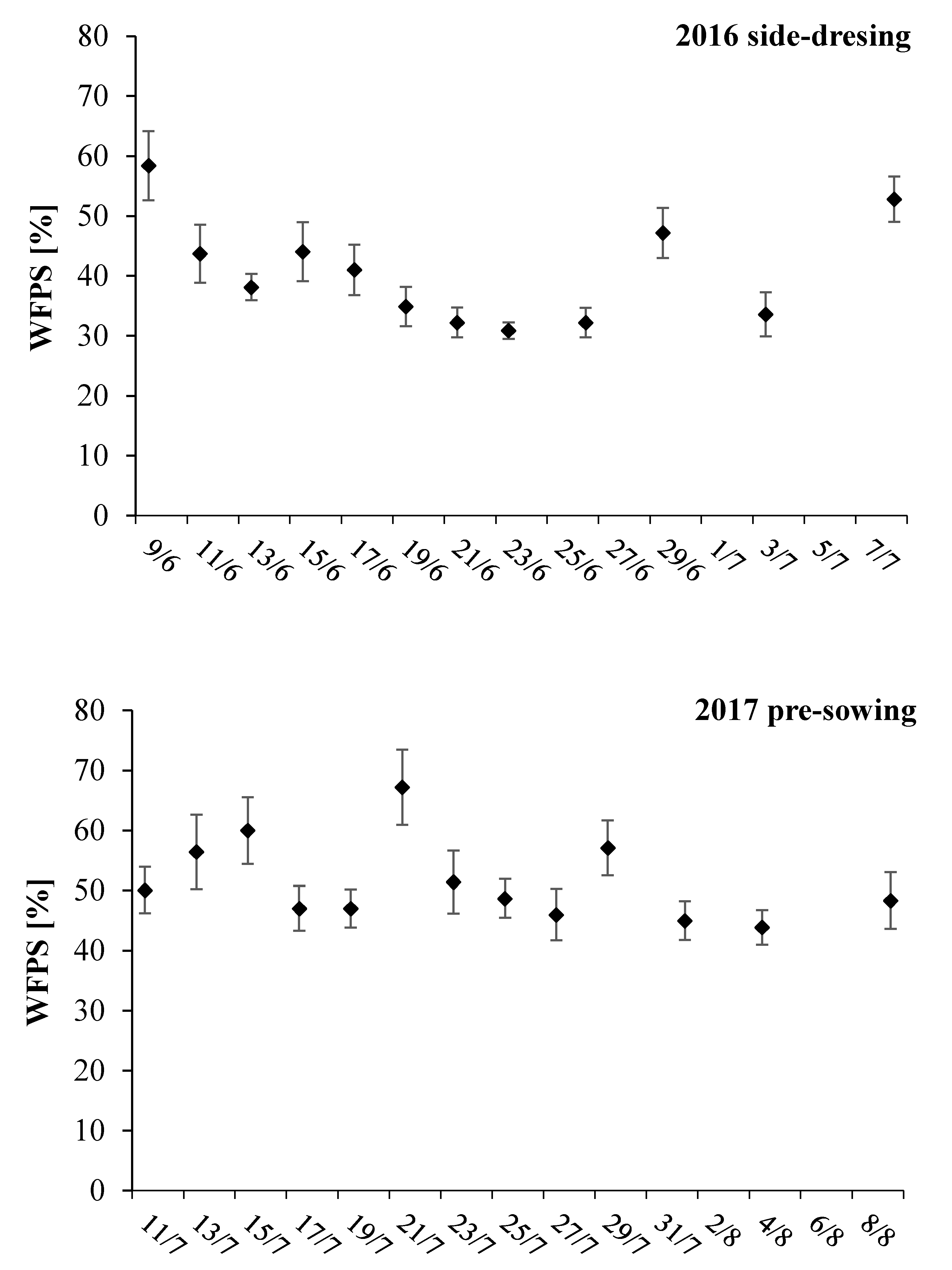
3.3. Bulk Density, NO3-N Content, and WFPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf (accessed on 24 July 2019).
- Butler, J.H.; Montzka, S.A. The NOAA Annual Greenhouse Gas Index (AGGI). Updated: Spring 2019. Available online: https://www.esrl.noaa.gov/gmd/aggi/aggi.html (accessed on 25 July 2019).
