Immunomodulatory Effects Associated with Cladribine Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cell Isolation
2.3. Media and Reagents
2.4. In Vitro Stimulation of PBMCs and Functional Assays
2.4.1. Proliferation
2.4.2. Apoptosis
2.4.3. Activation
2.5. In Vitro Stimulation of Monocytes and Activation Assay
2.6. Flow Cytometry
2.7. Statistical Analysis
3. Results
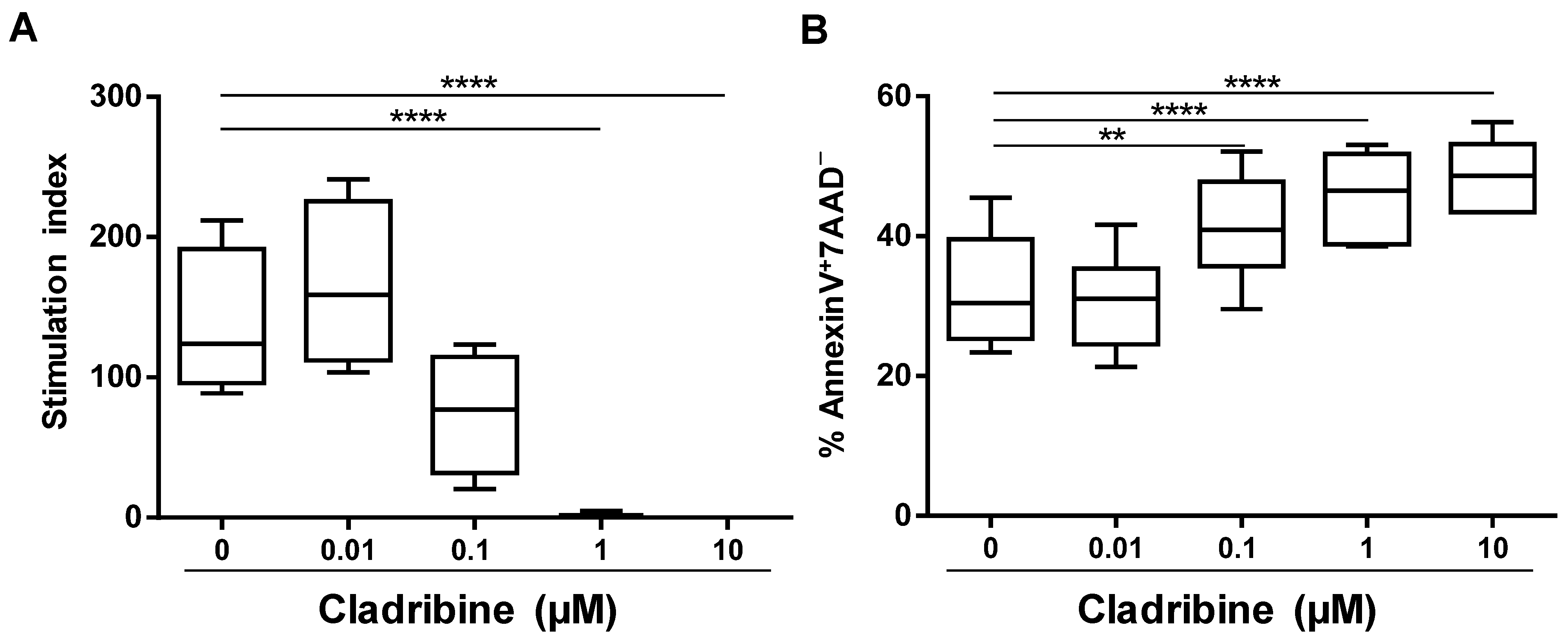
3.1. Assessment of the Functional In Vitro Dose of Cladribine
3.2. Inhibition of Cell Proliferation Caused by Cladribine Depends on Prodrug Activation
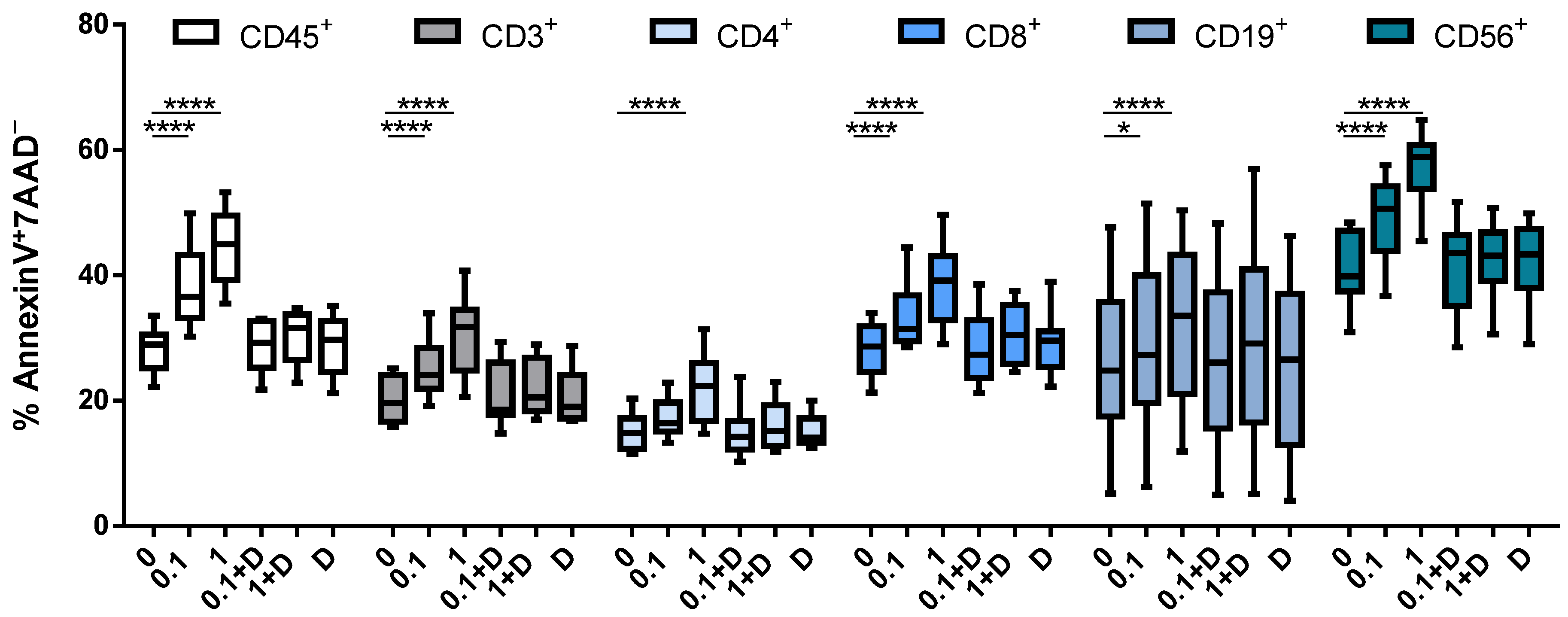
3.3. Cladribine Induces Apoptosis in a DCK-Dependent Manner
3.4. Cladribine Impairs Cell Activation Both Dependently and Independently of Deoxycytidine Kinase Activity
3.5. Cladribine Shows a Marginal Effect on Monocyte Activation
3.6. Cladribine Does Not Affect the Differentiation Process of DCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beutler, E. Cladribine (2-chlorodeoxyadenosine). Lancet 1992, 340, 952–956. [Google Scholar] [CrossRef]
- Huynh, E.; Sigal, D.; Saven, A. Cladribine in the treatment of hairy cell leukemia: Initial and subsequent results. Leuk. Lymphoma 2009, 50 (Suppl. 1), 12–17. [Google Scholar] [CrossRef]
- Romine, J.S.; Sipe, J.C.; Koziol, J.A.; Zyroff, J.; Beutler, E. A double-blind, placebo-controlled, randomized trial of cladribine in relapsing-remitting multiple sclerosis. Proc. Assoc. Am. Physicians 1999, 111, 35–44. [Google Scholar] [CrossRef]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Soelberg Sorensen, P.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. CLARITY Study Group. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Comi, G.; Cook, S.D.; Giovannoni, G.; Rammohan, K.; Rieckmann, P.; Soelberg Sørensen, P.; Vermersch, P.; Hamlett, A.; Viglietta, V.; Greenberg, S. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J. Neurol. 2013, 260, 1136–1146. [Google Scholar] [CrossRef]
- Giovannoni, G.; Soelberg Sørensen, P.; Cook, S.D.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [Green Version]
- Carson, D.A.; Wasson, D.B.; Taetle, R.; Yu, A. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood 1983, 62, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Liliemark, J. The clinical pharmacokinetics of cladribine. Clin. Pharm. 1997, 32, 120–131. [Google Scholar] [CrossRef]
