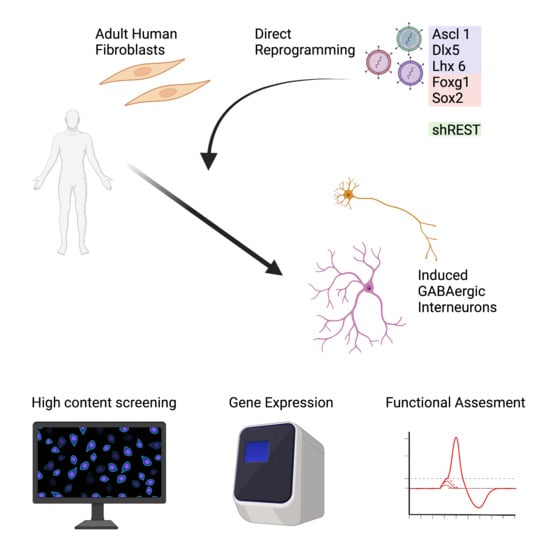
Reprogramming Human Adult Fibroblasts into GABAergic Interneurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fibroblast Culture
2.2. Viral Vectors
2.3. Neuronal Reprogramming
2.4. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.5. Immunocytochemistry
2.6. Microscopy
2.7. High-Content Screening
2.8. Electrophysiology
2.9. Statistical Analysis
3. Results
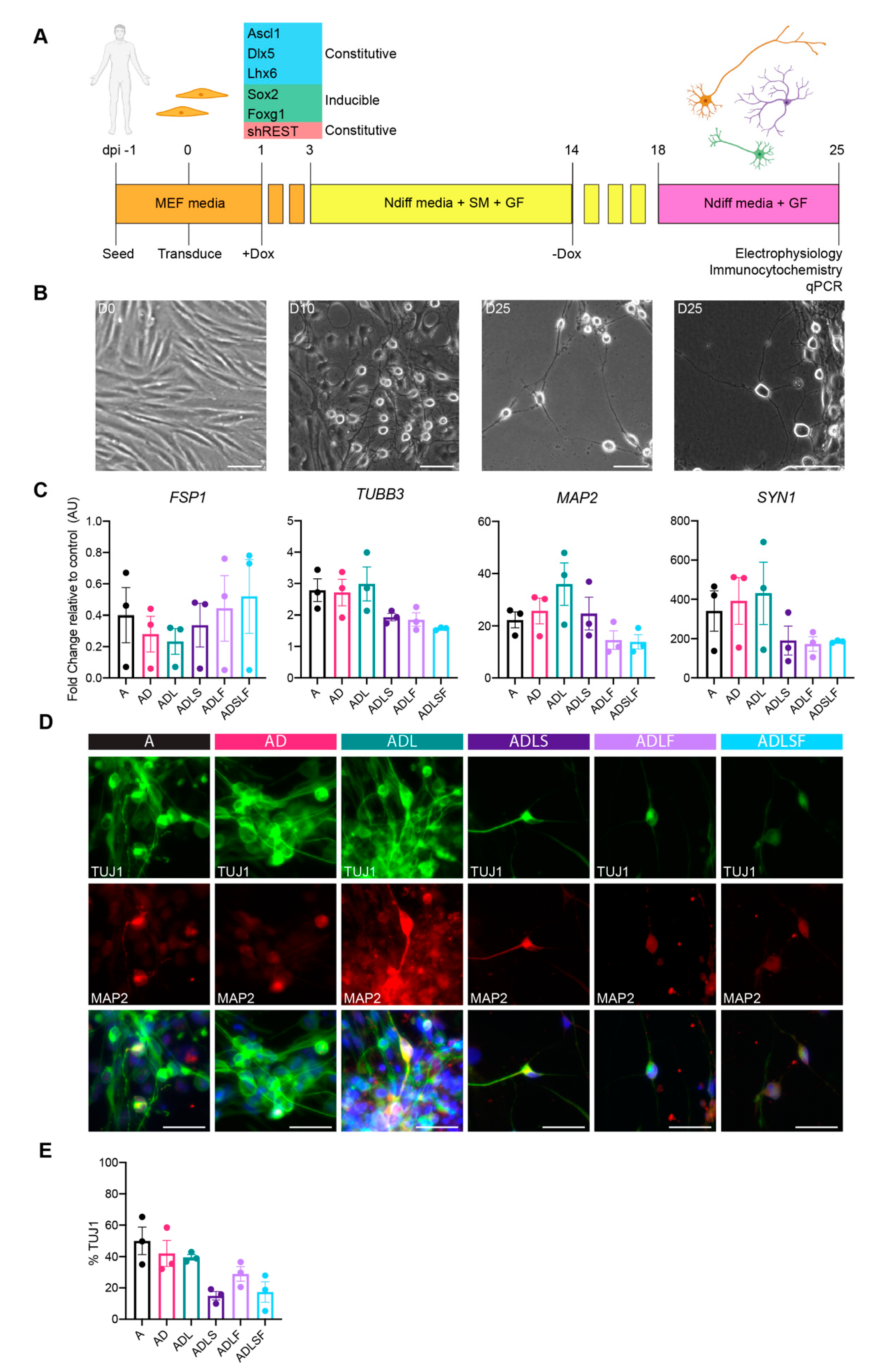
3.1. Adult Fibroblast to Neuron Conversion Using Different Combination of Factors
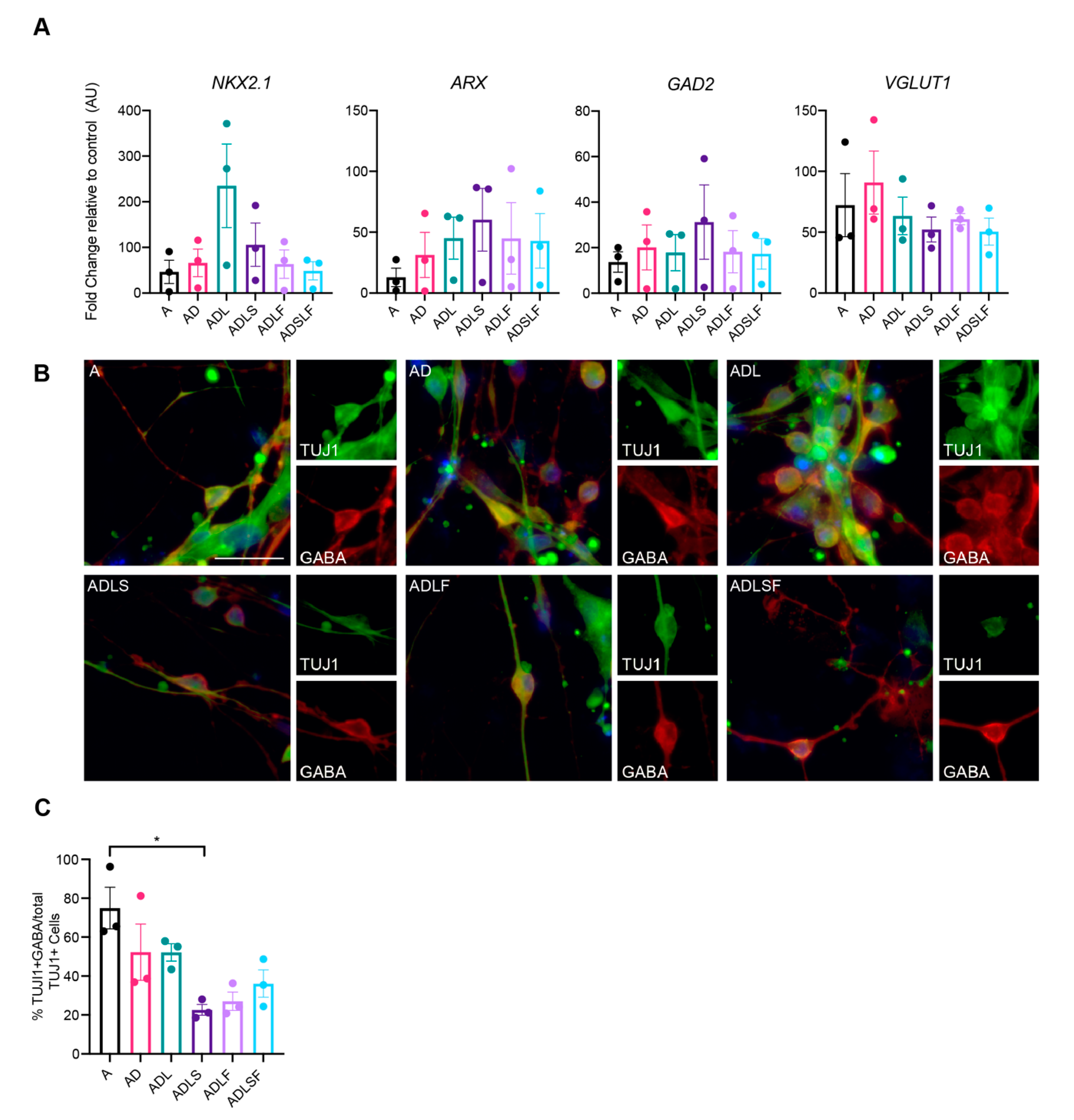
3.2. Human Adult Fibroblasts Convert into GABAergic iNs
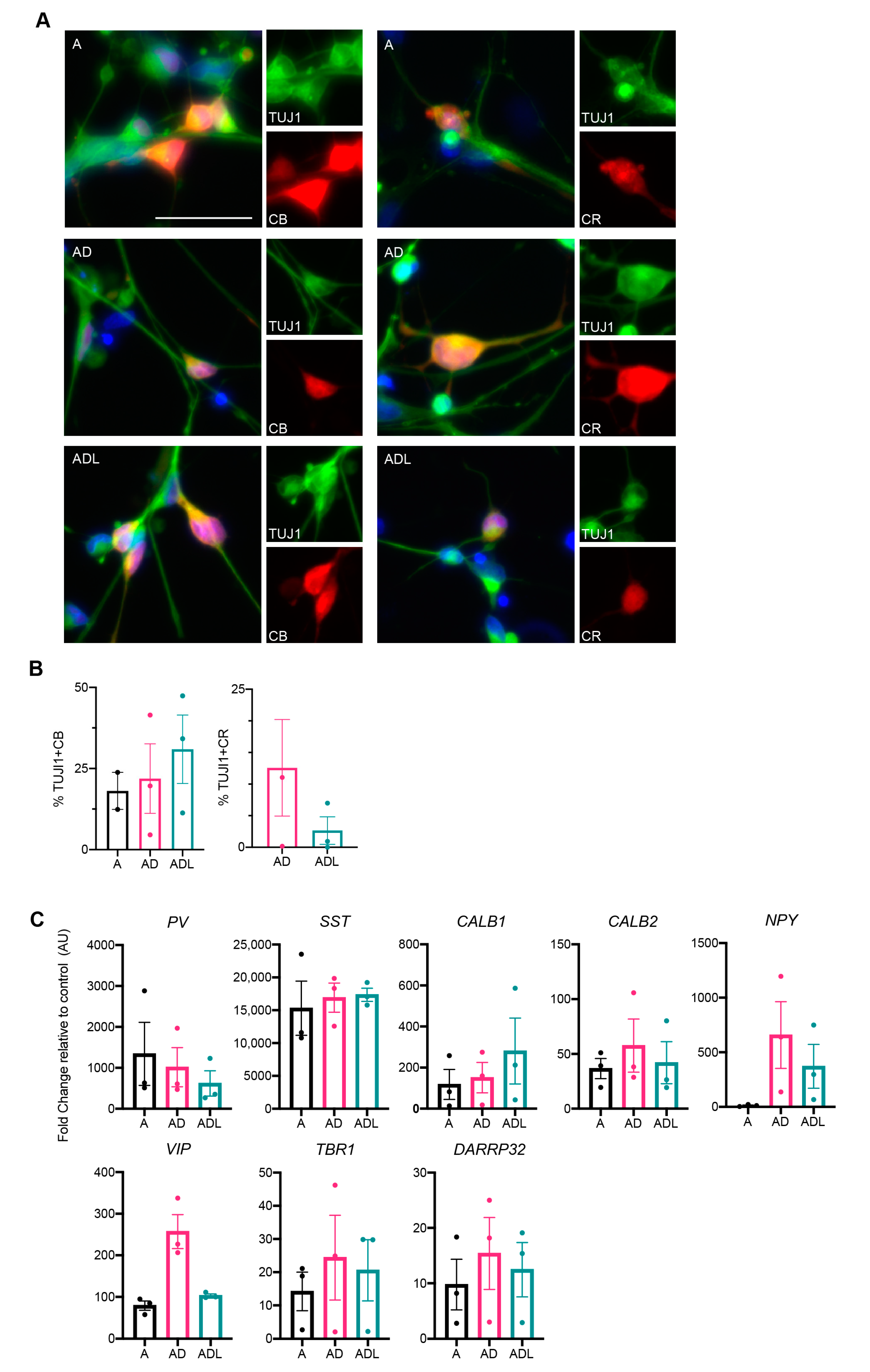
3.3. Molecular Characterization of Human-Induced GABAergic Neurons
3.4. Conversion into Neurons with Functional Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vignoles, R.; Lentini, C.; D’Orange, M.; Heinrich, C. Direct Lineage Reprogramming for Brain Repair: Breakthroughs and Challenges. Trends Mol. Med. 2019, 25, 897–914. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Pircs, K.; Barker, R.A.; Jakobsson, J.; Parmar, M. Direct Neuronal Reprogramming for Disease Modeling Studies Using Patient-Derived Neurons: What Have We Learned? Front. Neurosci. 2017, 11, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, J.; Paquola, A.C.; Ku, M.; Hatch, E.; Böhnke, L.; Ladjevardi, S.; McGrath, S.; Campbell, B.; Lee, H.; Herdy, J.R.; et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell 2015, 17, 705–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, R.A.; Götz, M.; Parmar, M. New approaches for brain repair—from rescue to reprogramming. Nat. Cell Biol. 2018, 557, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Qian, H.; Hu, J.; Zhou, B.; Zhou, Y.; Hu, X.; Karakhanyan, A.; Pang, Z.; Fu, X.-D. Sequential regulatory loops as key gatekeepers for neuronal reprogramming in human cells. Nat. Neurosci. 