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a Conceptual Model of Soil Emissions of Nitrous and Nitric Oxides: Using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils. BioScience 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Shcherbak, I.; Neville, M.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Dalal, R.; Allen, D. Greenhouse gas emissions from natural ecosystems. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Cowan, N.J.; Levy, P.E.; Famulari, D.; Anderson, M.; Drewer, J.; Carozzi, M.; Reay, D.S.; Skiba, U.M. The influence of tillage on N2O fluxes from an intensively managed grazed grassland in Scotland. Biogeosciences 2016, 13, 4811–4821. [Google Scholar] [CrossRef]
- Perego, A.; Wu, L.; Gerosa, G.; Finco, A.; Chiazzese, M.; Amaducci, S. Field evaluation combined with modelling analysis to study fertilizer and tillage as factors affecting N2O emissions: A case study in the Po valley (Northern Italy). Agric. Ecosyst. Environ. 2016, 225, 72–85. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosys. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef]
- Volpi, I.; Laville, P.; Bonari, E.; o di Nasso, N.N.; Bosco, S. Improving the management of mineral fertilizers for nitrous oxide mitigation: The effect of nitrogen fertilizer type, urease and nitrification inhibitors in two different textured soils. Geoderma 2017, 307, 181–188. [Google Scholar] [CrossRef]
- Zhu, J.; Mulder, J.; Wu, L.P.; Meng, X.X.; Wang, Y.H.; Dörsch, P. Spatial and temporal variability of N2O emissions in a subtropical forest catchment in China. Biogeosciences 2013, 10, 1309–1321. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Dobbie, K.E.; McTaggart, I.P.; Smith, K.A. Nitrous oxide emissions from intensive agricultural systems: Variations between crops and seasons, key driving variables, and mean emission factors. J. Geophys. Res. Atmos. 1999, 104, 26891–26899. [Google Scholar] [CrossRef]
- Skiba, U.; Jones, S.K.; Drewer, J.; Helfter, C.; Anderson, M.; Dinsmore, K.; McKenzie, R.; Nemitz, E.; Sutton, M.A. Comparison of soil greenhouse gas emissions from extensive and intensive grazing in a temperate maritime climate. Biogeosciences 2013, 10, 1231–1241. [Google Scholar] [CrossRef]
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Koszel, M.; Kocira, A.; Lorencowicz, E. The evaluation of the use of biogas plant digestate as a fertilizer in alfalfa and spring wheat cultivation. Fresenius Environ. Bull. 2016, 25, 3258–3264. [Google Scholar]
- Tambone, F.; Terruzzi, L.; Scaglia, B.; Adani, F. Composting of the solid fraction of digestate derived from pig slurry: Biological processes and compost properties. Waste Manag. 2015, 35, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Sørensen, P.; Velthof, G.; Amon, B.; Pinto, M.; Rodhe, L.; Salomon, E.; Hutchings, N.; Burczyk, P.; Reid, J. An assessment of the variation of manure nitrogen efficiency throughout Europe and an appraisal of means to increase manure-N efficiency. Adv. Agron. 2013, 119, 371–442. [Google Scholar]
- Prasad, R.; Power, J.F. Nitrification Inhibitors for Agriculture, Health, and the Environment. Adv. Agron. 1995, 233–281. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Berry, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I.M. Scope and Strategies for Regulation of Nitrification in Agricultural Systems—Challenges and Opportunities. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Merino, P.; Menéndez, S.; Pinto, M.; González-Murua, C.; Estavillo, J.M. 3,4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application. Soil Use Manag. 2005, 21, 53–57. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Cardenas, L.M.; Camp, V.; Thorman, R.E.; Williams, J.R.; Rollett, A.J.; Chambers, B.J. An assessment of nitrification inhibitors to reduce nitrous oxide emissions from UK agriculture. Environ. Res. Lett. 2014, 9, 115006. [Google Scholar] [CrossRef] [Green Version]