- Baker, D.; Herrod, S.S.; Alvarez-Gonzalez, C.; Zalewski, L.; Albor, C.; Schmierer, K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e360. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Prajeeth, C.K.; Gudi, V.; Bénardais, K.; Voss, E.V.; Stangel, M. 2-Chlorodeoxyadenosine (cladribine) induces apoptosis in human monocyte-derived dendritic cells. Clin. Exp. Immunol. 2013, 173, 288–297. [Google Scholar] [CrossRef]
- Mitosek-Szewczyk, K.; Tabarkiewicz, J.; Wilczynska, B.; Lobejko, K.; Berbecki, J.; Nastaj, M.; Dworzanska, E.; Kolodziejczyk, B.; Stelmasiak, Z.; Rolinski, J. Impact of cladribine therapy on changes in circulating dendritic cell subsets, T cells and B cells in patients with multiple sclerosis. J. Neurol. Sci. 2013, 332, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Johnson, L.A.; Jacobson, P.A.; Kachler, S.; Kirstein, M.N.; Lamba, J.; Klotz, K.N. Cytotoxic purine nucleoside analogues bind to A1, A2A, and A3 adenosine receptors. Naunyn Schmiedebergs Arch. Pharm. 2012, 38, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Wasserman, W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharm. 2000, 362, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Gupta, M. Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharm. J. 2013, 21, 245–453. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharm. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [Green Version]
- Sitkovsky, M.V. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem. Pharm. 2003, 65, 493–501. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Merck Serono Australia Pty Ltd. Movectro Tablets Product Information. December 2011. Available online: https://www.tga.gov.au/file/auspar-movectropdf (accessed on 1 September 2018).
- Korsen, M.; Bragado Alonso, S.; Peix, L.; Bröker, B.M.; Dressel, A. Cladribine Exposure Results in a Sustained Modulation of the Cytokine Response in Human Peripheral Blood Mononuclear Cells. PLoS ONE 2015, 10, e0129182. [Google Scholar] [CrossRef]
- Carlini, F.; Ivaldi, F.; Gualandi, F.; Boschert, U.; Centonze, D.; Matarese, G.; Salvetti, M.; Kerlero de Rosbo, N.; Uccelli, A. Different Susceptibility of T and B Cells to Cladribine Depends On Their Levels of Deoxycytidine Kinase Activity Linked to Activation Status. J. Neuroimmune Pharmacol. 2021. [Google Scholar] [CrossRef]
- Stuve, O.; Soelberg Soerensen, P.; Leist, T.; Giovannoni, G.; Hyvert, Y.; Damian, D.; Dangond, F.; Boschert, U. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: An extended analysis of surface markers. Adv. Neurol. Disord. 2019, 12, 1756286419854986. [Google Scholar] [CrossRef] [Green Version]
