Therapeutic Potential of Annexin A1 Modulation in Kidney and Cardiovascular Disorders
Abstract
:1. Introduction
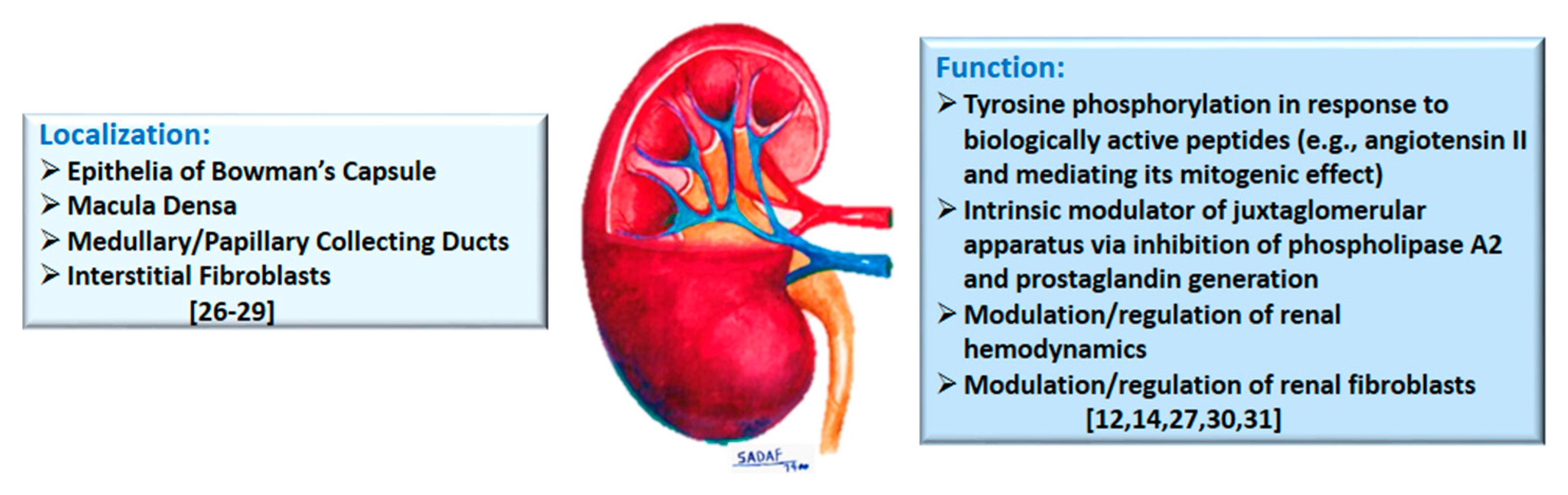
2. Annexins in the Kidney
3. Role of Annexin A1 in Pathologies of the Kidney
3.1. Annexin A1 and Immune/Inflammatory Responses in Kidney Pathologies
3.2. Annexin A1 and Acute Kidney Injury
3.3. Annexin A1 and Immunosuppressive Agents
3.4. Annexin A1 and Renal Injury of Diverse Etiopathogenesis
3.5. Annexin A1 and Renal Fibrosis
3.6. Annexin A1 and Metabolic Diseases
3.7. Annexin A1 and Kidney Stone
3.8. Annexin A1 and Renal Cancer
4. Recent Developments on the Role of Annexin A1 in the Cardiovascular System
| Condition | Finding | Citation(s) |
|---|---|---|
| Lupus Nephritis | Increased serum IgG2 against Annexin A1 | [35] |
| Glomerulonephritis with polyangiitis | Increased Annexin A1 expression in neutrophils | [36] |
| Kidney injuries of diverse pathogenesis (e.g., diabetes mellitus, pyemic secondary AKI, Adriamycin-induced nephrotoxicity, etc.) | Increased kidney, serum (or plasma) and/or urinary Annexin A1 | [41,42,43,44,45,46] |
| Patients with acute heart failure associated with impaired kidney function | Increased plasma Annexin A1 associated with worse congestion, morbidity and mortality | [75] |
| Renal cancer | Marker of poor prognosis Predictor of poor sunitinib response | [54,55,56,57] |
| Model/Condition | Findings | Citation(s) |
|---|---|---|
| Human coronary atherosclerotic plaques | Expression of annexin-I in macrophages, with foam cell phenotype, in tunica intima and adventitia High expression of annexin-I in macrophages in plaque areas, containing TUNEL positive cells, is suggestive of phagocytosis of apoptotic cells | [58] |
| Patients with carotid artery stenosis (undergoing carotid endarterectomy) | Higher expression of Annexin A1 in carotid plaques of asymptomatic than symptomatic patients thereby suggestive of its protective role in atherosclerosis | [67] |
| Patients with or without recent acute cerebrovascular symptoms | Increased Annexin A1 expression in plaque-derived smooth muscle cells of the asymptomatic group | [59] |
| Administration of nanoparticles containing Ac2-26, with a lesion-targeting collagen-IV motif, to mice with established atherosclerosis | Decreased lesion size in association with reduced oxidative stress and necrotic area but increased plaque stability and lesional interleukin-10 | [64] |
| Treatment of mice prone to develop atherosclerosis with hrANXA1 | No effect on initiation of plaque formation but attenuated progression of existing plaques of aortic arch and subclavian artery | [65] |
| Administration of Ac2-26 to mice with early stage atherosclerosis ApoE−/−, Fpr2−/− and ApoE−/−Anexxin-A1−/− mice | Curtailed early atherogenesis in a receptor-dependent manner associated with decreased lesional size and macrophage accumulation Display increased atherosclerotic lesion size as well as myeloid cell adhesion and recruitment | [66] |
| Model/Condition | Findings | Citation(s) |
|---|---|---|
| Effect Ac2-26 on sepsis-induced cardiomyocyte apoptosis | Attenuated cell death | [71] |
