Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Biologic Reagents
2.2. Plasmid Constructions
2.3. HPH and HepaRG Cell Culture and Treatment
2.4. RT-PCR Analysis
2.5. Western Blot Analysis
2.6. PXR Knockdown in HPH
2.7. Transfection Assays in Hepatoma Cells and HPH
2.8. Electrophoretic Mobility Shift Assays
2.9. Statistical Analysis
3. Results
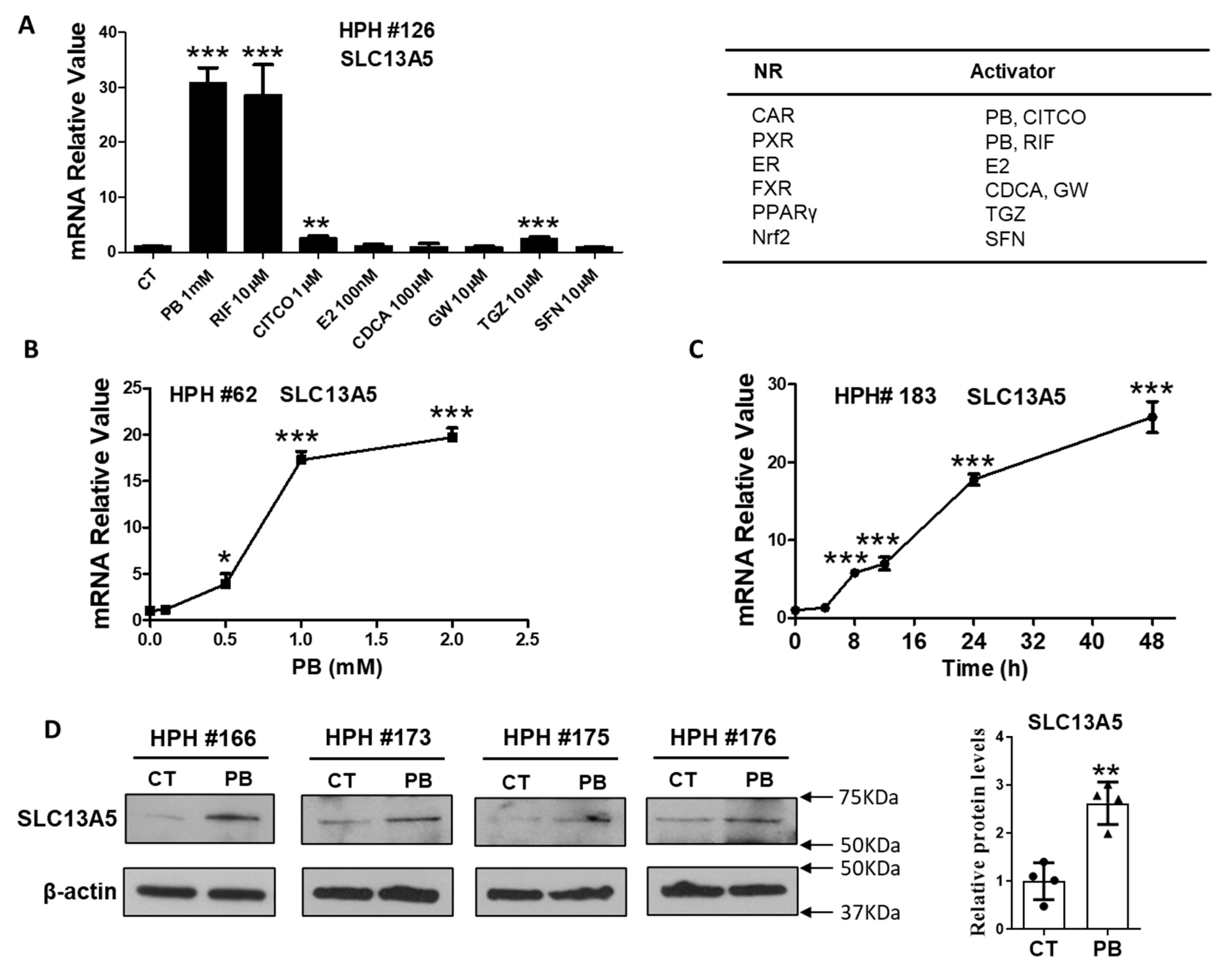
3.1. PB Induces the Expression of SLC13A5 Gene in HPH
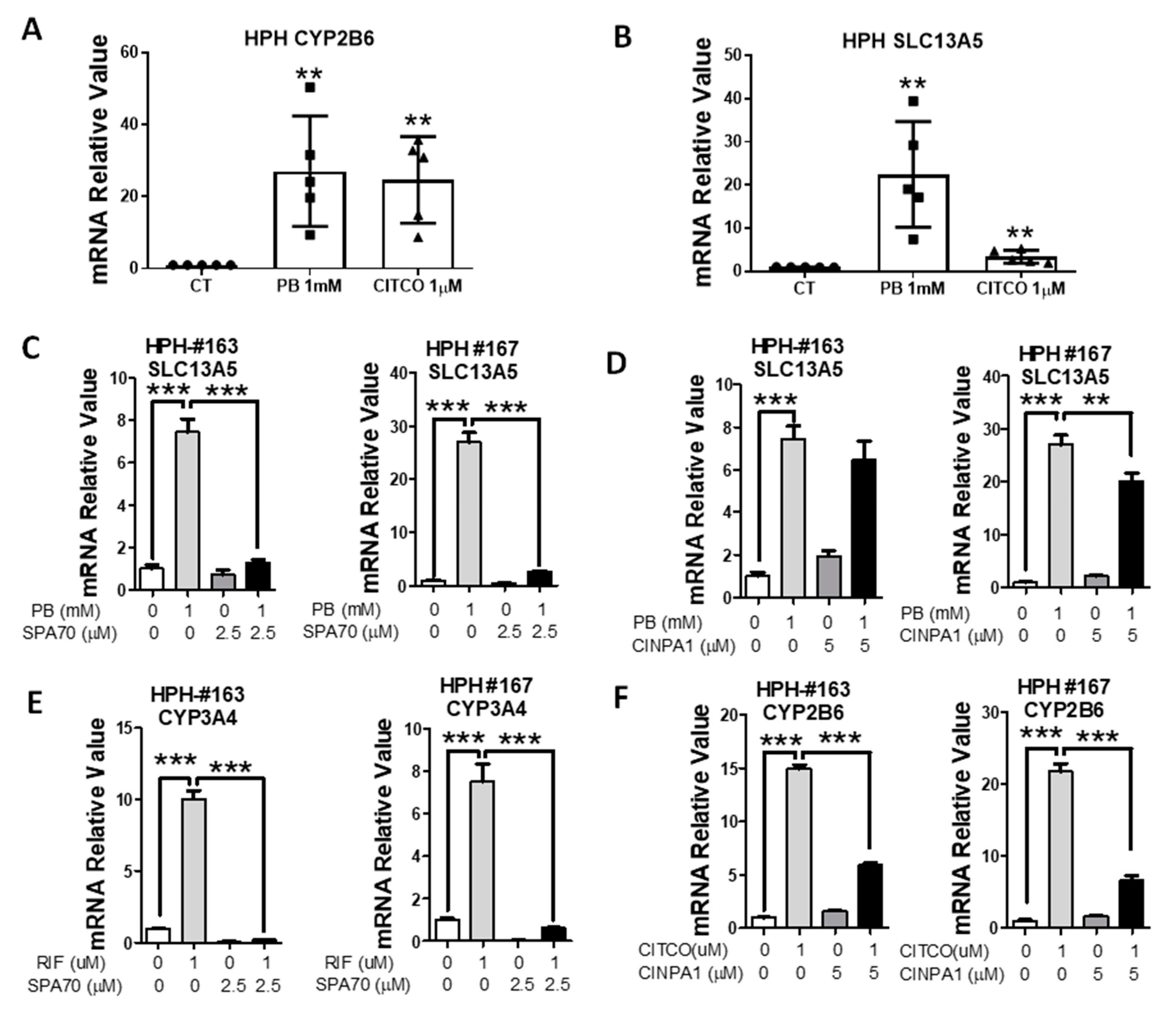
3.2. PB-Mediated Induction of SLC13A5 Was Repressed by Selective PXR but Not CAR Inhibitors
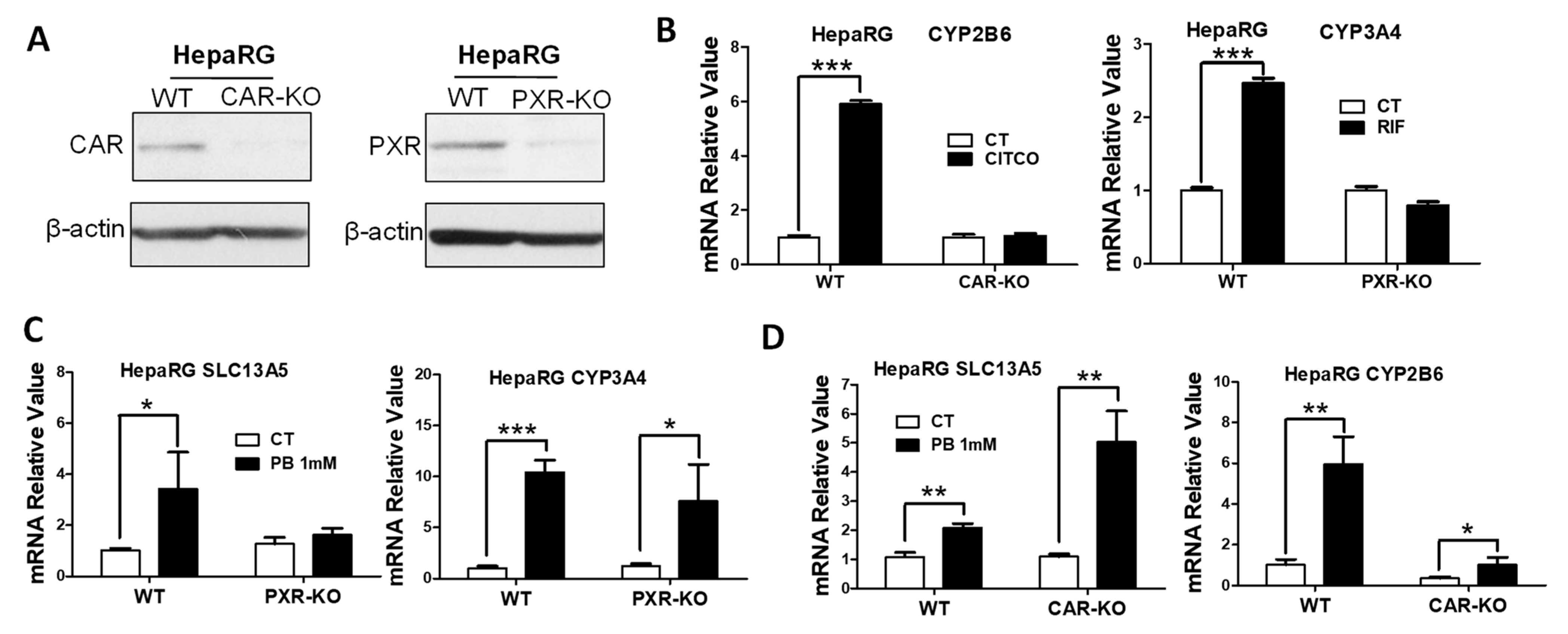
3.3. PB Induces the SLC13A5 Expression in CAR-KO but Not PXR-KO HepaRG Cells
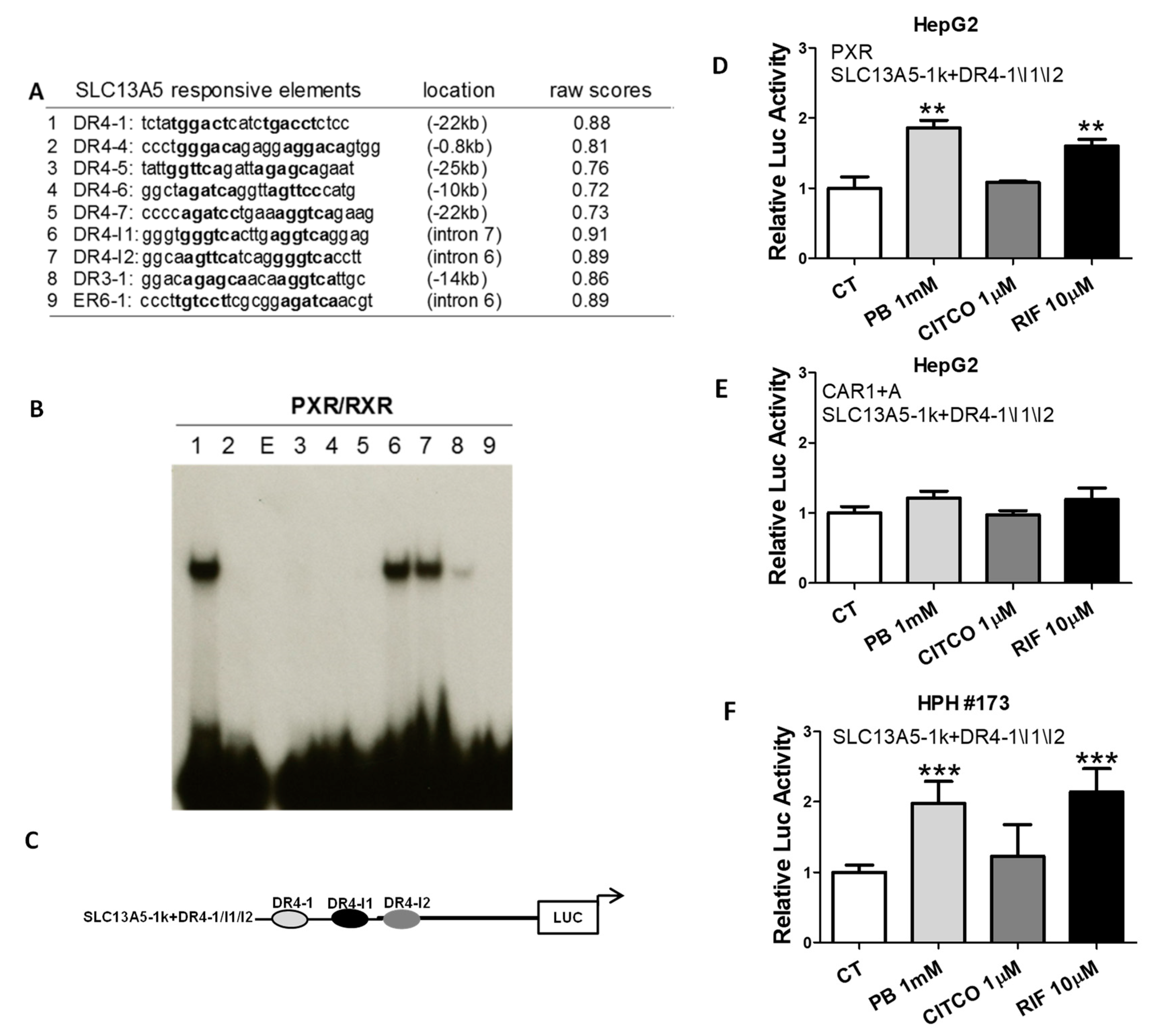
3.4. Identification of Novel PXR-Response Elements in the SLC13A5 Gene
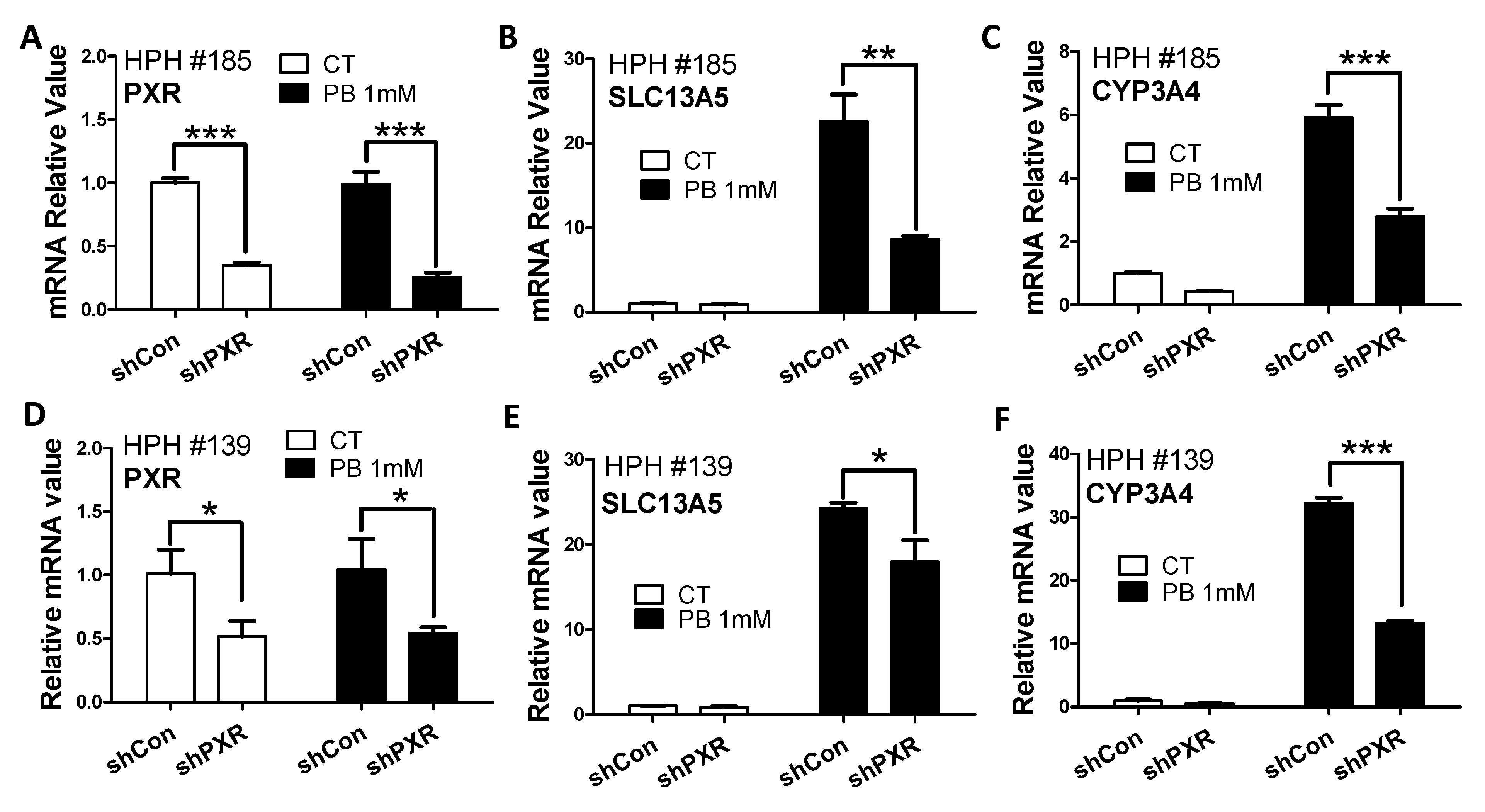
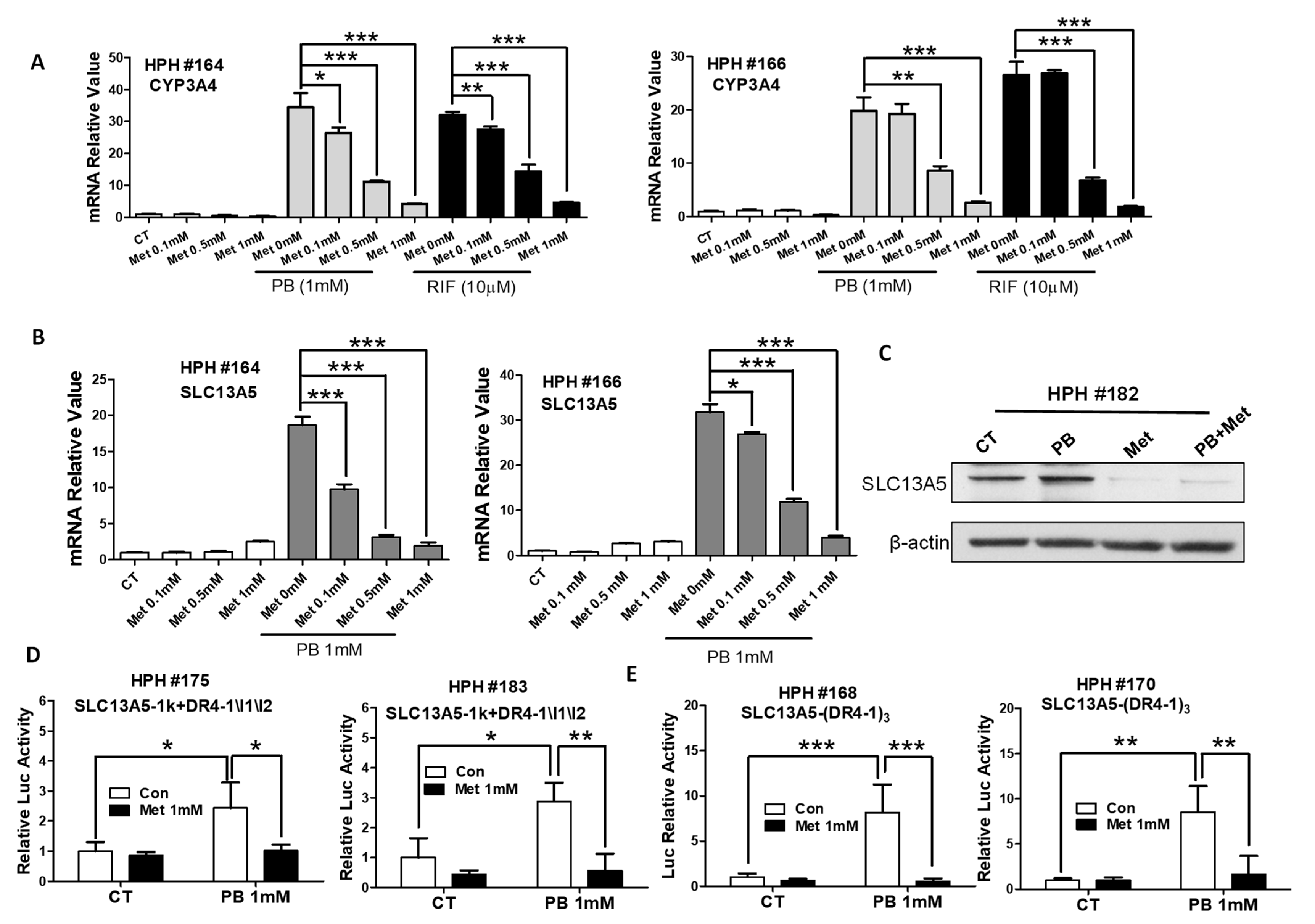
3.5. Metformin Represses PB-Induced SLC13A5 Expression through PXR Inhibition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, K.; Zhuang, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J. Biol. Chem. 2002, 277, 39469–39476. [Google Scholar] [CrossRef] [Green Version]
- Rogina, B.; Reenan, R.A.; Nilsen, S.P.; Helfand, S.L. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science 2000, 290, 2137–2140. [Google Scholar] [CrossRef]
- Inoue, K.; Fei, Y.J.; Zhuang, L.; Gopal, E.; Miyauchi, S.; Ganapathy, V. Functional features and genomic organization of mouse NaCT, a sodium-coupled transporter for tricarboxylic acid cycle intermediates. Biochem. J. 2004, 378, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Birkenfeld, A.L.; Lee, H.Y.; Guebre-Egziabher, F.; Alves, T.C.; Jurczak, M.J.; Jornayvaz, F.R.; Zhang, D.; Hsiao, J.J.; Martin-Montalvo, A.; Fischer-Rosinsky, A.; et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 2011, 14, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantila