Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Tissue Collection
2.2. Cell Lines, Antibodies, and Reagents
2.3. Cell Culture and Conditioned Media (CM) Collection
2.4. Treatment of MDA231 Cells and HMECs with DPMSCs
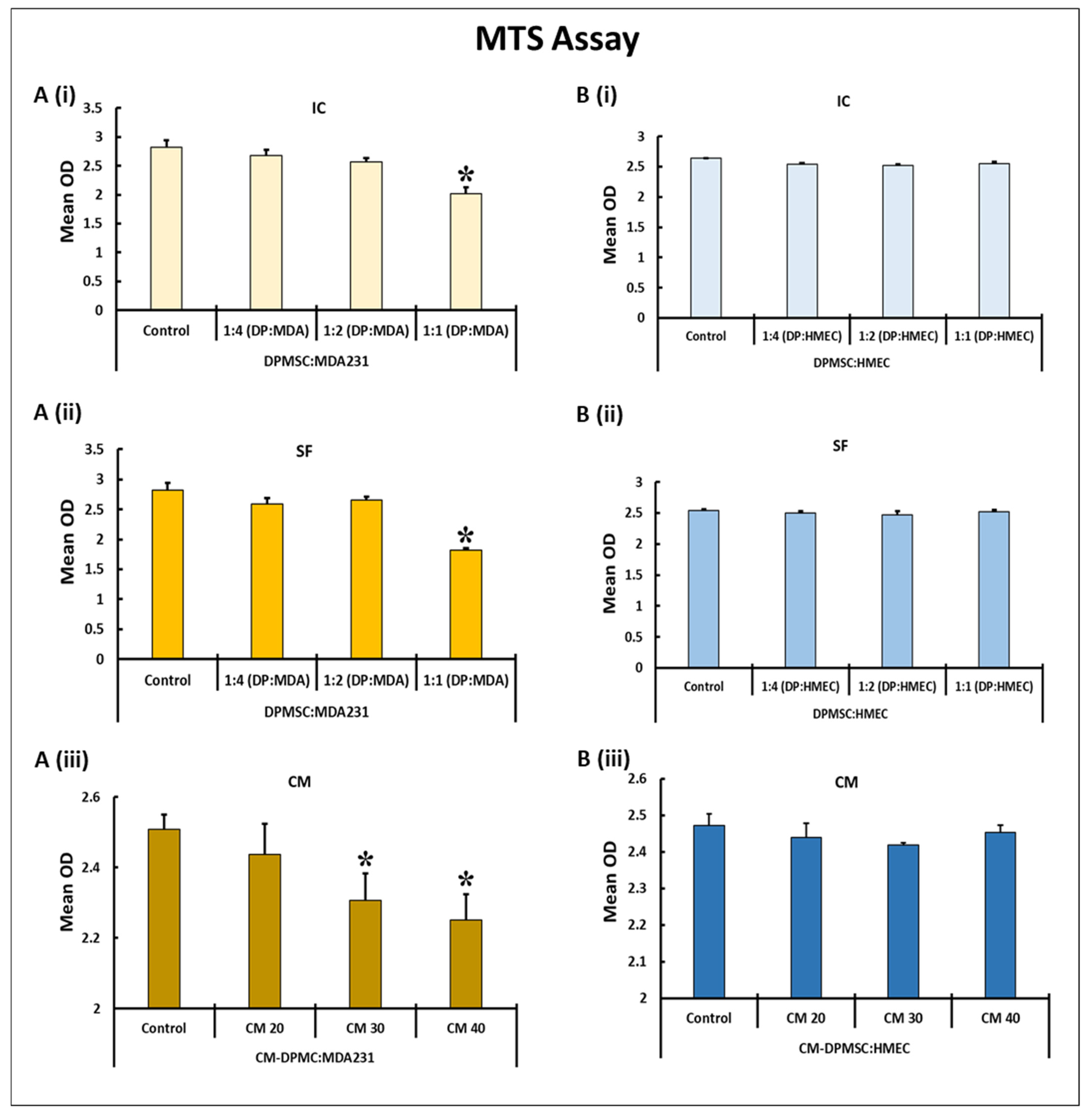
2.5. Cellular Proliferation by MTS Assay
2.6. xCELLigence: Impedence-Based Assays
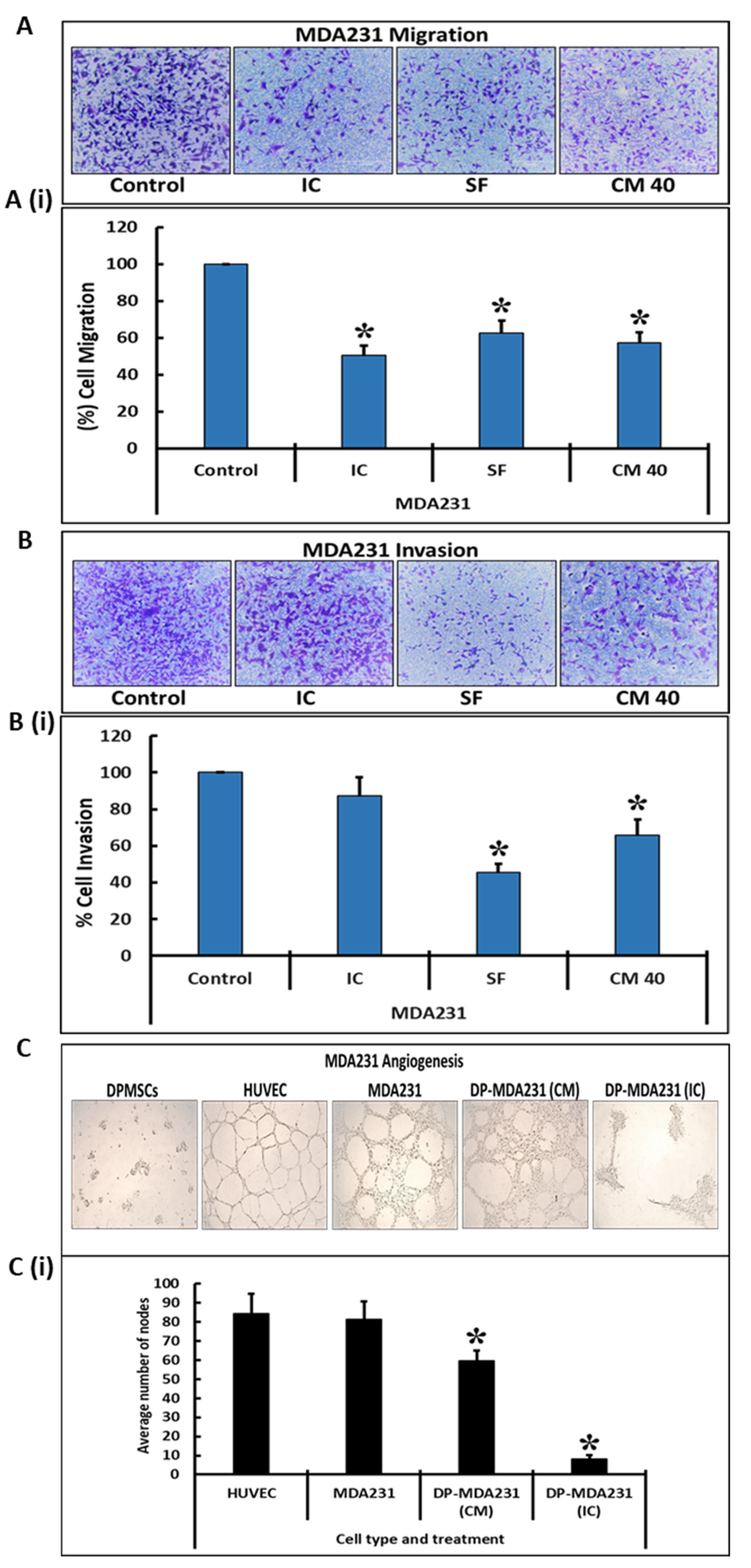
2.7. Cell Migration, Invasion, and Angiogenesis Assays
2.8. RNA Expression Profiling by Real-Time PCR Analysis (RT-qPCR)
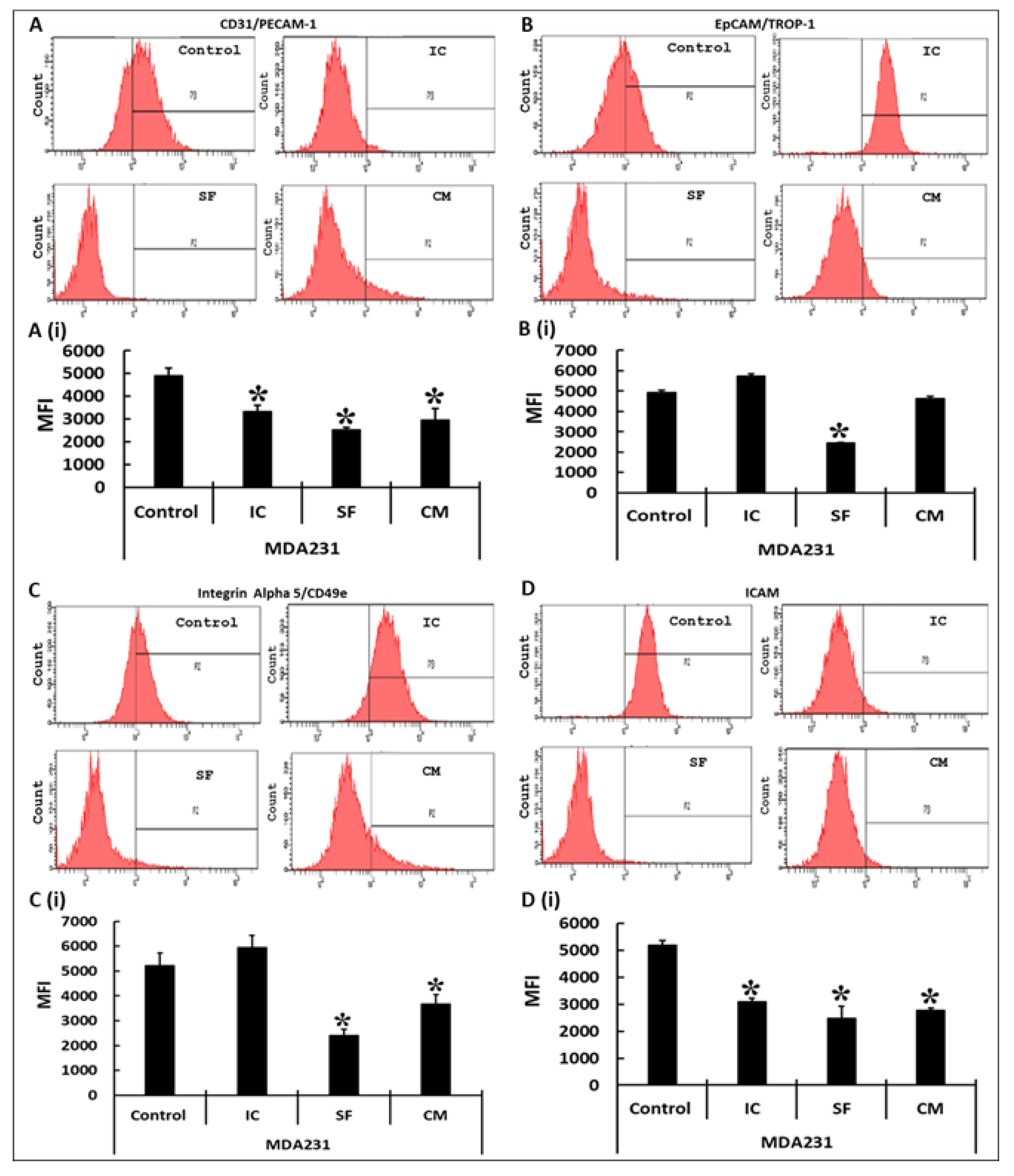
2.9. Flow Cytometry
2.10. Immunoblotting
2.11. Data Analyses
3. Results
3.1. Temporal and Dose–Response Standardization
3.2. Impact of DPMSCs (CM and Cells) on the Adhesion and Proliferation of MDA231 Cells
3.3. DPMSCs Restrain the Invasive Characteristics of MDA231 Cells
3.4. Modulation of Functionally Relevant Effectors in MDA231 Cells in Response to DPMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Goffin, J.; Lacchetti, C.; Ellis, P.M.; Ung, Y.C.; Evans, W.K. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: A systematic review. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef]
- Sermer, D.; Brentjens, R. CAR T-cell therapy: Full speed ahead. Hematol. Oncol. 2019, 37 (Suppl. S1), 95–100. [Google Scholar] [CrossRef]
- Gomes, J.P.A.; Assoni, A.F.; Pelatti, M.; Coatti, G.; Okamoto, O.K.; Zatz, M. Deepening a Simple Question: Can MSCs Be Used to Treat Cancer? Anticancer Res. 2017, 37, 4747–4758. [Google Scholar] [CrossRef] [PubMed]
- Javan, M.R.; Khosrojerdi, A.; Moazzeni, S.M. New Insights into Implementation of Mesenchymal Stem Cells in Cancer Therapy: Prospects for Anti-angiogenesis Treatment. Front. Oncol. 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Kamga, P.T.; Simo, R.T.; Vecchio, L.; Seke Etet, P.F.; Muller, J.M.; Bassi, G.; Lukong, E.; Goel, R.K.; Amvene, J.M.; et al. Mesenchymal stromal cells’ role in tumor microenvironment: Involvement of signaling pathways. Cancer Biol. Med. 2017, 14, 129–141. [Google Scholar] [CrossRef]
- Phinney, D.G.; Sensebé, L. Mesenchymal stromal cells: Misconceptions and evolving concepts. Cytotherapy 2013, 15, 140–145. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Atiya, H.; Frisbie, L.; Pressimone, C.; Coffman, L. Mesenchymal Stem Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Z.; Zhao, T.; Zhao, Z.; Shi, M.; Zhao, R.C.; Ye, L.; Zhang, X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008, 18, 500–507. [Google Scholar] [CrossRef]