| Treatment of rats, subjected to acute myocardial ischemia-reperfusion injury (IRI), with hrANXA1 | Reduced infarct size in association with reduced leukocyte extravasation, myeloperoxidase activity, TNF-α and macrophage inflammatory protein-1a | [60] |
| Treatment of rats, subjected to acute myocardial IRI, with Ac2-26 | Reduced myeloperoxidase activity and interleukin-1β in the infarcted heart; protective effects were abrogated with an antagonist of Fpr receptors | [61] |
| Ac2-26 treatment of wild-type and Fpr null mice subjected to cardiac IRI | Cardioprotection in both wild-type and Fpr null mice | [62] |
| Effects of of Annexin A1(2-50) peptide in wild-type, Fpr1−/−, and Fpr2−/−/ALX−/− mice. Effects of the metabolically stable form of this peptide (i.e., CR-Annexin A1(2-50) in the murine model of cardiac IRI | Reduced leukocyte adhesion in wild-type and Fpr1−/−, but not Fpr2−/−/ALX−/−, mice Reduced infarct size and incidence of 24-h death | [63] |
| Effects of hrANXA1 in ANXA1 deficient STZ mice | Ameliorated cardiac injury | [46] |
| ANXA1 overexpression in the rat model of myocardial infarction | Reduced infarct size and improved functional outcome in association with reduced levels of pro-inflammatory factors, neutrophil infiltration and their apoptosis | [72] |
| Model/Condition | Findings | Citation(s) |
|---|---|---|
| Extracellular vesicles aggregates and microcalcification | Promotion by Annexin A1 | [68] |
| Acute aortic dissection | Possible protection by promoting Annexin A1 signaling | [69] |
| Murine model of metabolic syndrome | Beneficial effects of hrANXA1 against metabolic and microvascular abnormalities | [70] |
| Vascular complication of metabolic syndrome | Beneficial impact of small molecule agonist of Annexin A1 receptors | [38] |
| Murine model of rheumatoid arthritis with diastolic dysfunction | Treatment with hrANXA1 prevented progression of heart disease and also reversed established diastolic dysfunction in association with curtailing cardiac inflammation and fibrosis. | [73] |
| Acute coronary syndrome patients | Rosuvastatin therapy increased serum Annexin A1 level | [74] |
5. Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [Green Version]
- Cantacessi, C.; Seddon, J.M.; Miller, T.L.; Leow, C.Y.; Thomas, L.; Mason, L.; Willis, C.; Walker, G.; Loukas, A.; Gasser, R.B.; et al. A genome-wide analysis of annexins from parasitic organisms and their vectors. Sci. Rep. 2013, 3, 2893. [Google Scholar] [CrossRef] [Green Version]
- Gerke, V.; Moss, S.E. Annexins: From Structure to Function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef]
- Markoff, A.; Gerke, V. Expression and functions of annexins in the kidney. Am. J. Physiol.-Renal Physiol. 2005, 289, F949–F956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloix, J.F.; Colard, O.; Rothhut, B.; Russo-Marie, F. Characterization and partial purification of ‘renocortins’: Two polypeptides formed in renal cells causing the anti-phospholipase-like action of glucocorticoids. Br. J. Pharmacol. 1983, 79, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.O.; Fernandez, M.P. Annexin gene structures and molecular evolutionary genetics. Cell. Mol. Life Sci. 1997, 53, 508–515. [Google Scholar] [CrossRef]