Roosa, S.M.; Liu, Y.; Turner, C.H. Gene expression patterns in bone following mechanical loading. J. Bone Min. Res. 2011, 26, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, K.; Zhuang, L.; Ganapathy, V. Human Na+ -coupled citrate transporter: Primary structure, genomic organization, and transport function. Biochem. Biophys. Res. Commun. 2002, 299, 465–471. [Google Scholar] [CrossRef]
- Kopel, J.J.; Bhutia, Y.D.; Sivaprakasam, S.; Ganapathy, V. Consequences of NaCT/SLC13A5/mINDY deficiency: Good versus evil, separated only by the blood-brain barrier. Biochem. J. 2021, 478, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Gopal, E.; Miyauchi, S.; Martin, P.M.; Ananth, S.; Srinivas, S.R.; Smith, S.B.; Prasad, P.D.; Ganapathy, V. Expression and functional features of NaCT, a sodium-coupled citrate transporter, in human and rat livers and cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G402–G408. [Google Scholar] [CrossRef]
- Willmes, D.M.; Kurzbach, A.; Henke, C.; Schumann, T.; Zahn, G.; Heifetz, A.; Jordan, J.; Helfand, S.L.; Birkenfeld, A.L. The longevity gene INDY (I’m Not Dead Yet) in metabolic control: Potential as pharmacological target. Pharmacology 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Rogers, R.P.; Rogina, B. The role of INDY in metabolism, health and longevity. Front. Genet. 2015, 6, 204. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B. Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine. HSOA J. Hum. Endocrinol. 2016, 1, 005. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H. Molecular Mechanisms of the SLC13A5 Gene Transcription. Metabolites 2021, 11, 706. [Google Scholar] [CrossRef]