- Laugel, B.; Borlat, F.; Galibert, L.; Vicari, A.; Weissert, R.; Chvatchko, Y.; Bruniquel, D. Cladribine inhibits cytokine secretion by T cells independently of deoxycytidine kinase activity. J. Neuroimmunol 2011, 240–241, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. A2A receptors in inflammation and injury: Lessons learned from transgenic animals. J. Leukoc. Biol. 2008, 83, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Linden, J. Regulation of leukocyte function by adenosine receptors. Adv. Pharmacol. 2011, 61, 95–114. [Google Scholar] [CrossRef] [Green Version]
- Kraus, S.H.P.; Luessi, F.; Trinschek, B.; Lerch, S.; Hubo, M.; Poisa-Beiro, L.; Paterka, M.; Jonuleit, H.; Zipp, F.; Jolivel, V. Cladribine exerts an immunomodulatory effect on human and murine dendritic cells. Int. Immunopharmacol. 2014, 18, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Savinetti, I.; Papagna, A.; Foti, M. Human Monocytes Plasticity in Neurodegeneration. Biomedicines 2021, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Lunda, B.T.; Ashikian, N.; Ta, H.Q.; Chakryan, Y.; Manoukian, K.; Groshen, S.; Gilmore, W.; Cheema, G.S.; Stohl, W.; Burnett, M.E.; et al. Increased CXCL8 (IL-8) expression in Multiple Sclerosis. J. Neuroimmunol. 2004, 155, 161–171. [Google Scholar] [CrossRef]
- Mahad, D.J.; Ransohoff, R.M. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Semin Immunol. 2003, 15, 23–32. [Google Scholar] [CrossRef]
- Comabella, M.; Imitola, J.; Weiner, H.L.; Khoury, S.J. Interferon-h treatment alters peripheral blood monocytes chemokine production in MS patients. J. Neuroimmunol. 2002, 126, 205–212. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Delohery, T.; Zhang, D.; Arendt, C.; Jones, C. The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J. Neuroimmunol. 2013, 265, 82–90. [Google Scholar] [CrossRef]
- Diebold, M.; Sievers, C.; Bantug, G.; Sanderson, N.; Kappos, L.; Kuhle, J.; Lindberg, R.L.P.; Derfuss, T. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J. Autoimmun. 2018, 86, 39–50. [Google Scholar] [CrossRef]
- Carrera, C.J.; Terai, C.; Lotz, M.; Curd, J.G.; Piro, L.D.; Beutler, E.; Carson, D.A. Potent toxicity of 2-chlorodeoxyadenosine toward human monocytes in vitro and in vivo. A novel approach to immunosuppressive therapy. J. Clin. Invest. 1990, 86, 1480–1488. [Google Scholar] [CrossRef] [Green Version]