- Araújo, T.G.; Mota, S.T.S.; Ferreira, H.S.V.; Ribeiro, M.A.; Goulart, L.R.; Vecchi, L. Annexin A1 as a Regulator of Immune Response in Cancer. Cells 2021, 10, 2245. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef] [Green Version]
- Solito, E.; Mulla, A.; Morris, J.F.; Christian, H.C.; Flower, R.J.; Buckingham, J.C. Dexamethasone Induces Rapid Serine-Phosphorylation and Membrane Translocation of Annexin 1 in a Human Folliculostellate Cell Line via a Novel Nongenomic Mechanism Involving the Glucocorticoid Receptor, Protein Kinase C, Phosphatidylinositol 3-Kinase, and Mitogen-Activated Protein Kinase. Endocrinology 2003, 144, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef]
- Croxtall, J.D.; Choudhury, Q.; Newman, S.; Flower, R.J. Lipocortin 1 and the control of cPLA2 activity in A549 cells: Glucocorticoids block EGF stimulation of cPLA2 phosphorylation. Biochem. Pharmacol. 1996, 52, 351–356. [Google Scholar] [CrossRef]
- Solito, E.; Raguenes-Nicol, C.; De Coupade, C.; Bisagni-Faure, A.; Russo-Marie, F. U937 cells deprived of endogenous annexin 1 demonstrate an increased PLA2activity. Br. J. Pharmacol. 1998, 124, 1675–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, L.; Solito, E. Annexin 1: More than an anti-phospholipase protein. Inflamm. Res. 2004, 53, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hannon, R.; Croxtall, J.D.; Getting, S.J.; Roviezzo, F.; Yona, S.; Paul-Clark, M.J.; Gavins, F.N.E.; Perretti, M.; Morris, J.F.; Buckingham, J.C.; et al. Aberrant inflammation and resistance to glucocorticoids in Annexin 1−/− Mouse. FASEB J. 2002, 17, 253–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vago, J.P.; Tavares, L.P.; Riccardi, C.; Teixeira, M.M.; Sousa, L.P. Exploiting the pro-resolving actions of glucocorticoid-induced proteins Annexin A1 and GILZ in infectious diseases. Biomed. Pharmacother. 2021, 133, 111033. [Google Scholar] [CrossRef]
- Ricci, E.; Ronchetti, S.; Pericolini, E.; Gabrielli, E.; Cari, L.; Gentili, M.; Roselletti, E.; Migliorati, G.; Vecchiarelli, A.; Riccardi, C. Role of the glucocorticoid-induced leucine zipper gene in dexamethasone-induced inhibition of mouse neutrophil migration via control of annexin A1 expression. FASEB J. 2017, 31, 3054–3065. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef]
- He, H.-Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Babbin, B.A.; Lee, W.Y.; Parkos, C.A.; Winfree, L.M.; Akyildiz, A.; Perretti, M.; Nusrat, A. Annexin I Regulates SKCO-15 Cell Invasion by Signaling through Formyl Peptide Receptors. J. Biol. Chem. 2006, 281, 19588–19599. [Google Scholar] [CrossRef] [Green Version]