- Nakamura, K.; Ito, Y.; Kawano, Y.; Kurozumi, K.; Kobune, M.; Tsuda, H.; Bizen, A.; Honmou, O.; Niitsu, Y.; Hamada, H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004, 11, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Hertens, E.; Galli, P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell. Mol. Life Sci. 1999, 55, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Xu, W.; Zhu, W.; Hu, J.; Qian, H.; Yin, Q.; Jiang, R.; Yan, Y.; Mao, F.; Yang, H.; et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol. Int. 2008, 32, 8–15. [Google Scholar] [CrossRef]
- Otsu, K.; Das, S.; Houser, S.D.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009, 113, 4197–4205. [Google Scholar] [CrossRef]
- Gondi, C.S.; Veeravalli, K.K.; Gorantla, B.; Dinh, D.H.; Fassett, D.; Klopfenstein, J.D.; Gujrati, M.; Rao, J.S. Human umbilical cord blood stem cells show PDGF-D-dependent glioma cell tropism in vitro and in vivo. Neuro-Oncol. 2010, 12, 453–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akimoto, K.; Kimura, K.; Nagano, M.; Takano, S.; To’a Salazar, G.; Yamashita, T.; Ohneda, O. Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 2013, 22, 1370–1386. [Google Scholar] [CrossRef]
- Basmaeil, Y.; Al Subayyil, A.; Abumaree, M.; Khatlani, T. Conditions Mimicking the Cancer Microenvironment Modulate the Functional Outcome of Human Chorionic Villus Mesenchymal Stem/Stromal Cells in vitro. Front. Cell Dev. Biol. 2021, 9, 650125. [Google Scholar] [CrossRef]
- Abomaray, F.M.; Al Jumah, M.A.; Alsaad, K.O.; Jawdat, D.; Al Khaldi, A.; Alaskar, A.S.; Al Harthy, S.; Al Subayyil, A.M.; Khatlani, T.; Alawad, A.O.; et al. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta. Stem Cells Int. 2016, 2016, 5184601. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Abomaray, F.M.; Alshehri, N.A.; Almutairi, A.; AlAskar, A.S.; Kalionis, B.; Al Jumah, M.A. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Parietalis of Human Term Placenta. Reprod. Sci. 2016, 23, 1193–1207. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; AlTalabani, A.A.; Knawy, B.A. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. Rep. 2013, 9, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Abomaray, F.M.; Alshabibi, M.A.; AlAskar, A.S.; Kalionis, B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 2017, 59, 87–95. [Google Scholar] [CrossRef]
