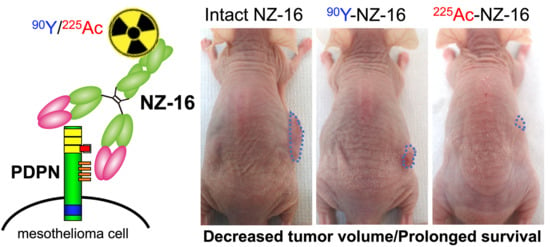
Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma
Abstract
Share and Cite
Sudo, H.; Tsuji, A.B.; Sugyo, A.; Kaneko, M.K.; Kato, Y.; Nagatsu, K.; Suzuki, H.; Higashi, T. Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells 2021, 10, 2503. https://doi.org/10.3390/cells10102503
Sudo H, Tsuji AB, Sugyo A, Kaneko MK, Kato Y, Nagatsu K, Suzuki H, Higashi T. Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells. 2021; 10(10):2503. https://doi.org/10.3390/cells10102503
Chicago/Turabian StyleSudo, Hitomi, Atsushi B. Tsuji, Aya Sugyo, Mika K. Kaneko, Yukinari Kato, Kotaro Nagatsu, Hisashi Suzuki, and Tatsuya Higashi. 2021. "Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma" Cells 10, no. 10: 2503. https://doi.org/10.3390/cells10102503
APA StyleSudo, H., Tsuji, A. B., Sugyo, A., Kaneko, M. K., Kato, Y., Nagatsu, K., Suzuki, H., & Higashi, T. (2021). Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells, 10(10), 2503. https://doi.org/10.3390/cells10102503