- De Jong, R.; Leoni, G.; Drechsler, M.; Soehnlein, O. The advantageous role of annexin A1 in cardiovascular disease. Cell Adhes. Migr. 2017, 11, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Ansari, J.; Kaur, G.; Gavins, F.N.E. Therapeutic Potential of Annexin A1 in Ischemia Reperfusion Injury. Int. J. Mol. Sci. 2018, 19, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredman, G.; Tabas, I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. Am. J. Pathol. 2017, 187, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Recio, C.; Maione, F.; Iqbal, A.J.; Mascolo, N.; De Feo, V. The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front. Pharmacol. 2017, 7, 526. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhang, S.; Guo, Z.; Xing, D.; Chen, W. The crosstalk of ABCA1 and ANXA1: A potential mechanism for protection against atherosclerosis. Mol. Med. 2020, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caso, V.M.; Manzo, V.; Pecchillo Cimmino, T.; Conti, V.; Caso, P.; Esposito, G.; Russo, V.; Filippelli, A.; Ammendola, R.; Cattaneo, F. Regulation of Inflammation and Oxidative Stress by Formyl Peptide Receptors in Cardiovascular Disease Progression. Life 2021, 11, 243. [Google Scholar] [CrossRef]
- McKanna, J.A.; Chuncharunee, A.; Munger, K.A.; Breyer, J.A.; Cohen, S.; Harris, R.C. Localization of p35 (annexin I, lipocortin I) in normal adult rat kidney and during recovery from ischemia. J. Cell. Physiol. 1992, 153, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.P.; Gayral-Taminh, M.; Fauvel, J.; Delobbe, I.; Mignon-Conté, M.; Conté, J.J.; Chap, H. Sustained effect of angiotensin II on tyrosine phosphorylation of annexin I in glomerular mesangial cells. J. Biol. Chem. 1993, 268, 12805–12811. [Google Scholar] [CrossRef]
- Neymeyer, H.; Labes, R.; Reverte, V.; Saez, F.; Stroh, T.; Dathe, C.; Hohberger, S.; Zeisberg, M.; Müller, G.A.; Salazar, J.; et al. Activation of annexin A1 signalling in renal fibroblasts exerts antifibrotic effects. Acta Physiol. 2015, 215, 144–158. [Google Scholar] [CrossRef]
- Dang, V.D.; Jella, K.K.; Ragheb, R.R.T.; Denslow, N.D.; Alli, A.A. Lipidomic and proteomic analysis of exosomes from mouse cortical collecting duct cells. FASEB J. 2017, 31, 5399–5408. [Google Scholar] [CrossRef] [Green Version]
- Vervoordeldonk, M.J.; Schalkwijk, C.G.; Vishwanath, B.S.; Aarsman, A.J.; van den Bosch, H. Levels and localization of group II phospholipase A2 and annexin I in interleukin- and dexamethasone-treated rat mesangial cells: Evidence against annexin mediation of the dexamethasone-induced inhibition of group II phospholipases A2. Biochim. Biophys. Acta (BBA)-Bioenerg. 1994, 1224, 541–550. [Google Scholar] [CrossRef]
- Seidel, S.; Neymeyer, H.; Kahl, T.; Röschel, T.; Mutig, K.; Flower, R.; Schnermann, J.; Bachmann, S.; Paliege, A. Annexin A1 modulates macula densa function by inhibiting cyclooxygenase 2. Am. J. Physiol.-Renal Physiol. 2012, 303, F845–F854. [Google Scholar] [CrossRef] [Green Version]
- Abboud, H.E. Mesangial cell biology. Exp. Cell Res. 2012, 318, 979–985. [Google Scholar] [CrossRef]