- von Loeffelholz, C.; Lieske, S.; Neuschafer-Rube, F.; Willmes, D.M.; Raschzok, N.; Sauer, I.M.; Konig, J.; Fromm, M.F.; Horn, P.; Chatzigeorgiou, A.; et al. The human longevity gene homolog INDY and interleukin-6 interact in hepatic lipid metabolism. Hepatology 2017, 66, 616–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, D.; Choi, E.Y.; Lapidus, R.; Zhang, L.; Huang, S.M.; Shapiro, P.; Wang, H. Silencing of solute carrier family 13 member 5 disrupts energy homeostasis and inhibits proliferation of human hepatocarcinoma cells. J. Biol. Chem. 2017, 292, 13890–13901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevenon, J.; Milh, M.; Feillet, F.; St-Onge, J.; Duffourd, Y.; Juge, C.; Roubertie, A.; Heron, D.; Mignot, C.; Raffo, E.; et al. Mutations in SLC13A5 cause autosomal-recessive epileptic encephalopathy with seizure onset in the first days of life. Am. J. Hum. Genet. 2014, 95, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hardies, K.; de Kovel, C.G.; Weckhuysen, S.; Asselbergh, B.; Geuens, T.; Deconinck, T.; Azmi, A.; May, P.; Brilstra, E.; Becker, F.; et al. Recessive mutations in SLC13A5 result in a loss of citrate transport and cause neonatal epilepsy, developmental delay and teeth hypoplasia. Brain J. Neurol. 2015, 138, 3238–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuschafer-Rube, F.; Lieske, S.; Kuna, M.; Henkel, J.; Perry, R.J.; Erion, D.M.; Pesta, D.; Willmes, D.M.; Brachs, S.; von Loeffelholz, C.; et al. The mammalian INDY homolog is induced by CREB in a rat model of type 2 diabetes. Diabetes 2014, 63, 1048–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuschafer-Rube, F.; Schraplau, A.; Schewe, B.; Lieske, S.; Krutzfeldt, J.M.; Ringel, S.; Henkel, J.; Birkenfeld, A.L.; Puschel, G.P. Arylhydrocarbon receptor-dependent mIndy (Slc13a5) induction as possible contributor to benzo[a]pyrene-induced lipid accumulation in hepatocytes. Toxicology 2015, 337, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Garzel, B.; Yang, H.; Sueyoshi, T.; Li, Q.; Shu, Y.; Zhang, J.; Hu, B.; Heyward, S.; et al. SLC13A5 is a novel transcriptional target of the pregnane X receptor and sensitizes drug-induced steatosis in human liver. Mol. Pharmacol. 