- Bahattab, E.; Khatlani, T.; Abomaray, F.M.; Messaoudi, S.A.; Abumaree, M.H. Cancer Conditioned Medium Modulates Functional and Phenotypic Properties of Human Decidua Parietalis Mesenchymal Stem/Stromal Cells. Tissue Eng. Regen. Med. 2019, 16, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Al Subayyil, A.; Basmaeil, Y.S.; Alenzi, R.; Khatlani, T. Human Placental Mesenchymal Stem/Stromal cells (pMSCs) inhibit agonist-induced platelet functions reducing atherosclerosis and thrombosis phenotypes. J. Cell. Mol. Med. 2021, 25, 9268–9280. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Vistejnova, L.; Dvorakova, J.; Hasova, M.; Muthny, T.; Velebny, V.; Soucek, K.; Kubala, L. The comparison of impedance-based method of cell proliferation monitoring with commonly used metabolic-based techniques. Neuro Endocrinol. Lett. 2009, 30 (Suppl. S1), 121–127. [Google Scholar]
- Stefanowicz-Hajduk, J.; Ochocka, J.R. Real-time cell analysis system in cytotoxicity applications: Usefulness and comparison with tetrazolium salt assays. Toxicol. Rep. 2020, 7, 335–344. [Google Scholar] [CrossRef]
- Dowling, C.M.; Herranz Ors, C.; Kiely, P.A. Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells. Biosci. Rep. 2014, 34, e00126. [Google Scholar] [CrossRef] [PubMed]
- Basmaeil, Y.; Al Rashid, M.; Khatlani, T.; AlShabibi, M.; Bahattab, E.; Abdullah, M.L.; Abomaray, F.; Kalionis, B.; Massoudi, S.; Abumaree, M. Preconditioning of Human Decidua Basalis Mesenchymal Stem/Stromal Cells with Glucose Increased Their Engraftment and Anti-diabetic Properties. Tissue Eng. Regen. Med. 2020, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Boyer, T.G.; Lee, W.H. Lessons learned from BRCA1 and BRCA2. Oncogene 2000, 19, 6159–6175. [Google Scholar] [CrossRef]
- Albergaria, A.; Paredes, J.; Sousa, B.; Milanezi, F.; Carneiro, V.; Bastos, J.; Costa, S.; Vieira, D.; Lopes, N.; Lam, E.W.; et al. Expression of FOXA1 and GATA-3 in breast cancer: The prognostic significance in hormone receptor-negative tumours. Breast Cancer Res. 2009, 11, R40. [Google Scholar] [CrossRef] [PubMed]
- Pamidimukkala, N.; Puts, G.S.; Kathryn Leonard, M.; Snyder, D.; Dabernat, S.; De Fabo, E.C.; Noonan, F.P.; Slominski, A.; Merlino, G.; Kaetzel, D.M. Nme1 and Nme2 genes exert metastasis-suppressor activities in a genetically engineered mouse model of UV-induced melanoma. Br. J. Cancer 2021, 124, 161–165. [Google Scholar] [CrossRef]
- Csolle, M.P.; Ooms, L.M.; Papa, A.; Mitchell, C.A. PTEN and Other PtdIns(3,4,5)P(3) Lipid Phosphatases in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 9189. [Google Scholar] [CrossRef]