- Bronson, K.F.; Mosier, A.R.; Bishnoi, S.R. Nitrous Oxide Emissions in Irrigated Corn as Affected by Nitrification Inhibitors. Soil Sci. Soc. Am. J. 1992, 56, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Habibullah, H.; Nelson, K.; Motavalli, P. Management of Nitrapyrin and Pronitridine Nitrification Inhibitors with Urea Ammonium Nitrate for Winter Wheat Production. Agronomy 2018, 8, 204. [Google Scholar] [CrossRef]
- Huérfano, X.; Menéndez, S.; Bolaños-Benavides, M.M.; González-Murua, C.; Estavillo, J.M. 3,4-dimethylpyrazole phosphate (DMPP) reduces N2O Emissions from a Tilled Grassland in the Bogotá Savanna. Agronomy 2019, 9, 102. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; von Locquenghien, K.H.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Tindaon, F.; Benckiser, G.; Ottow, J.C. Evaluation of ecological doses of the nitrification inhibitors 3, 4-dimethylpyrazole phosphate (DMPP) and 4-chloromethylpyrazole (ClMP) in comparison to dicyandiamide (DCD) in their effects on dehydrogenase and dimethyl sulfoxide reductase activity in soils. Biol. Fertil. Soils 2012, 48, 643–650. [Google Scholar]
- Andreae, M. ENTEC (DMPP—E in neuer Ammoniumstabilisator: Ökotoxikologische Bewertung. In Düngen mit einer neuen Technologie—Innovation in der Düngung. Wissenschaftliches Kolloquium Agrarzentrum der BASF Limburgerhof; BASF: Ludwigshafen, Germany, 1999; Volume 7, pp. 3–10. [Google Scholar]
- Rodrigues, J.M.; Lasa, B.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. 3,4-Dimethylpyrazole phosphate and 2-(N-3, 4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 2018, 624, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Severin, M.; Fuß, R.; Well, R.; Hähndel, R.; Van den Weghe, H. Greenhouse gas emissions after application of digestate: Short-term effects of nitrification inhibitor and application technique effects. Arch. Agron. Soil Sci. 2016, 62, 1007–1020. [Google Scholar] [CrossRef]
- Dittert, K.; Bol, R.; King, R.; Chadwick, D.; Hatch, D. Use of a novel nitrification inhibitor to reduce nitrous oxide emission from15N-labelled dairy slurry injected into soil. Rapid Commun. Mass Spectrom. 2001, 15, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Weiske, A.; Benckiser, G.; Herbert, T.; Ottow, J. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide emissions and methane oxidation during 3 years of repeated application in field experiments. Biol. Fertil. Soils 2001, 34, 109–117. [Google Scholar] [CrossRef]
- Fangueiro, D.; Ribeiro, H.; Vasconcelos, E.; Coutinho, J.; Cabral, F. Treatment by acidification followed by solid–liquid separation affects slurry and slurry fractions composition and their potential of N mineralization. Bioresour. Technol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, C.C.; Rolston, D.E. Measurement of small nitrous oxide concentrations by gas chromatography [Denitrification, helium ionization]. Soil Sci. Soc. Am. J. 1976, 40, 324–327. [Google Scholar]
- Matthias, A.D.; Yarger, D.N.; Weinbeck, R.S. A numerical evaluation of chamber methods for determining gas emissions. Geophys. Res. Lett. 1978, 5, 765–768. [Google Scholar] [CrossRef]
- Denmead, O.T. Approaches to measuring emissions of methane and nitrous oxide between landscapes and the atmosphere. Plant Soil 2008, 309, 5–24. [Google Scholar] [CrossRef]