2015, 87, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, T.; Pitot, H.C.; Miller, E.C.; Miller, J.A. Promotion by dietary phenobarbital of hepatocarcinogenesis by 2-methyl-N,N-dimethyl-4-aminoazobenzene in the rat. Cancer Res. 1979, 39, 112–115. [Google Scholar] [PubMed]
- Feldman, D.; Swarm, R.L.; Becker, J. Elimination of excess smooth endoplasmic reticulum after phenobarbital administration. J. Histochem. Cytochem. 1980, 28, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luisier, R.; Lempiainen, H.; Scherbichler, N.; Braeuning, A.; Geissler, M.; Dubost, V.; Muller, A.; Scheer, N.; Chibout, S.D.; Hara, H.; et al. Phenobarbital induces cell cycle transcriptional responses in mouse liver humanized for constitutive androstane and pregnane x receptors. Toxicol. Sci. 2014, 139, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Negishi, M.; Kobayashi, K.; Sakuma, T.; Sueyoshi, T. Nuclear receptor phosphorylation in xenobiotic signal transduction. J. Biol. Chem. 2020, 295, 15210–15225. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, S.; Yamamoto, Y.; Ueda, A.; Moore, R.; Sueyoshi, T.; Negishi, M. Phenobarbital induction of drug/steroid-metabolizing enzymes and nuclear receptor CAR. Biochim. Biophys. Acta 2003, 1619, 239–242. [Google Scholar] [CrossRef]
- Miles, J.S.; Spurr, N.K.; Gough, A.C.; Jowett, T.; McLaren, A.W.; Brook, J.D.; Wolf, C.R. A novel human cytochrome P450 gene (P450IIB): Chromosomal localization and evidence for alternative splicing. Nucleic Acids Res. 1988, 16, 5783–5795. [Google Scholar] [CrossRef] [Green Version]