- Doan, T.B.; Cheung, V.; Clyne, C.D.; Hilton, H.N.; Eriksson, N.; Young, M.J.; Funder, J.W.; Muscat, G.E.O.; Fuller, P.J.; Clarke, C.L.; et al. A tumour suppressive relationship between mineralocorticoid and retinoic acid receptors activates a transcriptional program consistent with a reverse Warburg effect in breast cancer. Breast Cancer Res. 2020, 22, 122. [Google Scholar] [CrossRef]
- DiPippo, A.J.; Patel, N.K.; Barnett, C.M. Cyclin-Dependent Kinase Inhibitors for the Treatment of Breast Cancer: Past, Present, and Future. Pharmacotherapy 2016, 36, 652–667. [Google Scholar] [CrossRef]
- Andreeva, A.V.; Kutuzov, M.A. Cadherin 13 in cancer. Genes. Chromosom. Cancer 2010, 49, 775–790. [Google Scholar] [CrossRef]
- Gasco, M.; Shami, S.; Crook, T. The p53 pathway in breast cancer. Breast Cancer Res. 2002, 4, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Omoto, Y.; Iwase, H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 2015, 106, 337–343. [Google Scholar] [CrossRef]
- Furuuchi, K.; Tada, M.; Yamada, H.; Kataoka, A.; Furuuchi, N.; Hamada, J.I.; Takahashi, M.; Todo, S.; Moriuchi, T. Somatic mutations of the APC gene in primary breast cancers. Am. J. Pathol. 2000, 156, 1997–2005. [Google Scholar] [CrossRef]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.P.; Riggins, R.B.; Shajahan, A.N.; Klimach, U.; Wang, A.; Crawford, A.C.; Zhu, Y.; Zwart, A.; Wang, M.; Clarke, R. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 4013–4027. [Google Scholar] [CrossRef]
- Garcia, M.; Platet, N.; Liaudet, E.; Laurent, V.; Derocq, D.; Brouillet, J.P.; Rochefort, H. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells 1996, 14, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Leonessa, F.; Clarke, R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr. Relat. Cancer 2003, 10, 43–73. [Google Scholar] [CrossRef]
- Syed Khaja, A.S.; Dizeyi, N.; Kopparapu, P.K.; Anagnostaki, L.; Härkönen, P.; Persson, J.L. Cyclin A1 Modulates the Expression of Vascular Endothelial Growth Factor and Promotes Hormone-Dependent Growth and Angiogenesis of Breast Cancer. PLoS ONE 2013, 8, e72210. [Google Scholar] [CrossRef]
- Gumireddy, K.; Li, A.; Kossenkov, A.V.; Cai, K.Q.; Liu, Q.; Yan, J.; Xu, H.; Showe, L.; Zhang, L.; Huang, Q. ID1 promotes breast cancer metastasis by S100A9 regulation. Mol. Cancer Res. 2014, 12, 1334–1343. [Google Scholar] [CrossRef]