- Allcock, G.H.; Allegra, M.; Flower, R.J.; Perretti, M. Neutrophil accumulation induced by bacterial lipopolysaccharide: Effects of dexamethasone and annexin 1. Clin. Exp. Immunol. 2001, 123, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Brancaleone, V.; Mitidieri, E.; Flower, R.J.; Cirino, G.; Perretti, M. Annexin A1 Mediates Hydrogen Sulfide Properties in the Control of Inflammation. J. Pharmacol. Exp. Ther. 2014, 351, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruschi, M.; Sinico, R.A.; Moroni, G.; Pratesi, F.; Migliorini, P.; Galetti, M.; Murtas, C.; Tincani, A.; Madaio, M.; Radice, A.; et al. Glomerular Autoimmune Multicomponents of Human Lupus Nephritis In Vivo: α-Enolase and Annexin AI. J. Am. Soc. Nephrol. 2014, 25, 2483–2498. [Google Scholar] [CrossRef] [Green Version]
- Everts-Graber, J.; Martin, K.R.; Thieblemont, N.; Mocek, J.; Roccabianca, A.; Chafey, P.; Le Gall, M.; Tacnet-Delorme, P.; Reutelingsperger, C.P.; Naccache, J.-M.; et al. Proteomic analysis of neutrophils in ANCA-associated vasculitis reveals a dysregulation in proteinase 3-associated proteins such as Annexin-A1 involved in apoptotic cell clearance. Kidney Int. 2019, 96, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.A.; Qin, C.X.; Jelinic, M.; O’Sullivan, K.; Deo, M.; Walsh, J.; Li, M.; Parry, L.J.; Ritchie, R.H.; Leo, C.H. The Novel Small-molecule Annexin-A1 Mimetic, Compound 17b, Elicits Vasoprotective Actions in Streptozotocin-induced Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 1384. [Google Scholar] [CrossRef] [Green Version]
- Facio, F.N., Jr.; Sena, A.A.; Araújo, L.P.; Mendes, G.E.; de Castro, I.; Luz, M.A.M.; Yu, L.; Oliani, S.M.; Burdmann, E.A. Annexin 1 mimetic peptide protects against renal ischemia/reperfusion injury in rats. J. Mol. Med. 2010, 89, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Suliman, H.; Ma, Q.; Zhang, Z.; Ren, J.; Morris, B.T.; Crowley, S.D.; Ulloa, L.; Privratsky, J.R. Annexin A1 Tripeptide Mimetic Increases Sirtuin-3 and Augments Mitochondrial Function to Limit Ischemic Kidney Injury. Front. Physiol. 2021, 12, 683098. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Meng, L.; Cao, G.-K.; Zhang, Y. Evaluation of CRRT effects on pyemic secondary AKI by serum cartilage glycoprotein 39 and Annexin A1. Exp. Ther. Med. 2016, 12, 2997–3001. [Google Scholar] [CrossRef] [Green Version]
- Araujo, L.P.; Truzzi, R.R.; Mendes, G.E.; Luz, M.A.; Burdmann, E.A.; Oliani, S.M. Interaction of the Anti-Inflammatory Annexin A1 Protein and Tacrolimus Immunosuppressant in the Renal Function of Rats. Am. J. Nephrol. 2010, 31, 527–533. [Google Scholar] [CrossRef]
- Araujo, L.P.; Truzzi, R.R.; Mendes, G.E.F.; Luz, M.A.M.; Burdmann, E.A.; Oliani, S.M. Annexin A1 protein attenuates cyclosporine-induced renal hemodynamics changes and macrophage infiltration in rats. Inflamm. Res. 2011, 61, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ka, S.-M.; Tsai, P.-Y.; Chao, T.-K.; Yang, S.-M.; Hung, Y.-J.; Chen, J.-S.; Shui, H.-A.; Chen, A. Urine Annexin A1 as an Index for Glomerular Injury in Patients. Dis. Markers 2014, 2014, 854163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcott, C.A.; Glenn, J.P.; Shade, R.E.; Kammerer, C.M.; Hinojosa-Laborde, C.; Fink, G.D.; Haywood, J.R.; Cox, L.A. A custom rat and baboon hypertension gene array to compare experimental models. Exp. Biol. Med. 2012, 237, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purvis, G.S.D.; Chiazza, F.; Chen, J.; Azevedo-Loiola, R.; Martin, L.; Kusters, D.H.M.; Reutelingsperger, C.; Fountoulakis, N.; Gnudi, L.; Yaqoob, M.M.; et al. Annexin A1 attenuates microvascular complications through restoration of Akt signalling in a murine model of type 1 diabetes. Diabetologia 2018, 61, 482–495. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, C.; Chang, D.-Y.; Zhan, R.; Sun, J.; Cui, S.-H.; Eddy, S.; Nair, V.; Tanner, E.; Brosius, F.C.; et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 2021, 100, 107–121. [Google Scholar] [CrossRef]
- Wu, L.; Liu, C.; Chang, D.-Y.; Zhan, R.; Zhao, M.; Lam, S.M.; Shui, G.; Zhao, M.-H.; Zheng, L.; Chen, M. The Attenuation of Diabetic Nephropathy by Annexin A1 via Regulation of Lipid Metabolism through AMPK/PPARα/CPT1b Pathway. Diabetes 2021, 70, 2192–2203. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Fong-Ngern, K.; Peerapen, P.; Thongboonkerd, V. High Calcium Enhances Calcium Oxalate Crystal Binding Capacity of Renal Tubular Cells via Increased Surface Annexin A1 but Impairs Their Proliferation and Healing. J. Proteome Res. 2012, 11, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. Caffeine prevents kidney stone formation by translocation of apical surface annexin A1 crystal-binding protein into cytoplasm: In vitro evidence. Sci. Rep. 2016, 6, 38536. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Protective Cellular Mechanism of Estrogen against Kidney Stone Formation: A Proteomics Approach and Functional Validation. Proteomics 2019, 19, e1900095. [Google Scholar] [CrossRef]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-A1–A Blessing or a Curse in Cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef]
- Kim, S.B.; Yang, W.S.; Lee, S.O.; Lee, K.P.; Park, J.S.; Na, D.S. Lipocortin-1 Inhibits Proliferation of Cultured Human Mesangial Cells. Nephron 1996, 74, 39–44. [Google Scholar] [CrossRef]
- Zimmermann, U.; Woenckhaus, C.; Teller, S.; Venz, S.; Langheinrich, M.; Protzel, C.; Maile, S.; Junker, H.; Giebel, J. Expression of annexin AI in conventional renal cell carcinoma (CRCC) correlates with tumour stage, Fuhrman grade, amount of eosinophilic cells and clinical outcome. Histol. Histopathol. 2007, 22, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Niinivirta, M.; Enblad, G.; Edqvist, P.-H.; Pontén, F.; Dragomir, A.; Ullenhag, G.J. Tumoral ANXA1 Is a Predictive Marker for Sunitinib Treatment of Renal Cancer Patients. J. Cancer 2017, 8, 3975–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanoi, M.; Yamanoi, K.; Fujii, C.; Fukuda, M.N.; Nakayama, J. Annexin A1 expression is correlated with malignant potential of renal cell carcinoma. Int. J. Urol. 2018, 26, 284–290. [Google Scholar] [CrossRef]
- Sadashiv, R.; Bannur, B.M.; Shetty, P.; Dinesh, U.S.; Vishwanatha, J.K.; Deshpande, S.K.; Bargale, A.; Sarathkumar, E.; Ruikar, K. Comparative expression analysis of phospholipid binding protein annexin a1 in nephrogenesis and kidney cancer. J. Basic Clin. Physiol. Pharmacol. 2019, 31, 20190179. [Google Scholar] [CrossRef]