- Honkakoski, P.; Zelko, I.; Sueyoshi, T.; Negishi, M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell Biol. 1998, 18, 5652–5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Welch, M.A.; Li, Z.; Mackowiak, B.; Heyward, S.; Swaan, P.W.; Wang, H. Mechanistic Insights of Phenobarbital-Mediated Activation of Human but Not Mouse Pregnane X Receptor. Mol. Pharmacol. 2019, 96, 345–354. [Google Scholar] [CrossRef]
- Mackowiak, B.; Hodge, J.; Stern, S.; Wang, H. The Roles of Xenobiotic Receptors: Beyond Chemical Disposition. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Regulation of CAR and PXR Expression in Health and Disease. Cells 2020, 9, 2395. [Google Scholar] [CrossRef]
- Chen, T.; Tompkins, L.M.; Li, L.; Li, H.; Kim, G.; Zheng, Y.; Wang, H. A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J. Pharmacol. Exp. Ther. 2010, 332, 106–115. [Google Scholar] [CrossRef]
- Li, L.; Chen, T.; Stanton, J.D.; Sueyoshi, T.; Negishi, M.; Wang, H. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol. Pharm. 2008, 74, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Faucette, S.R.; Zhang, T.C.; Moore, R.; Sueyoshi, T.; Omiecinski, C.J.; LeCluyse, E.L.; Negishi, M.; Wang, H. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J. Pharmacol. Exp. Ther. 2007, 320, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Wang, Y.M.; Chai, S.C.; Lv, L.; Zheng, J.; Wu, J.; Zhang, Q.; Wang, Y.D.; Griffin, P.R.; Chen, T. SPA70 is a potent antagonist of human pregnane X receptor. Nat. Commun. 2017, 8, 741. [Google Scholar] [CrossRef] [Green Version]
- Cherian, M.T.; Lin, W.; Wu, J.; Chen, T. CINPA1 is an inhibitor of constitutive androstane receptor that does not activate pregnane X receptor. Mol. Pharmacol. 2015, 87, 878–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grime, K.; Ferguson, D.D.; Riley, R.J. The use of HepaRG and human hepatocyte data in predicting CYP induction drug-drug interactions via static equation and dynamic mechanistic modelling approaches. Curr. Drug Metab. 2010, 11, 870–885. [Google Scholar] [CrossRef]