- Yang, J.-D.; Ma, L.; Zhu, Z. SERPINE1 as a cancer-promoting gene in gastric adenocarcinoma: Facilitates tumour cell proliferation, migration, and invasion by regulating EMT. J. Chemother. 2019, 31, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Bahattab, E.; Alsadoun, A.; Al Dosaimani, A.; Abomaray, F.M.; Khatlani, T.; Kalionis, B.; El-Muzaini, M.F.; Alawad, A.O.; Alaskar, A.S. Characterization of the interaction between human decidua parietalis mesenchymal stem/stromal cells and natural killer cells. Stem Cell Res. Ther. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef]
- Moh, M.C.; Shen, S. The roles of cell adhesion molecules in tumor suppression and cell migration: A new paradox. Cell Adh. Migr. 2009, 3, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Schaffner, F.; Ray, A.M.; Dontenwill, M. Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers 2013, 5, 27–47. [Google Scholar] [CrossRef]
- Benedicto, A.; Romayor, I.; Arteta, B. Role of liver ICAM-1 in metastasis. Oncol. Lett. 2017, 14, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, A.; Schardt, C.; Rotsch, M.; Zehrer, M.; Wolf, M.; Havemann, K.; Heymanns, J. Soluble intercellular adhesion molecule-1 in patients with lung cancer and benign lung diseases. J. Cancer Res. Clin. Oncol. 1997, 123, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Maruo, Y.; Gochi, A.; Kaihara, A.; Shimamura, H.; Yamada, T.; Tanaka, N.; Orita, K. ICAM-1 expression and the soluble ICAM-1 level for evaluating the metastatic potential of gastric cancer. Int. J. Cancer 2002, 100, 486–490. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Borawska, M.H. Soluble intercellular adhesion molecule-1 (sICAM-1): An overview. Eur. Cytokine Netw. 2004, 15, 91–98. [Google Scholar]
- Yang, C.; Lei, D.; Ouyang, W.; Ren, J.; Li, H.; Hu, J.; Huang, S. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. BioMed Res. Int. 2014, 2014, 109389. [Google Scholar] [CrossRef]
- Jiao, H.; Guan, F.; Yang, B.; Li, J.; Shan, H.; Song, L.; Hu, X.; Du, Y. Human umbilical cord blood-derived mesenchymal stem cells inhibit C6 glioma via downregulation of cyclin D1. Neurol. India 2011, 59, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Velpula, K.K.; Dasari, V.R.; Tsung, A.J.; Gondi, C.S.; Klopfenstein, J.D.; Mohanam, S.; Rao, J.S. Regulation of glioblastoma progression by cord blood stem cells is mediated by downregulation of cyclin D1. PLoS ONE 2011, 6, e18017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kološa, K.; Motaln, H.; Herold-Mende, C.; Koršič, M.; Lah, T.T. Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant. 2015, 24, 631–644. [Google Scholar] [CrossRef]