- Rochette, P. Towards a standard non-steady-state chamber methodology for measuring soil N2O emissions. Anim. Feed Sci. Technol. 2011, 166, 141–146. [Google Scholar] [CrossRef]
- Perego, A.; Giussani, A.; Sanna, M.; Fumagalli, M.; Carozzi, M.; Alfieri, L.; Brenna, S.; Acutis, M. The ARMOSA simulation crop model: Overall features, calibration and validation results. Ital. J. Agrometeorol. 2013, 3, 23–38. [Google Scholar]
- Fumagalli, M.; Perego, A.; Acutis, M. Modelling nitrogen leaching from sewage sludge application to arable land in the Lombardy region (northern Italy). Sci. Total Environ. 2013, 461, 509–518. [Google Scholar] [CrossRef]
- Acutis, M.; Alfieri, L.; Giussani, A.; Provolo, G.; Di Guardo, A.; Colombini, S.; Bertoncini, G.; Castelnuovo, M.; Sali, G.; Moschini, M.; et al. ValorE: An integrated and GIS-based decision support system for livestock manure management in the Lombardy region (northern Italy). Land Use Policy 2014, 41, 149–162. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Blackwell Wissenschafts-Verlag: Hoboken, NJ, USA, 1997. [Google Scholar]
- Chadwick, D.R.; Cardenas, L.; Misselbrook, T.H.; Smith, K.A.; Rees, R.M.; Watson, C.J.; McGeough, K.L.; Williams, J.R.; Cloy, J.M.; Thorman, R.E.; et al. Optimizing chamber methods for measuring nitrous oxide emissions from plot-based agricultural experiments. Eur. J. Soil Sci. 2014, 65, 295–307. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L. Enclosure-based measurement of trace gas exchange: Applications and sources of error. In Biogenic Trace Gases: Measuring Emissions from Soil and Water; Matson, P., Ed.; Blackwell Science: Oxford, UK, 1995. [Google Scholar]
- Rochette, P.; Eriksen-Hamel, N.S. Chamber Measurements of Soil Nitrous Oxide Flux: Are Absolute Values Reliable? Soil Sci. Soc. Am. J. 2008, 72, 331–342. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; Harvey, M.J. Nitrous oxide chamber methodology guidelines. In Global Research Alliance on Agricultural Greenhouse Gases; Version 1.1.; de Klein, C.A.M., Harvey, M., Eds.; Ministry for Primary Industries: Wellington, New Zealand, 2015; ISBN 978-0-478-40584-2. [Google Scholar]
- Efron, B.; Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1986, 1, 54–75. [Google Scholar] [CrossRef]
- Ding, W.; Yagi, K.; Akiyama, H.; Sudo, S.; Nishimura, S. Time-lagged induction of N2O emission and its trade-off with NO emission from a nitrogen fertilized Andisol. Soil Sci. Plant Nutr. 2007, 53, 362–372. [Google Scholar] [CrossRef]
- Macadam, X.M.B.; del Prado, A.; Merino, P.; Estavillo, J.M.; Pinto, M.; González-Murua, C. Dicyandiamide and 3,4-dimethyl pyrazole phosphate decrease N2O emissions from grassland but dicyandiamide produces deleterious effects in clover. J. Plant Physiol. 2003, 160, 1517–1523. [Google Scholar] [CrossRef]
- Menéndez, S.; Merino, P.; Pinto, M.; González-Murua, C.; Estavillo, J.M. 3,4-Dimethylpyrazol Phosphate Effect on Nitrous Oxide, Nitric Oxide, Ammonia, and Carbon Dioxide Emissions from Grasslands. J. Environ. Qual. 2006, 35, 973–981. [Google Scholar] [CrossRef]
- Serna, M.D.; Bañuls, J.; Quiñones, A.; Primo-Millo, E.; Legaz, F. Evaluation of 3,4-dimethylpyrazole phosphate as a nitrification inhibitor in a Citrus-cultivated soil. Biol. Fertil. Soils 2000, 32, 41–46. [Google Scholar] [CrossRef]
- Díez-López, J.A.; Hernaiz-Algarra, P.; Arauzo-Sánchez, M.; Carrasco-Martín, I. Effect of a nitrification inhibitor (DMPP) on nitrate leaching and maize yield during two growing seasons. Span. J. Agric. Res. 2008, 2, 294–303. [Google Scholar] [CrossRef]
- Cardenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Gregory, A.S.; Matthews, G.P.; Whalley, W.R.; Misselbrook, T.H.; Scholefield, D.; Well, R. Effect of soil saturation on denitrification in a grassland soil. Biogeosciences 2017, 14, 4691–4710. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Escuer Gatius, J.; Shanskiy, M.; Mander, U.; Kauer, K.; Astover, A.; Soosaar, K. Effectiveness of a Nitrification Inhibitor to Reduce N2O Emissions—A Case Study in Estonia; European Geoscience Union General Assembly: Vienna, Austria, 2019. [Google Scholar]
- Grundmann, G.L.; Rolston, D.E. A water function approximation to degree of anaerobiosis associated with denitrification. Soil Sci. 1987, 144, 437–441. [Google Scholar] [CrossRef]
- Davidson, E.A.; Vitousek, P.M.; Matson, P.A.; Riley, R.; García-Méndez, G.; Maass, J.M. Soil emissions of nitric oxide in a seasonally dry tropical forest of Mexico. J. Geophys. Res. Atmos. 1991, 96, 15439–15445. [Google Scholar] [CrossRef]
- Torralbo, F.; Menéndez, S.; Barrena, I.; Estavillo, J.M.; Marino, D.; González-Murua, C. Dimethyl pyrazol-based nitrification inhibitors effect on nitrifying and denitrifying bacteria to mitigate N2O emission. Sci. Rep. 2017, 7, 13810. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.; Mary, B.; Renault, P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol. Biochem. 2004, 36, 687–699. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M. Nitrous oxide emissions from the soil under different fertilization systems on a long-term experiment. Plant Soil Environ. 2014, 60, 481–488. [Google Scholar] [CrossRef] [Green Version]


| Variable | Year | |
|---|---|---|
| 2016 | 2017 | |
| Sand (%) | 56.5 | 43.7 |
| Silt (%) | 24.9 | 38.8 |
| Clay (%) | 18.6 | 18.5 |
| pH | 6.8 | 6.7 |
| Total N (g N kg−1) | 1.76 | 2.26 |
| Years | Treatment | Application Method | Total Supplied N (kg N ha−1) | Supplied NH4-N (kg NH4-N ha−1) | Cumulative N2O-N Emissions (kg N2O-N ha−1) | EF (%) |
|---|---|---|---|---|---|---|
| 2016 | D | Inter-row injection | 61 | 27.8 | 1.1 ± 0.2a | 1.8 ± 0.3a |
| V | 0.52 ± 0.1b | 0.9 ± 0.2b | ||||
| 2017 | D | Tines harrow injection | 238 | 167.5 | 9.22 ± 1.2a | 3.9 ± 0.5a |
| V | 1.73 ± 0.5b | 0.7 ± 0.2b |
| Year | Depth (cm) | Bulk Density (Mg m−3) | ||
|---|---|---|---|---|
| N0 | D | V | ||
| 2016 | 0–5 | 1.17 ± 0.5 | 1.33 ± 0.6 | 1.28 ± 0.6 |
| 5–10 | 1.48 ± 0.2 | 1.43 ± 0.2 | 1.42 ± 0.2 | |
| 10–15 | 1.37 ± 0.5 | 1.48 ± 0.6 | 1.52 ± 0.6 | |
| 15–20 | 1.53 ± 0.5 | 1.59 ± 0.5 | 1.45 ± 0.4 | |
| 20–25 | 1.83 ± 0.2 | 1.85 ± 0.2 | 1.77 ± 0.2 | |
| 2017 | 0–5 | 1.24 ± 0.5 | 1.13 ± 0.4 | 1.21 ± 0.5 |
| 5–10 | 1.39 ± 0.5 | 1.27 ± 0.5 | 1.29 ± 0.5 | |
| 10–15 | 1.45 ± 0.5 | 1.35 ± 0.4 | 1.47 ± 0.5 | |
| 15–20 | 1.60 ± 0.3 | 1.50 ± 0.3 | 1.55 ± 0.3 | |
| 20–25 | 1.57 ± 0.8 | 1.41 ± 0.7 | 1.65 ± 0.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiodini, M.E.; Perego, A.; Carozzi, M.; Acutis, M. The Nitrification Inhibitor Vizura® Reduces N2O Emissions When Added to Digestate before Injection under Irrigated Maize in the Po Valley (Northern Italy). Agronomy 2019, 9, 431. https://doi.org/10.3390/agronomy9080431
Chiodini ME, Perego A, Carozzi M, Acutis M. The Nitrification Inhibitor Vizura® Reduces N2O Emissions When Added to Digestate before Injection under Irrigated Maize in the Po Valley (Northern Italy). Agronomy. 2019; 9(8):431. https://doi.org/10.3390/agronomy9080431
Chicago/Turabian StyleChiodini, Marcello Ermido, Alessia Perego, Marco Carozzi, and Marco Acutis. 2019. "The Nitrification Inhibitor Vizura® Reduces N2O Emissions When Added to Digestate before Injection under Irrigated Maize in the Po Valley (Northern Italy)" Agronomy 9, no. 8: 431. https://doi.org/10.3390/agronomy9080431