- Mackowiak, B.; Li, L.; Welch, M.A.; Li, D.; Jones, J.W.; Heyward, S.; Kane, M.A.; Swaan, P.W.; Wang, H. Molecular Basis of Metabolism-Mediated Conversion of PK11195 from an Antagonist to an Agonist of the Constitutive Androstane Receptor. Mol. Pharm. 2017, 92, 75–87. [Google Scholar] [CrossRef]
- Jackson, J.P.; Li, L.; Chamberlain, E.D.; Wang, H.; Ferguson, S.S. Contextualizing Hepatocyte Functionality of Cryopreserved HepaRG Cell Cultures. Drug Metab. Dispos. Biol. Fate Chem. 2016, 44, 1463–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Mackowiak, B.; Brayman, T.G.; Mitchell, M.; Zhang, L.; Huang, S.M.; Wang, H. Genome-wide analysis of human constitutive androstane receptor (CAR) transcriptome in wild-type and CAR-knockout HepaRG cells. Biochem. Pharmacol. 2015, 98, 190–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Faucette, S.; Sueyoshi, T.; Moore, R.; Ferguson, S.; Negishi, M.; LeCluyse, E.L. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J. Biol. Chem. 2003, 278, 14146–14152. [Google Scholar] [CrossRef] [Green Version]
- Krausova, L.; Stejskalova, L.; Wang, H.; Vrzal, R.; Dvorak, Z.; Mani, S.; Pavek, P. Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochem. Pharmacol. 2011, 82, 1771–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willson, T.M.; Kliewer, S.A. PXR, CAR and drug metabolism. Nat. Rev. Drug Discov. 2002, 1, 259–266. [Google Scholar] [CrossRef]
- Wada, T.; Gao, J.; Xie, W. PXR and CAR in energy metabolism. Trends Endocrinol. Metab. TEM 2009, 20, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W.; Chua, S.S.; Wei, P.; Moore, D.D. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science 2002, 298, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Moore, R.; Goldsworthy, T.L.; Negishi, M.; Maronpot, R.R. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004, 64, 7197–7200. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Faucette, S.; Moore, R.; Sueyoshi, T.; Negishi, M.; LeCluyse, E. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J. Biol. Chem. 2004, 279, 29295–29301. [Google Scholar] [CrossRef] [Green Version]