- Waschbisch, A.; Schröder, S.; Schraudner, D.; Sammet, L.; Weksler, B.; Melms, A.; Pfeifenbring, S.; Stadelmann, C.; Schwab, S.; Linker, R.A. Pivotal Role for CD16+ Monocytes in Immune Surveillance of the Central Nervous System. J. Immunol. 2016, 196, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Chuluundorj, D.; Harding, S.A.; Abernethy, D.; La Flamme, A.C. Expansion and preferential activation of the CD14(+)CD16(+) monocyte subset during multiple sclerosis. Immunol. Cell Biol. 2014, 92, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Chuluundorj, D.; Harding, S.A.; Abernethy, D.; La Flamme, A.C. Glatiramer acetate treatment normalized the monocyte activation profile in MS patients to that of healthy controls. Immunol. Cell Biol. 2017, 95, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moratalla, N.; Gonzalez, A.; Aymerich, M.S.; Lopez-Zabalza, M.J.; Pio, R.; de Castro, P.; Santiago, E. Monocyte inducible nitric oxide synthase in multiple sclerosis: Regulatory role of nitric oxide. Nitric Oxide 1997, 1, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Shortman, K.; Naik, S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007, 7, 19–30. [Google Scholar] [CrossRef]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef]
- Martin, R.; Sospedra, M.; Rosito, M.; Engelhardt, B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 2016, 46, 2078–2090. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fissolo, N.; Calvo-Barreiro, L.; Eixarch, H.; Boschert, U.; Espejo, C.; Montalban, X.; Comabella, M. Immunomodulatory Effects Associated with Cladribine Treatment. Cells 2021, 10, 3488. https://doi.org/10.3390/cells10123488
Fissolo N, Calvo-Barreiro L, Eixarch H, Boschert U, Espejo C, Montalban X, Comabella M. Immunomodulatory Effects Associated with Cladribine Treatment. Cells. 2021; 10(12):3488. https://doi.org/10.3390/cells10123488
Chicago/Turabian StyleFissolo, Nicolás, Laura Calvo-Barreiro, Herena Eixarch, Ursula Boschert, Carmen Espejo, Xavier Montalban, and Manuel Comabella. 2021. "Immunomodulatory Effects Associated with Cladribine Treatment" Cells 10, no. 12: 3488. https://doi.org/10.3390/cells10123488
APA StyleFissolo, N., Calvo-Barreiro, L., Eixarch, H., Boschert, U., Espejo, C., Montalban, X., & Comabella, M. (2021). Immunomodulatory Effects Associated with Cladribine Treatment. Cells, 10(12), 3488. https://doi.org/10.3390/cells10123488