2016, 19, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G.; et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nat. Cell Biol. 2011, 476, 224–227. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zang, T.; Zou, Y.; Chang, J.C.; Gibson, J.R.; Huber, K.M.; Zhang, C.-L. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 2013, 4, 2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfisterer, U.; Kirkeby, A.; Torper, O.; Wood, J.; Nelander, J.; Dufour, A.; Björklund, A.; Lindvall, O.; Jakobsson, J.; Parmar, M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 10343–10348. [Google Scholar] [CrossRef] [Green Version]
- Drouin-Ouellet, J.; Lau, S.; Brattås, P.L.; Rylander Ottosson, D.; Pircs, K.; Grassi, D.A.; Collins, L.M.; Vuono, R.; Andersson Sjöland, A.; Westergren-Thorsson, G.; et al. REST suppression mediates neural conversion of adult human fibroblasts via microRNA-dependent and -independent pathways. EMBO Mol. Med. 2017, 9, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Verret, L.; Mann, E.; Hang, G.B.; Barth, A.M.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Petryszyn, S.; Parent, A.; Parent, M. The calretinin interneurons of the striatum: Comparisons between rodents and primates under normal and pathological conditions. J. Neural Transm. 2017, 125, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef]
- Colasante, G.; Lignani, G.; Rubio, A.; Medrihan, L.; Yekhlef, L.; Sessa, A.; Massimino, L.; Giannelli, S.G.; Sacchetti, S.; Caiazzo, M.; et al. Rapid Conversion of Fibroblasts into Functional Forebrain GABAergic Interneurons by Direct Genetic Reprogramming. Cell Stem Cell 2015, 17, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Giacomoni, J.; Bruzelius, A.; Stamouli, C.-A.; Ottosson, D.R. Direct Conversion of Human Stem Cell-Derived Glial Progenitor Cells into GABAergic Interneurons. Cells 2020, 9, 2451. [Google Scholar] [CrossRef] [PubMed]