- Pinne, M.; Ponce, E.; Raucy, J.L. Transactivation Assays that Identify Indirect and Direct Activators of Human Pregnane X Receptor (PXR, NR1I2) and Constitutive Androstane Receptor (CAR, NR1I3). Drug Metab. Lett. 2017, 11, 128–137. [Google Scholar] [CrossRef]
- Jiang, Y.; Yao, X.; Fan, S.; Gao, Y.; Zhang, H.; Huang, M.; Bi, H. Lipidomic profiling reveals triacylglycerol accumulation in the liver during pregnane X receptor activation-induced hepatomegaly. J. Pharm. Biomed. Anal. 2021, 195, 113851. [Google Scholar] [CrossRef] [PubMed]
- Jeske, J.; Windshugel, B.; Thasler, W.E.; Schwab, M.; Burk, O. Human pregnane X receptor is activated by dibenzazepine carbamate-based inhibitors of constitutive androstane receptor. Arch. Toxicol. 2017, 91, 2375–2390. [Google Scholar] [CrossRef]
- de Boussac, H.; Gondeau, C.; Briolotti, P.; Duret, C.; Treindl, F.; Romer, M.; Fabre, J.M.; Herrero, A.; Ramos, J.; Maurel, P.; et al. Epidermal Growth Factor Represses Constitutive Androstane Receptor Expression in Primary Human Hepatocytes and Favors Regulation by Pregnane X Receptor. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 223–236. [Google Scholar] [CrossRef]
- Ding, X.; Staudinger, J.L. The ratio of constitutive androstane receptor to pregnane X receptor determines the activity of guggulsterone against the Cyp2b10 promoter. J. Pharmacol. Exp. Ther. 2005, 314, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Garzel, B.; Heyward, S.; Moeller, T.; Shapiro, P.; Wang, H. Metformin represses drug-induced expression of CYP2B6 by modulating the constitutive androstane receptor signaling. Mol. Pharmacol. 