- Bagnato, C.; Thumar, J.; Mayya, V.; Hwang, S.I.; Zebroski, H.; Claffey, K.P.; Haudenschild, C.; Eng, J.K.; Lundgren, D.H.; Han, D.K. Proteomics Analysis of Human Coronary Atherosclerotic Plaque: A Feasibility Study of Direct Tissue Proteomics by Liquid Chromatography and Tandem Mass Spectrometry* S. Mol. Cell. Proteom. 2007, 6, 1088–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viiri, L.E.; Full, L.E.; Navin, T.J.; Begum, S.; Didangelos, A.; Astola, N.; Berge, R.K.; Seppälä, I.; Shalhoub, J.; Franklin, I.J.; et al. Smooth muscle cells in human atherosclerosis: Proteomic profiling reveals differences in expression of Annexin A1 and mitochondrial proteins in carotid disease. J. Mol. Cell. Cardiol. 2012, 54, 65–72. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, M.; Di Filippo, C.; La, M.; Solito, E.; McLean, P.G.; JFlower, R.J.; Oliani, S.M.; Perretti, M. Lipocortin 1 reduces myocardial ischemia-reperfusion injury by affecting local leukocyte recruitment. FASEB J. 2000, 14, 1867–1869. [Google Scholar] [CrossRef]
- La, M.; D’Amico, M.; Bandiera, S.; Di Filippo, C.; Oliani, S.M.; Gavins, F.N.E.; Flower, R.J.; Perretti, M. Annexin 1 peptides protect against experimental myocardial ischemia-reperfusion: Analysis of their mechanism of action. FASEB J. 2001, 15, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.N.E.; Kamal, A.M.; D’Amico, M.; Oliani, S.M.; Perretti, M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005, 19, 100–102. [Google Scholar] [CrossRef]
- Dalli, J.; Consalvo, A.P.; Ray, V.; Di Filippo, C.; D’Amico, M.; Mehta, N.; Perretti, M. Proresolving and Tissue-Protective Actions of Annexin A1–Based Cleavage-Resistant Peptides Are Mediated by Formyl Peptide Receptor 2/Lipoxin A4 Receptor. J. Immunol. 2013, 190, 6478–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredman, G.; Kamaly, N.; Spolitu, S.; Milton, J.; Ghorpade, D.; Chiasson, R.; Kuriakose, G.; Perretti, M.; Farokhzad, O.; Tabas, I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015, 7, 275ra20. [Google Scholar] [CrossRef] [Green Version]
- Kusters, D.H.M.; Chatrou, M.L.; Willems, B.A.G.; De Saint-Hubert, M.; Bauwens, M.; Van Der Vorst, E.; Bena, S.; Biessen, E.A.L.; Perretti, M.; Schurgers, L.J.; et al. Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR-/- Mice on Western Type Diet. PLoS ONE 2015, 10, e0130484. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, M.; De Jong, R.; Rossaint, J.; Viola, J.R.; Leoni, G.; Wang, J.M.; Grommes, J.; Hinkel, R.; Kupatt, C.; Weber, C.; et al. Annexin A1 Counteracts Chemokine-Induced Arterial Myeloid Cell Recruitment. Circ. Res. 2015, 116, 827–835. [Google Scholar] [CrossRef]
- Cheuk, B.L.Y.; Cheng, S.W.K. Annexin A1 Expression in Atherosclerotic Carotid Plaques and its Relationship with Plaque Characteristics. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.A.; Buffolo, F.; Schlotter, F.; Atkins, S.K.; Lee, L.H.; Halu, A.; Blaser, M.C.; Tsolaki, E.; Higashi, H.; Luther, K.; et al. Annexin A1–dependent tethering promotes extracellular vesicle aggregation revealed with single–extracellular vesicle analysis. Sci. Adv. 2020, 6, eabb1244. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, Z.; Cao, H.; Chen, Y.; Li, J.; Zhuang, X.; Ma, D.; Ji, L.; Li, W.; Xu, S.; et al. Anxa1 in smooth muscle cells protects against acute aortic dissection. Cardiovasc. Res. 2021, cvab109. [Google Scholar] [CrossRef] [PubMed]