- Georgievska, B.; Jakobsson, J.; Persson, E.; Ericson, C.; Kirik, D.; Lundberg, C. Regulated Delivery of Glial Cell Line-Derived Neurotrophic Factor into Rat Striatum, Using a Tetracycline-Dependent Lentiviral Vector. Hum. Gene Ther. 2004, 15, 934–944. [Google Scholar] [CrossRef]
- Richner, M.; Victor, M.B.; Liu, Y.; Abernathy, D.; Yoo, A.S. MicroRNA-based conversion of human fibroblasts into striatal medium spiny neurons. Nat. Protoc. 2015, 10, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Shrigley, S.; Pircs, K.; Barker, R.A.; Parmar, M.; Drouin-Ouellet, J. Simple Generation of a High Yield Culture of Induced Neurons from Human Adult Skin Fibroblasts. J. Vis. Exp. 2018, 2018, e56904. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.S.; Silver, R.A. NeuroMatic: An Integrated Open-Source Software Toolkit for Acquisition, Analysis and Simulation of Electrophysiological Data. Front. Neuroinform. 2018, 12, 14. [Google Scholar] [CrossRef]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2005, 9, 173–179. [Google Scholar] [CrossRef]
- Lim, L.; Mi, D.; Llorca, A.; Marín, O. Development and functional diversification of cortical interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef] [Green Version]
- Elias, L.A.; Potter, G.B.; Kriegstein, A.R. A Time and a Place for Nkx2-1 in Interneuron Specification and Migration. Neuron 2008, 59, 679–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nat. Cell Biol. 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Südhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nat. Cell Biol. 2011, 476, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qiu, B.; Guan, W.; Wang, Q.; Wang, M.; Li, W.; Gao, L.; Shen, L.; Huang, Y.; Xie, G.; et al. Direct Conversion of Normal and Alzheimer’s Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell 2015, 17, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlenius, H.; Chanda, S.; Webb, A.E.; Yousif, I.; Karmazin, J.; Prusiner, S.B.; Brunet, A.; Südhof, T.C.; Wernig, M. FoxO3 regulates neuronal reprogramming of cells from postnatal and aging mice. Proc. Natl. Acad. Sci. USA 2016, 113, 8514–8519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victor, M.B.; Richner, M.; Olsen, H.E.; Lee, S.W.; Monteys, A.M.; Ma, C.; Huh, C.J.; Zhang, B.; Davidson, B.; Yang, X.W.; et al. Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat. Neurosci. 2018, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasawa, C.; Narita, M.; Tamura, H. Regional and temporal regulation and role of somatostatin receptor subtypes in the mouse brain following systemic kainate-induced acute seizures. Neurosci. Res. 2019, 149, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Early network dysfunction in Alzheimer’s disease. Science 2019, 365, 540–541. [Google Scholar] [CrossRef]
- Lozovaya, N.; Eftekhari, S.; Cloarec, R.; Gouty-Colomer, L.A.; Dufour, A.; Riffault, B.; Billon-Grand, M.; Pons-Bennaceur, A.; Oumar, N.; Burnashev, N.; et al. GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat. Commun. 2018, 9, 1422. [Google Scholar] [CrossRef]
- Masserdotti, G.; Gillotin, S.; Sutor, B.; Drechsel, D.; Irmler, M.; Jørgensen, H.F.; Sass, S.; Theis, F.J.; Beckers, J.; Berninger, B.; et al. Transcriptional Mechanisms of Proneural Factors and REST in Regulating Neuronal Reprogramming of Astrocytes. Cell Stem Cell 2015, 17, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Ouyang, K.; Huang, J.; Zhou, Y.; Ouyang, H.; Li, H.; Wang, G.; Wu, Q.; Wei, C.; Bi, Y.; et al. Direct Conversion of Fibroblasts to Neurons by Reprogramming PTB-Regulated MicroRNA Circuits. Cell 2013, 152, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Parent, A. Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: A comparative study in the rat, monkey and human. Brain Res. 2000, 863, 182–191. [Google Scholar] [CrossRef]