- Ganta, C.; Chiyo, D.; Ayuzawa, R.; Rachakatla, R.; Pyle, M.; Andrews, G.; Weiss, M.; Tamura, M.; Troyer, D. Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res. 2009, 69, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, K.; Yee, F.C.; Cheyyatraivendran, S.; Biswas, A.; Choolani, M.; Bongso, A. Human umbilical cord Wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J. Cell. Biochem. 2012, 113, 2027–2039. [Google Scholar] [CrossRef]
- Dzobo, K.; Vogelsang, M.; Thomford, N.E.; Dandara, C.; Kallmeyer, K.; Pepper, M.S.; Parker, M.I. Wharton’s Jelly-Derived Mesenchymal Stromal Cells and Fibroblast-Derived Extracellular Matrix Synergistically Activate Apoptosis in a p21-Dependent Mechanism in WHCO1 and MDA MB 231 Cancer Cells In Vitro. Stem Cells Int. 2016, 2016, 4842134. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, C.; Chen, X.; Tao, C.; Cheng, H.; Lu, X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol. Lett. 2018, 15, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Khalil, C.; Moussa, M.; Azar, A.; Tawk, J.; Habbouche, J.; Salameh, R.; Ibrahim, A.; Alaaeddine, N. Anti-proliferative effects of mesenchymal stem cells (MSCs) derived from multiple sources on ovarian cancer cell lines: An in-vitro experimental study. J. Ovarian Res. 2019, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef]
- Najar, M.; Fayyad-Kazan, H.; Faour, W.H.; Badran, B.; Journe, F.; Lagneaux, L. Breast cancer cells and bone marrow mesenchymal stromal cells: A regulated modulation of the breast tumor in the context of immune response. Inflamm. Res. 2017, 66, 129–139. [Google Scholar] [CrossRef]
- Wagner, M.; Klussmann, J.P.; Fangmann, R.; Linder, R.; Elewa, M.E.; Eidt, S.; Rose, V.M.; Jungehulsing, M.; Schulze, H.J. Cyclin-dependent kinase-inhibitor 1 (CDKN1A) in the squamous epithelium of the oropharynx: Possible implications of molecular biology and compartmentation. Anticancer Res. 2001, 21, 333–345. [Google Scholar]
- Seo, J.; Seong, D.; Lee, S.R.; Oh, D.-B.; Song, J. Post-Translational Regulation of ARF: Perspective in Cancer. Biomolecules 2020, 10, 1143. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Goswami, S.; Lapidus, K.; Wells, A.L.; Wyckoff, J.B.; Sahai, E.; Singer, R.H.; Segall, J.E.; Condeelis, J.S. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004, 64, 8585–8594. [Google Scholar] [CrossRef]