- Purvis, G.S.; Collino, M.; Loiola, R.A.; Baragetti, A.; Chiazza, F.; Brovelli, M.; Sheikh, M.H.; Collotta, D.; Cento, A.; Mastrocola, R.; et al. Identification of AnnexinA1 as an Endogenous Regulator of RhoA, and Its Role in the Pathophysiology and Experimental Therapy of Type-2 Diabetes. Front. Immunol. 2019, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zheng, Y.-L.; Hu, R.-H.; Zhu, L.; Hu, C.-C.; Cheng, F.; Li, S.; Li, J.-G. Annexin A1 Mimetic Peptide AC2-26 Inhibits Sepsis-induced Cardiomyocyte Apoptosis through LXA4/PI3K/AKT Signaling Pathway. Curr. Med. Sci. 2018, 38, 997–1004. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, B.; Jiang, J.; Wang, Y.; Wu, Y. Up-regulation of ANXA1 suppresses polymorphonuclear neutrophil infiltration and myeloperoxidase activity by activating STAT3 signaling pathway in rat models of myocardial ischemia-reperfusion injury. Cell. Signal. 2019, 62, 109325. [Google Scholar] [CrossRef]
- Chen, J.; Norling, L.V.; Mesa, J.G.; Silva, M.P.; Burton, S.E.; Reutelingsperger, C.; Perretti, M.; Cooper, D. Annexin A1 attenuates cardiac diastolic dysfunction in mice with inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2020385118. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Samy, O.M. Statin therapy improves serum Annexin A1 levels in patients with acute coronary syndrome: A case-controlled study. Int. J. Crit. Illn. Inj. Sci. 2021, 11, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Adel, F.W.; Rikhi, A.; Wan, S.-H.; Iyer, S.R.; Chakraborty, H.; McNulty, S.; Tang, W.W.; Felker, G.M.; Givertz, M.M.; Chen, H.H. Annexin A1 is a Potential Novel Biomarker of Congestion in Acute Heart Failure. J. Card. Fail. 2020, 26, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.S. Role of GILZ in the Kidney and the Cardiovascular System: Relevance to Cardiorenal Complications of COVID-19. J. Pharmacol. Exp. Ther. 2020, 375, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Baban, B.; Marchetti, C.; Khodadadi, H.; Malik, A.; Emami, G.; Lin, P.-C.; Arbab, A.S.; Riccardi, C.; Mozaffari, M.S. Glucocorticoid-Induced Leucine Zipper Promotes Neutrophil and T-Cell Polarization with Protective Effects in Acute Kidney Injury. J. Pharmacol. Exp. Ther. 2018, 367, 483–493. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozaffari, M.S. Therapeutic Potential of Annexin A1 Modulation in Kidney and Cardiovascular Disorders. Cells 2021, 10, 3420. https://doi.org/10.3390/cells10123420
Mozaffari MS. Therapeutic Potential of Annexin A1 Modulation in Kidney and Cardiovascular Disorders. Cells. 2021; 10(12):3420. https://doi.org/10.3390/cells10123420
Chicago/Turabian StyleMozaffari, Mahmood S. 2021. "Therapeutic Potential of Annexin A1 Modulation in Kidney and Cardiovascular Disorders" Cells 10, no. 12: 3420. https://doi.org/10.3390/cells10123420