- Tóth, K.; Maglóczky, Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front. Neuroanat. 2014, 8, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birtele, M.; Sharma, Y.; Kidnapillai, S.; Lau, S.; Stoker, T.B.; Barker, R.A.; Ottosson, D.R.; Drouin-Ouellet, J.; Parmar, M. Dual modulation of neuron-specific microRNAs and the REST complex promotes functional maturation of human adult induced neurons. FEBS Lett. 2019, 593, 3370–3380. [Google Scholar] [CrossRef] [PubMed]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.-W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed Differentiation and Functional Maturation of Cortical Interneurons from Human Embryonic Stem Cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindpani, K.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A.; Guzmán, B.C.-F. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzelius, A.; Kidnapillai, S.; Drouin-Ouellet, J.; Stoker, T.; Barker, R.A.; Rylander Ottosson, D. Reprogramming Human Adult Fibroblasts into GABAergic Interneurons. Cells 2021, 10, 3450. https://doi.org/10.3390/cells10123450
Bruzelius A, Kidnapillai S, Drouin-Ouellet J, Stoker T, Barker RA, Rylander Ottosson D. Reprogramming Human Adult Fibroblasts into GABAergic Interneurons. Cells. 2021; 10(12):3450. https://doi.org/10.3390/cells10123450
Chicago/Turabian StyleBruzelius, Andreas, Srisaiyini Kidnapillai, Janelle Drouin-Ouellet, Tom Stoker, Roger A. Barker, and Daniella Rylander Ottosson. 2021. "Reprogramming Human Adult Fibroblasts into GABAergic Interneurons" Cells 10, no. 12: 3450. https://doi.org/10.3390/cells10123450
APA StyleBruzelius, A., Kidnapillai, S., Drouin-Ouellet, J., Stoker, T., Barker, R. A., & Rylander Ottosson, D. (2021). Reprogramming Human Adult Fibroblasts into GABAergic Interneurons. Cells, 10(12), 3450. https://doi.org/10.3390/cells10123450