- Liu, J.; Han, G.; Liu, H.; Qin, C. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: A possible role of Wnt and Akt signaling. PLoS ONE 2013, 8, e62844. [Google Scholar] [CrossRef]
- Visweswaran, M.; Arfuso, F.; Dilley, R.J.; Newsholme, P.; Dharmarajan, A. The inhibitory influence of adipose tissue-derived mesenchymal stem cell environment and Wnt antagonism on breast tumour cell lines. Int. J. Biochem. Cell Biol. 2018, 95, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Yonemitsu, Y. Cancer neovascularization and proinflammatory microenvironments. Curr. Cancer Drug Targets 2008, 8, 253–265. [Google Scholar] [CrossRef]
- Dasari, V.R.; Kaur, K.; Velpula, K.K.; Dinh, D.H.; Tsung, A.J.; Mohanam, S.; Rao, J.S. Downregulation of Focal Adhesion Kinase (FAK) by cord blood stem cells inhibits angiogenesis in glioblastoma. Aging (Albany NY) 2010, 2, 791–803. [Google Scholar] [CrossRef]
- Ho, I.A.W.; Toh, H.C.; Ng, W.H.; Teo, Y.L.; Guo, C.M.; Hui, K.M.; Lam, P.Y.P. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 2013, 31, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Zilfou, J.T.; Lowe, S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009, 1, a001883. [Google Scholar] [CrossRef]
- Tamrakar, S.; Rubin, E.; Ludlow, J.W. Role of pRB dephosphorylation in cell cycle regulation. Front. Biosci. 2000, 5, D121–D137. [Google Scholar] [CrossRef]
- Vélez-Cruz, R.; Johnson, D.G. The Retinoblastoma (RB) Tumor Suppressor: Pushing Back against Genome Instability on Multiple Fronts. Int. J. Mol. Sci. 2017, 18, 1776. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell. Mol. Life Sci. 2013, 70, 4009–4021. [Google Scholar] [CrossRef] [PubMed]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Donato, A.L.; Huang, Q.; Liu, X.; Li, F.; Zimmerman, M.A.; Li, C.-Y. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. J. Investig. Dermatol. 2014, 134, 1686–1692. [Google Scholar] [CrossRef] [PubMed]


| Tumor Suppressor Genes | ||||||
| Gene Symbol | Gene Name | Function | Expression | References | ||
| IC | SF | CM | ||||
| BRCA1 | Breast cancer gene 1 | Tumor suppressor | Up | Up | Up | [31] |
| BRCA2 | Breast cancer gene 2 | Tumor suppressor | Up | Up | Up | [31] |
| FOXA1 | Forkhead box protein A1 | Tumor suppressor | Up | Up | Up | [32] |
| GATA3 | GATA binding protein 3 | Tumor suppressor | Up | Up | Up | [32] |