2014, 85, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, E.; Zhu, Z.; He, S.; Chu, D.; Ge, D.; Zhan, Y.; Liu, W.; Yang, J.; Xiong, J. Involvement of pregnane X receptor in the suppression of carboxylesterases by metformin in vivo and in vitro, mediated by the activation of AMPK and JNK signaling pathway. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 102, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Kopel, J.; Higuchi, K.; Ristic, B.; Sato, T.; Ramachandran, S.; Ganapathy, V. The Hepatic Plasma Membrane Citrate Transporter NaCT (SLC13A5) as a Molecular Target for Metformin. Sci. Rep. 2020, 10, 8536. [Google Scholar] [CrossRef] [PubMed]
- Kopel, J.; Grooms, A.; Ganapathy, V.; Clothier, J. Metformin, valproic acid, and starvation induce seizures in a patient with partial SLC13A5 deficiency: A case of pharmaco-synergistic heterozygosity. Psychiatr. Genet. 2021, 31, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Z.; Spelbrink, E.M.; Nye, K.L.; Hsu, E.R.; Porter, B.E. Epilepsy and EEG Phenotype of SLC13A5 Citrate Transporter Disorder. Child. Neurol. Open 2020, 7, 2329048X20931361. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, L.; Heyward, S.; Men, S.; Xu, M.; Sueyoshi, T.; Wang, H. Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes. Cells 2021, 10, 3381. https://doi.org/10.3390/cells10123381
Li Z, Li L, Heyward S, Men S, Xu M, Sueyoshi T, Wang H. Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes. Cells. 2021; 10(12):3381. https://doi.org/10.3390/cells10123381
Chicago/Turabian StyleLi, Zhihui, Linhao Li, Scott Heyward, Shuaiqian Men, Meishu Xu, Tatsuya Sueyoshi, and Hongbing Wang. 2021. "Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes" Cells 10, no. 12: 3381. https://doi.org/10.3390/cells10123381
APA StyleLi, Z., Li, L., Heyward, S., Men, S., Xu, M., Sueyoshi, T., & Wang, H. (2021). Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes. Cells, 10(12), 3381. https://doi.org/10.3390/cells10123381