| NME1 | NME/NM23 nucleoside diphosphate kinase 1 | Tumor suppressor | Up | Up | Up | [33] |
| PTEN | Phosphatase and tensin homolog | Tumor suppressor | Up | Up | Up | [34] |
| RARB | Retinoic acid receptor b | Tumor suppressor | Up | Up | Up | [35] |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | Tumor suppressor | Up | Up | NSC | [36] |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A | Tumor suppressor | Up | NSC | Up | [36] |
| CDKN1C | Cyclin-dependent kinase inhibitor 1C | Tumor suppressor | NSC | Up | Up | [36] |
| CDH13 | Cadherin 13, H-cadherin | Tumor suppressor | Up | NSC | Up | [37] |
| TP53 | Tumor protein p53 | Tumor suppressor | Up | NSC | Up | [38] |
| ESR2 | Estrogen receptor beta | Tumor suppressor | NSC | Up | NSC | [39] |
| APC | Adenomatous polyposis coli | Tumor suppressor | NSC | NSC | Up | [40] |
| Oncogenes | ||||||
| Gene Symbol | Gene Name | Function | Expression | References | ||
| IC | SF | CM | ||||
| MUC1 | Mucin 1 | Oncogene | Down | NSC | Down | [41] |
| XBP1 | X-box binding protein 1 | Oncogene | Down | NSC | NSC | [42] |
| CTSD | Cathepsin-D | Oncogene | NSC | Down | Down | [43] |
| ABCB1 | ATP-binding cassette sub-family B member 1 | Oncogene | NSC | Down | NSC | [44] |
| CCNA1 | Cyclin A1 | Oncogene | NSC | NSC | Down | [45] |
| ID1 | Inhibitor of differentiation/DNA binding 1 | Oncogene | NSC | NSC | Down | [46] |
| SERPINE1 | Plasminogen activator inhibitor-1 | Oncogene | NSC | NSC | Down | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basmaeil, Y.; Bahattab, E.; Al Subayyil, A.; Kulayb, H.B.; Alrodayyan, M.; Abumaree, M.; Khatlani, T. Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro. Cells 2021, 10, 3493. https://doi.org/10.3390/cells10123493
Basmaeil Y, Bahattab E, Al Subayyil A, Kulayb HB, Alrodayyan M, Abumaree M, Khatlani T. Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro. Cells. 2021; 10(12):3493. https://doi.org/10.3390/cells10123493
Chicago/Turabian StyleBasmaeil, Yasser, Eman Bahattab, Abdullah Al Subayyil, Haya Bin Kulayb, Maha Alrodayyan, Mohammad Abumaree, and Tanvir Khatlani. 2021. "Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro" Cells 10, no. 12: 3493. https://doi.org/10.3390/cells10123493
APA StyleBasmaeil, Y., Bahattab, E., Al Subayyil, A., Kulayb, H. B., Alrodayyan, M., Abumaree, M., & Khatlani, T. (2021). Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro. Cells, 10(12), 3493. https://doi.org/10.3390/cells10123493






